Abstract
Tetramethylenedisulfotetramine (TMDT) is a highly lethal neuroactive rodenticide responsible for many accidental and intentional poisonings in mainland China. Ease of synthesis, water solubility, potency, and difficulty to treat make TMDT a potential weapon for terrorist activity. We characterized TMDT-induced convulsions and mortality in male C57BL/6 mice. TMDT (ip) produced a continuum of twitches, clonic, and tonic-clonic seizures decreasing in onset latency and increasing in severity with increasing dose; 0.4 mg/kg was 100% lethal. The NMDA antagonist, ketamine (35 mg/kg) injected ip immediately after the first TMDT-induced seizure, did not change number of tonic-clonic seizures or lethality, but increased the number of clonic seizures. Doubling the ketamine dose decreased tonic-clonic seizures and eliminated lethality through a 60 min observation period. Treating mice with another NMDA antagonist, MK-801, 0.5 or 1 mg/kg ip, showed similar effects as low and high doses of ketamine, respectively, and prevented lethality, converting status epilepticus EEG activity to isolated interictal discharges. Treatment with these agents 15 min prior to TMDT administration did not increase their effectiveness. Post-treatment with the GABAA receptor allosteric enhancer diazepam (5 mg/kg) greatly reduced seizure manifestations and prevented lethality 60 min post-TMDT, but ictal events were evident in EEG recordings and, hours post-treatment, mice experienced status epilepticus and died. Thus, TMDT is a highly potent and lethal convulsant for which single-dose benzodiazepine treatment is inadequate in managing electrographic seizures or lethality. Repeated benzodiazepine dosing or combined application of benzodiazepines and NMDA receptor antagonists are more likely to be effective in treating TMDT poisoning.
INTRODUCTION
In recent years, there have been numerous reports from mainland China of accidental or intentional poisonings with the rodenticide tetramethylene disulfotetramine (TMDT), which has neurotoxic and convulsant properties (Whitlow et al., 2005). First patented by Farbenfabriken Bayer in 1953, TMDT found utility as a rodenticide treatment of tree seedlings used by the U.S. Department of Interior (Croddy, 2004). However, the hazards of manufacture and persistence in the environment were recognized as shortcomings to its use (Bullard, 1966; Dimock, 1957). Indeed, TMDT is included in the World Health Organization’s (WHO) list of “extremely hazardous” pesticides (Whitlow et al., 2005). Although it has been banned for over two decades, TMDT is illicitly available in China because of its effectiveness, and has been found as an active ingredient in rodenticidal products such as “Dushuqiang,” “Meishuming,” and “Shanbudao” (Whitlow, et al., 2005). The inadvertent ingestion of TMDT-containing rat poison by a toddler in New York City’s Chinatown raised awareness of the dangers of this agent domestically (2003; Barrueto et al., 2003).
Ingestion of TMDT produces nausea, vomiting, twitching and agitation, which can be followed by generalized tonic-clonic seizures. Severe poisoning leads to status epilepticus, and often death (Lu et al., 2008). Initial symptoms usually appear within 30–60 min, and death from respiratory and multi-organ failure can occur as early as 1 to 2 hours (h) thereafter (Zhang et al., 2011). Exposure may also occur through inhalation, or through wounds or lesions in the skin (Haskell and Voss, 1957). Between 1991 and 2010, there were as many as 14,000 documented cases of TMDT poisonings in China, with nearly 1,000 resulting in death (Li et al., 2012).
The convulsant actions of TMDT have been attributed to its ability to bind to GABAA receptors. Similar to picrotoxin (PTX), TMDT potently competes for the [3H]-ethynylbicyclochloroorthobenzoate (3H-EBOB) binding site within the chloride ionophore (Ratra et al., 2001), and has been shown to antagonize GABA-stimulated chloride uptake in membrane vesicles from cerebral cortex (Obata et al., 1988). A recent report has highlighted the similarities and differences in convulsant action between TMDT and picrotoxin (Zolkowska et al., 2012). Nevertheless, understanding of the mechanisms of toxicity of TMDT and its treatment is poor.
TMDT is a tasteless, odorless white powder with slight solubility in water (De Jager et al., 2009). These qualities, along with its high potency and ease of manufacture from simple starting materials (Croddy, 2004; Haskell and Voss, 1957), could make it an attractive tool for use by terrorists. Moreover, there is no uniform treatment regimen for TMDT poisoning (Flomenbaum et al., 2006) and current modalities are clearly inadequate (Lu et al., 2008; Poon et al., 2005). Accordingly, TMDT is considered a higher priority chemical threat and an agricultural chemical of concern by the National Institutes of Health (Jett and Yeung, 2010).
In this report, we characterize the TMDT syndrome, with special emphasis on its convulsant actions and related death. We further evaluate the acute and long-term alterations of EEG by TMDT and address the antidotal effects of diazepam (DZP), MK-801, and ketamine (KET) in mice. We found that all three agents are able to inhibit TMDT-induced motor seizures (convulsions), and that agents that are NMDA receptor blockers may improve long-term survival, a property not shared by DZP, a noncompetitive GABAA receptor agonist.
MATERIALS AND METHODS
Chemicals
Dimethylsulfoxide (DMSO), DZP and MK-801 were obtained from Sigma-Aldrich (St. Louis, MO), and KET hydrochloride from Tocris Bioscience (Minneapolis, MN). KET, DZP, and MK-801 were always administered in a volume of 10 ml/kg. TMDT ≥98% purity (CAS 80-12-6, MW=240.26), was kindly provided by Dr. Lowri S. deJager, Center for Food Safety and Applied Nutrition, US Food and Drug Administration. TMDT was stored in a secure location at room temperature. Multiple small aliquots (1–2 mg) were weighed out in one session to minimize hazards of the compound handling, and nitrile gloves, facemask and lab coat were worn. Stocks of TMDT were prepared in DMSO at a concentration of 10 mg/ml. Prior to use, they were diluted in normal saline for injection to final concentration 0.05 mg/ml, resulting in an 0.5% [DMSO] vehicle solution. Both stocks and injection solutions remained stable (based on activity) for 30 days when stored at 4°C.
Animals
Adult male C57BL/6 mice (25 g) were obtained from Charles River Laboratories (Wilmington, MA). Mice were kept in our AAALAC-accredited animal facilities, housed four to five per standard plexiglass cage containing bedding of aspen shavings, a cotton nestlet, and covered with a filter top. Animals were given food and water ad libitum on a regular light cycle (on at 07:00 and off at 19:00), and were allowed to acclimate at least 48 h prior to experimentation. All experiments were approved by the Institutional Animal Care and Use Committee of the New York Medical College and conformed to the Revised Guide for the Care and Use of Laboratory Animals (Committee for the Update of the Guide for the Care and Use of Laboratory Animals, 2011). All efforts were made to minimize pain and the number of animals used while maintaining sufficient statistical power.
For testing, mice were placed individually in clear plexiglass cages. Testing took place between 1100 h and 1600 h. On the day of experiment, the animals were randomly split into the testing and control groups. Power Analysis based on our previous experiments suggested that group sizes of 5–8 provide sufficient statistical power. TMDT and anticonvulsants were administered intraperitoneally (ip). Animals were observed for occurrence of motor seizures (convulsions) during the course of 1 h, starting immediately after TMDT administration. Specifically, we recorded the latency of onset and number of twitches, clonic motor seizures, and tonic-clonic motor seizures following administration of TMDT. Incidence of death, and latency to death were recorded as well. We tested anticonvulsant agents either 15 min prior to TMDT administration or immediately after the first clonic seizure began.
EEG recordings
Cortical electrodes were implanted under combined KET/xylazine anesthesia (100/7 mg/kg, ip), the accepted standard in our facilities which provides sufficient level of analgesia/anesthesia for the surgical procedure. A screw placed in the nasal bone served as the reference electrode; a second screw was positioned behind lambda to serve as ground. Four silver ball electrodes were placed epidurally in a bilateral arrangement above the frontal and occipital cortices. Electrodes and screws were covered with dental acrylic. Mice were returned to their home cages for approximately one week recovery following surgery.
Baseline EEG was recorded for 10 minutes, prior to injection of mice with 0.4 mg/kg TMDT. EEG was continuously recorded until the first clonic motor seizure occurred. Mice were then injected ip with diazepam (5 mg/kg), MK-801 (1 mg/kg), or saline vehicle, and EEG recording continued for 24 h. Simultaneous and synchronized video were also captured for evaluation of EEG and behavioral correlates using a Pinnacle Solutions, Inc. (Kansas City, MO, U.S.A.) 3-channel EEG/video system.
Statistics
Multiple groups were evaluated using ANOVA with post hoc Dunnett’s test for multiple comparisons. In drug treatments where endpoints were completely suppressed in some subjects, we used nonparametric Kaplan-Meier survival analysis with a censored variable and Mantel-Cox log rank test. Finally, for incidence comparisons, we used chi-square (multiple groups) or Fisher’s exact (two groups) tests. Level of significance was preset to p < 0.05.
RESULTS
Characterization of TMDT-induced Motor Seizures
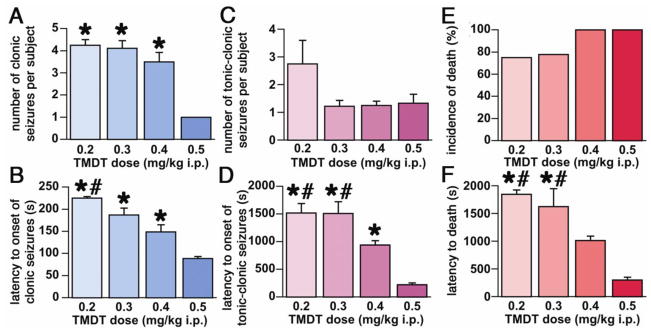
We first characterized behavioral changes and expression and progression of seizures following TMDT administration. Mice were injected ip with TMDT in doses ranging from 0.2 to 0.5 mg/kg body weight. TMDT produced a consistent pattern of behaviors similar to that reported for other antagonists of the GABAA receptor complex, such as PTX (Velíšková, 2006). First signs of convulsive activity were twitches of the body and an erect and upright tail (Straub-like tail). There was a non-significant trend towards decreased latency to the first twitch as a function of dose (0.2 mg/kg: 142.5±17 s vs 0.5 mg/kg: 87.5±2.5 s; data not shown). Multiple twitches were followed by unilateral or bilateral clonic seizures consisting of forelimb clonus with rearing while the animal was keeping righting reflex. Mice experienced one or several clonic seizures before a tonic-clonic seizure ensued. The number of clonic seizures preceding tonic-clonic seizures was also dose-dependent, indicating that, with higher TMDT doses, the more rapidly occurring tonic-clonic seizures masked the clonic seizures (Kruskal-Wallis H=8.686; tied p=0.0267; Figure 1A). Latency to the first clonic seizure displayed dose dependency with the interval for the 0.4 and 0.5 mg/kg groups being significantly shorter than the 0.2 mg/kg group (ANOVA F(3,20)=7.647; p=0.0013; Figure 1B). Tonic-clonic seizures were characterized by a sequence of wild running, loss of righting reflex, tonic flexion and extension of the limbs, and finally clonic movements of all limbs. The 0.2 mg/kg dose of TMDT was associated with repeated occurrence of tonic-clonic seizures; thus mice administered 0.2 mg/kg TMDT sustained more than twice as many tonic-clonic seizures as the other treatment groups (not significant, Kruskal-Wallis test; H=3.639; tied p=0.1969; Figure 1C). This suggests that tonic-clonic seizures produced by the higher TMDT doses studied are more lethal. Latency to the first tonic-clonic seizure mirrored the dose-dependence of twitches and clonic seizures. Tonic-clonic seizures developed more rapidly with higher doses of TMDT (ANOVA F(3,19)=8.703; p=0.0008; Figure 1D). Death rate was high after all tested doses of TMDT without any significant difference (Chi-square=3.163; p=0.3672; Figure 1E). Latency to death decreased significantly with increasing dose of TMDT, falling from 30.8 min for the 0.2 mg/kg dose to 5.1 min for 0.5 mg/kg dose (ANOVA F(3,17)=6.054; p=0.0054, Figure 1F).
Figure 1. Behavioral characterization of the TMDT-induced syndrome.
1A. Number of clonic motor seizures per mouse (mean ± S.E.M.) as a function of dose of TMDT. With the highest TMDT dose (0.5 mg/kg), tonic-clonic seizures occurred so early that they completely overlapped any additional clonic seizures. (*p<0.05 compared to 0.5 mg/kg TMDT; Kruskal-Wallis test).
1B. Dose-dependent latency to onset of clonic seizures (mean ± S.E.M.; *p<0.05 compared group 0.5 mg/kg TMDT; #p<0.05 compared to 0.4 mg/kg TMDT. ANOVA with post-hoc Fisher PLSD test).
1C. Number of tonic-clonic motor seizures per mouse (mean ± S.E.M.) as a function of TMDT dose. Tonic-clonic seizures appeared to developed more frequently after the lowest dose (0.2 mg/kg), but Kruskal-Wallis nonparametric tests did not return significance.
1D. Dose-dependent latency to onset of tonic-clonic seizures (mean ± S.E.M.). *p<0.05 compared to 0.5 mg/kg TMDT (#p<0.05 compared to 0.4 mg/kg TMDT. ANOVA with post-hoc Fisher PLSD test).
1E. Death rate (in %) after treatments with different doses of TMDT, illustrating the high lethality of TMDT even at the lowest dose tested.
1F. Dose-dependent latency of death (mean ± S.E.M.; *p<0.05 compared to 0.5 mg/kg TMDT; #p<0.05 compared to 0.4 mg/kg TMDT. ANOVA with post-hoc Fisher PLSD test).
Pharmacologic Intervention in TMDT-Induced Motor Seizures
We treated mice with agents with potential to antagonize the seizure activity associated with TMDT exposure. Agents were administered ip either as a “rescue therapy” immediately after the first episode of clonic motor seizure (post-treatment), or as a preventative, 15 min prior to TMDT treatment (pretreatment). We selected a dose of 0.4 mg/kg TMDT for testing anticonvulsant treatments because this dose caused rapid and reliable seizures as well as significant lethal outcome.
Post-treatments
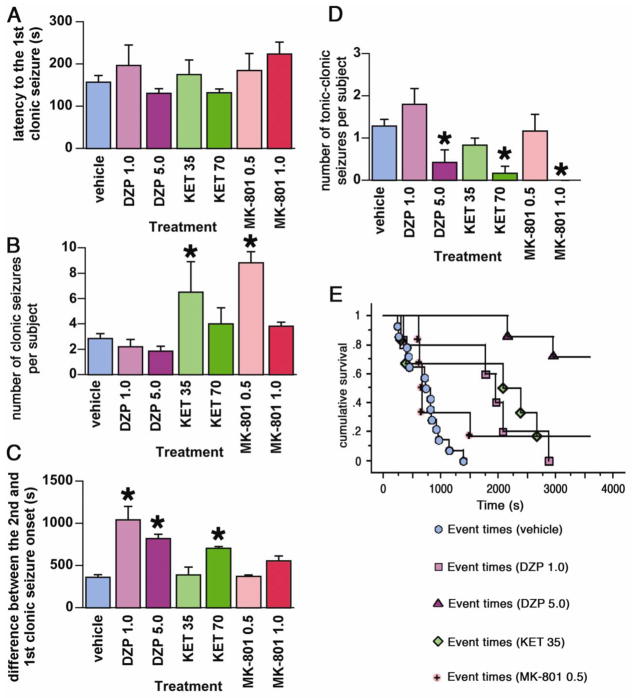
The data in Figure 2A demonstrate that the onset to the first clonic motor seizure after TMDT administration did not vary significantly between post-treatment groups, validating our ability to compare therapeutic efficacies across treatment groups.
Figure 2. Post-treatment effects of DZP, KET, and MK-801 on TMDT syndrome.
2A. Latency to onset of the first clonic seizures (mean ± S.E.M.) induced by TMDT plus post-treatment with one of the following: vehicle; DZP 1.0 or 5.0 mg/kg, KET 35 or 70 mg/kg, MK-801 0.5 or 1.0 mg/kg. All treatments were administered immediately after the first clonic seizure. There was no statistically significant difference in TMDT clonic seizure onset prior to drug post-treatment.
2B. Number of clonic seizures (mean ± S.E.M.) in mice injected with 0.4 mg/kg TMDT and one of the postreatments. KET 35 mg/kg and MK-801 0.5 mg/kg increased the number of clonic seizures (Kruskal-Wallis test; *p<0.05).
2C. Mean ± S.E.M. time difference between the onset of the first and second clonic seizure. DZP (both 1.0 and 5.0 mg/kg) and KET (70 mg/kg) significantly extended this time difference compared to vehicle-injected controls (*p<0.05; ANOVA with post hoc Dunnett’s test).
2D. Number of tonic-clonic seizures (mean ± S.E.M.) in mice injected with 0.4 mg/kg TMDT for each post-treatment. Higher doses of DZP (5.0 mg/kg), KET (70 mg/kg), and MK-801 (1.0 mg/kg) significantly decreased number of tonic-clonic seizures occurring during the observation period compared to vehicle (*p<0.05; Kruskal-Wallis test).
2E. Non-parametric survival analysis of the latency to onset of tonic-clonic seizures in all post-treatment groups in which tonic clonic seizures occurred.
Diazepam (DZP)
Treatment with 1 mg/kg DZP immediately after the first clonic seizure, significantly delayed the onset of the next clonic seizure (Figure 2C), and that of the first tonic-clonic seizure (Figure 2E), however, there was no change in the number of clonic or tonic-clonic seizures observed following this treatment dose (Figures 2B and D). While all of the vehicle-treated mice died, 40% of the DZP 1 mg/kg mice survived the 60-min observation period (Table 1). Increasing the DZP dose to 5 mg/kg induced ataxia and hypoactivity, but dramatically improved the outcome of exposure to TMDT. This dose of DZP reduced the number of TMDT-induced tonic-clonic seizures (Figure 2E, Table 1), and complete survival within the 60-min observation period was achieved (Table 1). Of the seven mice in this group, three had additional clonic seizures, and two had just a single tonic-clonic seizure after administration of 5 mg/kg DZP (Table 1).
Table 1.
Effect of anticonvulsant treatments to mice immediately following the first TMDT-induced clonic seizure on the incidence of TMDT-induced tonic-clonic seizures and lethality 60 min later
| Post-TMDT treatment group | tonic-clonic seizures | death |
|---|---|---|
|
| ||
| control | 14/14 | 14/14 |
| DZP 1 mg/kg | 5/5 | 3/5 |
| DZP 5 mg/kg | 2/7* | 0/7* |
| KET 35 mg/kg | 5/6 | 5/6 |
| KET 70 mg/kg | 1/6* | 0/6* |
| MK801 0.5 mg/kg | 5/6 | 0/6* |
| MK801 1 mg/kg | 0/6* | 0/6* |
|
| ||
| Chi-square p | p=0.0001 | p=0.0083 |
When drugs were administered immediately following to the occurrence of the first TMDT-induced clonic seizure (POST-TREATMENT), the occurrence of a clonic seizure was always a condition of treatment, therefore we evaluate here only the incidences of tonic-clonic seizures and death. Fractions (n/N) indicate the number of mice (n) presenting with the phenomenon out of total number of mice in the group (N). Asterisks denote largest cell contributions.
Ketamine (KET)
In contrast to DZP, administration of 35 mg/kg KET after the first clonic episode did not delay the onset for the subsequent clonic seizures, and actually led to a greater number of clonic seizures, compared to vehicle treatment (Figure 2B and 2D). The quality of these seizures changed as well with KET treatment, with mice displaying popcorn-like behavior, i.e., paroxysmal hindlimb seizures that propel mice into the air. While the appearance of the first tonic-clonic seizure was significantly delayed, the numbers of tonic-clonic seizures remained unchanged (Figure 2D and 2E. Likewise, post-treatment with this dose of KET did not significantly increase survival of the TMDT-exposed mice (Table 1). Unlike the 35 mg/kg group, administration of 70 mg/kg of KET did not increase the number of TMDT-induced clonic seizures relative to vehicle-treated mice, although the seizures were often associated with popcorn-like jumping behavior (Figure 2B). All mice at this dose displayed obvious signs of ataxia. Importantly, this regimen provided significant protection against the more severe actions of TMDT, dramatically reducing the number of tonic-clonic seizures and allowing for 100% survival (Figure 2 and Table 1).
MK-801
Post-treatment with 0.5 mg/kg MK-801 was generally unable to control seizures initiated by TMDT. Like mice post-treated with 35 mg/kg KET, 0.5 mg/kg of MK-801 did not delay the onset of subsequent clonic seizures, which were also associated with popcorn-like behavior and significantly increased in number (Figure 2B and 2C). Furthermore, of the mice post-treated with the 0.5 mg/kg dose, 83% experienced tonic-clonic seizures. Despite this, all survived throughout the observation period (Table 1).
In contrast to the lower dose of MK-801, 1 mg/kg MK-801 did not increase number of TMDT-induced clonic seizures compared to vehicle-injected controls (Figure 2B). Moreover, tonic-clonic seizures were eliminated in mice given this dose of MK-801, and no deaths were observed in this group (Figure 2 and Table 1).
Pretreatments
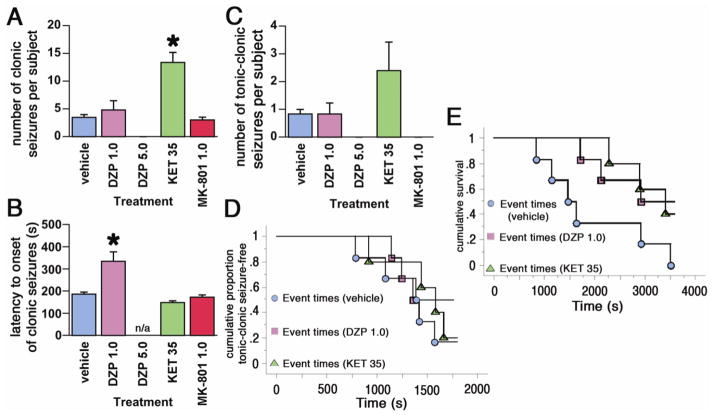
As mentioned above, separate groups of mice were administered pharmacological treatments 15 min prior to TMDT treatment (Figure 3).
Figure 3. Pre-treatment effects of DZP, KET, and MK-801 on TMDT syndrome.
3A. Number of clonic seizures (mean ± S.E.M.) in mice injected with 0.4 mg/kg TMDT and one of the following: vehicle (controls), DZP 1.0 or 5.0 mg/kg, KET 35 mg/kg or MK-801 1.0 mg/kg. Pre-treatment (15 min prior to TMDT injection) with KET increased the number of clonic seizures (*p<0.05; Kruskal-Wallis test). Treatment with 5.0 mg/kg of DZP completely blocked any motor activity, including clonic seizures.
3B. Latency to onset of clonic seizures (mean ± S.E.M.) induced by administration of TMDT 15 min after each drug pre-treatment. Only DZP 1.0 mg/kg was effective in delaying seizures (*p<0.05; ANOVA with post-hoc Dunnet’s test; n/a – not applicable, there were no seizures).
3C. Number of tonic-clonic seizures (mean ± S.E.M.) in mice injected with 0.4 mg/kg TMDT 15 minutes after each pharmacological pre-treatment. While DZP 5.0 mg/kg completely suppressed seizures and ketamine moderately increased their occurrence, Kruskal-Wallis test did not return any significant differences.
3D. Kaplan-Meier analysis of latency to onset of tonic-clonic seizures in mice injected with 0.4 mg/kg TMDT for each pharmacological pre-treatment. Neither DZP 1.0 mg/kg nor KET 35 mg/kg pre-treatment affected latency to onset of tonic-clonic seizures.
3E. Kaplan-Meier analysis of latency to death in mice injected with 0.4 mg/kg TMDT for each pharmacological pre-treatment. Neither DZP 1.0 mg/kg nor KET 35 mg/kg pre-treatment had any effect.
Diazepam (DZP)
Pretreatment with DZP 1 mg/kg ip produced a weak protective action against TMDT-induced seizures, not unlike that seen with post-treatment at this dose. No differences were recorded in the number of clonic seizures per mouse relative to the vehicle pretreated group, however, there was significant delay of onset of clonic seizures after this dose of DZP (Figure 3A and B). Similarly, there was no difference in number of tonic-clonic seizures per mouse. Three of six mice pretreated with 1 mg/kg DZP survived through the 60 min observation period (although one died at 63 min), while all six of the vehicle pretreated mice died within 1 h (Figure 3E). As expected, pretreatment with 5 mg/kg DZP markedly improved outcome of exposure to TMDT, while also inducing ataxia and hypoactivity. This DZP dose prevented development of TMDT-induced clonic and tonic-clonic seizures in all pretreated mice and none of the mice died (Figure 3 and Table 2).
Table 2.
Effect of 15 min PRETREATMENT of mice with anticonvulsant agents on the incidence of TMDT-induced tonic-clonic seizures and lethality 60 min later
| Pre-TMDT treatment group | clonic seizures | tonic-clonic seizures | death |
|---|---|---|---|
|
| |||
| control | 6/6 | 5/6 | 6/6 |
| DZP 1 mg/kg | 4/6 | 3/6 | 3/6 |
| DZP 5 mg/kg | 0/6* | 0/6* | 0/6* |
| KET 35 mg/kg | 5/5 | 4/5 | 3/5 |
| MK801 1 mg/kg | 5/5 | 0/5* | 0/5* |
|
| |||
| Chi-square p | p=0.0003 | p=0.0043 | p=0.0063 |
Fractions (n/N) indicate the number of mice (n) presenting with the phenomenon out of total number of mice in the group (N). Asterisks denote largest cell contributions.
Ketamine (KET)
A 15-minute pretreatment of mice with 35 mg/kg KET ip prior to TMDT administration increased the occurrence of seizures per mouse (Figure 3A). Numbers of both clonic, and tonic-clonic seizures were higher in the KET pretreated animals relative to vehicle control, reaching significance for clonic seizures (*p<0.05; Kruskal-Wallis test, Figure 3A and C). KET-treated animals showed obvious signs of ataxia, but were also very active, many displaying climbing and jumping behaviors, after their exposure to TMDT. Mean latency to the first tonic-clonic seizure was unchanged (Figure 3D), but lethality over the observation period was 60%, compared to 100% in the vehicle-treated group (Figure 3E and Table 2).
MK-801
Mice pretreated with 1 mg/kg MK801 exhibited no significant change in onset of clonic seizures or number of clonic seizures per mouse (Figure 3A and B). However, this treatment eliminated all tonic-clonic seizures, and resulted in 100% survival over the 1 h observation period after TMDT treatment (Figure 3E and Table 2).
Effect of TMDT Upon Cortical EEG
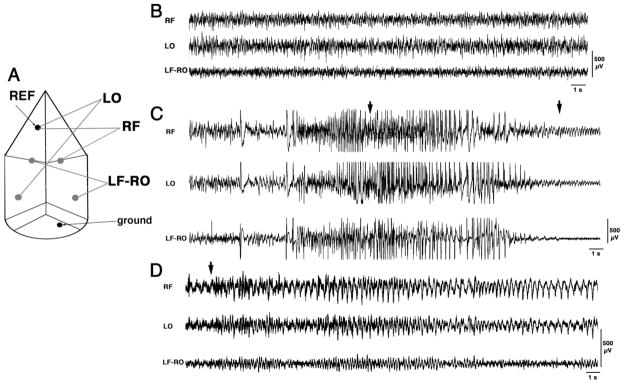
In six mice, we followed the development of the TMDT syndrome with simultaneous EEG and videorecordings after an ip dose of 0.4 mg/kg of TMDT, as in post-treatment paradigm described above (For electrode arrangement see Figure 4A). Two mice were administered saline, two were administered 5 mg/kg DZP, and two were administered 1 mg/kg MK-801 ip immediately after experiencing their first TMDT-induced clonic motor seizure. These DZP and MK-801 post-treatments were chosen because they provided the most effective reductions of both seizure manifestations and death within 60 min of TMDT treatment of those that we had tested. During baseline EEG recordings prior to TMDT injection, all channels displayed low-amplitude asynchronized fast activity corresponding to the awake state of the animals (Figure 4B). After TMDT injection, EEG activity became more synchronized and high-amplitude discharges developed within 2 minutes. Eventually, these discharges transitioned into true polyspike- and spike-and-wave rhythm. This ictal activity corresponded behaviorally to a clonic motor seizure (Figure 4C). In saline post-treated animals, the amplitude of spike-and-wave discharges later decreased, which behaviorally corresponded to a tonic-clonic seizure (Figure 4D). It should be noted that this cortical EEG ictal activity emerged several seconds after the onset of motor convulsions, possibly indicating origin of the seizures within subcortical structures that spread to cortical regions. The saline post-treated mice displayed the twitches, clonic seizures, and tonic-clonic seizures that we had observed previously. These mice died within 20 min, which was soon after a tonic-clonic seizure developed. Unlike the saline post-treated animals, mice post-treated with 5 mg/kg DZP notably displayed no subsequent behavioral signs of during the initial 60 after TMDT administration. Nevertheless, EEG ictal activity was still present. Thus, while 5 mg/kg DZP was able to entirely suppress behavioral (motor) seizures, it did not suppress ongoing TMDT-induced EEG ictal activity (EEG seizures) within the brain. However, this suppression lasted only about 2 h, after which both mice experienced repeated clonic seizures as well as several tonic-clonic seizures with corresponding EEG discharges. These mice perished as a result of status epilepticus, which occurred between 4–8 h after TMDT challenge (Figure 5A). In contrast, the two mice post-treated with 1 mg/kg MK-801, while displaying both clonic motor and EEG seizures, never exhibited tonic-clonic motor seizures. With time, the EEG seizure activity decreased and, by the post-seizure time when DZP post-treated mice were dying in status epilepticus, EEG of MK-801 post-treated mice showed only isolated interictal discharges without any behavioral correlates (Figure 5B).
Figure 4. EEG characterization of the TMDT syndrome.
4A. Schematics of the position of EEG electrodes and channel montages. LF, LO – left frontal and occipital, RF, RO right frontal and occipital. REF- reference electrode for unipolar montages. Ground electrode was positioned in the occipital bone.
4B. Typical baseline EEG prior to administration of TMDT. Three channels were recorded simultaneously. RF and LO (signals from the right frontal and left occipital cortices) were recorded in unipolar mode (each versus reference electrode; REF). The signal from left frontal (LF) and right occipital (RO) cortices was recorded as bipolar LF-RO.
4C. Typical EEG recordings during a clonic seizure. Individual high-amplitude discharges preceded the occurrence of the clonic seizure, after which multiple spikes and spike and wave complexes developed. Clonic motor seizure started at the first arrow and lasted for approximately 12 s (end marked by the second arrow).
4D. Typical EEG recordings during a tonic-clonic seizure. Please note that the activity had a lower amplitude than activity accompanying a clonic seizure in 4B. From a preceding clonic seizure, the mouse progressed directly to a tonic-clonic seizure (onset of motor seizure marked by an arrow). The arrow points to the onset of wild run lasting for less than 2 s, followed by a fall during the tonic phase of the seizure and a long clonus of all four limbs exceeding the duration of this sample recording. Except for the phase of run and tonic stretch, which were accompanied by EEG polyspikes, the usual correlate was a relatively low-amplitude spike-and-wave rhythm.
(All calibrations 500 μV, time mark 1s).
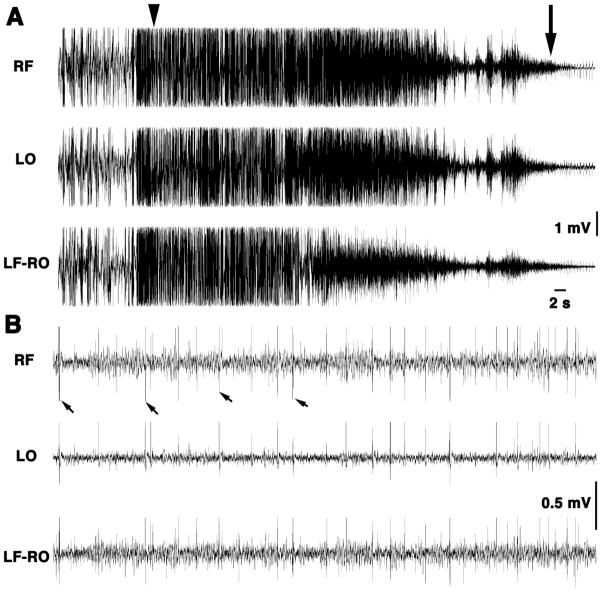
Figure 5. Long-term EEG/videorecordings reveal delayed seizures and death after TMDT injection with DZP post-treatment.
5A. This mouse was injected with 0.4 mg/kg TMDT and, with the occurrence of the first clonic seizure, received 5 mg/kg of DZP ip. Both EEG and motor activity were continuously recorded from before the TMDT injection through death approximately 9 h post-TMDT administration. EEG recordings document ictal activity of a clonic seizure transitioning into a tonic-clonic seizure (arrowhead), which ended lethally. Motor convulsions ceased to be present at the arrow. Please note high amplitude discharges associated with tonic-clonic seizure (actually preceding the seizure by several seconds). Decreasing amplitude of EEG discharges was associated with diminishing motor activity ending lethally. (All calibrations 1 mV; time mark 2 s).
5B. This mouse was also injected with 0.4 mg/kg TMDT and, with the occurrence of the first clonic seizure, received 1 mg/kg of MK-801 ip. EEG shown here was recorded at corresponding time after TMDT injection as in Figure 5A. However, the EEG of the mouse post-treated with MK-801 shows only individual discrete interictal discharges (some marked with arrows) without any behavioral correlates. The mouse survived 24 hours of recording. (All calibrations 0.5 mV; time mark 2 s).
DISCUSSION
This study characterizes, in an animal model, the extremely hazardous nature of TMDT, and also explores potential measures that may be taken to manage TMDT-induced toxicity. We find that TMDT displays significant seizure-producing activity and lethality at low doses in mice. This syndrome resembles that of other inhibitors of GABAA receptor-mediated chloride conductance, and can be reduced in severity by both GABAA agonists and NMDA receptor antagonists, with some important distinctions. These were revealed in experiments where mice were treated with these agents either 15 minutes before exposure, or immediately after the first TMDT-induced clonic seizure. Finally, we documented characteristic changes in the EEG, which correlate with behavioral manifestations of TMDT-induced seizures, and the influence of DZP or MK-801 treatment upon these sequelae. These data provide more clarity regarding the level of effectiveness of these two agents - and also the duration of action of TMDT.
Our results showing the high lethality of TMDT are consistent with previous observations (Casida et al., 1976; Voss et al., 1961), which estimated a 24-h median lethal dose of 0.21 to 0.24 mg/kg after ip administration to Swiss strain male mice. The present results are roughly in concurrence, with our lowest administered dose, 0.2 mg/kg ip, having led to a 1 h mortality of 75% in the C57BL/6 mouse strain. TMDT is thus highly toxic, and considerably more toxic than the analogous GABAA chloride channel blocker, PTX, whose convulsive dose in mice is approximately 9 mg/kg (Milbrath et al., 1979). The potency differences between these two agents were recently corroborated by Zolkowska and colleagues (Zolkowska et al., 2012), demonstrating that TMDT is approximately 40 times more potent than PTX for producing a similar onset and frequency of clonic seizures. Interestingly, in competitive radioligand binding assays against 3H-EBOB, and in 36Cl− uptake assays, these two agents were found to be roughly equipotent (Obata et al., 1988; Ratra and Casida, 2001). This disparity in the relative potencies of TMDT and PTX between in vitro membrane assays and convulsant activity in vivo could suggest different levels of receptor selectivity for the two agents in the CNS and/or a disparity in pharmacokinetics. However, the convulsant potencies of TMDT and PTX are comparable when administered intraventricularly (Zolkowska et al., 2012), which points to pharmacokinetics as a more likely explanation for differing activities after peripheral administration. We speculate that the differences are due to the more rapid detoxification and elimination of PTX compared to TMDT. Notably, blood levels of PTX fall rapidly after intravenous administration to rabbits (Das, 1939a; Das, 1939b; Duff and Dille, 1939) or rats (Soto-Otero et al., 1989), and the inhibition of drug metabolizing enzymes in mice potentiates the convulsant and lethal actions of PTX (Sasaki et al., 1982). In contrast, TMDT was found to be eliminated from mice at a rate of one-quarter of the LD50 dose daily {Voss et al., 1961}, have a half-life of 57 h after intravenous administration in rabbits, and persist in the body of poisoned victims for up to 6 months (Chau et al., 2005). TMDT appears to be a poor substrate for hepatic mixed function oxidation in mice (Cole et al., 1991). In our hands, the effects of ip TMDT were highly dose-dependent, with latency of seizure events decreasing and seizure activity increasing with increasing dose. Time to lethality fell six-fold from the lowest to the highest dose tested.
The anticonvulsant agents tested to counteract TMDT-induced seizure activity were from two major categories: GABAA receptor positive allosteric modulators, represented by DZP, and NMDA receptor antagonists, represented by MK-801 and KET. All were found to be effective at subhypnotic doses, i.e., those that did not result in loss of righting reflex. DZP delayed the onset and decreased the numbers of both clonic and tonic-clonic seizures. These effects were dependent on the dose of DZP and whether it was administered prior to or after TMDT administration. Post-treatment of mice with 5 mg/kg DZP immediately after the first TMDT-induced clonic seizure, led to a significant reduction in subsequent motor seizures, while pretreatment with the same dose eliminated all signs of seizure activity within the 1 h observation period. The results of these pretreatment experiments strongly suggest that TMDT acts via benzodiazepine-sensitive GABAA receptors, but post-treatment is less able to eliminate TMDT seizures once abnormal activity has been established. Assessment of therapeutic potential in the post-treatment paradigm is important because it more realistically reflects the scenario encountered with TMDT poisoning.
The actions of KET and MK-801 against TMDT-induced seizures showed clear distinctions from that of DZP. First, the ataxia observed was accompanied by increased motor activity, and clonic episodes were often exacerbated and prolonged relative to vehicle-treatment. The increased motor activity is not surprising, and typical of these agents (Schmidt and Kretschmer, 1997). Secondly, the continuum of behaviors from low to high doses of these two compounds went from increasing frequency of clonic seizures to a reduction in the number and severity of tonic-clonic, but not clonic, seizures. These observations are in accord with our previous reports on the effects of MK-801 and KET on PTX-induced seizures in rats (Velíšková et al., 1990; Velíšková and Velíšek, 1992). It appears that NMDA receptor antagonists raise the seizure threshold for tonic-clonic seizures and thus unmask ongoing clonic seizures (Velíšek et al., 1990; Velíšek and Mareš, 1990), while DZP has more equivalent inhibitory effects on both clonic and tonic-clonic seizures. Third, we could not detect any difference in outcome between post-treatment and pretreatment dosing regimens in regard to seizure onsets, incidences, or death with NMDA receptor antagonists. This may be because KET and MK-801 act primarily upon a component of the seizure pathway downstream of cortical GABAA receptors, where TMDT-induced seizure activity may originate. These results give us insight into how the mechanism of action of NMDA receptor antagonists differs from that of DZP.
While the above analysis suggests that GABAA positive modulators like DZP might be treatments of choice against TMDT-induced seizures, this was not borne out by our observations of cortical EEG activity. In TMDT and saline treated mice, EEG activity characteristic of clonic seizures preceded their physical manifestation, while with tonic-clonic seizures, EEG activity followed the seizure, supporting the fact that the locus of the latter seizure type is in the lower centers of the brain, rather than cortex (Browning and Nelson, 1986). Behavioral manifestations of seizures in TMDT-treated mice were eliminated by DZP 5 mg/kg, but ictal activity was readily apparent on the EEG, and these mice later died, hours after the anticonvulsant treatment. On the other hand, mice treated with MK-801 1 mg/kg after TMDT exposure, while displaying clonic motor seizures and some ictal activity on the EEG (sometimes without corresponding motor activity), survived. This coincides with earlier observations where we observed TMDT and 5 mg/kg DZP-treated mice die several hours after administration, while TMDT and MK-801-treated mice survived (unpublished observations). From these results, we conclude, firstly, that the extreme persistence of the actions of TMDT make more transient stimulation of the GABAA receptor complex insufficient to ameliorate delayed mortality. The slow elimination of TMDT from the body may allow it to persist longer than the effective window for both the anticonvulsant and antiseizure actions of DZP. However, the pharmacokinetics of DZP and MK-801 are comparable in rodents (Musteata et al., 2008; Vezzani et al., 1989; Walker et al., 1998; Wegener et al., 2011) so drug levels alone are inadequate to explain the differences we observed between these two anticonvulsant treatments. Indeed, there are several case reports indicating that benzodiazepine therapy does not adequately protect TMDT-poisoned victims (Barrueto et al., 2003; Chau et al., 2005; Li et al., 2011). A second explanation for the refractoriness of these seizures to benzodiazepines is that, once seizures develop, they recruit glutamatergic excitatory mechanisms and synaptic plasticity that take them beyond GABAA receptor control. Yen et al. (2004) suggest that NMDA receptor antagonists may be more effective than GABAergic drugs during status epilepticus due to enhanced sensitivity of NMDA receptors to glutamate and resulting synaptic plasticity. Other reports have attributed pharmacoresistance to benzodiazepines during status epilepticus to both internalization of synaptic GABAA receptors and an increase in synaptic NMDA receptors (Kramer, 2012; Wasterlain et al., 2009). Using a pilocarpine model for acquired epilepsy, Raza et al. (2004) found that MK-801 can block the triphasic (injury, epileptogenesis, and chronic) alterations in Ca++ dynamics that contribute to epileptogenesis in this model. In a report of TMDT poisoning (Chau et al., 2005), intractable status epilepticus was controlled by KET administration. KET and MK-801 are both use-dependent channel blockers that need to enter the open channel for effective blockade, and become trapped therein, resulting in activity-dependent, slowly recovering receptor inhibition (Traynelis et al., 2010). At appropriate doses, KET can also potentiate GABAergic conductance by binding receptors containing α6 and δ subunits (Hevers et al., 2008), receptors which do not bind benzodiazepines (D’Hulst et al., 2009), and which may contribute to the anticonvulsant potency of KET. Thus, NMDA receptor antagonists may offer a therapeutic modality that GABAA modulation alone cannot provide.
In conclusion, TMDT is a persistent, potent, and lethal convulsant which produces seizures akin to GABAA chloride channel antagonists such as PTX. Single dose treatment with DZP antagonized convulsive behaviors, but did not prevent brain ictal EEG activity or lethality caused by TMDT. Conversely, NMDA receptor antagonists, while not blocking the full spectrum of TMDT convulsant activity, did effectively inhibit tonic-clonic motor seizures, and may increase long-term survival. Further research is needed to determine longer-term (>24 h) effects of TMDT upon behavior and EEG, combined with assessment of treatment regimens that address both the quality and persistence of TMDT seizures and eventual lethality. NMDA receptor antagonists, used alone or in combination with GABAA positive modulators, deserve further investigation and may have a role to play in the treatment of TMDT-induced seizures.
Highlights.
TMDT produces convulsions and lethality at low doses in mice.
Diazepam pre- or post-treatments inhibit TMDT-induced convulsions & death.
Low dose ketamine & MK-801 increase TMDT clonic, but reduce tonic-clonic convulsions & death.
Diazepam stops convulsions, but ictal EEG events persist to cause lethality hrs later.
Diazepam may more effectively block TMDT via repeat dose or combined with ketamine/MK-801.
Acknowledgments
FUNDING
This work was supported by the U.S. National Institutes of Health [NS056093 to J.V., NS072966 to L.V., and NS044421 to P.K.S.]; the U.S. Department of Defense [PR100634P1 to P.K.S.]; the Migraine Research Foundation [to P.K.S.]; and the New York Medical College Intramural Grant Fund [to M.P.S.]
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Jana Velíšková, Email: jana_veliskova@nymc.edu.
Patric K. Stanton, Email: patric_stanton@nymc.edu.
Libor Velíšek, Email: libor_velisek@nymc.edu.
References
- Barrueto F, Jr, Furdyna PM, Hoffman RS, Hoffman RJ, Nelson LS. Status epilepticus from an illegally imported Chinese rodenticide: “tetramine”. J Toxicol Clin Toxicol. 2003;41:991–994. doi: 10.1081/clt-120026523. [DOI] [PubMed] [Google Scholar]
- Browning RA, Nelson DK. Modification of electroshock and pentylenetetrazol seizure patterns in rats after precollicular transections. Exp Neurol. 1986;93:546–556. doi: 10.1016/0014-4886(86)90174-3. [DOI] [PubMed] [Google Scholar]
- Bullard RW. Residue, Recovery, Determination of Translocated Tetramine in Foliage by Hydrogen-Flame Gas Chromatography. J Agric Food Chem. 1966;14:137–139. [Google Scholar]
- Casida JE, Eto M, Moscioni AD, Engel JL, Milbrath DS, Verkade JG. Structure-toxicity relationships of 2,6,7-trioxabicyclo(2.2.2)octanes and related compounds. Toxicol Appl Pharmacol. 1976;36:261–279. doi: 10.1016/0041-008x(76)90006-5. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control Prevention (CDC) Poisoning by an illegally imported Chinese rodenticide containing tetramethylenedisulfotetramine--New York City, 2002. MMWR Morb Mortal Wkly Rep. 2003;52:199–201. [PubMed] [Google Scholar]
- Chau CM, Leung AK, Tan IK. Tetramine poisoning. Hong Kong Med J. 2005;11:511–514. [PubMed] [Google Scholar]
- Cole LM, Sanders M, Palmer CJ, Casida JE. Structure-Biodegradability Relationships of Insecticidal 1,4-Disubstituted-2,6,7-trioxabicyclo2[. 2.2]octanes. J Ag Food Chem. 1991;39:560–565. [Google Scholar]
- Croddy E. Rat poison and food security in the People’s Republic of China: focus on tetramethylene disulfotetramine (tetramine) Arch Toxicol. 2004;78:1–6. doi: 10.1007/s00204-003-0509-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Hulst C, Atack JR, Kooy RF. The complexity of the GABAA receptor shapes unique pharmacological profiles. Drug Discov Today. 2009;14:866–875. doi: 10.1016/j.drudis.2009.06.009. [DOI] [PubMed] [Google Scholar]
- Das SC. Antagonism of Evipan by Picrotoxin, Coramine, and Cardiazol. Exp Physiol. 1939;29:355–365. [Google Scholar]
- De Jager LS, Perfetti GA, Diachenko GW. Comparison of membrane assisted solvent extraction, stir bar sorptive extraction, and solid phase microextraction in analysis of tetramine in food. J Sep Sci. 2009;32:1081–1086. doi: 10.1002/jssc.200800576. [DOI] [PubMed] [Google Scholar]
- Dimock EJ. A comparison of two rodent repellents in broadcast seeding Douglas-fir. USDA Forest Service Old Series Research Notes (No 20) 1957:1–17. [Google Scholar]
- Duff DM, Dille JM. Distribution and Rate of Elimination of Picrotoxin. J Pharmacol Exp Ther. 1939;67:353–357. [Google Scholar]
- Flomenbaum N, Goldfrank L, Hoffman R, Howland M, Lewin N, Nelson L. Goldfrank’s Toxicologic Emergencies. 8. McGraw-Hill; New York: 2006. [Google Scholar]
- Haskell AR, Voss E. The pharmacology of tetramine (tetraethylenedisulfotetramine) J Am Pharm Assoc Am Pharm Assoc (Baltim) 1957;46:239–242. doi: 10.1002/jps.3030460412. [DOI] [PubMed] [Google Scholar]
- Hevers W, Hadley SH, Luddens H, Amin J. Ketamine, but not phencyclidine, selectively modulates cerebellar GABA(A) receptors containing alpha6 and delta subunits. J Neurosci. 2008;28:5383–5393. doi: 10.1523/JNEUROSCI.5443-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jett DA, Yeung DT. The CounterACT Research Network: basic mechanisms and practical applications. Proc Am Thorac Soc. 2010;7:254–256. doi: 10.1513/pats.201001-003SM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer AH. Early ketamine to treat refractory status epilepticus. Neurocrit Care. 2012;16:299–305. doi: 10.1007/s12028-011-9668-7. [DOI] [PubMed] [Google Scholar]
- Li JM, Gan J, Zeng TF, Sander JW, Zhou D. Tetramethylenedisulfotetramine intoxication presenting with de novo Status Epilepticus: A case series. NeuroToxicology. 2012;33:207–211. doi: 10.1016/j.neuro.2011.10.008. [DOI] [PubMed] [Google Scholar]
- Lu Y, Wang X, Yan Y, Xiao Z, Stephani U. Nongenetic cause of epileptic seizures in 2 otherwise healthy Chinese families: tetramine--case presentation and literature survey. Clin Neuropharmacol. 2008;31:57–61. doi: 10.1097/WNF.0b013e3180d09983. [DOI] [PubMed] [Google Scholar]
- Milbrath DS, Engel JL, Verkade JG, Casida JE. Structure--toxicity relationships of 1-substituted-4-alkyl-2,6,7-trioxabicyclo[2.2.2. ]octanes. Toxicol Appl Pharmacol. 1979;47:287–293. doi: 10.1016/0041-008x(79)90323-5. [DOI] [PubMed] [Google Scholar]
- Musteata FM, de Lannoy I, Gien B, Pawliszyn J. Blood sampling without blood draws for in vivo pharmacokinetic studies in rats. J Pharm Biomed Anal. 2008;47:907–912. doi: 10.1016/j.jpba.2008.03.028. [DOI] [PubMed] [Google Scholar]
- Obata T, Yamamura HI, Malatynska E, Ikeda M, Laird H, Palmer CJ, Casida JE. Modulation of gamma-aminobutyric acid-stimulated chloride influx by bicycloorthocarboxylates, bicyclophosphorus esters, polychlorocycloalkanes and other cage convulsants. J Pharmacol Exp Ther. 1988;244:802–806. [PubMed] [Google Scholar]
- Poon WT, Chan K, Lo MH, Yip KK, Lee T, Chan AY. A case of tetramine poisoning: a lethal rodenticide. Hong Kong Med J. 2005;11:507–509. [PubMed] [Google Scholar]
- Ratra GS, Kamita SG, Casida JE. Role of human GABA(A) receptor beta3 subunit in insecticide toxicity. Toxicol Appl Pharmacol. 2001;172:233–240. doi: 10.1006/taap.2001.9154. [DOI] [PubMed] [Google Scholar]
- Raza M, Blair RE, Sombati S, Carter DS, Deshpande LS, DeLorenzo RJ. Evidence that injury-induced changes in hippocampal neuronal calcium dynamics during epileptogenesis cause acquired epilepsy. Proc Natl Acad Sci U S A. 2004;101:17522–17527. doi: 10.1073/pnas.0408155101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki K, Furusawa S, Takayanagi G. Effect of doxapram on the action of other drugs and the hepatic drug-metabolizing system in mice. Jpn J Pharmacol. 1982;32:699–707. doi: 10.1254/jjp.32.699. [DOI] [PubMed] [Google Scholar]
- Schmidt WJ, Kretschmer BD. Behavioural pharmacology of glutamate receptors in the basal ganglia. Neurosci Biobehav Rev. 1997;21:381–92. doi: 10.1016/s0149-7634(96)00044-9. [DOI] [PubMed] [Google Scholar]
- Soto-Otero R, Mendez-Alvarez E, Sierra-Paredes G, Galan-Valiente J, Aguilar-Veiga E, Sierra-Marcuno G. Simultaneous determination of the two components of picrotoxin in serum by reversed-phase high-performance liquid chromatography with application to a pharmacokinetic study in rats. J Pharm Biomed Anal. 1989;7:369–375. doi: 10.1016/0731-7085(89)80104-9. [DOI] [PubMed] [Google Scholar]
- Traynelis SF, Wollmuth LP, McBain CJ, Menniti FS, Vance KM, Ogden KK, Hansen KB, Yuan H, Myers SJ, Dingledine R. Glutamate receptor ion channels: structure, regulation, and function. Pharmacol Rev. 2010;62:405–496. doi: 10.1124/pr.109.002451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velíšek L, Kusá R, Kulovaná M, Mareš P. Excitatory amino acid antagonists and pentylenetetrazol-induced seizures during ontogenesis: I. The effects of 2-amino-7-phosphonoheptanoate. Life Sci. 1990;46:1349–1357. doi: 10.1016/0024-3205(90)90334-n. [DOI] [PubMed] [Google Scholar]
- Velíšek L, Mareš P. Anticonvulsant action of ketamine in laboratory animals. In: Domino EF, editor. Status of Ketamine in Anesthesiology. NPP Books; Ann Arbor, MI: 1990. pp. 541–549. [Google Scholar]
- Velíšková J. Behavioral Characterization of Seizures in Rats. In: Pitkanen A, Schwartzkroin PA, Moshe SL, editors. Models of Seizures and Epilepsy. Elsevier Press; Burlington VT: 2006. pp. 601–611. [Google Scholar]
- Velíšková J, Velíšek L, Mares P, Rokyta R. Ketamine suppresses both bicuculline- and picrotoxin-induced generalized tonic-clonic seizures during ontogenesis. Pharmacol Biochem Behav. 1990;37:667–674. doi: 10.1016/0091-3057(90)90544-r. [DOI] [PubMed] [Google Scholar]
- Velíšková J, Velíšek LS. Picrotoxin-induced tonic-clonic seizures and lethality are decreased by MK-801 in developing rats. Pharmacol Biochem Behav. 1992;43:291–295. doi: 10.1016/0091-3057(92)90670-b. [DOI] [PubMed] [Google Scholar]
- Vezzani A, Serafini R, Stasi MA, Caccia S, Conti I, Tridico RV, Samanin R. Kinetics of MK-801 and its effect on quinolinic acid-induced seizures and neurotoxicity in rats. J Pharmacol Exp Ther. 1989;249:278–283. [PubMed] [Google Scholar]
- Voss E, Haskell AR, Gartenberg L. Reduction of tetramine toxicity by sedatives and anticonvulsants. J Pharm Sci. 1961;50:858–860. doi: 10.1002/jps.2600501014. [DOI] [PubMed] [Google Scholar]
- Walker MC, Tong X, Brown S, Shorvon SD, Patsalos PN. Comparison of single- and repeated-dose pharmacokinetics of diazepam. Epilepsia. 1998;39:283–289. doi: 10.1111/j.1528-1157.1998.tb01374.x. [DOI] [PubMed] [Google Scholar]
- Wasterlain CG, Liu H, Naylor DE, Thompson KW, Suchomelova L, Niquet J, Mazarati AM, Baldwin RA. Molecular basis of self-sustaining seizures and pharmacoresistance during status epilepticus: The receptor trafficking hypothesis revisited. Epilepsia. 2009;50(Suppl 12):16–8. doi: 10.1111/j.1528-1167.2009.02375.x. [DOI] [PubMed] [Google Scholar]
- Wegener N, Nagel J, Gross R, Chambon C, Greco S, Pietraszek M, Gravius A, Danysz W. Evaluation of brain pharmacokinetics of (+)MK-801 in relation to behaviour. Neurosci Lett. 2011;503:68–72. doi: 10.1016/j.neulet.2011.08.012. [DOI] [PubMed] [Google Scholar]
- Whitlow KS, Belson M, Barrueto F, Nelson L, Henderson AK. Tetramethylenedisulfotetramine: old agent and new terror. Ann Emerg Med. 2005;45:609–13. doi: 10.1016/j.annemergmed.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Yen W, Williamson J, Bertram EH, Kapur J. A comparison of three NMDA receptor antagonists in the treatment of prolonged status epilepticus. Epilepsy Res. 2004;59:43–50. doi: 10.1016/j.eplepsyres.2004.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Su M, Tian DP. Tetramine poisoning: A case report and review of the literature. Forensic Sci Int. 2011;204:e24–7. doi: 10.1016/j.forsciint.2010.06.025. [DOI] [PubMed] [Google Scholar]
- Zolkowska D, Banks CN, Dhir A, Inceoglu B, Sanborn JR, McCoy MR, Bruun DA, Hammock BD, Lein PJ, Rogawski MA. Characterization of Seizures Induced by Acute and Repeated Exposure to Tetramethylenedisulfotetramine. J Pharmacol Exp Ther. 2012;341:435–46. doi: 10.1124/jpet.111.190579. [DOI] [PMC free article] [PubMed] [Google Scholar]