Abstract
AIM: To determine whether bispectral index (BIS) monitoring is useful for propofol administration for deep sedation during endoscopic retrograde cholangiopancreatography (ERCP).
METHODS: Fifty-nine consecutive patients with a variety of reasons for ERCP who underwent the procedure at least twice between 1 July 2010 and 30 November 2010. This was a randomized cross-over study, in which each patient underwent ERCP twice, once with BIS monitoring and once with control monitoring. Whether BIS monitoring was done during the first or second ERCP procedure was random. Patients were intermittently administered a mixed regimen including midazolam, pethidine, and propofol by trained nurses. The nurse used a routine practice to monitor sedation using the Modified Observer’s Assessment of Alertness/Sedation (MOAA/S) scale or the BIS monitoring. The total amount of midazolam and propofol used and serious side effects were compared between the BIS and control groups.
RESULTS: The mean total propofol dose administered was 53.1 ± 32.2 mg in the BIS group and 54.9 ± 30.8 mg in the control group (P = 0.673). The individual propofol dose received per minute during the ERCP procedure was 2.90 ± 1.83 mg/min in the BIS group and 3.44 ± 2.04 mg in the control group (P = 0.103). The median value of the MOAA/S score during the maintenance phase of sedation was comparable for the two groups. The mean BIS values throughout the procedure (from insertion to removal of the endoscope) were 76.5 ± 8.7 for all 59 patients in using the BIS monitor. No significant differences in the frequency of < 80% oxygen saturation, hypotension (< 80 mmHg), or bradycardia (< 50 beats/min) were observed between the two study groups. Four cases of poor cooperation occurred, in which the procedure should be stopped to add the propofol dose. After adding the propofol, the procedure could be conducted successfully (one case in the BIS group, three cases in the control group). The endoscopist rated patient sedation as excellent for all patients in both groups. All patients in both groups rated their level of satisfaction as high (no discomfort). During the post-procedural follow-up in the recovery area, no cases of clinically significant hypoxic episodes were recorded in either group. No other postoperative side effects related to sedation were observed in either group.
CONCLUSION: BIS monitoring trend to slighlty reduce the mean propofol dose. Nurse-administered propofol sedation under the supervision of a gastroenterologist may be considered an alternative under anesthesiologist.
Keywords: Conscious sedation, Bispectral index monitors, Pancreatic neoplasm, Endoscopic retrograde cholangiopancreatography
INTRODUCTION
Electroencephalography (EEG)-guided sedation has been used by anesthesiologists to achieve optimal titration of sedatives[1]. Bispectral index (BIS) monitoring is an EEG-based method that quantifies the depth of anesthesia by analyzing the EEG and uses a complex algorithm to generate an index score, providing an objective measurement of the level of consciousness in sedated patients[1]. The value of BIS monitoring as an adjunct to endoscopic sedation has been tested in a limited number of endoscopic studies[2-6]. The EEG-guided method was originally evaluated for facilitation of sedation in endoscopic retrograde cholangiopancreatography (ERCP)[7-10].
Propofol can rapidly and easily induce deep sedation, and the depth of sedation can be adjusted[11]. The Division of Drug Risk Evaluation in the United States has stated that “doses of propofol should be kept as low as effectively possible and patients who are sedated with propofol should be monitored properly”[12]. A combination of propofol and midazolam significantly reduces the total propofol amount required and consequently reduces the risk of apnea; however, recovery time is prolonged as compared with propofol alone[8]. According to Athens international statements[12] and the American Society of Anesthesiologists Physical Status Classification (ASA grade), patients of classes I, II and often III can be safely sedated to the level of conscious sedation by nurses qualified in cardiopulmonary resuscitation for esophagogastroduo-denoscopy and colonoscopy, but there are no data for sedation by nurses during ERCP.
In this study, we hypothesized that if we employed BIS monitoring to achieve the desired level of deep sedation using the minimal doses of propofol, then the risk of respiratory depression would be reduced. In particular, a combination of propofol and midazolam, administered by trained nurses with BIS monitoring, was used to evaluate the usefulness of this sedation method during ERCP.
We determined whether BIS monitoring is a useful adjunct technique for the administration of propofol titrated for deep sedation, as measured by differences in the dose of propofol administered during ERCP.
MATERIALS AND METHODS
This study was approved by the hospital ethics committee, and informed consent was obtained from participating patients. Fifty-nine consecutive patients with a variety of reasons for ERCP and who underwent the procedure at least twice between 1 July 2010 and 30 Nov 2010 were prospectively included in this study. Associated medical illnesses were graded according to ASA grade[13]. Exclusion criteria were age < 20 years, critical illness (ASA grade IV or V), pregnancy, chronic use of benzodiazepines or opiates, and history of allergy to eggs. No patient was excluded after randomization.
All procedures were therapeutic and were performed by one gastroenterologist (Jung MK, who has performed > 500 ERCP procedures per annum for 5 years). Trained three nurses administered the sedative agents and carried out the anesthetic protocol, which was generated by an anesthesiologist (Jeon YH, who has performed > 1000 general anesthesia procedures per annum for 15 years). This was a randomized cross-over study, in which each patient underwent ERCP twice, once with BIS monitoring and once with control monitoring. Whether BIS monitoring was done during the first or second ERCP procedure was random (Figure 1). The endoscopist, trained nurse, radiologist, and assistant nurse had access to the randomization scheme when the patient was admitted to the endoscopy suite. The independent research fellow (Park HG) who performed all pre- and post-procedural assessments was blinded to the randomization scheme.
Figure 1.
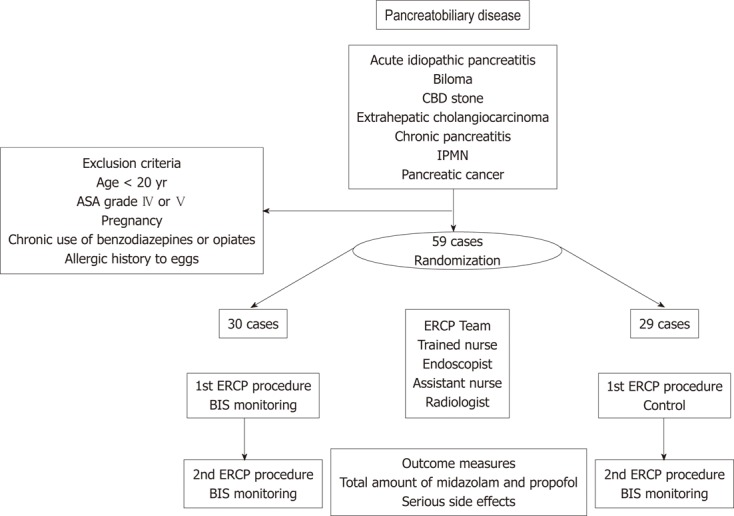
Flow chart of the enrolled patients. ASA grade: American Society of Anesthesiologists Physical Status Classification; BIS: Bispectral index; CBD: Common bile duct; ERCP: Endoscopic retrograde cholangiopancreatography; IPMN: Intraductal papillary mucinous neoplasm.
The sedation end point was conscious sedation[14]. Patient received the fixed dose midazolam, propofol and pethidine before the procedure. Trained nurse reassessed the sedation grade using BIS monitor or the modified Observer’s Assessment of Alertness/Sedation (MOAA/S) scale every minute[3]. Patients were intermittently administered a mixed regimen including midazolam, pethidine, and propofol by trained nurse. The end point of sedation was a MOAA/S score of 1. For the BIS monitoring group, the nurse was instructed to use BIS as the primary end point for titration of propofol and to target the BIS value to between 65 and 80. Patients were moved to the recovery area immediately after the procedure if their vital signs were stable. All patients were routinely kept in the hospital for at least 24 h after the ERCP procedure. If patients awoke or were hyperactive during sedation, this was considered a significant adverse event. And the procedure should be stopped to add the propofol dose. After adding the propofol, patient could be controlled. the procedure could be conducted successfully.
BIS monitoring
All patients were continuously monitored for heart rate, oxygen saturation (pulse oximetry), and blood pressure (automated blood pressure cuff, serial measurements every 3 min).
Baseline vital signs were recorded immediately before the procedure. All patients were given supplemental intranasal oxygen (5 L/min). Respiratory depression was considered significant when oxygen saturation was < 80% for > 15 s with oxygen supplementation. A drop in systolic arterial blood pressure below 80 mmHg or a heart rate below 50 beats/min was considered a significant adverse event. All patients were monitored in the recovery area by electrocardiography, pulse oximetry, and blood pressure recording. All patients were monitored for BIS scores using the BIS VIEW device (BIS Monitoring System) and a specific BIS Quatro Sensor (Aspect Medical System, Newton, MA, United States). During the procedure, BIS scores were monitored every 3 min.
Statistical analysis
The total amount of midazolam, propofol used and serious side effects between the BIS and control groups were compared. Continuous data were compared using the paired Student’s t-test. Categorical variables were tested using the χ2 test. The criterion for statistical significance was P < 0.05. SPSS Version 15 (SPSS, Chicago, IL, United States) was used for analyses. Data are presented as the mean ± SD.
RESULTS
Baseline characteristics of the 59 patients are presented in Table 1. The mean total propofol dose administered was 53.1 ± 32.2 mg in the BIS group and 54.9 ± 30.8 mg in the control group (P = 0.673; Table 2). The individual propofol dose administered per minute during the ERCP procedure was 2.90 ± 1.83 mg/min in the BIS group and 3.44 ± 2.04 mg/min in the control group (P = 0.103). The median value of the MOAA/S score during the maintenance phase of sedation was comparable between the two groups. The mean BIS values throughout the procedure (from insertion to removal of the endoscope) were 76.5 ± 8.7.
Table 1.
Baseline characteristics of patients n (%)
| Characteristics | Value |
| Gender | |
| Male | 36 (61) |
| Female | 23 (39) |
| Etiology | |
| Acute idiopathic pancreatitis | 3 (5.1) |
| Biloma | 1 (1.7) |
| Common bile duct stone | 34 (57.6) |
| Cholangiocarcinoma | 10 (16.9) |
| Chronic pancreatitis | 4 (6.8) |
| Intraductal papillary mucinous neoplasm | 2 (3.4) |
| Pancreatic cancer | 5 (8.5) |
Table 2.
Comparative results of paired examinations
| BIS | Control | P value | |
| Midazolam (mg) | 1.64 ± 0.87 | 1.61 ± 0.70 | 0.788 |
| Propofol (mg) | 53.1 ± 32.2 | 54.9 ± 30.8 | 0.673 |
| Procedure duration (min) | 21.0 ± 10.5 | 18.6 ± 9.6 | 0.187 |
| Propofol dose/min (mg/min) | 2.90 ± 1.83 | 3.44 ± 2.04 | 0.103 |
| Procedure failed cases | 1 | 3 | 0.309 |
| Poor cooperation | 2 | 2 | |
| Mean BIS score | 76.5 ± 8.7 | NA |
NA: Not available; BIS: Bispectral index.
No significant differences in the frequency of < 80% oxygen saturation, hypotension (< 80 mmHg), or bradycardia (< 50 beats/min) were observed between the two study groups. Four cases of poor cooperation occurred, in which the procedure should be stopped to add the propofol dose. After adding the propofol, the procedure could be conducted successfully (one case in the BIS group, three cases in the control group).
The endoscopist rated the patient sedation as excellent for all the patients in both groups. All patients in both groups rated their level of satisfaction as high (no discomfort). During the post-procedural follow-up in the recovery area, no cases of clinically significant hypoxic episodes were recorded in either group. No other postoperative side effects related to sedation were observed in either group.
DISCUSSION
Sedation and analgesia reduce pain, discomfort, and stress in patients undergoing unpleasant and prolonged procedures such as ERCP and contribute to better patient tolerance and compliance[15]. Moreover, sedation and analgesia reduce the danger of injuries during ERCP due to a lack of cooperation by the patient and facilitate the endoscopist’s task[16]. Since the first report of endoscopic cannulation of major papilla in 1968[17], ERCP has evolved from being a simple diagnostic procedure to becoming a therapeutic procedure with increased duration and complexity, requiring a high degree of patient cooperation. Reports have indicated that complications such as duodenal perforation and pancreatitis result as a consequence of poor patient cooperation manifested by restlessness and anxiety during the procedure[18]. Successful ERCP procedures have been performed with the patient either moderately or deeply sedated or under general anesthesia. Patel et al[19] reported that even when the target level of sedation was moderate, deep sedation episodes of all sedation occurred in 35% of ERCP patients. ERCP is thus recognized as an independent risk factor of deep sedation.
Propofol is a lipophilic anesthetic agent with rapid distribution and elimination times, and it does not have a cumulative effect after infusion. Its therapeutic spectrum is much narrower than that of midazolam, so careful monitoring is much more demanding to differentiate between moderate and deep sedation and general anesthesia. Propofol has been evaluated in a variety of regimens in ERCP and has been shown to provide the same or superior sedation quality as midazolam with the advantage of better patient cooperation and shorter recovery time[20-24]. One large multicenter study from North America demonstrated that the leading cause of death from ERCP was cardiopulmonary complications[25]. In a large audit of upper endoscopy procedures from the United Kingdom, cardiopulmonary complications resulted in mortality in one in 2000 procedures[26]. Sedation-related complications were attributed to high doses of sedatives and inadequate monitoring. In a retrospective analysis, Sharma et al[27] showed that the incidence of cardiopulmonary complications in ERCP (2.1%) was almost double that during colonoscopy (1.1%) and more than triple that during upper endoscopy (0.6%).
The computer-generated BIS ranges from 0 (coma) to 100 (fully awake) and reflects the level of sedation regardless of a patient’s clinical characteristics and the type of sedative drug used. The main aim of this study was to determine whether BIS monitoring could be a useful adjunct technique to the administration of propofol that was titrated to achieve conscious sedation, as measured by a difference in the propofol dose administered during ERCP. The primary outcome observed was a slightly lower mean dose of propofol in the group of patients who were deeply sedated with the use of BIS monitoring.
As the primary end point, we chose the amount of propofol given, because we believe that this will be of significant interest for endoscopists. More specifically, our guiding hypothesis was that if we achieved the desired level of sedation using a minimal dose of propofol with BIS monitoring, then the risk of respiratory depression would be reduced.
The method of sedation during ERCP procedures involves a formulation of benzodiazepines in combination with opioids and propofol. The level of sedation is a continuum, and deep sedation is logically associated with an increased frequency of inadequate ventilation or airway obstruction[28].
The Practice Committee of the American Society for Gastrointestinal Endoscopy has stated that “the use of EEG monitoring may have a role in the future for the delivery of sedation during selected endoscopic procedures”[29]. EEG monitoring, which is a more complex technique than BIS monitoring with respect to interpretation, enables more effective titration of the propofol dose for sedation during ERCP[10]. More specifically, Wehrmann et al[10] found that the mean propofol dose was significantly lower in the group of patients sedated with EEG-guided monitoring as compared with that of the control group. The results from a previous colonoscopy study suggested that BIS monitoring may be useful in preventing over-sedation as well as in reducing the propofol dose during the maintenance phase of the sedation[2]. A randomized controlled trial published 2 years later by the same group did not confirm these suggestions[3]. This may be explained by the notably shorter time required for a colonoscopy and, in particular, the especially shorter maintenance phase during a colonoscopy, during which over-sedation is less likely to occur. BIS monitoring may be valuable during ERCP during which patients are sedated with the conventional regimen of benzodiazepines plus opioids[7]. More specifically, in that study the total dose of midazolam was significantly lower in the BIS group as compared with the control group[7]. In a recent study, BIS monitoring during propofol sedation during endoscopic submucosal dissection did not lead to a reduction in the dose of propofol required but did lead to higher satisfaction scores from the patients and endoscopists[30]. In this study, BIS monitoring during midazolam and propofol sedation during ERCP was safe and effective when sedatives were intermittently infused by well-trained nurses.
The role of BIS monitoring for conscious sedation targeted to the moderate level has not been established[4]. BIS monitoring is less accurate for detecting deep sedation episodes during endoscopy where a particular level of conscious sedation is desired[4,19]. In contrast, a meta-analysis of ambulatory surgery studies showed that the use of BIS monitoring significantly reduces the amount of anesthetic administered by 19%[31]. Furthermore, titrating propofol with BIS monitoring during balanced anesthesia can decrease the amount of propofol required[32]. Moreover, BIS monitoring may prevent awareness during general anesthesia[33], although conflicting data have been reported[34].
There are some limitations to our study. First, the nurse was not blinded to the absence or presence of BIS monitoring during ERCP. It was impossible for this study to be scheduled in a blinded fashion. Second, it is well established that BIS values lag behind actual sedation scores during induction of sedation as well as during recovery[2]. However, it is noteworthy that all patients in the BIS group were deeply sedated during the maintenance phase (BIS score of 65-80).
In conclusion, BIS monitoring trend to slightly reduce the mean propofol dose, when the BIS index is used as the primary target for sedation during ERCP procedures. Approaches such as nurse-administered propofol sedation under the supervision of a gastroenterologist may be considered an alternative under the anesthesiologist.
COMMENTS
Background
Electroencephalography (EEG)-guided sedation has been used by anesthesiologists to achieve optimal titration of sedatives. Bispectral index (BIS) monitoring is an EEG-based method that quantifies the depth of anesthesia by analyzing the EEG and uses a complex algorithm to generate an index score, providing an objective measurement of the level of consciousness in sedated patients.
Research frontiers
Successful endoscopic retrograde cholangiopancreatography (ERCP) procedures have been performed with the patient either moderately or deeply sedated or under general anesthesia. Even when the target level of sedation was moderate, deep sedation episodes of all sedation occurred in 35% of ERCP patients. ERCP is thus recognized as an independent risk factor of deep sedation.
Innovations and breakthroughs
BIS monitoring trend to slightly reduce the mean propofol dose. Nurse-administered propofol sedation under the supervision of a gastroenterologist may be considered an alternative under anesthesiologist.
Peer review
The authors studied the usefulness of BIS moniter in the sedation of ERCP. The findings reported are of clinical importance.
Footnotes
Peer reviewer: Takahiro Nakazawa, MD, Department of Gastroenterology and Metabolism, Nagoya City University Graduate School of Medical Sciences, 1 Kawasumi, Mizuho-cho, Mizuho-ku, Nagoya 467-8601, Japan
S- Editor Gou SX L- Editor A E- Editor Xiong L
References
- 1.Soehle M, Ellerkmann RK, Grube M, Kuech M, Wirz S, Hoeft A, Bruhn J. Comparison between bispectral index and patient state index as measures of the electroencephalographic effects of sevoflurane. Anesthesiology. 2008;109:799–805. doi: 10.1097/ALN.0b013e3181895fd0. [DOI] [PubMed] [Google Scholar]
- 2.Chen SC, Rex DK. An initial investigation of bispectral monitoring as an adjunct to nurse-administered propofol sedation for colonoscopy. Am J Gastroenterol. 2004;99:1081–1086. doi: 10.1111/j.1572-0241.2004.03279.x. [DOI] [PubMed] [Google Scholar]
- 3.Drake LM, Chen SC, Rex DK. Efficacy of bispectral monitoring as an adjunct to nurse-administered propofol sedation for colonoscopy: a randomized controlled trial. Am J Gastroenterol. 2006;101:2003–2007. doi: 10.1111/j.1572-0241.2006.00806.x. [DOI] [PubMed] [Google Scholar]
- 4.Qadeer MA, Vargo JJ, Patel S, Dumot JA, Lopez AR, Trolli PA, Conwell DL, Stevens T, Zuccaro G. Bispectral index monitoring of conscious sedation with the combination of meperidine and midazolam during endoscopy. Clin Gastroenterol Hepatol. 2008;6:102–108. doi: 10.1016/j.cgh.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 5.Cohen LB. Patient monitoring during gastrointestinal endoscopy: why, when, and how? Gastrointest Endosc Clin N Am. 2008;18:651–653, vii. doi: 10.1016/j.giec.2008.06.015. [DOI] [PubMed] [Google Scholar]
- 6.Bower AL, Ripepi A, Dilger J, Boparai N, Brody FJ, Ponsky JL. Bispectral index monitoring of sedation during endoscopy. Gastrointest Endosc. 2000;52:192–196. doi: 10.1067/mge.2000.107284. [DOI] [PubMed] [Google Scholar]
- 7.Al-Sammak Z, Al-Falaki MM, Gamal HM. Predictor of sedation during endoscopic retrograde cholangiopancreatography--bispectral index vs clinical assessment. Middle East J Anesthesiol. 2005;18:141–148. [PubMed] [Google Scholar]
- 8.Paspatis GA, Chainaki I, Manolaraki MM, Vardas E, Theodoropoulou A, Tribonias G, Konstantinidis K, Karmiris K, Chlouverakis G. Efficacy of bispectral index monitoring as an adjunct to propofol deep sedation for ERCP: a randomized controlled trial. Endoscopy. 2009;41:1046–1051. doi: 10.1055/s-0029-1215342. [DOI] [PubMed] [Google Scholar]
- 9.Krugliak P, Ziff B, Rusabrov Y, Rosenthal A, Fich A, Gurman GM. Propofol versus midazolam for conscious sedation guided by processed EEG during endoscopic retrograde cholangiopancreatography: a prospective, randomized, double-blind study. Endoscopy. 2000;32:677–682. doi: 10.1055/s-2000-9021. [DOI] [PubMed] [Google Scholar]
- 10.Wehrmann T, Grotkamp J, Stergiou N, Riphaus A, Kluge A, Lembcke B, Schultz A. Electroencephalogram monitoring facilitates sedation with propofol for routine ERCP: a randomized, controlled trial. Gastrointest Endosc. 2002;56:817–824. doi: 10.1067/mge.2002.129603. [DOI] [PubMed] [Google Scholar]
- 11.Wysowski DK, Pollock ML. Reports of death with use of propofol (Diprivan) for nonprocedural (long-term) sedation and literature review. Anesthesiology. 2006;105:1047–1051. doi: 10.1097/00000542-200611000-00027. [DOI] [PubMed] [Google Scholar]
- 12.Cohen LB, Ladas SD, Vargo JJ, Paspatis GA, Bjorkman DJ, Van der Linden P, Axon AT, Axon AE, Bamias G, Despott E, et al. Sedation in digestive endoscopy: the Athens international position statements. Aliment Pharmacol Ther. 2010;32:425–442. doi: 10.1111/j.1365-2036.2010.04352.x. [DOI] [PubMed] [Google Scholar]
- 13.Keats AS. The ASA classification of physical status--a recapitulation. Anesthesiology. 1978;49:233–236. doi: 10.1097/00000542-197810000-00001. [DOI] [PubMed] [Google Scholar]
- 14.Rex DK. Review article: moderate sedation for endoscopy: sedation regimens for non-anaesthesiologists. Aliment Pharmacol Ther. 2006;24:163–171. doi: 10.1111/j.1365-2036.2006.02986.x. [DOI] [PubMed] [Google Scholar]
- 15.Froehlich F, Schwizer W, Thorens J, Köhler M, Gonvers JJ, Fried M. Conscious sedation for gastroscopy: patient tolerance and cardiorespiratory parameters. Gastroenterology. 1995;108:697–704. doi: 10.1016/0016-5085(95)90441-7. [DOI] [PubMed] [Google Scholar]
- 16.Freeman ML. Adverse outcomes of ERCP. Gastrointest Endosc. 2002;56:S273–S282. doi: 10.1067/mge.2002.129028. [DOI] [PubMed] [Google Scholar]
- 17.McCune WS, Shorb PE, Moscovitz H. Endoscopic cannulation of the ampulla of vater: a preliminary report. Ann Surg. 1968;167:752–756. doi: 10.1097/00000658-196805000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martindale SJ. Anaesthetic considerations during endoscopic retrograde cholangiopancreatography. Anaesth Intensive Care. 2006;34:475–480. doi: 10.1177/0310057X0603400401. [DOI] [PubMed] [Google Scholar]
- 19.Patel S, Vargo JJ, Khandwala F, Lopez R, Trolli P, Dumot JA, Conwell DL, Zuccaro G. Deep sedation occurs frequently during elective endoscopy with meperidine and midazolam. Am J Gastroenterol. 2005;100:2689–2695. doi: 10.1111/j.1572-0241.2005.00320.x. [DOI] [PubMed] [Google Scholar]
- 20.Chen WX, Lin HJ, Zhang WF, Gu Q, Zhong XQ, Yu CH, Li YM, Gu ZY. Sedation and safety of propofol for therapeutic endoscopic retrograde cholangiopancreatography. Hepatobiliary Pancreat Dis Int. 2005;4:437–440. [PubMed] [Google Scholar]
- 21.Fanti L, Agostoni M, Casati A, Guslandi M, Giollo P, Torri G, Testoni PA. Target-controlled propofol infusion during monitored anesthesia in patients undergoing ERCP. Gastrointest Endosc. 2004;60:361–366. doi: 10.1016/s0016-5107(04)01713-4. [DOI] [PubMed] [Google Scholar]
- 22.Jung M, Hofmann C, Kiesslich R, Brackertz A. Improved sedation in diagnostic and therapeutic ERCP: propofol is an alternative to midazolam. Endoscopy. 2000;32:233–238. doi: 10.1055/s-2000-96. [DOI] [PubMed] [Google Scholar]
- 23.Kongkam P, Rerknimitr R, Punyathavorn S, Sitthi-Amorn C, Ponauthai Y, Prempracha N, Kullavanijaya P. Propofol infusion versus intermittent meperidine and midazolam injection for conscious sedation in ERCP. J Gastrointestin Liver Dis. 2008;17:291–297. [PubMed] [Google Scholar]
- 24.Seifert H, Schmitt TH, Gültekin T, Caspary WF, Wehrmann T. Sedation with propofol plus midazolam versus propofol alone for interventional endoscopic procedures: a prospective, randomized study. Aliment Pharmacol Ther. 2000;14:1207–1214. doi: 10.1046/j.1365-2036.2000.00787.x. [DOI] [PubMed] [Google Scholar]
- 25.Metzner J, Posner KL, Domino KB. The risk and safety of anesthesia at remote locations: the US closed claims analysis. Curr Opin Anaesthesiol. 2009;22:502–508. doi: 10.1097/ACO.0b013e32832dba50. [DOI] [PubMed] [Google Scholar]
- 26.Quine MA, Bell GD, McCloy RF, Charlton JE, Devlin HB, Hopkins A. Prospective audit of upper gastrointestinal endoscopy in two regions of England: safety, staffing, and sedation methods. Gut. 1995;36:462–467. doi: 10.1136/gut.36.3.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sharma VK, Nguyen CC, Crowell MD, Lieberman DA, de Garmo P, Fleischer DE. A national study of cardiopulmonary unplanned events after GI endoscopy. Gastrointest Endosc. 2007;66:27–34. doi: 10.1016/j.gie.2006.12.040. [DOI] [PubMed] [Google Scholar]
- 28.Zuccaro G. Sedation and analgesia for GI endoscopy. Gastrointest Endosc. 2006;63:95–96. doi: 10.1016/j.gie.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 29.Lichtenstein DR, Jagannath S, Baron TH, Anderson MA, Banerjee S, Dominitz JA, Fanelli RD, Gan SI, Harrison ME, Ikenberry SO, et al. Sedation and anesthesia in GI endoscopy. Gastrointest Endosc. 2008;68:205–216. doi: 10.1016/j.gie.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 30.Imagawa A, Fujiki S, Kawahara Y, Matsushita H, Ota S, Tomoda T, Morito Y, Sakakihara I, Fujimoto T, Taira A, et al. Satisfaction with bispectral index monitoring of propofol-mediated sedation during endoscopic submucosal dissection: a prospective, randomized study. Endoscopy. 2008;40:905–909. doi: 10.1055/s-2008-1077641. [DOI] [PubMed] [Google Scholar]
- 31.Liu SS. Effects of Bispectral Index monitoring on ambulatory anesthesia: a meta-analysis of randomized controlled trials and a cost analysis. Anesthesiology. 2004;101:311–315. doi: 10.1097/00000542-200408000-00010. [DOI] [PubMed] [Google Scholar]
- 32.Gan TJ, Glass PS, Windsor A, Payne F, Rosow C, Sebel P, Manberg P. Bispectral index monitoring allows faster emergence and improved recovery from propofol, alfentanil, and nitrous oxide anesthesia. BIS Utility Study Group. Anesthesiology. 1997;87:808–815. doi: 10.1097/00000542-199710000-00014. [DOI] [PubMed] [Google Scholar]
- 33.Myles PS, Leslie K, McNeil J, Forbes A, Chan MT. Bispectral index monitoring to prevent awareness during anaesthesia: the B-Aware randomised controlled trial. Lancet. 2004;363:1757–1763. doi: 10.1016/S0140-6736(04)16300-9. [DOI] [PubMed] [Google Scholar]
- 34.Avidan MS, Zhang L, Burnside BA, Finkel KJ, Searleman AC, Selvidge JA, Saager L, Turner MS, Rao S, Bottros M, et al. Anesthesia awareness and the bispectral index. N Engl J Med. 2008;358:1097–1108. doi: 10.1056/NEJMoa0707361. [DOI] [PubMed] [Google Scholar]


