Abstract
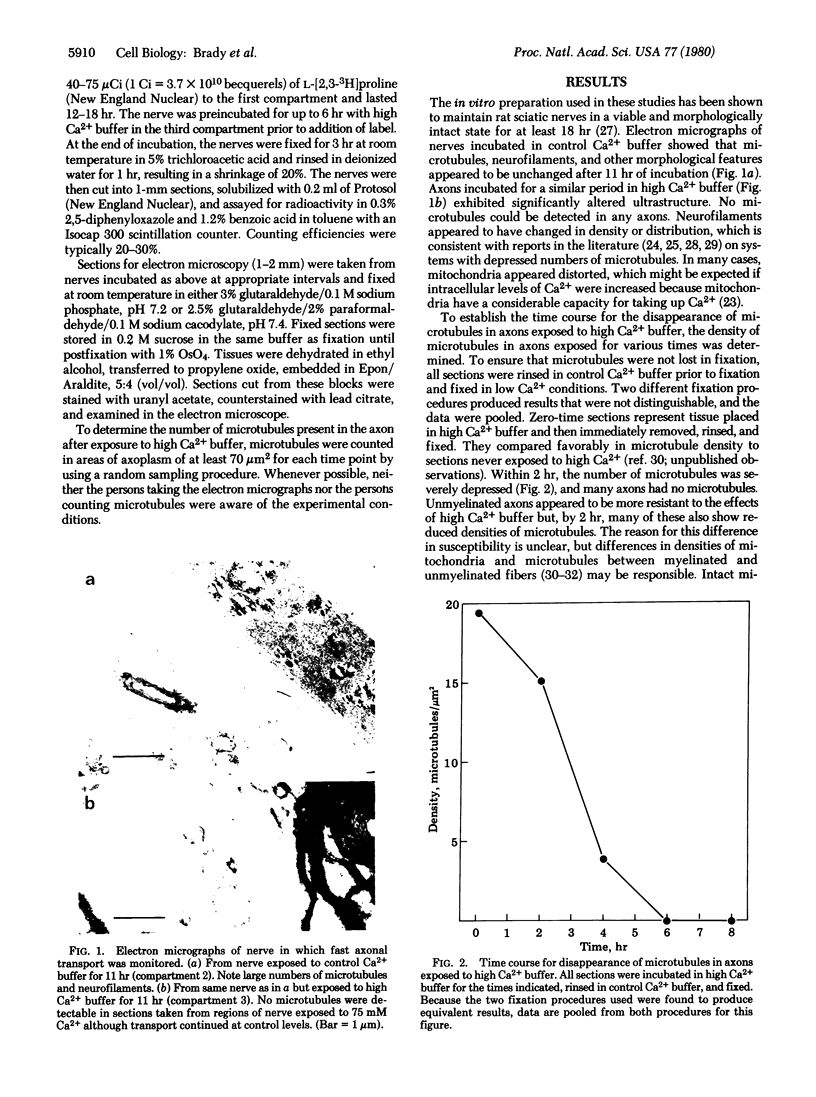
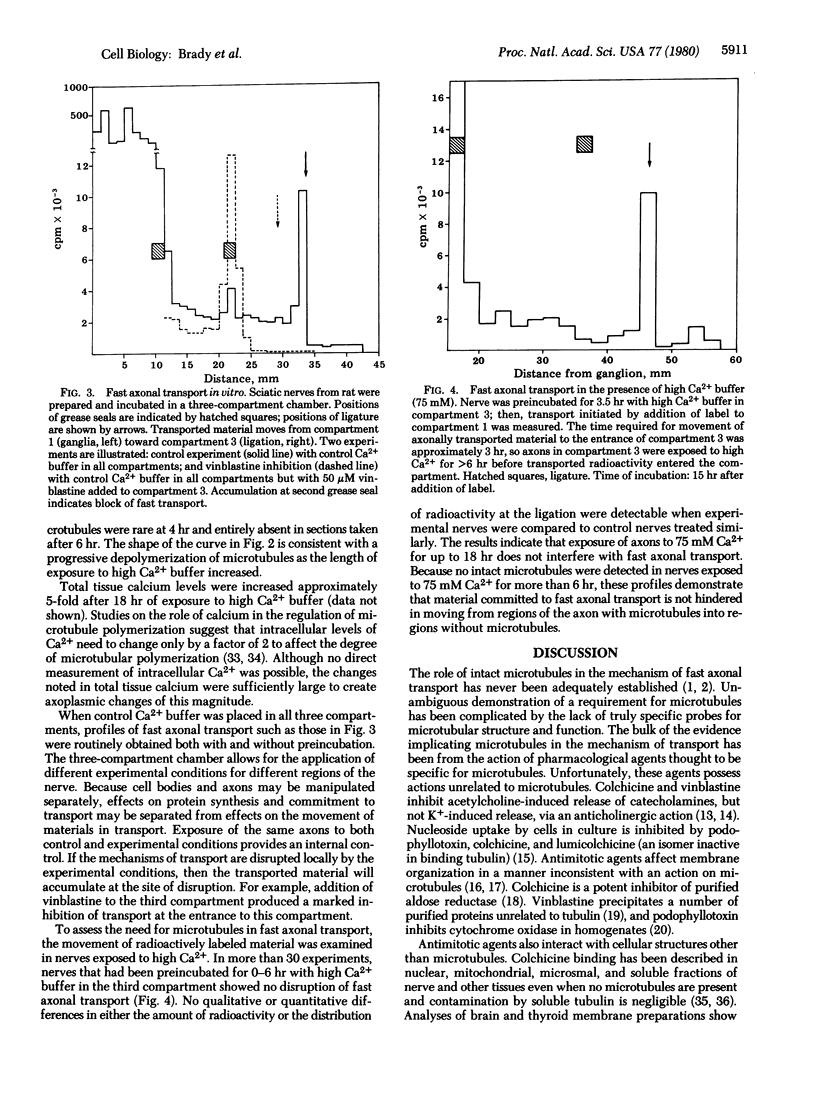
Microtubules have long been associated with the mechanism of fast axoplasmic transport, although experimental evidence to support an involvement has been equivocal. Electron microscopic studies demonstrated that incubation of the axons of excised rat sciatic nerves in media containing 75 mM Ca2+ caused complete loss of microtubules within 6 hr. To evaluate the role of microtubules in fast anterograde transport, studies of transport in nerves exposed to these conditions were undertaken. Prior to measurement of axoplasmic transport, nerves ligated distal to the dorsal root ganglia were preincubated in vitro in 75 mM Ca2+ for 0-6 hr. Fast axonal transport was subsequently monitored by measuring the amount of trichloroacetic acid-insoluble radioactivity that accumulated at the ligature after incubation for 12-18 hr with L-[3H]proline. Nerves in which microtubules had been depolymerized by preincubation in high Ca2+ maintained control levels of transport. We conclude that intact microtubules are not required for fast anterograde axoplasmic transport.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe T., Haga T., Kurokawa M. Rapid transport of phosphatidylcholine occurring simultaneously with protein transport in the frog sciatic nerve. Biochem J. 1973 Nov;136(3):731–740. doi: 10.1042/bj1360731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banker G., Crain B., Cotman C. W. Molecular weights of the polypeptide chains of synaptic plasma membranes. Brain Res. 1972 Jul 20;42(2):508–513. doi: 10.1016/0006-8993(72)90551-3. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya B., Volff J. Membrane-bound tubulin in brain and thyroid tissue. J Biol Chem. 1975 Oct 10;250(19):7639–7646. [PubMed] [Google Scholar]
- Byers M. R. Structural correlates of rapid axonal transport: evidence that microtubules may not be directly involved. Brain Res. 1974 Jul 19;75(1):97–113. doi: 10.1016/0006-8993(74)90773-2. [DOI] [PubMed] [Google Scholar]
- Carafoli E., Lehninger A. L. A survey of the interaction of calcium ions with mitochondria from different tissues and species. Biochem J. 1971 May;122(5):681–690. doi: 10.1042/bj1220681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoso J. A., Watson D. F., Heller-Bettinger I. E., Samson F. E. Maytansine action on fast axoplasmic transport and the ultrastructure of vagal axons. Cancer Res. 1978 Jun;38(6):1633–1637. [PubMed] [Google Scholar]
- Douglas W. W., Sorimachi M. Colchicine inhibits adrenal medullary secretion evoked by acetylcholine without affecting that evoked by potassium. Br J Pharmacol. 1972 May;45(1):129–132. [PMC free article] [PubMed] [Google Scholar]
- Droz B., Koenig H. L., Biamberardino L. D., Di Giamberardino L. Axonal migration of protein and glycoprotein to nerve endings. I. Radioautographic analysis of the renewal of protein in nerve endings of chicken ciliary ganglion after intracerebral injection of (3H)lysine. Brain Res. 1973 Sep 28;60(1):93–127. doi: 10.1016/0006-8993(73)90852-4. [DOI] [PubMed] [Google Scholar]
- Droz B., Rambourg A., Koenig H. L. The smooth endoplasmic reticulum: structure and role in the renewal of axonal membrane and synaptic vesicles by fast axonal transport. Brain Res. 1975 Jul 25;93(1):1–13. doi: 10.1016/0006-8993(75)90282-6. [DOI] [PubMed] [Google Scholar]
- Edström A. Effects of Ca2+ and Mg2+ on rapid axonal transport of proteins in vitro in frog sciatic nerves. J Cell Biol. 1974 Jun;61(3):812–818. doi: 10.1083/jcb.61.3.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez H. L., Burton P. R., Samson F. E. Axoplasmic transport in the crayfish nerve cord. The role of fibrillar constituents of neurons. J Cell Biol. 1971 Oct;51(1):176–192. doi: 10.1083/jcb.51.1.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friede R. L., Ho K. C. The relation of axonal transport of mitochondria with microtubules and other axoplasmic organelles. J Physiol. 1977 Feb;265(2):507–519. doi: 10.1113/jphysiol.1977.sp011727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friede R. L., Samorajski T. Axon caliber related to neurofilaments and microtubules in sciatic nerve fibers of rats and mice. Anat Rec. 1970 Aug;167(4):379–387. doi: 10.1002/ar.1091670402. [DOI] [PubMed] [Google Scholar]
- Furcht L. T., Scott E. Effect of vinblastine sulfate, colchicine and lumicolchicine on membrane organization of normal and transformed cells. Exp Cell Res. 1975 Dec;96(2):271–282. doi: 10.1016/0014-4827(75)90257-8. [DOI] [PubMed] [Google Scholar]
- Gabbay K. H., Tze W. J. Inhibition of glucose-induced release of insulin by aldose reductase inhibitors. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1435–1439. doi: 10.1073/pnas.69.6.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haga T., Abe T., Kurokawa M. Polymerization and depolymerization of microtubules in vitro as studied by flow birefringence. FEBS Lett. 1974 Mar 1;39(3):291–295. doi: 10.1016/0014-5793(74)80133-x. [DOI] [PubMed] [Google Scholar]
- Heller J., Ostwald T. J., Bok D. The osmotic behavior of rod photoreceptor outer segment discs. J Cell Biol. 1971 Mar;48(3):633–649. doi: 10.1083/jcb.48.3.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heslop J. P. Axonal flow and fast transport in nerves. Adv Comp Physiol Biochem. 1975;6:75–163. doi: 10.1016/b978-0-12-011506-8.50008-1. [DOI] [PubMed] [Google Scholar]
- Hinkley R. E., Jr, Samson F. E., Jr The effects of an elevated temperature, colchicine, and vinblastine on axonal microtubules of the crayfish (Procambarus clarkii). J Exp Zool. 1974 Jun;188(3):321–336. doi: 10.1002/jez.1401880308. [DOI] [PubMed] [Google Scholar]
- KELLY M., HARTWELL J. L. The biological effects and the chemical composition of podophyllin: a review. J Natl Cancer Inst. 1954 Feb;14(4):967–1010. [PubMed] [Google Scholar]
- Kasa P. Acetylcholinesterase transport in the central and peripheral nervous tissue: the role of tubules in the enzyme transport. Nature. 1968 Jun 29;218(5148):1265–1267. doi: 10.1038/2181265a0. [DOI] [PubMed] [Google Scholar]
- Kornguth S. E., Sunderland E. Isolation and partial characterization of a tubulin-like protein from human and swine synaptosomal membranes. Biochim Biophys Acta. 1975 May 30;393(1):100–114. doi: 10.1016/0005-2795(75)90220-2. [DOI] [PubMed] [Google Scholar]
- Lagnado J. R., Lyons C., Wickremasinghe G. The subcellular distribution of colchicine-binding protein(s) ( microtubule protein') in rat brain. Biochem J. 1971 May;122(5):56P–57P. doi: 10.1042/bj1220056pb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liwnicz B. H., Kristensson K., Wiśniewski H. M., Shelanski M. L., Terry R. D. Observations on axoplasmic transport in rabbits with aluminum-induced neurofibrillary tangles. Brain Res. 1974 Nov 22;80(3):413–420. doi: 10.1016/0006-8993(74)91026-9. [DOI] [PubMed] [Google Scholar]
- Mizel S. B., Wilson L. Nucleoside transport in mammalian cells. Inhibition by colchicine. Biochemistry. 1972 Jul 4;11(14):2573–2578. doi: 10.1021/bi00764a003. [DOI] [PubMed] [Google Scholar]
- Ochs S. Fast transport of materials in mammalian nerve fibers. Science. 1972 Apr 21;176(4032):252–260. doi: 10.1126/science.176.4032.252. [DOI] [PubMed] [Google Scholar]
- Ochs S., Worth R. M., Chan S. Y. Calcium requirement for axoplasmic transport in mammalian nerve. Nature. 1977 Dec 22;270(5639):748–750. doi: 10.1038/270748a0. [DOI] [PubMed] [Google Scholar]
- Ochs S., Worth R. Batrachotoxin block of fast axoplasmic transport in mammalian nerve fibers. Science. 1975 Mar 21;187(4181):1087–1089. doi: 10.1126/science.46619. [DOI] [PubMed] [Google Scholar]
- Paulson J. C., McClure W. O. Microtubules and axoplasmic transport. Inhibition of transport by podophyllotoxin: an interaction with microtubule protein. J Cell Biol. 1975 Nov;67(2PT1):461–467. doi: 10.1083/jcb.67.2.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulson J. C., McClure W. O. Microtubules and axoplasmic transport. Brain Res. 1974 Jun 20;73(2):333–337. doi: 10.1016/0006-8993(74)91053-1. [DOI] [PubMed] [Google Scholar]
- Ranish N., Ochs S. Fast axoplasmic transport of acetylcholinesterase in mammalian nerve fibres. J Neurochem. 1972 Nov;19(11):2641–2649. doi: 10.1111/j.1471-4159.1972.tb01323.x. [DOI] [PubMed] [Google Scholar]
- Samorajski T., Friede R. L. Size-dependent distribution of axoplasm, Schwann cell cytoplasm, and mitochondri in the peripheral nerve fibers of mouse. Anat Rec. 1968 Jul;161(3):281–292. doi: 10.1002/ar.1091610302. [DOI] [PubMed] [Google Scholar]
- Schlaepfer W. W., Bunge R. P. Effects of calcium ion concentration on the degeneration of amputated axons in tissue culture. J Cell Biol. 1973 Nov;59(2 Pt 1):456–470. doi: 10.1083/jcb.59.2.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaepfer W. W. Experimental alterations of neurofilaments and neurotubules by calcium and other ions. Exp Cell Res. 1971 Jul;67(1):73–80. doi: 10.1016/0014-4827(71)90622-7. [DOI] [PubMed] [Google Scholar]
- Schliwa M. The role of divalent cations in the regulation of microtubule assembly. In vivo studies on microtubules of the heliozoan axopodium using the ionophore A23187. J Cell Biol. 1976 Sep;70(3):527–540. doi: 10.1083/jcb.70.3.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt F. O. Fibrous proteins--neuronal organelles. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1092–1101. doi: 10.1073/pnas.60.4.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer P. S., Schaumburg H. H., Raleigh R. L., Terhaar C. J. Nervous system degeneration produced by the industrial solvent methyl n-butyl ketone. Arch Neurol. 1975 Apr;32(4):219–222. doi: 10.1001/archneur.1975.00490460035002. [DOI] [PubMed] [Google Scholar]
- Stadler J., Franke W. W. Characterization of the colchicine binding of membrane fractions from rat and mouse liver. J Cell Biol. 1974 Jan;60(1):297–303. doi: 10.1083/jcb.60.1.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens R. E., Edds K. T. Microtubules: structure, chemistry, and function. Physiol Rev. 1976 Oct;56(4):709–777. doi: 10.1152/physrev.1976.56.4.709. [DOI] [PubMed] [Google Scholar]
- Teichberg S., Holtzman E. Axonal agranular reticulum and synaptic vesicles in cultured embryonic chick sympathetic neurons. J Cell Biol. 1973 Apr;57(1):88–108. doi: 10.1083/jcb.57.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theiler R. F., McClure W. O. A comparison of axonally transported proteins in the rat sciatic nerve by in vitro and in vivo techniques. J Neurochem. 1977 Feb;28(2):321–330. doi: 10.1111/j.1471-4159.1977.tb07751.x. [DOI] [PubMed] [Google Scholar]
- Trifaró J. M., Collier B., Lastowecka A., Stern D. Inhibition by colchicine and by vinblastine of acetylcholine-induced catecholamine release from the adrenal gland: an anticholinergic action, not an effect upon microtubules. Mol Pharmacol. 1972 Mar;8(2):264–267. [PubMed] [Google Scholar]
- Tsukita S., Ishikawa H. The movement of membranous organelles in axons. Electron microscopic identification of anterogradely and retrogradely transported organelles. J Cell Biol. 1980 Mar;84(3):513–530. doi: 10.1083/jcb.84.3.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisenberg R. C. Microtubule formation in vitro in solutions containing low calcium concentrations. Science. 1972 Sep 22;177(4054):1104–1105. doi: 10.1126/science.177.4054.1104. [DOI] [PubMed] [Google Scholar]
- Wilson D. L., Stone G. C. Axoplasmic transport of proteins. Annu Rev Biophys Bioeng. 1979;8:27–45. doi: 10.1146/annurev.bb.08.060179.000331. [DOI] [PubMed] [Google Scholar]
- Wilson L., Bryan J., Ruby A., Mazia D. Precipitation of proteins by vinblastine and calcium ions. Proc Natl Acad Sci U S A. 1970 Jul;66(3):807–814. doi: 10.1073/pnas.66.3.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wunderlich F., Müller R., Speth V. Direct evidence for a colchicine-induced impairment in the mobility of membrane components. Science. 1973 Dec 14;182(4117):1136–1138. doi: 10.1126/science.182.4117.1136. [DOI] [PubMed] [Google Scholar]