Abstract
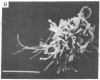
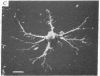
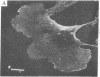
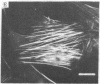
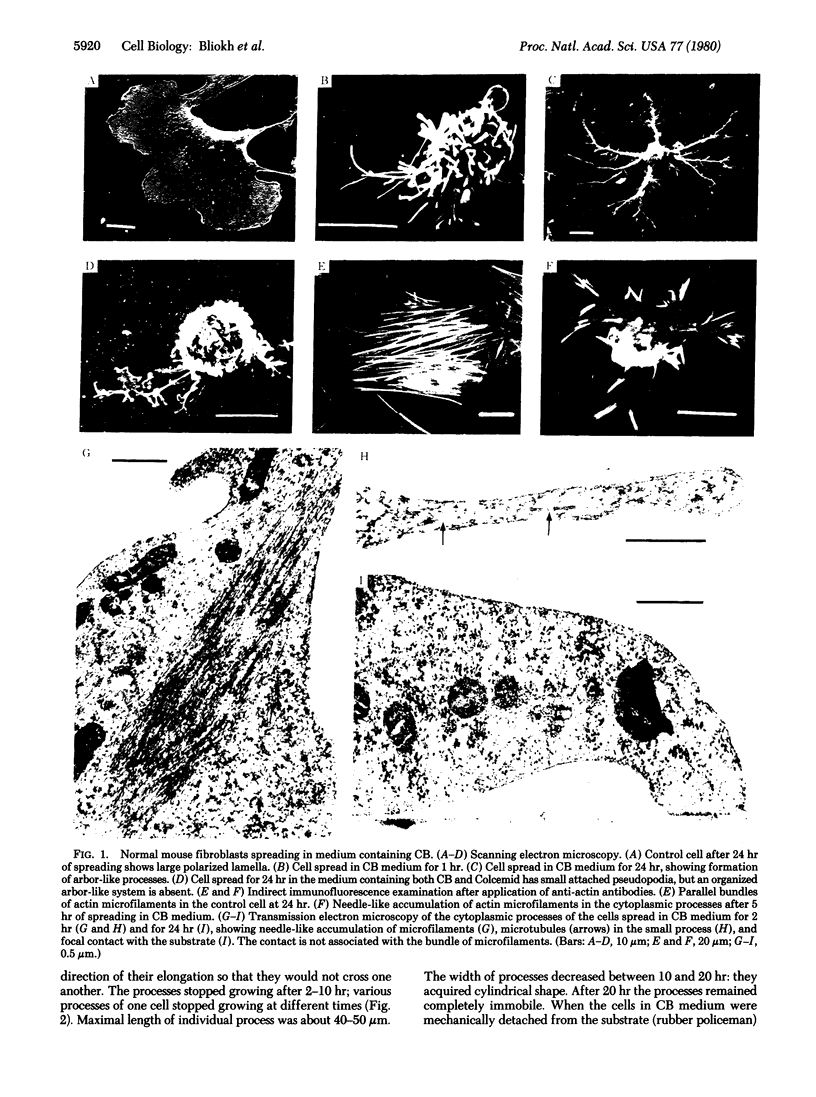
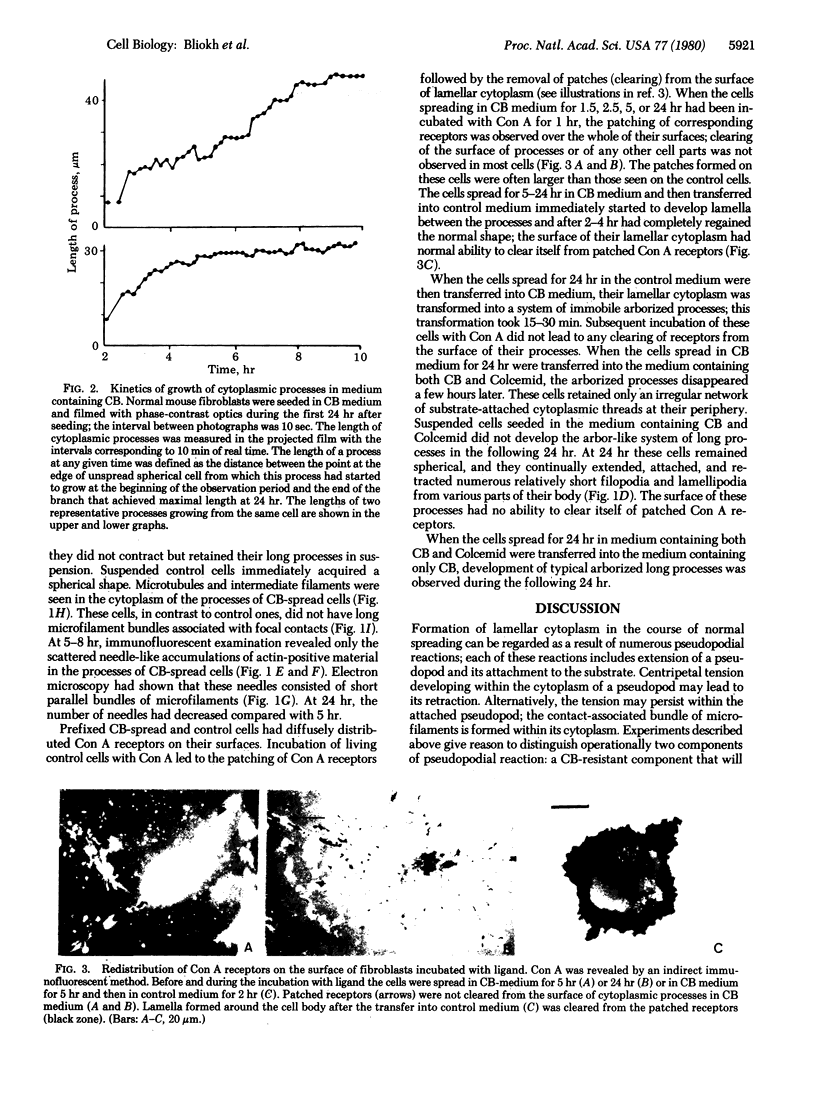
Normal cultured mouse fibroblasts spreading on solid substrate extend and attach numerous pseudopods; lamellar cytoplasm is eventually formed from the attached pseudopods. Fibroblasts spreading in the presence of cytochasin B (CB) from de novo a system of arbor-like branched processes rather than lamellar cytoplasm. The growing and fully formed arbor-like processes, in contrast to normal lamellar cytoplasm, have low contractility and are unable to clear patched concanavalin A receptors from their surfaces; their attachement sites are not associated with microfilament bundles. The cells spreading in medium containing CB and Colcemid do not form well-organized branched structures but extend and attach numerous unstable pseudopods. It is suggested that normal formation of lamellar cytoplasm can be regarded as a combination of several functionally different processes: (a) of rudimentary pseudopodial reactions resistant to CB and Colcemid; (b) of CB-sensitive lamellization; and (c) of Colcemid-sensitive stabilization.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abercrombie M., Heaysman J. E., Pegrum S. M. Locomotion of fibroblasts in culture. V. Surface marking with concanavalin A. Exp Cell Res. 1972 Aug;73(2):536–539. doi: 10.1016/0014-4827(72)90090-0. [DOI] [PubMed] [Google Scholar]
- Bershadsky A. D., Tint I. S., Gelfand V. I., Rosenblat V. A., Vasiliev J. M., Gelfand I. M. Microtubular system in cultured mouse epithelial cells. Cell Biol Int Rep. 1978 Jul;2(4):345–351. doi: 10.1016/0309-1651(78)90020-6. [DOI] [PubMed] [Google Scholar]
- Carter S. B. Effects of cytochalasins on mammalian cells. Nature. 1967 Jan 21;213(5073):261–264. doi: 10.1038/213261a0. [DOI] [PubMed] [Google Scholar]
- Croop J., Holtzer H. Response of myogenic and fibrogenic cells to cytochalasin B and to colcemid. I. Light microscope observations. J Cell Biol. 1975 May;65(2):271–285. doi: 10.1083/jcb.65.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath J. P., Dunn G. A. Cell to substratum contacts of chick fibroblasts and their relation to the microfilament system. A correlated interference-reflexion and high-voltage electron-microscope study. J Cell Sci. 1978 Feb;29:197–212. doi: 10.1242/jcs.29.1.197. [DOI] [PubMed] [Google Scholar]
- Ivanova O. Y., Margolis L. B., Vasiliev J. M. Effect of colcemid on the spreading of fibroblasts in culture. Exp Cell Res. 1976 Aug;101(1):207–219. doi: 10.1016/0014-4827(76)90431-6. [DOI] [PubMed] [Google Scholar]
- Lazarides E., Weber K. Actin antibody: the specific visualization of actin filaments in non-muscle cells. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2268–2272. doi: 10.1073/pnas.71.6.2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin D. C., Lin S. Actin polymerization induced by a motility-related high-affinity cytochalasin binding complex from human erythrocyte membrane. Proc Natl Acad Sci U S A. 1979 May;76(5):2345–2349. doi: 10.1073/pnas.76.5.2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn M., Weber K. Tubulin-specific antibody and the expression of microtubules in 3T3 cells after attachment to a substratum. Further evidence for the polar growth of cytoplasmic microtubules in vivo. Exp Cell Res. 1976 Dec;103(2):331–340. doi: 10.1016/0014-4827(76)90270-6. [DOI] [PubMed] [Google Scholar]
- Spooner B. S., Yamada K. M., Wessells N. K. Microfilaments and cell locomotion. J Cell Biol. 1971 Jun;49(3):595–613. doi: 10.1083/jcb.49.3.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasiliev J. M., Gelfand I. M., Dominina L. V., Dorfman N. A., Pletyushkina O. Y. Active cell edge and movements of concanavalin A receptors of the surface of epithelial and fibroblastic cells. Proc Natl Acad Sci U S A. 1976 Nov;73(11):4085–4089. doi: 10.1073/pnas.73.11.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasiliev J. M., Gelfand I. M., Domnina L. V., Ivanova O. Y., Komm S. G., Olshevskaja L. V. Effect of colcemid on the locomotory behaviour of fibroblasts. J Embryol Exp Morphol. 1970 Nov;24(3):625–640. [PubMed] [Google Scholar]
- Vasiliev J. M., Gelfand I. M. Mechanisms of morphogenesis in cell cultures. Int Rev Cytol. 1977;50:159–274. doi: 10.1016/s0074-7696(08)60099-6. [DOI] [PubMed] [Google Scholar]
- Weber K., Rathke P. C., Osborn M., Franke W. W. Distribution of actin and tubulin in cells and in glycerinated cell models after treatment with cytochalasin B (CB). Exp Cell Res. 1976 Oct 15;102(2):285–297. doi: 10.1016/0014-4827(76)90044-6. [DOI] [PubMed] [Google Scholar]
- Wehland J., Osborn M., Weber K. Cell-to-substratum contacts in living cells: a direct correlation between interference-reflexion and indirect-immunofluorescence microscopy using antibodies against actin and alpha-actinin. J Cell Sci. 1979 Jun;37:257–273. doi: 10.1242/jcs.37.1.257. [DOI] [PubMed] [Google Scholar]