Abstract
Purpose.
Although cortical visual impairment (CVI) is the leading cause of bilateral vision impairment in children in Western countries, little is known about the effects of CVI on visual function. The aim of this study was to compare visual evoked potential measures of contrast sensitivity and grating acuity in children with CVI with those of age-matched typically developing controls.
Methods.
The swept parameter visual evoked potential (sVEP) was used to measure contrast sensitivity and grating acuity in 34 children with CVI at 5 months to 5 years of age and in 16 age-matched control children. Contrast thresholds and spatial frequency thresholds (grating acuities) were derived by extrapolating the tuning functions to zero amplitude. These thresholds and maximal suprathreshold response amplitudes were compared between groups.
Results.
Among 34 children with CVI, 30 had measurable but reduced contrast sensitivity with a median threshold of 10.8% (range 5.0%–30.0% Michelson), and 32 had measurable but reduced grating acuity with median threshold 0.49 logMAR (9.8 c/deg, range 5–14 c/deg). These thresholds were significantly reduced, compared with age-matched control children. In addition, response amplitudes over the entire sweep range for both measures were significantly diminished in children with CVI compared with those of control children.
Conclusions.
Our results indicate that spatial contrast sensitivity and response amplitudes are strongly affected by CVI. The substantial degree of loss in contrast sensitivity suggests that contrast is a sensitive measure for evaluating vision deficits in patients with CVI.
This study compared visual evoked potential measures of contrast sensitivity and grating acuity in children with cortical visual impairment with those of age-matched typically developing controls.
Introduction
Cortical visual impairment (CVI) is the leading cause of bilateral visual impairment in children in Western countries.1 CVI often occurs perinatally with bilateral cerebral damage to the optic radiations or visual cortex or both.2–4 The most common etiology for CVI is perinatal hypoxia and ischemia, with premature birth being an important contributory event in many cases. With nearly 12% of children in the United States born prematurely, it is easy to understand how CVI is emerging as an important and common cause of bilateral vision impairment in children.5,6
No specific medical treatment for CVI is available. Therefore, management of CVI involves efforts to prevent it7,8 or to rehabilitate children once neurological damage occurs. Rehabilitation in part includes optimal presentation of visual targets to impaired children and takes advantage of the fact that children with CVI almost always have some residual vision.4,9 Environmental lighting may be optimized to promote visual functioning,10 and the size of visual targets coupled with optimal contrast is also important. However, optimal target size and contrast in patients with CVI are still not well documented. Most children with CVI are either preverbal or nonverbal, making the quantitative diagnosis of reduced acuity, or reduced contrast sensitivity, a difficult task. Behavioral measures (e.g., optotype test, preferential looking) are sometimes not possible for patients with CVI, or may underestimate visual acuity.11–13
The swept parameter visual evoked potential (sVEP) provides a technique that can be used to assess visual function in infants and children with severe CVI.10–14 With sVEP measurements, a number of studies have demonstrated grating and vernier acuity deficits in CVI.2,10–13 Despite the importance of contrast sensitivity to visual function, this aspect of vision has not been fully evaluated,14,15 especially in young children with CVI.
To quantitatively estimate visual function for children with CVI, we used the sVEP to evaluate spatial contrast sensitivity in young children with CVI. In this experiment, we compared sVEP spatial frequency threshold (grating acuity) and contrast threshold, and response amplitudes in young children with CVI, with age-matched healthy control children.
Methods
Participants
sVEP measurements were recorded from 34 patients with CVI ranging in age from 5 months to 5 years (mean ± SD: 1.94 ± 1.37) and 16 age-matched healthy controls (mean ± SD: 2.19 ± 1.55). Controls were typically developing children recruited through letters mailed to parents. Names were obtained through the state registry. Infants with eye or systemic illness, or prematurity, were excluded from the control group. The age difference between the two groups was not significant (P = 0.615). Children with CVI were diagnosed clinically on the basis of reduced visual acuity in both eyes, with the diagnosis corroborated by neuroimaging, history, and physical examination to exclude with certainty any coexisting eye disease. Pupillary reactions were normal. Multiple etiologies accounted for CVI in this cohort, including hypoxic-ischemic encephalopathy in most, as well as infection, hydrocephalus, and metabolic disorders (Table). The protocol was approved by the institutional review board of the California Pacific Medical Center and conforms to the tenets of the Declaration of Helsinki. Written informed consent was obtained from the parents of the CVI patients and control children, after the recording procedure was explained.
Table. .
CVI Patient Description
|
Study No. |
Sex |
Age, y |
Etiology of CVI |
| 1 | M | 2.66 | Metabolic defect |
| 2 | F | 1.90 | Perinatal hypoxia |
| 3 | M | 0.98 | Meningitis |
| 4 | F | 4.70 | Perinatal hypoxia, ischemia |
| 5 | M | 1.07 | Perinatal hypoxia, ischemia |
| 6 | F | 0.61 | Hydrocephalus |
| 7 | M | 3.37 | Unknown, presumed perinatal hypoxia |
| 8 | M | 1.37 | Congenital heart disease and hypoxia |
| 9 | M | 3.97 | Subarachnoid hemorrhage |
| 10 | F | 1.07 | Hypoxia ischemia |
| 11 | M | 0.63 | Unknown |
| 12 | F | 2.41 | Cerebrovascular accident in utero |
| 13 | M | 1.45 | Choroid plexus papilloma |
| 14 | M | 0.82 | Perinatal hypoxia, ischemia |
| 15 | M | 0.75 | Unknown, presumed perinatal hypoxia |
| 16 | M | 2.31 | Neurodegenerative disease |
| 17 | M | 0.86 | Cerebrovascular accident in utero |
| 18 | M | 0.63 | Perinatal hypoxia, ischemia |
| 19 | M | 2.18 | Unknown, presumed perinatal hypoxia |
| 20 | M | 2.36 | Perinatal hypoxia, ischemia |
| 21 | M | 5.67 | Unknown etiology |
| 22 | M | 2.56 | Perinatal hypoxia |
| 23 | M | 1.20 | Lissencephaly |
| 24 | M | 0.87 | Encephalitis |
| 25 | M | 1.60 | Perinatal hypoxia, ischemia |
| 26 | M | 1.82 | Perinatal hypoxia, ischemia |
| 27 | F | 0.82 | Perinatal hypoxia, ischemia |
| 28 | F | 2.57 | Unknown, presumed perinatal hypoxia |
| 29 | F | 0.94 | Hydrocephalus |
| 30 | F | 1.47 | Perinatal hypoxia |
| 31 | F | 0.45 | Perinatal hypoxia |
| 32 | F | 5.58 | Unknown, presumed perinatal hypoxia |
| 33 | M | 2.57 | Meningitis |
| 34 | F | 1.85 | Unknown, presumed perinatal hypoxia |
Stimuli and Apparatus
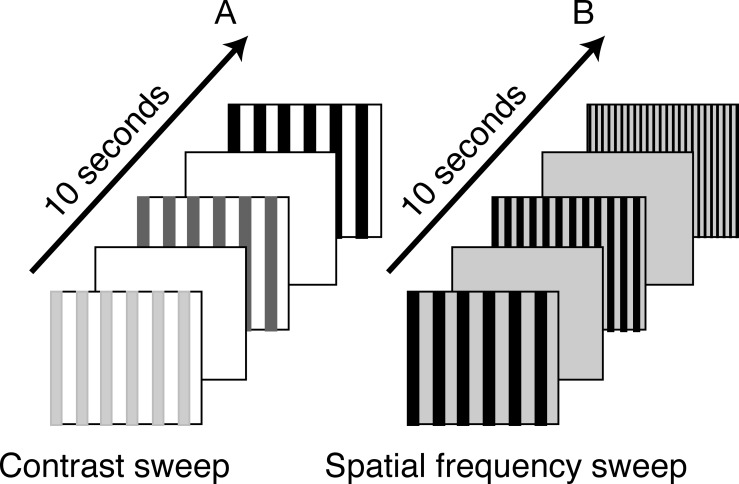
Measurements of spatial frequency threshold (grating acuity) and contrast sensitivity using the sVEP have been obtained previously in full-term, healthy infants and adults.16–18 In the present study, we used the same technique as in previous studies,16–18 but we selected a low spatial frequency (i.e., 1 c/deg) and a large contrast sweep range (e.g., 10%–80%) for contrast threshold measurement owing to the poor vision in children with CVI. We also used a low mean luminance (20 cd/m2) for spatial frequency threshold measurement based on a previous result indicating that visual acuity of children with CVI is best under lower luminance-viewing conditions.10,11 In brief, stimuli were presented on a multisynch video monitor (1600 × 1200 pixels; 60-Hz vertical refresh, video bandwidth, 150 MHz; MRHB2000, Richardson Electronics, Inc., Jasper, AL). The stimulus field was 18° × 25° for CVI patients at 70-cm viewing distance and 10° × 13° for healthy controls at 130-cm viewing distance. The contrast sweep stimulus (Fig. 1A) was a 1-c/deg black vertical cosine-wave grating presented on a 109 cd/m2 space-average luminance white background screen in a pattern on/off mode at a rate of 3.76 Hz. To compare contrast response functions (amplitudes) between groups, the same contrast sweep range (from 10%–80%) in 10-logarithmic steps over a 10-second period was used both for children with CVI and age-matched controls. Because this contrast sweep range (from 10%–80%) was a suitable range for children with CVI for estimating a threshold due to their poor vision, but was a supra-threshold for typically developing infants, it could not be used to estimate a threshold via extrapolation to zero amplitude. Thus, we used historical control data for contrast sensitivity thresholds from one of our previous studies,17 in which contrast sweep range was used from 0.25% to 20.00% for typically developing infants. The spatial frequency sweep stimulus (Fig. 1B) was a black vertical cosine-wave grating presented on 20-cd/m2 space-average luminance white background screen at 80% contrast in a pattern on/off at 3.76 Hz. Spatial frequency was swept from 1 to 12 c/deg for CVI patients and 2 to 28 c/deg for age-matched controls in 10 linear steps over a 10-second period.
Figure 1. .
Schematic depiction of the sVEP stimuli. Contrast sweep (A): 3.76-Hz onset-offset vertical cosine-wave grating (shown here as square-wave) with 1 c/deg spatial frequency presented on 109 cd/m2 space-average luminance white background screen was swept from 10% to 80% contrast in 10-logarithm steps. Spatial frequency sweep (B): 80% contrast vertical cosine-wave grating at 3.76-Hz onset-offset pattern presented on 20 cd/m2 luminance white background screen was swept from 1 to 12 c/deg for patients with CVI and 2 to 28 c/deg for age-matched healthy controls in 10 linear steps. The sweep duration for both measures was 10 seconds.
VEP Recording and Procedure
Grass E-6H gold-cup surface electrodes and Ten20 conductive EEG paste (DO Weaver and Co., Aurora, CO) were used to collect electroencephalogram (EEG) data. VEPs were recorded at O1, Oz, and O2 with respect to a reference at Cz (International 10-10 electrode placement system).19 Differential voltages were measured between the reference and each of the electrodes placed at O1, Oz, and O2. The EEG was amplified at a gain of 20,000, with amplitude band-pass-filter settings of 0.3 to 100.0 Hz at −6 dB (Model 12 A5; Grass Instruments, Quincy, MA).
During an experimental session, the participants were seated in one of their parent's lap or, in some cases, seated in their wheelchair in front of the monitor. The sVEP responses were measured under binocular viewing conditions in all observers. The experimenter attracted the participant's attention to the stimulus with small toys (approximately 1–2 cm in size) dangled over the center of the display. Recordings were interrupted when the participant was judged not to be attending to the stimulus and were resumed when the participant looked back at the screen. When interruptions occurred, the program interrupted the sweep but not the stimulus appearance or modulation. When the trial resumed after an interruption, data collection recommenced with the stimulus set to its value at 0.5 seconds before the interruption. No child in either the CVI or control category showed an aversion to the light emanating from the computer screen. In general, we used 6 to 8 trails per test stimulus for both control and CVI groups.
Data Analysis and sVEP Threshold Estimation
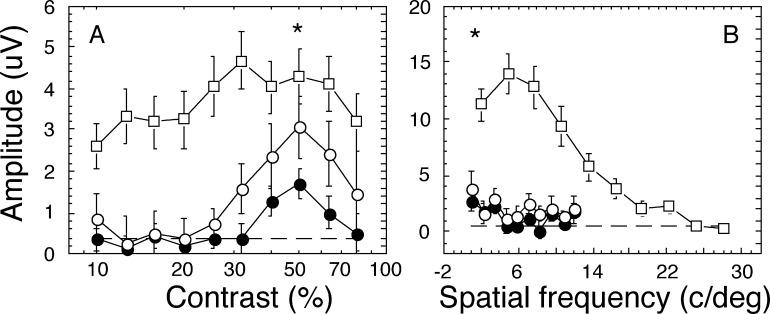
The swept parameter VEP technique has been described in detail previously.16,18 To measure the response functions, sVEP recordings for each 10-second trial were divided into 10 sequential epochs that corresponded to the swept stimulus values. For each epoch, a recursive least square (RLS) adaptive filter20 was used to generate a series of complex-valued spectral coefficients representing the amplitude and phase of response components tuned to various multiples (harmonics) of stimulus frequency (e.g., 3.76 Hz in the current study). These spectral coefficients for each epoch were averaged together across trials for each participant, recording derivation (electrode position), harmonic, and stimulus condition. Statistical significance for each epoch was quantified using P values derived from the circular T (T2 circ) statistic,21 which tests whether a given response amplitude is significantly different from zero, taking into account both response amplitude and phase consistency across trials. Group sVEP amplitudes were also averaged coherently across observers (data in Fig. 2).
Figure 2. .
Mean response functions for contrast and spatial frequency sVEP at Oz-Cz derivation. Vector-averaged sVEP response amplitudes as a function of swept stimulation parameters in all children with CVI (filled circles, n = 34), CVI with hypoxia only (open circles, n = 14) and age-matched control children (open squares, n = 16) are shown in (A) for contrast stimuli and in (B) for spatial frequency stimuli. Error bars are SEM. Dash lines are noise level. The t-test P values for the peak amplitude differences between all children with CVI (filled circle) and control children (open square) was less than 0.05, and shown as *. The response amplitudes over the entire swept range for both contrast and spatial frequency measures were severely reduced in children with CVI, compared with control children. For those with hypoxia only, their amplitudes were slightly larger than for all children with CVI, but remained the same for spatial frequency stimuli.
Response thresholds were estimated by regression to zero amplitude of the linear portion of the voltage versus log contrast function, or linear spatial frequency function. The range of epochs eligible for regression depended on the statistical significance and phase-consistency of the response according to a previously described algorithm.18 The regression range was limited to those epochs in which the criteria were met.10 Once the regression range was established, the threshold was determined by extrapolating the regression line to zero response amplitude (data in Figs. 3, 4).22 In the present study, we analyzed the first four harmonic components and five recording derivations (O1-Cz, Oz-Cz, O2-Cz, Oz-O1, and Oz-O2) for both spatial frequency and contrast sVEP measurements. However, we presented only those harmonics for which the mean response functions showed the strongest responses in children with CVI. A subgroup of infants with hypoxia as the cause for CVI was also analyzed.
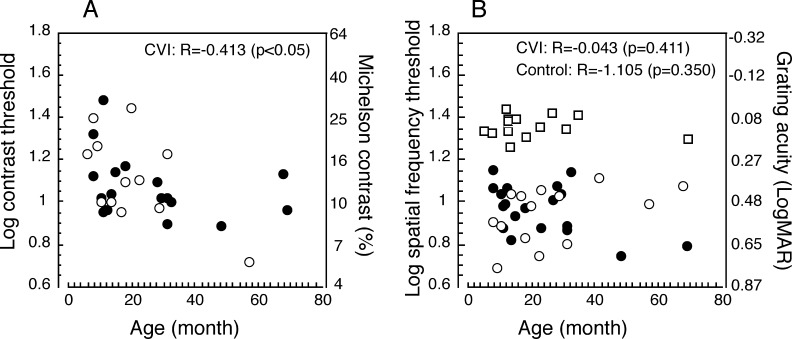
Figure 3. .
Correlation between sVEP thresholds and age. sVEP thresholds for children with CVI of various etiologies (filled circles, n = 18), CVI with hypoxia (open circles, n = 12 [A] and 14 [B]), and control (open squares, n = 16) for contrast stimuli (A) and for spatial frequency stimuli (B) as a function of age in months are shown. Contrast thresholds in children with CVI, including mixed etiologies and hypoxia were correlated with age (P < 0.01), suggesting that contrast sensitivity in children with CVI improves with age. There were no correlations between spatial frequency thresholds and age for children with CVI or control children. Note that nearly all of the causes of CVI would have occurred connatally.
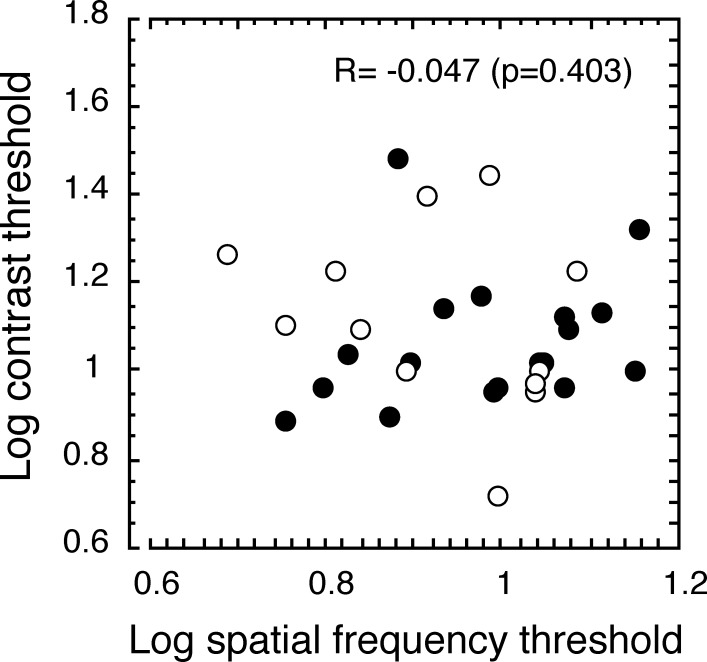
Figure 4. .
Correlation between sVEP contrast thresholds and grating acuities in children with CVI. The filled circles (n = 18) represent children with CVI of mixed etiologies, and the open circles (n = 12) represent children with CVI caused by hypoxia. sVEP contrast thresholds (y-axis) in children with CVI were not correlated with the sVEP grating acuities (x-axis).
Group differences in suprathreshold response amplitude were computed on the basis of individual participant response amplitudes and tested with two-tailed, heteroscedastic t-tests (Fig. 2).
Results
sVEP Response Functions
Figure 2A plots the mean response functions at the second harmonic component for contrast sVEP, and Figure 2B plots the first harmonic component for spatial frequency sVEP at Oz-Cz derivation, because these harmonic components showed the strongest responses in children with CVI, whereas all harmonic components showed equally strong responses in age-matched controls. The Oz-Cz derivation showed the largest response amplitude among all five recording derivations in both CVI and control groups. As children with hypoxic-ischemic encephalopathy (termed “hypoxia” in the rest of the article) represented most of subjects in this cohort of CVI, we also analyzed the data for children with hypoxic CVI as a subgroup.
As shown in Figures 2A and 2B, each response function showed a monotonic increase in amplitude as the stimulus values went from the invisible to visible range until the responses were saturated. In Figure 2A, the response functions for all children with CVI (termed as “all CVI,” n = 34, filled circles) and CVI with hypoxia only (n = 14, open circles) saturated at 50% contrast, whereas that of age-matched healthy controls saturated at 30% contrast, consistent with reduced contrast sensitivity in children with CVI. The response amplitudes over the entire swept range for both contrast (Fig. 2A) and spatial frequency (Fig. 2B) measures were diminished in children with CVI, compared with those of control children. The peak amplitudes at 50% contrast (Fig. 2A) and at 1 c/deg spatial frequency (Fig. 2B) swept values in all children with CVI showed the least amplitude differences between the groups but still were significantly lower than in the control children (P < 0.05). However, there was no statistically significant difference between the hypoxic and nonhypoxic CVI groups in terms of contrast at peak amplitude (P = 0.089).
Our results showed that the spatial contrast sensitivities and sVEP response amplitudes were strongly affected by CVI. For the CVI with hypoxia subgroup, the contrast response amplitudes between 20% and 80% contrast sweep range were slightly larger, compared with the entire group of children with CVI, implying that the other mixed etiologies (e.g., hydrocephalus, nonaccidental trauma, postnatal hypoxia, encephalitis) may cause more severe damage to the contrast response function. However, the spatial frequency response amplitudes for children with hypoxia were the same for the larger group.
sVEP Response Thresholds
Thresholds for an individual were determined as the minimum contrast (best) threshold for contrast measure and maximum threshold (highest spatial frequency) for the spatial frequency measure over the first four harmonic components and five recording derivations (O1-Cz, Oz-Cz, O2-Cz, Oz-O1, and Oz-O2). Among 34 children with CVI, 30 had measurable but reduced contrast thresholds with a median threshold of 1.10 log unit (10.8%, range 5.0%–30.0% Michelson), and 32 had measurable but reduced grating acuity with median threshold of 0.96 log unit (0.49 logarithm of the minimum angle of resolution [logMAR], 9.8 c/deg, range 5–14 c/deg). These thresholds were reduced relative to the mean contrast threshold of −0.3 log unit (0.5% Michelson) of 9-week-old healthy infants,17 and relative to the median grating acuity of 1.36 log unit (0.13 logMAR, 22.3 c/deg) of age-matched control children, all of whom had measureable threshold (P < 0.0001). Note that we were unable to measure contrast thresholds in age-matched control children because the contrast sweep range (10%–80%) was set for children with CVI, which was far beyond the contrast threshold for control children. Because the development of contrast sensitivity using the sVEP in infants and adults has been intensively studied,17,18 we compared the contrast threshold for healthy control children with the threshold of infants at 9 weeks old from a previous study,17 a conservative estimate of the difference.
Contrast thresholds were significantly correlated with age among those who had measurable thresholds (P < 0.01), implying that the contrast sensitivity in children with CVI improves with age (see Fig. 3). However, we did not find a significant correlation between grating acuity and age for children with CVI (P = 0.411) or for control children (P = 0.350) (see Fig. 3B). We also did not find a significant correlation between contrast thresholds and grating acuity thresholds in children with CVI (P = 0.403) (see Fig. 4).
Discussion
Despite the importance of contrast sensitivity to visual function, this aspect of vision has not been fully evaluated in patients with CVI. Previous investigations demonstrated grating4,12,14,23 and vernier2,13 acuity deficits in patients with CVI. Our results show that both contrast sensitivity and grating, as well as sVEP response amplitudes, are strongly affected by CVI. Interestingly, contrast sensitivity in children with CVI improved with age, but there was no significant improvement of grating acuity with age. Previous studies using a similar sVEP in 1- to 19-year-old children with CVI also showed reduced contrast sensitivity14 and reduced grating acuity (to 0.74 logMAR).12 In this controlled study, we found that both contrast sensitivity and grating acuity are substantially reduced in children with CVI, compared with age-matched controls. Children with CVI had a median contrast sensitivity of 10.8%, similar to the initial contrast threshold (10.0%) in those who improved with age in a previous study.14 The age range in this cohort of CVI was 5 months to 5 years (mostly between 1 and 4 years), which was younger than the range of 1 to 16 years in the previous study.14 This could explain reduced contrast thresholds in our study, because contrast thresholds might need a longer time to recover or to develop. We recorded a median grating acuity of 0.49 logMAR, which was better than 0.74 logMAR12 and 0.58 to 0.78 logMAR14 from previous studies. The low luminance background (20 cd/m2) used for grating acuity measures in our study might improve grating acuity.8,11 The grating acuity (0.49 logMAR) in the present study was similar to 0.44 logMAR from our previous study, which used the same low luminance background (20 cd/m2) in children with CVI. The same cohort showed 0.64 logMAR using a standard high-luminance background (109 cd/m2),8 which compares with values of 0.58 to 0.78 logMAR from other studies in which a high-luminance background was used.12,14
Our data show that the contrast sensitivity in CVI improves with age, but grating acuity does not, consistent with a previous study.14 However, we found that the relative contrast sensitivity deficit is worse than the grating acuity deficit in children with CVI. Contrast sensitivity loss in our cohort was reduced by a factor of 30, compared with contrast thresholds in healthy 9-week-old infants. Grating acuity thresholds were worse by a factor of 2.3, compared with age-matched control children. There was, however, no correlation between these two deficits.
In addition to contrast sensitivity and grating acuity loss, children with CVI also showed diminution of their evoked response amplitudes compared with controls over the entire spatial frequency and contrast sweep ranges. The most plausible explanation for this finding is that there simply are not as many neurons responding to the visual target. The most common insult leading to CVI in this cohort was hypoxia/ischemia, which results in a range of histological changes, particularly including neuronal loss and cortical architecture alteration.24,25 Other prominent causes of CVI are also expected to cause either white or gray matter loss.26 Compared with all children with CVI (mixed etiologies, n = 34, see Fig. 2A), the amplitudes of the contrast sweep range were slightly larger in the hypoxia-ischemia group (n = 14). This result suggests that etiologies other than perinatal hypoxia-ischemia (e.g., hydrocephalus, nonaccidental trauma, postnatal hypoxia, encephalitis) may have more of an effect on the contrast response function, although additional research is warranted, as children with hypoxic damage were a smaller number. Nevertheless, it is interesting that the spatial frequency response amplitudes in children with CVI caused by hypoxia showed the same reduction as the entire group of children with CVI.
In conclusion, our results demonstrate that the spatial contrast sensitivity and response amplitudes are strongly affected by CVI. Arguably the most important finding in this study is that most children with CVI have measurable contrast sensitivity and grating acuity, despite profound injury to their central nervous systems, making the point that CVI rarely results in complete blindness. This study supports standards used for rehabilitation of children with CVI. Deficits in contrast sensitivity and grating acuity should be addressed with the use of high-contrast materials with large-size visual targets.
Acknowledgments
We thank Margaret McGovern for her assistance in conducting the experiment for this report.
Footnotes
Supported by Grants EY015228 (WVG) and EY06579 (AMN) from the National Eye Institute, Bethesda, Maryland.
Disclosure: W.V. Good, None; C. Hou, None; A.M. Norcia, None
References
- 1.Steinkuller PG, Du L, Gilbert C, Foster A, Collins ML, Coats DK. Childhood blindness. J AAPOS. 1999;3:26–32 [DOI] [PubMed] [Google Scholar]
- 2.Skoczenski AM, Good WV. Vernier acuity is selectively affected in infants and children with cortical visual impairment. Dev Med Child Neurol. 2004;46:526–532 [DOI] [PubMed] [Google Scholar]
- 3.Good WV, Jan JE, Burden SK, Skoczenski A, Candy R. Recent advances in cortical visual impairment. Dev Med Child Neurol. 2001;43:56–60 [DOI] [PubMed] [Google Scholar]
- 4.Huo R, Burden SK, Hoyt CS, Good WV. Chronic cortical visual impairment in children: aetiology, prognosis, and associated neurological deficits. Br J Ophthalmol. 1999;83:670–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ananth CV, Joseph KS, Oyelese Y, Demissie K, Vintzileos AM. Trends in preterm birth and perinatal mortality among singletons: United States, 1989 through 2000. Obstet Gynecol. 2005;105:1084–1091 [DOI] [PubMed] [Google Scholar]
- 6.Martin JA, Kochanek KD, Strobino DM, Guyer B, MacDorman MF. Annual summary of vital statistics—2003. Pediatrics. 2005;115:619–634 [DOI] [PubMed] [Google Scholar]
- 7.Gunn AJ, Thoresen M. Hypothermic neuroprotection. NeuroRx. 2006;3:154–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edwards AD, Azzopardi DV. Therapeutic hypothermia following perinatal asphyxia. Arch Dis Child Fetal Neonatal Ed. 2006;91:F127–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Good WV, Jan JE, DeSa L, Barkovich AJ, Groenveld M, Hoyt CS. Cortical visual impairment in children. Surv Ophthalmol. 1994;38:351–364 [DOI] [PubMed] [Google Scholar]
- 10.Good WV, Hou C. Sweep visual evoked potential grating acuity thresholds paradoxically improve in low-luminance conditions in children with cortical visual impairment. Invest Ophthalmol Vis Sci. 2006;47:3220–3224 [DOI] [PubMed] [Google Scholar]
- 11.Good WV. Development of a quantitative method to measure vision in children with chronic cortical visual impairment. Trans Am Ophthalmol Soc. 2001;99:253–269 [PMC free article] [PubMed] [Google Scholar]
- 12.Watson T, Orel-Bixler D, Haegerstrom-Portnoy G. Early visual-evoked potential acuity and future behavioral acuity in cortical visual impairment. Optom Vis Sci. 2010;87:80–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Watson T, Orel-Bixler D, Haegerstrom-Portnoy G. VEP vernier, VEP grating, and behavioral grating acuity in patients with cortical visual impairment. Optom Vis Sci. 2009;86:774–780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Watson T, Orel-Bixler D, Haegerstrom-Portnoy G. Longitudinal quantitative assessment of vision function in children with cortical visual impairment. Optom Vis Sci. 2007;84:471–480 [DOI] [PubMed] [Google Scholar]
- 15.O'Connor AR, Fielder AR. Visual outcomes and perinatal adversity. Semin Fetal Neonatal Med. 2007;12:408–414 [DOI] [PubMed] [Google Scholar]
- 16.Norcia AM, Tyler CW. Spatial frequency sweep VEP: visual acuity during the first year of life. Vision Res. 1985;25:1399–1408 [DOI] [PubMed] [Google Scholar]
- 17.Norcia AM, Tyler CW, Hamer RD. Development of contrast sensitivity in the human infant. Vision Res. 1990;30:1475–1486 [DOI] [PubMed] [Google Scholar]
- 18.Norcia AM, Tyler CW, Hamer RD, Wesemann W. Measurement of spatial contrast sensitivity with the swept contrast VEP. Vision Res. 1989;29:627–637 [DOI] [PubMed] [Google Scholar]
- 19.Nuwer MR, Comi G, Emerson R, et al. IFCN standards for digital recording of clinical EEG. International Federation of Clinical Neurophysiology. Electroencephalogr Clin Neurophysiol. 1998;106:259–261 [DOI] [PubMed] [Google Scholar]
- 20.Tang Y, Norcia AM. An adaptive filter for steady-state evoked responses. Electroencephalogr Clin Neurophysiol. 1995;96:268–277 [DOI] [PubMed] [Google Scholar]
- 21.Victor JD, Mast J. A new statistic for steady-state evoked potentials. Electroencephalogr Clin Neurophysiol. 1991;78:378–388 [DOI] [PubMed] [Google Scholar]
- 22.Campbell FW, Maffei L. Electrophysiological evidence for the existence of orientation and size detectors in the human visual system. J Physiol. 1970;207:635–652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Birch EE, Bane MC. Forced-choice preferential looking acuity of children with cortical visual impairment. Dev Med Child Neurol. 1991;33:722–729 [DOI] [PubMed] [Google Scholar]
- 24.Johnston MV. Excitotoxicity in perinatal brain injury. Brain Pathol. 2005;15:234–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Robinson S, Li Q, Dechant A, Cohen ML. Neonatal loss of gamma-aminobutyric acid pathway expression after human perinatal brain injury. J Neurosurg. 2006;104:396–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pinteaux E, Trotter P, Simi A. Cell-specific and concentration-dependent actions of interleukin-1 in acute brain inflammation. Cytokine. 2009;45:1–7 [DOI] [PubMed] [Google Scholar]