Abstract
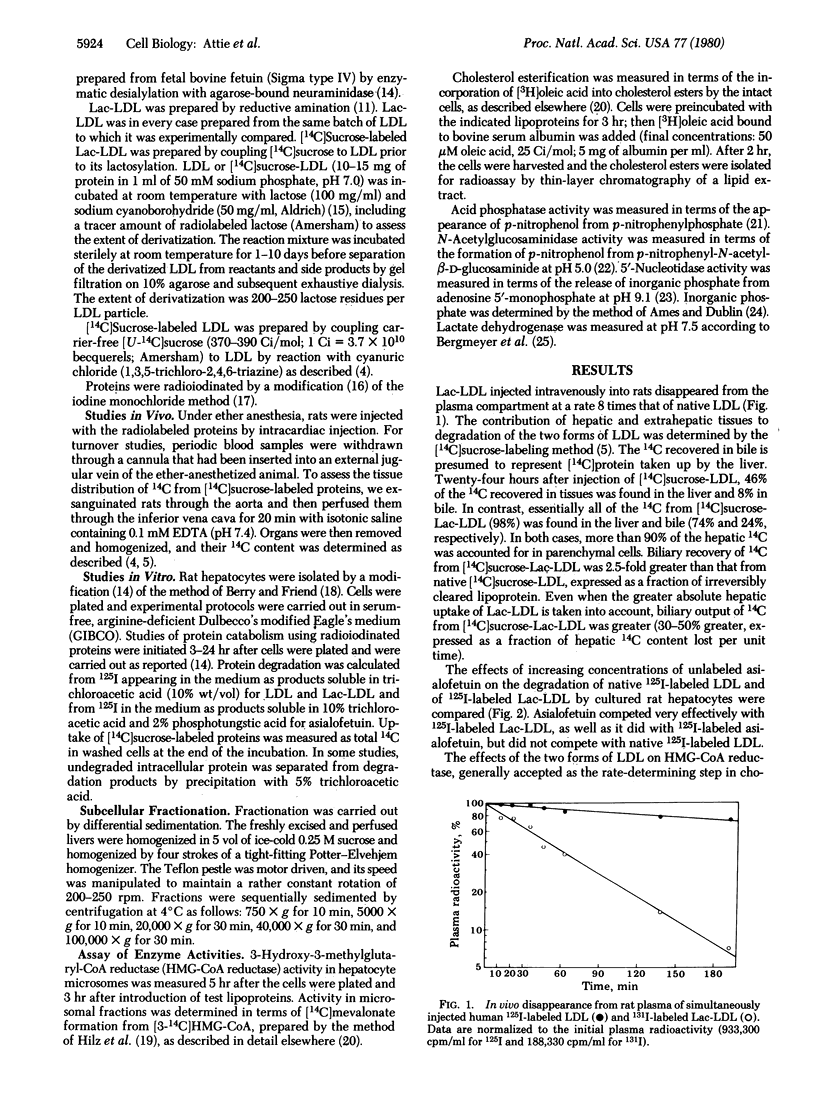
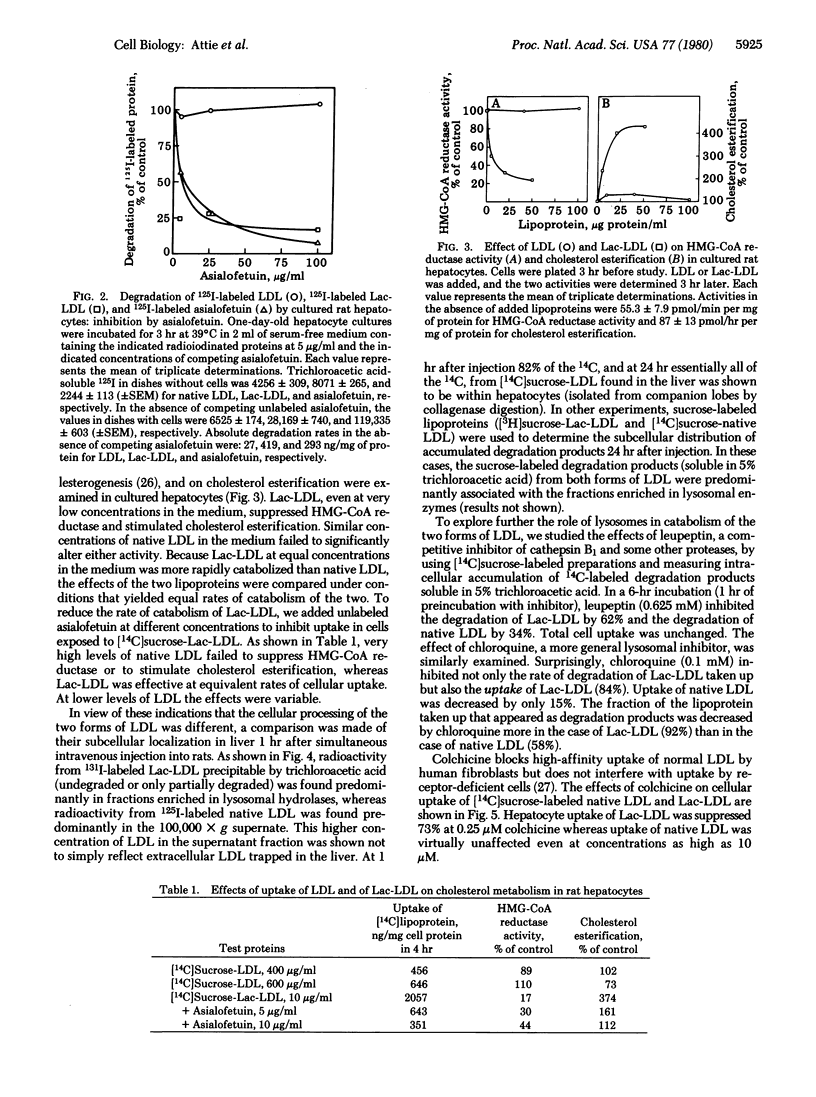
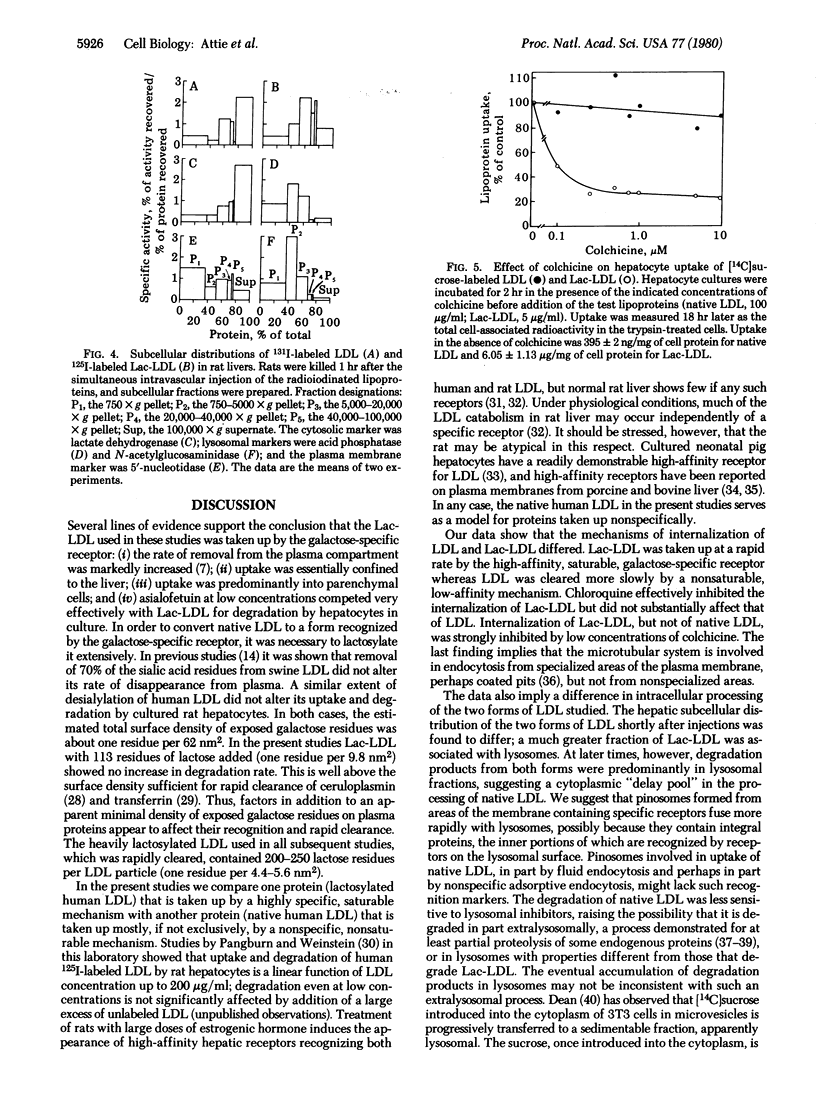
Human low density lipoprotein (LDL) covalently conjugated with 200-250 residues of lactose per LDL particle (Lac-LDL) was bound and rapidly taken up by the galactose-specific receptor of rat hepatocytes. Uptake of Lac-LDL was associated with inhibition of 3-hydroxy-3-methylglutaryl-CoA reductase and stimulation of cholesterol esterification. Uptake of native human LDL had no significant effects on these enzyme activities even when the rates of LDL uptake equaled those of Lac-LDL. When injected into rats, Lac-LDL was selectively removed by the liver (98% of injected dose). The hepatic subcellular distribution of simultaneously injected native 125I-labeled LDL and 131I-labeled Lac-LDL differed significantly, Lac-LDL was associated with fractions enriched in lysosomal hydrolases whereas native LDL was found predominantly in the supernatant fraction enriched in lactate dehydrogenase. Chloroquine (0.1 mM) markedly suppressed uptake of Lac-LDL by cultured rat hepatocytes (> 80%) but had only a small effect on uptake of native LDL. Leupeptin (0.625 mM) inhibited degradation of Lac-LDL more than it did degradation of native LDL. Colchicine (0.25 microM) dramatically suppressed uptake of Lac-LDL (> 70%) but did not affect native LDL uptake even at concentrations as high as 10 microM. Uptake of human LDL by rat hepatocytes occurs largely by nonspecific mechanisms, including fluid endocytosis, whereas Lac-LDL, as shown here, is taken up by a specific receptor-mediated mechanism. The results show further that native human LDL, representing an example of a protein taken up nonspecifically, is processed intracellularly by a pathway qualitatively distinct from that for Lac-LDL, an example of a protein taken up by a specific mechanism. Lac-LDL may serve as a vehicle for specifically delivering drugs, hormones, or radioactive compounds to hepatocytes for therapeutic or diagnostic purposes.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMES B. N., DUBIN D. T. The role of polyamines in the neutralization of bacteriophage deoxyribonucleic acid. J Biol Chem. 1960 Mar;235:769–775. [PubMed] [Google Scholar]
- Ashwell G., Morell A. G. The role of surface carbohydrates in the hepatic recognition and transport of circulating glycoproteins. Adv Enzymol Relat Areas Mol Biol. 1974;41(0):99–128. doi: 10.1002/9780470122860.ch3. [DOI] [PubMed] [Google Scholar]
- Attie A. D., Weinstein D. B., Freeze H. H., Pittman R. C., Steinberg D. Unaltered catabolism of desialylated low-density lipoprotein in the pig and in cultured rat hepatocytes. Biochem J. 1979 Jun 15;180(3):647–654. doi: 10.1042/bj1800647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachorik P. S., Kwiterovich P. O., Cooke J. C. Isolation of a porcine liver plasma membrane fraction that binds low density lipoproteins. Biochemistry. 1978 Nov 28;17(24):5287–5299. doi: 10.1021/bi00617a032. [DOI] [PubMed] [Google Scholar]
- Berry M. N., Friend D. S. High-yield preparation of isolated rat liver parenchymal cells: a biochemical and fine structural study. J Cell Biol. 1969 Dec;43(3):506–520. doi: 10.1083/jcb.43.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. I. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J Cell Biol. 1975 Dec;67(3):835–851. doi: 10.1083/jcb.67.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslow J. L., Lothrop D. A., Clowes A. W., Lux S. E. Lipoprotein regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity in rat liver cell cultures. J Biol Chem. 1977 Apr 25;252(8):2726–2733. [PubMed] [Google Scholar]
- Calandra S., Tarugi P., Battistini N., Ferrari R. Cholesterol synthesis in isolated rat hepatocytes: effect of homologous and heterologous serum lipoproteins. Metabolism. 1979 Aug;28(8):843–850. doi: 10.1016/0026-0495(79)90211-7. [DOI] [PubMed] [Google Scholar]
- Chao Y. S., Windler E. E., Chen G. C., Havel R. J. Hepatic catabolism of rat and human lipoproteins in rats treated with 17 alpha-ethinyl estradiol. J Biol Chem. 1979 Nov 25;254(22):11360–11366. [PubMed] [Google Scholar]
- Drevon C. A., Weinstein D. B., Steinberg D. Regulation of cholesterol esterification and biosynthesis in monolayer cultures of normal adult rat hepatocytes. J Biol Chem. 1980 Oct 10;255(19):9128–9137. [PubMed] [Google Scholar]
- Edwards P. A. Effect of plasma lipoproteins and lecithin-cholesterol dispersions on the activity of 3-hydroxy-3-methylglutaryl-coenzyme A reductase of isolated rat hepatocytes. Biochim Biophys Acta. 1975 Oct 21;409(1):39–50. doi: 10.1016/0005-2760(75)90078-8. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Brown M. S. The low-density lipoprotein pathway and its relation to atherosclerosis. Annu Rev Biochem. 1977;46:897–930. doi: 10.1146/annurev.bi.46.070177.004341. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Brunschede G. Y., Brown M. S. Inhibition of proteolytic degradation of low density lipoprotein in human fibroblasts by chloroquine, concanavalin A, and Triton WR 1339. J Biol Chem. 1975 Oct 10;250(19):7854–7862. [PubMed] [Google Scholar]
- Goldstein J. L., Dana S. E., Faust J. R., Beaudet A. L., Brown M. S. Role of lysosomal acid lipase in the metabolism of plasma low density lipoprotein. Observations in cultured fibroblasts from a patient with cholesteryl ester storage disease. J Biol Chem. 1975 Nov 10;250(21):8487–8495. [PubMed] [Google Scholar]
- HAVEL R. J., EDER H. A., BRAGDON J. H. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J Clin Invest. 1955 Sep;34(9):1345–1353. doi: 10.1172/JCI103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HILZ H., KNAPPE J., RINGELMANN E., LYNEN F. Methylglutaconase, eine neue Hydratase, die am Stoffwechsel verzweigter Carbonsäuren beteiligt ist. Biochem Z. 1958;329(6):476–489. [PubMed] [Google Scholar]
- Hubbard A. L., Stukenbrok H. An electron microscope autoradiographic study of the carbohydrate recognition systems in rat liver. II. Intracellular fates of the 125I-ligands. J Cell Biol. 1979 Oct;83(1):65–81. doi: 10.1083/jcb.83.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakoi L., Quarfordt S. H. Alterations of rat hepatic cholesterogenesis by heterologous lipoproteins. J Biol Chem. 1977 Oct 10;252(19):6856–6860. [PubMed] [Google Scholar]
- Knowles S. E., Ballard F. J. Selective control of the degradation of normal and aberrant proteins in Reuber H35 hepatoma cells. Biochem J. 1976 Jun 15;156(3):609–617. doi: 10.1042/bj1560609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovanen P. T., Basu S. K., Goldstein J. L., Brown M. S. Low density lipoprotein receptors in bovine adrenal cortex. II. Low density lipoprotein binding to membranes prepared from fresh tissue. Endocrinology. 1979 Mar;104(3):610–616. doi: 10.1210/endo-104-3-610. [DOI] [PubMed] [Google Scholar]
- Kovanen P. T., Brown M. S., Goldstein J. L. Increased binding of low density lipoprotein to liver membranes from rats treated with 17 alpha-ethinyl estradiol. J Biol Chem. 1979 Nov 25;254(22):11367–11373. [PubMed] [Google Scholar]
- Krieger M., Brown M. S., Faust J. R., Goldstein J. L. Replacement of endogenous cholesteryl esters of low density lipoprotein with exogenous cholesteryl linoleate. Reconstitution of a biologically active lipoprotein particle. J Biol Chem. 1978 Jun 25;253(12):4093–4101. [PubMed] [Google Scholar]
- LaBadie J. H., Chapman K. P., Aronson N. N., Jr Glycoprotein catabolism in rat liver: Lysosomal digestion of iodinated asialo-fetuin. Biochem J. 1975 Nov;152(2):271–279. doi: 10.1042/bj1520271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaRusso N. F., Fowler S. Coordinate secretion of acid hydrolases in rat bile. J Clin Invest. 1979 Oct;64(4):948–954. doi: 10.1172/JCI109561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFARLANE A. S. Efficient trace-labelling of proteins with iodine. Nature. 1958 Jul 5;182(4627):53–53. doi: 10.1038/182053a0. [DOI] [PubMed] [Google Scholar]
- Neff N. T., DeMartino G. N., Goldberg A. L. The effect of protease inhibitors and decreased temperature on the degradation of different classes of proteins in cultured hepatocytes. J Cell Physiol. 1979 Dec;101(3):439–457. doi: 10.1002/jcp.1041010311. [DOI] [PubMed] [Google Scholar]
- Nervi F. O., Dietschy J. M. Ability of six different lipoprotein fractions to regulate the rate of hepatic cholesterogenesis in vivo. J Biol Chem. 1975 Nov 25;250(22):8704–8711. [PubMed] [Google Scholar]
- Noble R. P. Electrophoretic separation of plasma lipoproteins in agarose gel. J Lipid Res. 1968 Nov;9(6):693–700. [PubMed] [Google Scholar]
- Ostlund R. E., Jr, Pfleger B., Schonfeld G. Role of microtubules in low density lipoprotein processing by cultured cells. J Clin Invest. 1979 Jan;63(1):75–84. doi: 10.1172/JCI109281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittman R. C., Attie A. D., Carew T. E., Steinberg D. Tissue sites of degradation of low density lipoprotein: application of a method for determining the fate of plasma proteins. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5345–5349. doi: 10.1073/pnas.76.10.5345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittman R. C., Green S. R., Attie A. D., Steinberg D. Radiolabeled sucrose covalently linked to protein. A device for quantifying degradation of plasma proteins catabolized by lysosomal mechanisms. J Biol Chem. 1979 Aug 10;254(15):6876–6879. [PubMed] [Google Scholar]
- Regoeczi E., Hatton M. W. Studies of the metabolism of asialotransferrins: the mechanism for the hypercatabolism of human asialotransferrin in the rabbit. Can J Biochem. 1974 Jul;52(7):645–651. doi: 10.1139/o74-092. [DOI] [PubMed] [Google Scholar]
- Silverstein S. C., Steinman R. M., Cohn Z. A. Endocytosis. Annu Rev Biochem. 1977;46:669–722. doi: 10.1146/annurev.bi.46.070177.003321. [DOI] [PubMed] [Google Scholar]
- Sniderman A. D., Carew T. E., Steinberg D. Turnover and tissue distribution of 125-I-labeled low density lipoprotein in swine and dogs. J Lipid Res. 1975 Jul;16(4):293–299. [PubMed] [Google Scholar]
- Touster O., Aronson N. N., Jr, Dulaney J. T., Hendrickson H. Isolation of rat liver plasma membranes. Use of nucleotide pyrophosphatase and phosphodiesterase I as marker enzymes. J Cell Biol. 1970 Dec;47(3):604–618. doi: 10.1083/jcb.47.3.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Den Hamer C. J., Morell A. G., Scheinberg I. H., Hickman J., Ashwell G. Physical and chemical studies on ceruloplasmin. IX. The role of galactosyl residues in the clearance of ceruloplasmin from the circulation. J Biol Chem. 1970 Sep 10;245(17):4397–4402. [PubMed] [Google Scholar]
- Wilson G. Effect of reductive lactosamination on the hepatic uptake of bovine pancreatic ribonuclease A dimer. J Biol Chem. 1978 Apr 10;253(7):2070–2072. [PubMed] [Google Scholar]



