Islet cell transplantation has recently emerged as a promising means to treat patients with type 1 diabetes. Despite significant success with the Edmonton protocol for intraportal naked islet transplantation (1), there is an active debate regarding whether or not the overall clinical success rates have met initial expectations (2,3). The next vital steps for improving islet cell transplantation protocols include developing a nontoxic and effective means to prevent graft rejection and islet cell death, as well as suitable imaging techniques to noninvasively probe islet engraftment and long-term survival.
Although it is currently unknown how long and what percentage of grafted cells survive, estimates suggest that up to only 30% of the initial β-cell mass remains after 2 weeks (4). It is believed that, aside from immunorejection, rapid islet cell death may occur from lack of oxygen before and after transplantation. One way to increase islet cell survival is to store them in perfluorocarbon emulsions that can act as an oxygen sink, leading to improved oxygenation (5). Another approach is to prevent immunorejection by encapsulating islets in semipermeable alginate capsules (6) or a combination of the two approaches (7). Although monitoring of islet engraftment following intraportal injection is possible by prelabeling naked cells (8,9) or encapsulated cells (10) with a superparamagnetic iron oxide (SPIO) contrast agent followed by magnetic resonance imaging (MRI), imaging of islet survival has been much more challenging (11).
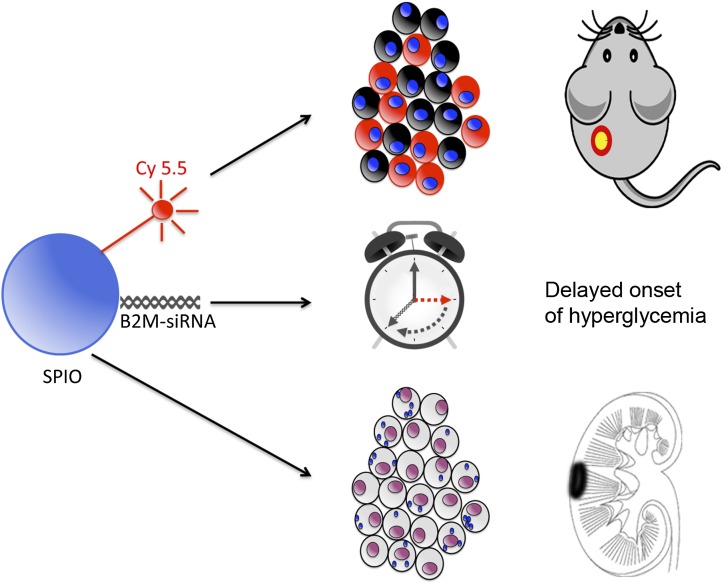
In this issue of Diabetes, Wang et al. (12) have used an elegant and clever approach for enhancing graft survival by prelabeling them with a novel theranostic nanoprobe. The formulation consists of small interference RNA (siRNA) targeting β2-microglobulin (B2M), the fluorescent dye Cy5.5 for optical detection, and SPIO nanoparticles for MRI detection (Fig. 1). The rationale for silencing B2M is to knock down functional expression of the major histocompatibility complex class I, of which B2M is a major component. A dysfunctional major histocompatibility complex class I expression will result in an impaired adaptive immune response by cytotoxic CD8+ T cells, one of the major factors driving islet immunorejection and graft failure (13,14). Cy 5.5 was incorporated primarily to assess the efficacy of B2M knockdown in vitro using fluorescent microscopy, and SPIO particles were used to evaluate graft survival in vivo through loss of signal as a surrogate marker (upon cell death, the magnetic nanoparticles are released and rapidly metabolized by host macrophages).
FIG. 1.
The theranostic nanoparticle formulation is composed of three components. The main core of the particle contains SPIO, representing the first diagnostic component as an magnetic resonance–visible hypointense contrast agent. The second diagnostic entity is the fluorescent Cy 5.5 molecule for optical detection. B2M-siRNA is the therapeutic effector molecule, inhibiting the expression of B2M and hence reducing the immunogenicity of transplanted islets. The theranostic particles are introduced into islet cells by simply adding them to the culture medium before transplantation. Cellular uptake is verified by Cy 5.5 fluorescence or SPIO-positive Prussian Blue staining. In vivo, labeled islets delay the onset of hyperglycemia in diabetic mice and can be visualized by optical imaging and MRI.
Freshly isolated cadaveric human islets were prelabeled by incubation with the theranostic targeted at silencing B2M (25 μg Fe/mL, 1.8 pmol siRNA/μg Fe) for 48 h in culture. These islets were then injected under the kidney capsule of immunodeficient B2M-deficient NOD/scid mice that lacked endogenous CD8+ T cells and that were made diabetic by intraperitoneal injection of streptozotocin, and then, at 1 week posttransplantation, splenocytes from immunocompetent NOD mice were adoptively transferred to compare the immunorejection and survival of islets with and without prelabeling of the theranostic probe (control particles contained scrambled siRNA in lieu of B2M siRNA). It was demonstrated that prelabeling with either probe did not result in loss of viability before transplantation. The B2M mRNA expression was reduced by 46% following incubation, with a concomitant decrease in protein expression as detected by Western blot and immunohistochemistry and a reduced number of activated CD8+ T cells in vitro.
In vivo, the hypointense MRI signals from the magnetic theranostic persisted longer when the islets were pretreated with the siRNA B2M probe. This correlated to a significant delay, up to 24 days in diabetes onset after the adoptive T-cell transfer as compared with 7 days for the controls. Prelabeling islets with the B2M-silencing theranostic probe was accompanied by a lower infiltration of CD8+ T cells, presumably as a result of the knockdown of B2M expression. Thus, the study by Wang et al. (12) has shown a proof-of-principle that xenografted islets can be protected from immediate immunorejection by simple prelabeling with an siRNA-based theranostic. It remains to be seen, however, if their approach will result in long-term islet protection. All grafts in this study eventually failed after 30 days posttransplantation, indicating that further therapeutic refinement will be needed. It is possible that knocking down a response by the adaptive immune system (CD8+ T cells) may be further enhanced by silencing ligands involved in binding of cells belonging to the innate immune system (e.g., natural killer cells, which are also involved in islet allograft rejection) (14).
Nonetheless, a noninvasive imaging technique to evaluate a long-term successful outcome of siRNA silencing that can be applied in patients, if successfully translated, is still not available at the present time. In preclinical studies, endowing islet cells with an optical gene allows short-term monitoring of islet cell survival (15), but this approach is not clinically translatable. Unfortunately, the indirect approach by Wang et al. (12) to endow the theranostic probe with magnetic properties to monitor graft volume in vivo may not be straightforward. Clinically, it has been shown that the number of hypointense spots on MRI does not correspond to the number of islets that were injected (16), and therefore, other techniques will need to be developed to noninvasively determine the efficacy and outcome of RNA silencing and immunomodulation.
ACKNOWLEDGMENTS
No potential conflicts of interest relevant to this article were reported.
Footnotes
See accompanying original article, p. 3247.
REFERENCES
- 1.Shapiro AM, Ricordi C, Hering BJ, et al. International trial of the Edmonton protocol for islet transplantation. N Engl J Med 2006;355:1318–1330 [DOI] [PubMed] [Google Scholar]
- 2.Khan MH, Harlan DM. Counterpoint: clinical islet transplantation: not ready for prime time. Diabetes Care 2009;32:1570–1574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mineo D, Pileggi A, Alejandro R, Ricordi C. Point: steady progress and current challenges in clinical islet transplantation. Diabetes Care 2009;32:1563–1569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davalli AM, Ogawa Y, Ricordi C, Scharp DW, Bonner-Weir S, Weir GC. A selective decrease in the beta cell mass of human islets transplanted into diabetic nude mice. Transplantation 1995;59:817–820 [PubMed] [Google Scholar]
- 5.Ricordi C, Fraker C, Szust J, et al. Improved human islet isolation outcome from marginal donors following addition of oxygenated perfluorocarbon to the cold-storage solution. Transplantation 2003;75:1524–1527 [DOI] [PubMed] [Google Scholar]
- 6.Tuch BE, Keogh GW, Williams LJ, et al. Safety and viability of microencapsulated human islets transplanted into diabetic humans. Diabetes Care 2009;32:1887–1889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barnett BP, Ruiz-Cabello J, Hota P, et al. Fluorocapsules for improved function, immunoprotection, and visualization of cellular therapeutics with MR, US, and CT imaging. Radiology 2011;258:182–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Toso C, Vallee JP, Morel P, et al. Clinical magnetic resonance imaging of pancreatic islet grafts after iron nanoparticle labeling. Am J Transplant 2008;8:701–706 [DOI] [PubMed] [Google Scholar]
- 9.Bulte JW. In vivo MRI cell tracking: clinical studies. AJR Am J Roentgenol 2009;193:314–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barnett BP, Arepally A, Stuber M, Arifin DR, Kraitchman DL, Bulte JW. Synthesis of magnetic resonance-, X-ray- and ultrasound-visible alginate microcapsules for immunoisolation and noninvasive imaging of cellular therapeutics. Nat Protoc 2011;6:1142–1151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arifin DR, Bulte JW. Imaging of pancreatic islet cells. Diabetes Metab Res Rev 2011;27:761–766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang P, Yigit MV, Ran C, et al. A theranostic small interfering RNA nanoprobe protects pancreatic islet grafts from adoptively transferred immune rejection. Diabetes 2012;61:3247–3254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mirenda V, Golshayan D, Read J, et al. Achieving permanent survival of islet xenografts by independent manipulation of direct and indirect T-cell responses. Diabetes 2005;54:1048–1055 [DOI] [PubMed] [Google Scholar]
- 14.Ramirez-Victorino F, Beilke JN, Gill RG. Both innate and adaptive major histocompatibility complex class I-dependent immunity impair long-term islet xenograft survival. Transplant Proc 2008;40:557–558 [DOI] [PubMed] [Google Scholar]
- 15.Teratani T, Matsunari H, Kasahara N, Nagashima H, Kawarasaki T, Kobayashi E. Islets from rats and pigs transgenic for photogenic proteins. Curr Diabetes Rev 2012;8:382–389 [DOI] [PubMed] [Google Scholar]
- 16.Saudek F, Jirák D, Girman P, et al. Magnetic resonance imaging of pancreatic islets transplanted into the liver in humans. Transplantation 2010;90:1602–1606 [DOI] [PubMed] [Google Scholar]