Abstract
Recruitment of activated immune cells into white adipose tissue (WAT) is linked to the development of insulin resistance and obesity, but the mechanism behind this is unclear. Here, we demonstrate that Y1 receptor signaling in immune cells controls inflammation and insulin resistance in obesity. Selective deletion of Y1 receptors in the hematopoietic compartment of mice leads to insulin resistance and inflammation in WAT under high fat–fed conditions. This is accompanied by decreased mRNA expression of the anti-inflammatory marker adiponectin in WAT and an increase of the proinflammatory monocyte chemoattractant protein-1 (MCP-1). In vitro, activated Y1-deficient intraperitoneal macrophages display an increased inflammatory response, with exacerbated secretion of MCP-1 and tumor necrosis factor, whereas addition of neuropeptide Y to wild-type macrophages attenuates the release of these cytokines, this effect being blocked by Y1 but not Y2 receptor antagonism. Importantly, treatment of adipocytes with the supernatant of activated Y1-deficient macrophages causes insulin resistance, as demonstrated by decreased insulin-induced phosphorylation of the insulin receptor and Akt as well as decreased expression of insulin receptor substrate 1. Thus, Y1 signaling in hematopoietic-derived cells such as macrophages is critical for the control of inflammation and insulin resistance in obesity.
Insulin resistance is commonly observed in obesity and is associated with progression to type 2 diabetes. The low-grade inflammation that develops in obesity is a major contributor to insulin resistance (1,2). Indeed, specific blockade of proinflammatory cytokines, such as monocyte chemoattractant protein 1 (MCP-1) and tumor necrosis factor (TNF), overexpressed in the white adipose tissue (WAT) of obese animals, improves insulin action in obese mice (3–6). Proinflammatory cytokines such as these are mostly secreted by activated immune cells (7–11), especially by macrophages recruited into the WAT, in proportion to the degree of obesity in both humans and mice (12,13). Thus, macrophages appear to play a key role in the development of resistance to the action of insulin. In keeping with this, specific modulation of macrophage-mediated inflammation influenced insulin resistance in diet-induced obese mice (14). Moreover, although a time-course study showed that macrophages were the last cells recruited into WAT during the development of obesity, complete lack of T and B cells did not improve insulin action in diet-induced obese mice (15), indicating that other cells besides T and B cells are also involved.
Macrophage functions are highly regulated by cytokines and hormones, but recent evidence suggests an important role for neuronal signals (16). One such molecule is neuropeptide Y (NPY), which is widely expressed in the central and peripheral nervous systems and is involved in the regulation of multiple physiological functions (17). However, NPY is also produced by nonneuronal cells, including adipocytes (18). Interestingly, NPY expression is upregulated in the WAT of obese rats, suggesting a local autocrine and/or paracrine function (19). NPY has been shown to have profound effects on the functions of macrophages, T cells, mast cells, and natural killer cells, mostly via signaling through Y1 (20), one of the five G-coupled receptors for NPY and the related peptides peptide YY (PYY) and pancreatic polypeptide. Interestingly, NPY has been shown to counteract the development of inflammatory diseases such as experimental autoimmune encephalomyelitis (21), and acting via Y1 has a direct anti-inflammatory effect on macrophages by stimulating the release of transforming growth factor-β (22). Thus, NPY could also potentially play a role in the development of insulin resistance via actions in the periphery, possibly via direct effects on immune cells.
The immune system is known to be involved in the development of insulin resistance, and NPY and Y1 signaling are known to regulate immune function. However, whether the action of NPY and Y1 signaling in immune cells is linked with alterations in inflammatory responses and the development of insulin resistance in obesity is unknown. As Y1 receptors on immune cells are key mediators of the anti-inflammatory effects of NPY (21,22), we hypothesized that lack of Y1 expression in immune cells would exacerbate the inflammation and thus the insulin resistance associated with diet-induced obesity in mice. To test our hypothesis, we used two different mouse models: mice with germline deletion of Y1 and mice with conditional deletion of Y1 from the hematopoietic compartment only, achieved by transferring bone marrow from Y1 knockout (KO) mice into lethally irradiated, wild-type recipient mice. Both mouse models were tested on a high-fat diet in order to determine the impact of Y1 deficiency on diet-induced obesity and insulin resistance, and subsequent in vitro studies were used to determine underlining mechanisms for observed differences.
RESEARCH DESIGN AND METHODS
Animals.
Generation of the Y1, Y2, and NPY PYY germline KO mice were published previously (23–25). Female C57BL/6 mice were purchased from Animal Resources Centre (Canning Vale, Western Australia, Australia). All KO lines were originally on a mixed C57BL/6-129SVJ background. They were backcrossed at least six times onto the C57BL/6 background, and all lines except for the NPY PYY double-KO line were maintained by breeding of heterozygous parents. The NPY PYY double-KO line was maintained by mating homozygous parents due to the low chance (1 in 16 offspring) of obtaining wild-type or double homozygous KO offspring from heterozygous breeding. All research and animal care procedures were approved by the Garvan Institute/St. Vincent’s Hospital Animal Ethics Committee and were in agreement with the Australian Code of Practice for the Care and Use of Animals for Scientific Purpose. The mice used were females and were group housed under conditions of controlled temperature (22°C) and illumination (12-h light cycle; lights on at 0700 h). Mice were fed ad libitum with a normal chow diet (6, 21, and 71% of calories from fat, protein, and carbohydrates; 2.6 kcal/g; Gordon’s Specialty Stock Feeds, Yanderra, New South Wales, Australia) or a high-fat diet (43, 17, and 40% of calories from fat, protein, and carbohydrates; 4.8 kcal/g; Specialty Feeds, Glen Forrest, Western Australia, Australia), unless otherwise stated. All animals had free access to water.
Fat mass determination.
Immediately after killing mice by cervical dislocation and decapitation, trunk blood was collected for subsequent serum analyses, and the left side of the inguinal, periovarian, and retroperitoneal, as well as the whole mesenteric, WAT depots were dissected out and weighed.
Blood glucose and serum insulin and adiponectin measurements.
Blood glucose was measured with AccuCheck blood glucose meter (Roche Diagnostics, Mannheim, Germany) from tail blood samples. Serum insulin and adiponectin were determined with an insulin ELISA kit (Crystal Chem, Inc., Downers Grove, IL) and a kit from R&D Systems Inc. (Minneapolis, MN), respectively.
Insulin tolerance test.
Insulin (Novo Nordisk Pharmaceuticals, Baulkham Hills, New South Wales, Australia) was injected intraperitoneally at a dose of 0.5 or 1 mU/g body weight after fasting for 5 h from 0900 to 1400 h. Blood glucose was measured in tail blood samples.
Glucose tolerance test.
Glucose (AstraZeneca, North Ryde, New South Wales, Australia) was injected intraperitoneally at a dose of 1 mg/g body weight after 16 h fasting. Blood glucose was measured in tail blood as described above.
Quantitative PCR.
Total RNA was isolated using TRIzol reagent (Invitrogen, Life Technologies, Mulgrave, Victoria, Australia), and cDNA was synthesized using SuperScript VILO cDNA Synthesis Kit (Invitrogen). Real-time PCR was conducted using SensiMix Probe (Bioline, Alexandria, New South Wales, Australia). All results were normalized with the housekeeping gene RPL13a and expressed as fold increase over control values as previously described (26).
Bone marrow chimeric mice.
Recipient mice at 6 weeks of age were lethally irradiated (950 rad). The following day, they were given an intravenous injection of 5 × 106 bone marrow cells isolated from Y1KO, Y2KO, or wild-type mice. Mice were put on a high-fat diet at 8 weeks after irradiation.
Genotyping bone marrow chimeric mice.
DNA was phenol/chloroform extracted from frozen spleen, muscle, periovarian WAT, liver, and tail tips and was subjected to PCR using the combination of oligonucleotides MY1H (5′-tggcaaaacaggtccctg-3′) and MY1-P5 (5′-ctagccagttggtaatgg-3′). The 520-bp band indicates deletion of the Y1 receptor gene, and the 750-bp band indicates presence of the wild-type gene.
Food intake measurement.
Mice were transferred to litter-free individual cages in order to accurately determine actual food intake independently of the amount of food spilled on the cage floor. Measurements indicate an average of 3 days.
Experiments on intraperitoneal macrophages.
Mice at 8–12 weeks of age were injected intraperitoneally with 2 mL 3% thioglycollate (Sigma-Aldrich, St. Louis, MO) 3 days before being killed by CO2 asphyxiation. Intraperitoneal macrophages were collected by peritoneal lavage with 10 mL sterile, cold PBS. Macrophages were purified by adherence to tissue culture plates for 2 h and cultured at 2 × 106 cells/mL. Macrophages were either incubated with 2 units/mL recombinant rat interferon-γ (IFN-γ) (R&D Systems) for 4 h followed by incubation for 24 h with or without 100 ng/mL lipopolysaccharide (LPS) (Sigma-Aldrich) in PBS vehicle, or with 400 μmol/L palmitate (Sigma-Aldrich) prepared as previously described (27) with BSA as control. Supernatants were harvested for further measurements of cytokine concentrations as described below. The Y1-specific antagonist peptide 1229U91 (1 μmol/L; Auspep Pty Ltd, Parkville, Victoria, Australia) was added with IFN-γ when specified. NPY (Auspep Pty Ltd) was added with LPS at 10 or 100 nmol/L for 24 h when specified. For studies on extracellular signal–related kinase 1/2 (Erk1/2) phosphorylation, macrophages were incubated in serum-free medium for 2 h and incubated with 100 nmol/L NPY at the indicated time points with or without pretreatment for at least 20 min with a 1 μmol/L concentration of the Y1-specific antagonist peptide 1229U91 or a 1 μmol/L concentration of the Y2-specific peptide antagonist BIIE0246 (Auspep Pty Ltd). Total and phosphorylated Erk levels were analyzed as described below. Macrophages were incubated with epidermal growth factor (EGF) (Sigma-Aldrich) for 5 min at 100 ng/mL as a positive control for Erk1/2 phosphorylation. For determination of arginase I activity, intraperitoneal macrophages (1 × 106/mL) were cultured with 10 ng/mL recombinant interleukin (IL)-4 (Peprotech Inc., Rocky Hill, NJ) for 48 h. Arginase I activity was monitored via a colorimetric assay (28). ELISAs for MCP-1, IL-6, and TNF were performed using kits from BD Biosciences (San Jose, CA).
Differentiation and treatment of 3T3L1 preadipocytes.
3T3L1 preadipocytes were cultured and differentiated (29). At day 12 postdifferentiation, adipocytes were incubated for 24 h with supernatants of macrophages from Y1KO or wild-type mice activated in the following three conditions: PBS for 28 h; 2 units/mL IFN-γ for 4 h and PBS for 24 h; or 2 units/mL IFN-γ for 4 h and 100 ng/mL LPS for 24 h. Cells were then incubated in serum-free, high-glucose Dulbecco’s modified Eagle’s medium for 2 h followed by 10 min in the same medium with the addition of either PBS or insulin at 7 nmol/L. Antibodies used for immunoblots were against total and phosphorylated Erk1/2 (p-T202/Y204) and total and phosphorylated Akt (p-T308), both purchased from Cell Signaling Technology (Arundel, Queensland, Australia). Other antibodies were against insulin receptor substrate 1 (IRS-1) (Millipore, Billerica, MA), IR β-subunit (p-Y1162/63; BD Biosciences, Franklin Lakes, NJ), glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Abacus ALS, East Brisbane, Queensland, Australia). Quantification was performed with ImageJ software (a public domain Java image processing program).
Statistical analyses.
Student t tests or repeated-measures ANOVA followed by Bonferroni post hoc tests were performed with Prism software (GraphPad Software, Inc., La Jolla, CA). Differences were regarded as statistically significant if P < 0.05.
RESULTS
Germline deletion of Y1 receptors exacerbates diet-induced obesity, insulin resistance, and inflammation.
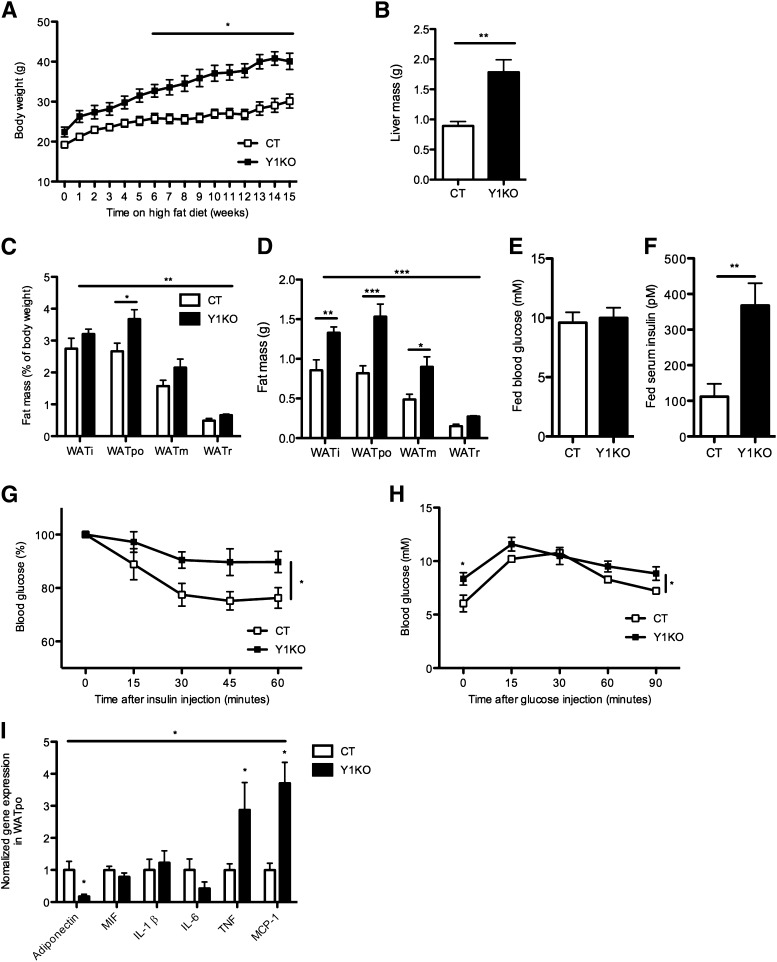
To study the role of Y1 receptor signaling in the regulation of insulin sensitivity in obesity, germline Y1 receptor KO mice were fed a high-fat diet for 15–17 weeks. Our high-fat diet induced obesity in both control and Y1KO mice, as indicated by significant diet-induced increases in body weight and adiposity. For instance, body weight in control mice at 25 weeks of age was 24.2 ± 0.4 g in chow-fed animals versus 30.1 ± 1.8 g in those on the high-fat diet. Corresponding body weights in Y1KO mice were 24.3 ± 0.8 g in chow-fed animals versus 40.1 ± 2.0 g in high fat–fed mice (means ± SEM of four to five mice per group; P < 0.05 for the effect of diet, genotype, and the interaction). From week 6 on the high-fat diet onwards, Y1KO mice were significantly heavier than control mice (Fig. 1A) despite similar food intake (0.065 ± 0.012 vs. 0.068 ± 0.005 g/g body weight per day in control and Y1KO mice, respectively; means ± SEM of five mice per group). This increased body weight may be partially due to an increase in nonfat mass, as indicated by the significant increase in liver mass (Fig. 1B) as well as a significant increase in relative and absolute weight of WAT (Fig. 1C and D). Under high fat–fed conditions, despite similar blood glucose levels (Fig. 1E), Y1KO mice presented markedly and significantly higher serum insulin levels (Fig. 1F). These differences were suggestive of an insulin-resistant status, which was confirmed by the significantly blunted hypoglycemic response to intraperitoneal insulin injection in Y1KO compared with wild-type mice (Fig. 1G). During the glucose tolerance test, blood glucose concentrations were significantly higher in Y1KO than in wild-type mice (Fig. 1H), albeit the area under the glucose tolerance curves (after subtracting baseline values) were comparable between genotypes (889 ± 46 vs. 798 ± 27 mmol/L ⋅ min for Y1KO and wild-type mice, respectively; means ± SEM of five mice per group).
FIG. 1.
Y1KO mice develop exacerbated obesity, metabolic alterations, and fat inflammation under high-fat feeding conditions. A: Body weight of Y1KO and wild-type mice (CT) fed on a high-fat diet from 10 to 25 weeks of age, represented as 0–15 weeks on the x-axis. B: Liver mass. Relative (as a percent of body weight) (C) and absolute (D) weight of dissected WAT depots from CT and Y1KO mice at 27 weeks of age, after 17 weeks on the high-fat diet. i, inguinal; po, periovarian; m, mesenteric; r, retroperitoneal. Blood glucose (E) and serum insulin (F) were measured in freely fed CT and Y1KO mice after 17 weeks on the high-fat diet. G: Blood glucose response (as percent of baseline values after 5 h fasting) to intraperitoneal insulin injection (0.5 units/kg) in CT and Y1KO mice after 16 weeks on the high-fat diet. H: Blood glucose concentrations in response to intraperitoneal glucose injection (1 g/kg) in CT and Y1KO mice after 17 weeks on the high-fat diet and after a 16-h fast. I: Analysis by real-time PCR of gene expression in WATpo from CT and Y1KO mice after 17 weeks on the high-fat diet. MIF, migration inhibitory factor. Data are means ± SEM of five female mice per group. *P < 0.05, **P < 0.01, and ***P < 0.001, vs. Y1KO vs. CT.
We aimed to determine whether obese and insulin-resistant high fat–fed Y1KO mice present indices of increased inflammation. Expression of mRNA for adiponectin, an anti-inflammatory adipokine known to be downregulated in insulin-resistant humans and animals (30), was decreased by more than sevenfold in the periovarian WAT (WATpo) of Y1KO compared with wild-type mice on the high-fat diet (Fig. 1I), with no difference in serum adiponectin levels between groups (36.9 ± 2.0 vs. 44.8 ± 3.4 μg/mL in control and Y1KO mice, respectively; means ± SEM of five mice per group). On the other hand, mRNA expression of the proinflammatory cytokines TNF and MCP-1 was significantly upregulated in Y1KO WATpo, whereas the mRNA expression of macrophage migration inhibitory factor, IL-1β, and IL-6, also known to be upregulated in obesity (31), was comparable between genotypes (Fig. 1I).
Lack of Y1 receptor in hematopoietic cells is sufficient to exacerbate insulin resistance and fat inflammation under a high-fat diet.
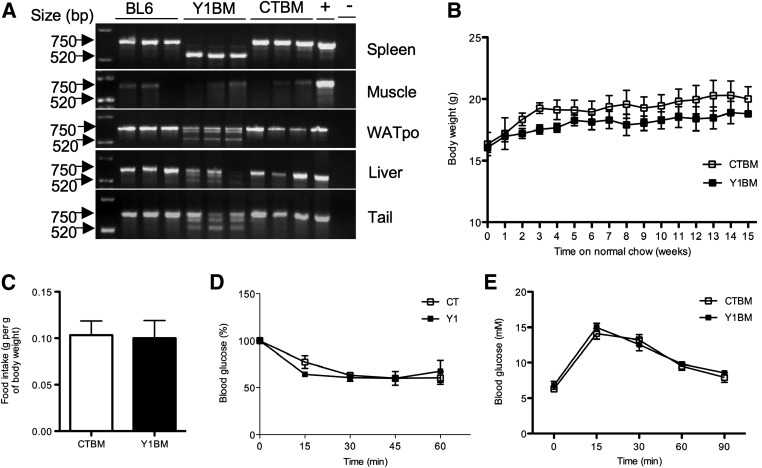
To determine whether the increased inflammation and exacerbated insulin resistance in Y1KO mice was due to impaired function of immune cells, we generated bone marrow chimeric mice specifically lacking Y1 in the hematopoietic compartment. After lethal irradiation, C57BL/6 mice were reconstituted with bone marrow isolated from either wild-type (CTBM) or Y1KO mice (Y1BM). As seen in Fig. 2A, the system was validated by demonstrating complete deletion of the Y1 gene in the spleen (which is mainly constituted of hematopoietic cells) and no deletion in the muscle (which is infiltrated with very few hemotopoietic cells), and a mixed population of Y1 receptor–deleted and Y1 receptor–intact cells were found in WATpo, liver, and tail, where both hematopoietic and nonhematopoietic cells are present. To check that deletion of Y1 in hematopoietic-derived cells would not adversely affect animal health, we monitored body weight and found no significant differences in absolute body weight in the Y1BM compared with CTBM mice (Fig. 2B), with no differences in normal chow intake (Fig. 2C). Compared with chow-fed CTBM control animals, chow-fed Y1BM mice showed comparable glycemic responses to insulin (Fig. 2D) and glucose (Fig. 2E).
FIG. 2.
Mice lacking Y1 receptor in the hematopoietic compartment remain healthy under normal chow feeding conditions. A: DNA from the indicated tissues was extracted from C57BL/6 (BL6) mice, or from irradiated C57BL/6 mice that had been reconstituted with bone marrow from either Y1KO or wild-type mice, termed Y1BM or CTBM, respectively. A PCR product of 750 bp indicates no Y1 gene deletion, and a 520-bp product indicates Y1 gene deletion. Mouse genomic DNA and water were used as positive (+) and negative (−) controls, respectively. B: Body weight of normal, chow-fed CTBM and Y1BM from 14 to 29 weeks of age, as indicated as 0–15 weeks on the x-axis. C: Food intake (grams per gram of mouse body weight) of 29-week-old CTBM and Y1BM mice on a normal chow diet. Blood glucose concentration in response to intraperitoneal injection of 1 unit/kg insulin after a 5-h fast (D) or 1 g/kg glucose after a 16-h fast (E) in normal, chow-fed CTBM and Y1BM mice at 29–30 weeks of age. Data are means ± SEM of three to four female mice per group.
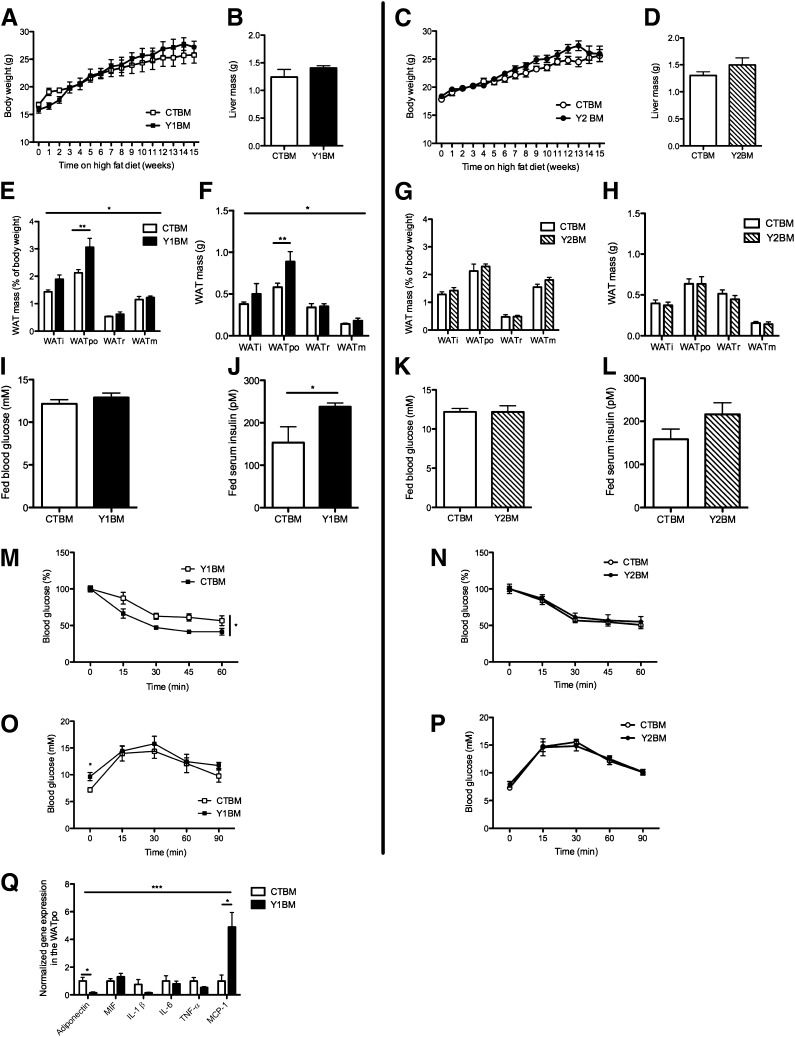
To determine the role of Y1 signaling in the hematopoietic compartment in diet-induced obesity and insulin resistance, mice reconstituted with Y1BM or CTBM were fed a high-fat diet for 15 weeks. Y1BM and CTBM mice had similar body weights (Fig. 3A) as well as liver masses (Fig. 3B), but Y1BM had significant increases in relative and absolute WAT weight (Fig. 3E and F) and serum insulin levels (Fig. 3J) with unchanged serum glucose levels (Fig. 3I) in the fed state, suggesting resistance to the action of insulin. As in germline Y1KO mice, Y1BM mice exhibited insulin resistance relative to CTBM, as indicated by a blunted decrease in blood glucose in response to intraperitoneal insulin injection (Fig. 3M), with elevated fasting serum glucose levels but a similar tolerance to intraperitoneal glucose injection (Fig. 3O). We also studied the impact of a lack of Y2, known to be expressed by immune cells (20,32), in a Y2-deficient bone marrow chimera model (Y2BM). Importantly, the lack of Y2 in hematopoietic-derived cells had no effect on any of the parameters shown (Fig. 3C, D, G, H, K, L, N, and P), demonstrating the specific role of Y1 in hematopoetic-derived cells in the regulation of glucose and insulin homeostasis. Similar to our finding in germline Y1KO mice, we found a significant decrease in the expression of adiponectin mRNA in the WATpo of Y1BM versus CTBM mice (Fig. 3Q), consistent with their impaired response to insulin and the fact that reduced adiponectin expression is associated with an insulin-resistant state (30). No differences in serum adiponectin concentrations were observed between groups (32.0 ± 4.3 vs. 33.9 ± 3.8 μg/mL in CTBM and Y1BM mice, respectively; means ± SEM of four mice per group). We also observed a significant increase in WATpo mRNA levels of MCP-1 in Y1BM mice, with no significant difference in expression of migration inhibitory factor, IL-1β, IL-6, or TNF between genotypes (Fig. 3Q). We did not find any significant differences between Y1BM and CTBM mice with respect to adipocyte size nor in the number of macrophages in WATpo (data not shown). Thus, the increased MCP-1 mRNA expression in Y1BM WATpo may be due to alterations in macrophage activation.
FIG. 3.
Lack of hematopoietic Y1 but not Y2 receptors exacerbates insulin resistance under high-fat conditions. Body weight (A and C) and liver weight (B and D) of irradiated C57BL/6 mice that had been reconstituted with bone marrow from either Y1KO, Y2KO, or wild-type mice, termed Y1BM, Y2BM, or CTBM, respectively. Mice were fed on a high-fat diet from 14 to 29 weeks of age, represented as 0–15 weeks on the x-axis. Relative (as a percent of body weight) (E and G) and absolute (F and H) weight of dissected WAT depots. Fed blood glucose (I and K) and fed serum insulin levels (J and L) of CTBM, Y1BM, and Y2BM mice at 31 weeks of age, after 17 weeks on the high-fat diet. i, inguinal; po, periovarian; m, mesenteric; r, retroperitoneal. Blood glucose response to intraperitoneal injection of 1 unit/kg insulin after 5 h of fasting (M and N) or 1 g/kg glucose after 16 h of fasting (O and P) in CTBM, Y1BM, and Y2BM mice at 30–31 weeks of age, after 16–17 weeks on the high-fat diet. Q: Analysis by real-time PCR of gene expression in WATpo from CTBM or Y1BM mice at 29 weeks of age, after 17 weeks on the high-fat diet. MIF, migration inhibitory factor. Data are means ± SEM of 4–10 female mice per group. *P < 0.05, **P < 0.01, and ***P < 0.001 Y1BM vs. CTBM.
Lack of Y1 signaling in macrophages exacerbates their response to inflammatory stimuli (M1 phenotype), with no effect on their alternatively activated phenotype (M2 phenotype).
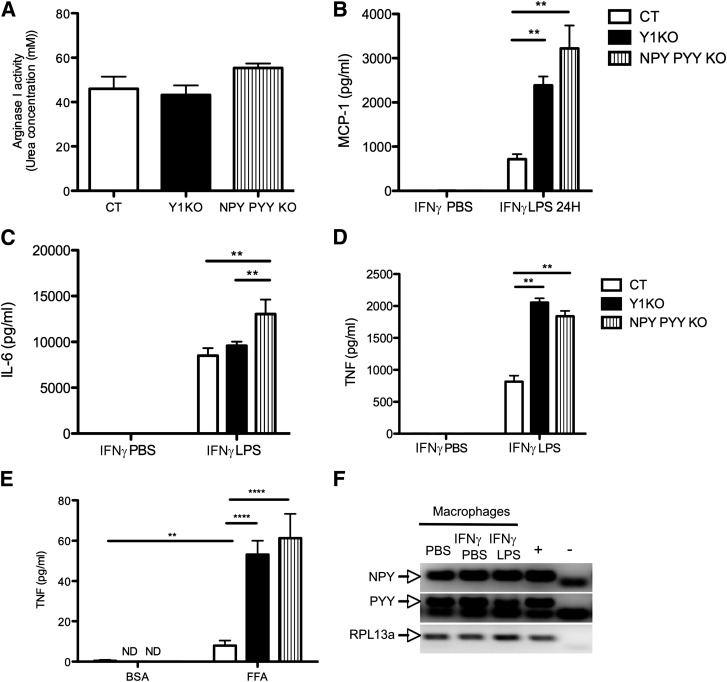
It has been suggested that a switch in macrophage phenotype from an alternatively activated, or M2, phenotype toward a proinflammatory, or M1, phenotype in WAT can contribute to the development of low-grade inflammation and insulin resistance (33). To elucidate if Y1 signaling in macrophages affects the balance between the M1 and M2 phenotypes, we studied intraperitoneal macrophages isolated from Y1KO mice or intraperitoneal macrophages lacking NPY and PYY, endogenous activators of Y1 (i.e., macrophages isolated from NPY PYY double-KO mice). The activity of arginase I, which can be induced by the cytokine IL-4, is used as a marker of the M2 phenotype (14). Macrophages from Y1KO and NPY PYY double-KO mice did not show a defect in arginase I activity (Fig. 4A), indicating no impairment in their M2 phenotype. To study the M1 phenotype, we stimulated intraperitoneal macrophages from wild-type, Y1KO, or NPY PYY double-KO mice with IFN-γ and LPS, a ligand for toll-like receptor 4 that promotes classical macrophage activation or the M1 phenotype (34). Both IFN-γ and Toll-like receptor 4 are known to contribute to WAT inflammation and progression to insulin resistance (35,36). Interestingly, we found that macrophages lacking Y1 signaling released significantly more MCP-1 (Fig. 4B), IL-6 (Fig. 4C), and/or TNF (Fig. 4D) than wild-type macrophages, suggesting a critical autologous role of NPY and/or PYY, acting via Y1, on the macrophage M1 inflammatory response. An increased inflammatory response was also observed when Y1KO or NPY PYY double-KO macrophages were activated with palmitate, as indicated by the enhanced secretion of TNF compared with control macrophages (Fig. 4E). After incubation of macrophages with BSA or palmitate, MCP-1 and IL-6 were not detectable (data not shown). The possible autologous effects of NPY and PYY to regulate the macrophage inflammatory response were corroborated by the presence of mRNA for both NPY and PYY in wild-type macrophages under basal (PBS), IFN-γ–primed (IFN-γ PBS), and activated conditions (IFN-γ LPS) (Fig. 4F).
FIG. 4.
Lack of Y1 receptor signaling in macrophages enhances the M1 but not the M2 phenotype. A: Arginase activity of intraperitoneal macrophages isolated from wild-type (CT), Y1KO, and NPY PYY KO mice after 48 h of activation with 10 ng/mL IL-4, as indicated by the resultant concentration of urea in the medium. Concentration of MCP-1 (B), IL-6 (C), and TNF (D) in the supernatants of intraperitoneal macrophages isolated from CT, Y1KO, and NPY PYY KO mice that had been incubated for 4 h with 2 units/mL IFN-γ followed by 24 h with either PBS (IFN-γ PBS) or 100 ng/mL LPS (IFN-γ LPS). E: Concentration of TNF in the supernatant of intraperitoneal CT, Y1KO, and NPY PYY KO macrophages stimulated for 24 h with either BSA or 400 μmol/L palmitate. ND, not detectable. Data are means ± SEM of three or more female mice per group, each measurement being made in triplicate at least twice. **P < 0.01 and ****P < 0.0001 for the comparison shown by horizontal bars. F: PCR illustrating the presence of NPY and PYY mRNA in wild-type intraperitoneal macrophages that had either been incubated in PBS, primed for 4 h with 2 units/mL IFN-γ followed by 24 h in PBS (IFN-γ PBS), or primed for 4 h with 2 units/mL IFN-γ followed by 24 h of 100 ng/mL LPS (IFN-γ LPS) (the RNA was extracted from triplicates pooled). Hypothalamic cDNA and water were used as positive (+) and negative (−) controls, respectively, and RPL13a was used as a housekeeping gene.
NPY has direct anti-inflammatory effects on macrophages via Y1 receptors.
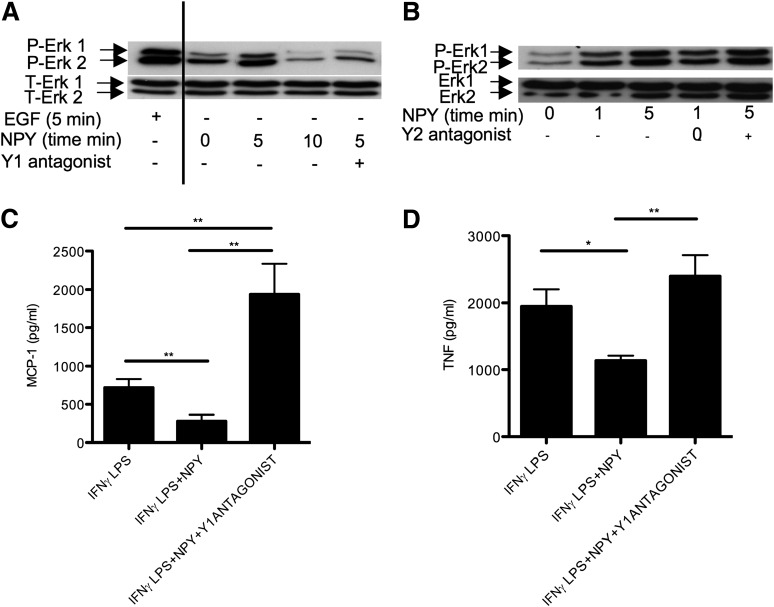
As lack of Y1 or the Y1 ligands NPY and PYY in macrophages is associated with an exacerbated inflammatory response, as described above, we hypothesized that NPY action via Y1 signaling would be a key controller of macrophage-induced inflammation. First, we demonstrated that NPY can efficiently signal in macrophages, since it time-dependently induced Erk1/2 phosphorylation in primary cultures of intraperitoneal macrophages (Fig. 5A and B), with a potency comparable to that of the positive control, EGF. Then, to determine if this effect was specifically mediated by Y1 signaling, we measured the impact of NPY on Erk1/2 phosphorylation in the presence of either a Y1- or Y2-specific antagonist. Pretreatment with the Y1 antagonist 1229U91, but not with the Y2 antagonist BIIE0246, strongly diminished NPY-induced Erk1/2 phosphorylation in macrophages (Fig. 5A and B), showing a dominant role of Y1 receptors in mediating the anti-inflammatory effects of NPY on macrophages. To further investigate the role of Y1 signaling in the inflammatory response, we activated macrophages with IFN-γ and LPS to induce the M1 phenotype, and then assessed the effect of NPY on the release of major inflammatory cytokines, in the presence or absence of the Y1 receptor antagonist 1229U91. Addition of NPY to activated macrophages had a strong anti-inflammatory effect, inhibiting MCP-1 (Fig. 5C) and TNF (Fig. 5D) release, and this effect of NPY was abolished by the addition of Y1 antagonist (Fig. 5C and D).
FIG. 5.
NPY signals in and attenuates the M1 phenotype of intraperitoneal macrophages via Y1 receptors. Levels of phosphorylated (P) and total (T) Erk 1 and 2 (P-Erk1, P-Erk2, T-Erk1, and T-Erk2) in intraperitoneal macrophages that had been either activated with 100 ng/mL EGF for 5 min (positive control) (A) or with 100 nmol/L NPY for the indicated times (minutes) (A and B), with or without pretreatment with a 1 μmol/L concentration of the specific Y1 receptor antagonist 1229U91 (A) or the specific Y2 receptor antagonist BIIE0246 (B). Concentration of MCP-1 (C) or TNF (D) in the supernatants of intraperitoneal macrophages either incubated for 4 h with 2 units/mL IFN-γ followed by 24 h of 100 ng/mL LPS (IFN-γ LPS), or for 4 h with 2 units/mL IFN-γ followed by 24 h of 100 ng/mL LPS with 10 nmol/L NPY, with or without concomitant treatment with a 1 μmol/L concentration of the specific Y1 receptor antagonist 1229U91 (IFN-γ LPS+NPY and IFN-γ LPS+NPY+Y1ANTAGONIST). Data are means ± SEM of three to four female mice per group. Measures were made in duplicate (A and B) or triplicate (C and D). *P < 0.05 and **P < 0.01 for the comparisons shown by horizontal bars.
Lack of Y1 signaling in macrophages promotes insulin resistance in adipocytes.
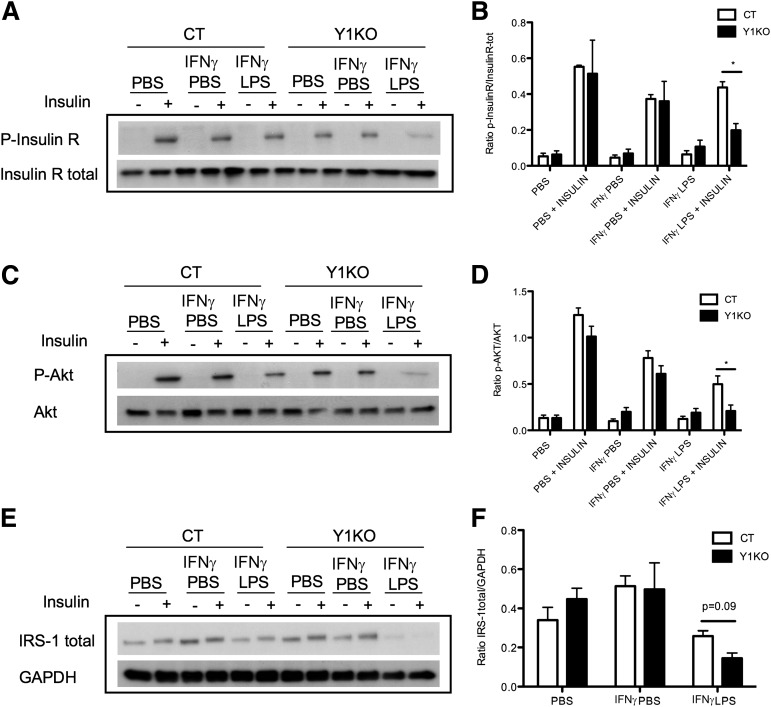
As lack of Y1 signaling in the hematopoietic compartment, notably in macrophages, was associated with exacerbated inflammation and resistance to insulin, as determined in vivo, we investigated whether lack of Y1 signaling in macrophages directly promotes insulin resistance. To test this hypothesis, we first activated intraperitoneal macrophages from either wild-type or Y1KO mice with IFN-γ and LPS (or with IFN-γ and PBS or PBS alone as controls), incubated differentiated 3T3L1 adipocytes with the supernatant from these cells, and assessed the impact on adipocyte response to insulin, as indicated by the phosphorylation and expression of key insulin signaling proteins. Under all of the conditions that did not cause macrophage activation, insulin was observed to induce phosphorylation of the insulin receptor (Fig. 6A and B), Akt (Fig. 6C and D), and IRS-1 (data not shown), consistent with a functional response to insulin in this system. Strikingly, in the presence of conditioned media from inflamed Y1KO macrophages that had been activated with IFN-γ and LPS, phosphorylation of the insulin receptor and Akt in adipocytes was significantly reduced (Fig. 6A–D). Moreover, the level of total IRS-1 protein was strongly reduced in adipocytes incubated with conditioned media from activated Y1KO macrophages versus those incubated with that from activated wild-type macrophages (Fig. 6E and F), showing that conditioned media from activated Y1KO macrophages altered key components of the insulin signaling pathway. Taken together, these findings provide the first evidence that “overinflamed” Y1KO macrophages can induce insulin resistance in adipocytes, and provide strong evidence that Y1 signaling in macrophages is critical in the control of insulin responses in adipose tissue.
FIG. 6.
Lack of Y1 signaling in macrophages leads to insulin resistance in differentiated 3T3L1 adipocytes. Western blot quantification of phosphorylated and total insulin receptor (P-InsulinR and InsulinR total) (A), phosphorylated and total Akt (P-Akt and Akt) (C), and total IRS-1 (E) protein levels in differentiated 3T3L1 adipocytes after treatment (+) or not (−) with 7 nmol/L insulin for 10 min. Adipocytes were preincubated for 24 h with the supernatants of intraperitoneal macrophages from wild-type (CT) or Y1KO mice that had been treated with PBS for 28 h (PBS), 2 units/mL IFN-γ for 4 h followed by 24 h of PBS (IFN-γ PBS), or 2 units/mL IFN-γ for 4 h followed by 24 h of 100 ng/mL LPS (IFN-γ LPS). The ratio of phosphorylated to total insulin receptor (p-InsulinR/InsulinR-tot) (B), phosphorylated to total Akt (p-Akt/Akt) (D), and total IRS-1 to the housekeeping gene GAPDH (IRS-1 total/GAPDH) (F), quantified by densitometry of Western blots at left and normalized with the results for PBS + insulin in each group. Data are means ± SEM of three to four female mice per group. Measurements were made in duplicate. *P < 0.05, CT vs. Y1KO.
DISCUSSION
In this study, we demonstrate that Y1 signaling in the hematopoietic compartment is critical for the development of insulin resistance under conditions of a high-fat diet. This effect was specific to Y1, as no effect of hematopoietic Y2 ablation on insulin action was seen. Moreover, we show that Y1 activation limits macrophage inflammation and its capability to induce insulin resistance in adipocytes in vitro, suggesting that signaling through Y1 in macrophages is a key mechanism of obesity-induced inflammation and insulin resistance.
Our data suggest that alterations in macrophage function contribute to the insulin-resistant phenotype observed in mice with germline or hematopoietic-specific Y1 ablation (Y1KO and Y1BM models, respectively). Indeed, both Y1KO and Y1BM mice demonstrated WAT inflammation, as indicated by increased WAT expression of the macrophage-derived proinflammatory cytokine MCP-1. Additionally, Y1-deficient macrophages demonstrated an increased proinflammatory (M1) phenotype, as indicated by increased IFN-γ plus LPS- or free fatty acid–induced secretion of MCP-1 or TNF, both of which have been shown to contribute to insulin resistance (37–40), with no difference in the M2 phenotype or the number of macrophages recruited to WAT. The effects of Y1 deficiency on macrophage MCP-1 or TNF secretion seem specific to Y1, since NPY inhibits MCP-1 and TNF secretion, and this effect is attenuated by Y1 antagonism. Finally, conditioned medium from activated macrophages from Y1-deficient mice induced exacerbated insulin resistance in cultured 3T3L1 adipocytes compared with that from wild-type macrophages, as demonstrated by reduced insulin-induced phosphorylation of the insulin receptor and Akt, as well as reduced total IRS-1 levels, classic hallmark features of insulin resistance (40). Although these findings suggest a key role for Y1 on macrophages in the regulation of insulin action in obesity, the contribution of Y1 on other hematopoietic-derived cells cannot be excluded. The hematopoietic compartment gives rise to macrophages, mast cells, and eosinophils, all of which express Y1 receptors (41) and have been implicated in obesity development (8,14,42). Thus, the precise role of Y1 signaling in the hematopoietic compartment in the development of insulin resistance in obesity warrants further investigation.
An exacerbated inflammatory response similar to that seen in Y1-deficient macrophages can be seen in macrophages isolated from NPY PYY double-KO mice. This finding, together with our observation of NPY and PYY expression in wild-type macrophages, indicates that an autologous activation mechanism via the NPY PYY system may be in place to control the function of macrophages. Previous results show an inverse correlation between NPY expression in mononuclear cells, including macrophages, and the development of acute inflammation in a context of graft rejection (43). As NPY is one of the preferential ligands for Y1, differences in Y1 signaling may underpin this observation. Other work demonstrates an increase in WAT NPY expression in obesity (19), suggesting that a local increase in Y1 signaling in immune cells recruited to WAT in obesity may act to inhibit inflammation and insulin resistance.
Although we show in this study that Y2 on hematopoietic cells does not play a role in diet-induced insulin resistance, other Y receptors on immune cells besides Y1 may be involved. Y4 and Y5 have been shown to have immunomodulatory effects (20,32,44,45). As such, Y4 and Y5, like Y1, could conceivably modulate fat inflammation and insulin sensitivity in response to a high-fat diet. Indeed, the increased secretion of IL-6 from activated macrophages lacking NPY and PYY, but not Y1KO, could be explained by an absence of signaling through Y4 and/or Y5.
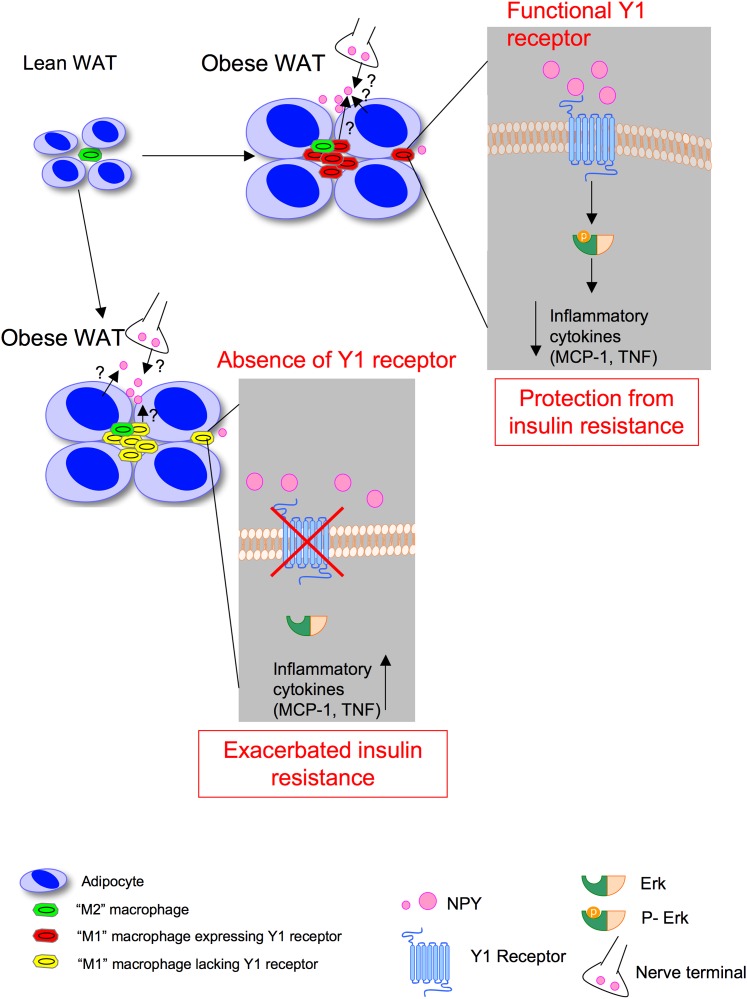
Overall, our results indicate that Y1 signaling in the hematopoetic compartment, notably in macrophages, is critical in the control of inflammation and insulin resistance in obesity, leading us to propose the model illustrated in Fig. 7. During the development of obesity (Fig. 7, top), activated, Y1-expressing immune cells, such as macrophages in WAT, respond to Y1 ligands such as NPY or PYY, thus leading to Erk1/2 phosphorylation and subsequent inhibition of the release of proinflammatory cytokines such as MCP-1 and TNF, thereby limiting the development of insulin resistance. However, under conditions where Y1 signaling in the hematopoietic compartment is impaired (Fig. 7, bottom), the anti-inflammatory effect of Y1 ligands such as NPY on macrophages and other immune cells will be compromised. The exacerbated release of proinflammatory cytokines such as MCP-1 and TNF will thus aggravate insulin resistance under obesogenic conditions. Further work would be required to demonstrate whether subtle alterations in Y1 expression within populations could contribute to the fact that some individuals develop insulin resistance on a high-fat diet whereas others do not.
FIG. 7.
Model showing how Y1 receptor signaling in immune cells such as macrophages may regulate insulin action. See DISCUSSION for explanation. (A high-quality color representation of this figure is available in the online issue.)
Together, this work provides the first evidence of the key role that Y1, specifically in hematopoietic-derived cells, plays in the regulation of insulin action in obesity, likely via associated alterations in inflammation. As such, increasing Y1 signaling specifically in immune cells might be an attractive target for “anti-inflammatory” approaches for the treatment of insulin resistance in obesity.
ACKNOWLEDGMENTS
This work is supported by a grant (427661) from the National Health and Medical Research Council (NHMRC) of Australia. E.Y., F.M., M.B., A.S., and H.H. are supported by scholarships or fellowships from the NHMRC. M.B. also received fellowship support from the Dutch Cancer Society and the Netherlands Organization for Scientific Research.
No potential conflicts of interest relevant to this article were reported.
L.M. designed the project and the experiments, performed the experiments, and wrote the manuscript. E.Y. and A.D.N. participated in the experiments. L.P. participated in the experiments and discussions about the project. S.L., Y.C.S., L.Z., M.B., S.G., and F.M. participated in discussions about the project. H.H. and A.S. founded the project, participated in the design of and discussions about the project, and wrote the manuscript. L.M. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors thank Stacey Walter (Garvan Institute of Medical Research) for her technical help.
REFERENCES
- 1.Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Annu Rev Immunol 2011;29:415–445 [DOI] [PubMed] [Google Scholar]
- 2.Rasouli N, Kern PA. Adipocytokines and the metabolic complications of obesity. J Clin Endocrinol Metab 2008;93(Suppl. 1):S64–S73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tamura Y, Sugimoto M, Murayama T, et al. Inhibition of CCR2 ameliorates insulin resistance and hepatic steatosis in db/db mice. Arterioscler Thromb Vasc Biol 2008;28:2195–2201 [DOI] [PubMed] [Google Scholar]
- 4.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science 1993;259:87–91 [DOI] [PubMed] [Google Scholar]
- 5.Uysal KT, Wiesbrock SM, Marino MW, Hotamisligil GS. Protection from obesity-induced insulin resistance in mice lacking TNF-alpha function. Nature 1997;389:610–614 [DOI] [PubMed] [Google Scholar]
- 6.Ventre J, Doebber T, Wu M, et al. Targeted disruption of the tumor necrosis factor-alpha gene: metabolic consequences in obese and nonobese mice. Diabetes 1997;46:1526–1531 [DOI] [PubMed] [Google Scholar]
- 7.Feuerer M, Herrero L, Cipolletta D, et al. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat Med 2009;15:930–939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu J, Divoux A, Sun J, et al. Genetic deficiency and pharmacological stabilization of mast cells reduce diet-induced obesity and diabetes in mice. Nat Med 2009;15:940–945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nishimura S, Manabe I, Nagasaki M, et al. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med 2009;15:914–920 [DOI] [PubMed] [Google Scholar]
- 10.Winer DA, Winer S, Shen L, et al. B cells promote insulin resistance through modulation of T cells and production of pathogenic IgG antibodies. Nat Med 2011;17:610–617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu L, Parekh VV, Gabriel CL, et al. Activation of invariant natural killer T cells by lipid excess promotes tissue inflammation, insulin resistance, and hepatic steatosis in obese mice. Proc Natl Acad Sci USA 2012;109:E1143–E1152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 2003;112:1796–1808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu H, Barnes GT, Yang Q, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest 2003;112:1821–1830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Odegaard JI, Ricardo-Gonzalez RR, Goforth MH, et al. Macrophage-specific PPARgamma controls alternative activation and improves insulin resistance. Nature 2007;447:1116–1120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duffaut C, Galitzky J, Lafontan M, Bouloumié A. Unexpected trafficking of immune cells within the adipose tissue during the onset of obesity. Biochem Biophys Res Commun 2009;384:482–485 [DOI] [PubMed] [Google Scholar]
- 16.Reyes-García MG, García-Tamayo F. A neurotransmitter system that regulates macrophage pro-inflammatory functions. J Neuroimmunol 2009;216:20–31 [DOI] [PubMed] [Google Scholar]
- 17.Herzog H. Neuropeptide Y and energy homeostasis: insights from Y receptor knockout models. Eur J Pharmacol 2003;480:21–29 [DOI] [PubMed] [Google Scholar]
- 18.Kos K, Harte AL, James S, et al. Secretion of neuropeptide Y in human adipose tissue and its role in maintenance of adipose tissue mass. Am J Physiol Endocrinol Metab 2007;293:E1335–E1340 [DOI] [PubMed] [Google Scholar]
- 19.Yang K, Guan H, Arany E, Hill DJ, Cao X. Neuropeptide Y is produced in visceral adipose tissue and promotes proliferation of adipocyte precursor cells via the Y1 receptor. FASEB J 2008;22:2452–2464 [DOI] [PubMed] [Google Scholar]
- 20.Bedoui S, Kawamura N, Straub RH, Pabst R, Yamamura T, von Hörsten S. Relevance of neuropeptide Y for the neuroimmune crosstalk. J Neuroimmunol 2003;134:1–11 [DOI] [PubMed] [Google Scholar]
- 21.Bedoui S, Miyake S, Lin Y, et al. Neuropeptide Y (NPY) suppresses experimental autoimmune encephalomyelitis: NPY1 receptor-specific inhibition of autoreactive Th1 responses in vivo. J Immunol 2003;171:3451–3458 [DOI] [PubMed] [Google Scholar]
- 22.Zhou JR, Xu Z, Jiang CL. Neuropeptide Y promotes TGF-beta1 production in RAW264.7 cells by activating PI3K pathway via Y1 receptor. Neurosci Bull 2008;24:155–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Howell OW, Scharfman HE, Herzog H, Sundstrom LE, Beck-Sickinger A, Gray WP. Neuropeptide Y is neuroproliferative for post-natal hippocampal precursor cells. J Neurochem 2003;86:646–659 [DOI] [PubMed] [Google Scholar]
- 24.Edelsbrunner ME, Herzog H, Holzer P. Evidence from knockout mice that peptide YY and neuropeptide Y enforce murine locomotion, exploration and ingestive behaviour in a circadian cycle-and gender-dependent manner. Behav Brain Res 2009;203:97–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sainsbury A, Schwarzer C, Couzens M, et al. Important role of hypothalamic Y2 receptors in body weight regulation revealed in conditional knockout mice. Proc Natl Acad Sci USA 2002;99:8938–8943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001;25:402–408 [DOI] [PubMed] [Google Scholar]
- 27.Schmitz-Peiffer C, Craig DL, Biden TJ. Ceramide generation is sufficient to account for the inhibition of the insulin-stimulated PKB pathway in C2C12 skeletal muscle cells pretreated with palmitate. J Biol Chem 1999;274:24202–24210 [DOI] [PubMed] [Google Scholar]
- 28.Pauleau AL, Rutschman R, Lang R, Pernis A, Watowich SS, Murray PJ. Enhancer-mediated control of macrophage-specific arginase I expression. J Immunol 2004;172:7565–7573 [DOI] [PubMed] [Google Scholar]
- 29.Larance M, Ramm G, Stöckli J, et al. Characterization of the role of the Rab GTPase-activating protein AS160 in insulin-regulated GLUT4 trafficking. J Biol Chem 2005;280:37803–37813 [DOI] [PubMed] [Google Scholar]
- 30.Maury E, Brichard SM. Adipokine dysregulation, adipose tissue inflammation and metabolic syndrome. Mol Cell Endocrinol 2010;314:1–16 [DOI] [PubMed] [Google Scholar]
- 31.Fain JN. Release of interleukins and other inflammatory cytokines by human adipose tissue is enhanced in obesity and primarily due to the nonfat cells. Vitam Horm 2006;74:443–477 [DOI] [PubMed] [Google Scholar]
- 32.Dimitrijević M, Stanojević S, Vujić V, Beck-Sickinger A, von Hörsten S. Neuropeptide Y and its receptor subtypes specifically modulate rat peritoneal macrophage functions in vitro: counter regulation through Y1 and Y2/5 receptors. Regul Pept 2005;124:163–172 [DOI] [PubMed] [Google Scholar]
- 33.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest 2007;117:175–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gordon S. Alternative activation of macrophages. Nat Rev Immunol 2003;3:23–35 [DOI] [PubMed] [Google Scholar]
- 35.O’Rourke RW, Metcalf MD, White AE, et al. Depot-specific differences in inflammatory mediators and a role for NK cells and IFN-gamma in inflammation in human adipose tissue. Int J Obes (Lond) 2009;33:978–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest 2006;116:3015–3025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sartipy P, Loskutoff DJ. Monocyte chemoattractant protein 1 in obesity and insulin resistance. Proc Natl Acad Sci USA 2003;100:7265–7270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kanda H, Tateya S, Tamori Y, et al. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest 2006;116:1494–1505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peraldi P, Hotamisligil GS, Buurman WA, White MF, Spiegelman BM. Tumor necrosis factor (TNF)-alpha inhibits insulin signaling through stimulation of the p55 TNF receptor and activation of sphingomyelinase. J Biol Chem 1996;271:13018–13022 [DOI] [PubMed] [Google Scholar]
- 40.Chakrabarti SK, Cole BK, Wen Y, Keller SR, Nadler JL. 12/15-lipoxygenase products induce inflammation and impair insulin signaling in 3T3-L1 adipocytes. Obesity (Silver Spring) 2009;17:1657–1663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wheway J, Mackay CR, Newton RA, et al. A fundamental bimodal role for neuropeptide Y1 receptor in the immune system. J Exp Med 2005;202:1527–1538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu D, Molofsky AB, Liang HE, et al. Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science 2011;332:243–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Holler J, Zakrzewicz A, Kaufmann A, et al. Neuropeptide Y is expressed by rat mononuclear blood leukocytes and strongly down-regulated during inflammation. J Immunol 2008;181:6906–6912 [DOI] [PubMed] [Google Scholar]
- 44.Mitić K, Stanojević S, Kuštrimović N, Vujić V, Dimitrijević M. Neuropeptide Y modulates functions of inflammatory cells in the rat: distinct role for Y1, Y2 and Y5 receptors. Peptides 2011;32:1626–1633 [DOI] [PubMed] [Google Scholar]
- 45.Painsipp E, Herzog H, Holzer P. Evidence from knockout mice that neuropeptide-Y Y2 and Y4 receptor signalling prevents long-term depression-like behaviour caused by immune challenge. J Psychopharmacol 2010;24:1551–1560 [DOI] [PMC free article] [PubMed] [Google Scholar]