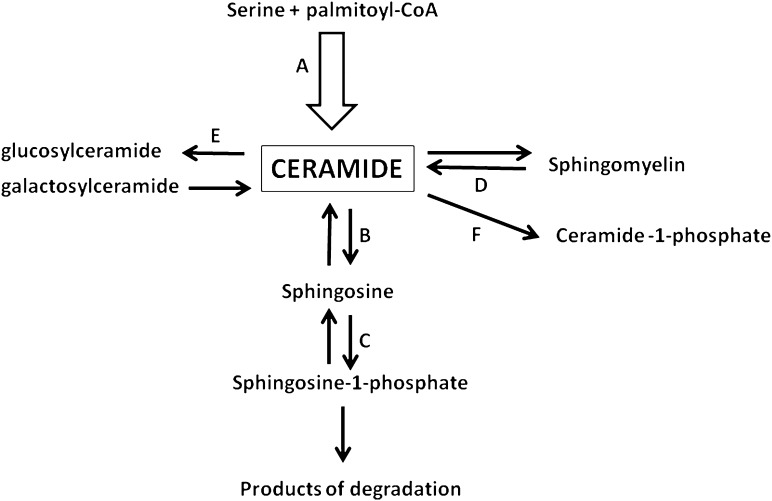
There is a growing amount of data indicating that ceramide is involved in the generation of insulin resistance (1–3). However, investigation into the biological function of ceramide is a complex issue. First, there are no ceramide receptors on the plasma membrane. Second, the membrane is impermeable for ceramides containing long-chain fatty acid residues and only such ceramides seem to exist in nature. There are 13 and 12 ceramides containing different long-chain fatty acid residues that have been identified in human and rat skeletal muscle, respectively (4,5). This means that none of them can be used to study a biological role of extracellular ceramide. Therefore, there remain two other options: 1) use short-chain (C2 or C6) ceramides, which penetrate the cell membrane or 2) manipulate with the level of intracellular ceramide. In the latter case, the activity of selected enzymes of its metabolism may be influenced either by different compounds or by genetic manipulations with the expression of the enzymes. However, there are several pathways of feeding into the ceramide pool in the cell. They are de novo synthesis, hydrolysis of sphingomyelin, catabolism of complex sphingolipids, acylation of sphingosine, and dephosphorylation of ceramide-1-phosphate (Fig. 1). Moreover, most reactions involved in ceramide metabolism are reversible (6,7). Ceramide is on the crossroads of sphingolipid metabolism. However, so far the contributions of different pathways to the ceramide pool have not been quantified in detail. Certainly, the most important source of ceramide for the balance of sphingolipids in the cell is its de novo synthesis. This pathway supplies, through ceramide, all other sphingolipid metabolic pathways (6,7).
FIG. 1.
The pathways of ceramide metabolism examined in relation to insulin sensitivity. A: De novo synthesis. B: Deacylation. C: Phosphorylation of sphingosine (the work of Bruce et al. [15]). D: Hydrolysis of sphingomyelin. E: Conversion to glucosylceramide. F: Phosphorylation. De novo synthesis pathway is distinguished since it supplies all pathways of sphingolipid metabolism with ceramide.
Studies with C2 and C6 ceramides showed that they produce insulin resistance in C2C12 and L6 myotubes (1–3). However, it should be added that it is not certain whether short-chain ceramides mimic fully the action of the long-chain ones. Other evidence of the involvement of ceramide in the generation of insulin resistance is indirect. It includes examination of a relationship between the level of muscle ceramide and insulin sensitivity. In vivo data obtained in rat and in most human studies (4,5,8) showed an existence of an inverse relationship between the level of ceramide in skeletal muscles and insulin sensitivity. Another approach was to study the role of different pathways of ceramide metabolism in the development of insulin resistance. It was shown that inhibition of the de novo synthesis pathway prevents accumulation of ceramide and reduction of insulin sensitivity in mice fed a high-fat diet and in db/db mice (Fig. 1) (9,10). Overexpression of acid ceramidase (the enzyme that deacylates ceramide) blocks saturated fatty acids–induced elevation in the level of ceramide in C2C12 myotubes with concomitant prevention in reduction of insulin sensitivity (11). On the contrary, inhibition of the enzyme markedly augmented both basal- and palmitate-induced level of ceramide in C2C12 cells (12). Deficiency of ceramide kinase, the enzyme that phosphorylates ceramide to ceramide-1-phosphate, increases insulin sensitivity in mice fed a high-fat diet (13). In this case, however, the role of a reduction in the level of ceramide-1-phosphate has not been elucidated. Blockade of the conversion of ceramide to glucosphingolipids increases insulin sensitivity. The latter effect could be caused by the inhibition of the formation of GM3, an intermediate on the glucosphingolipid metabolism pathway (1,14). The existing data on modulation of acid sphingomyelinase activity (the enzyme that catalyzes the conversion of sphigomyelin to ceramide) also indicate the role of ceramide in regulation of insulin sensitivity (1).
A novel approach to the problem is presented by Bruce et al. (15) in an article that appears in this issue of Diabetes. They used transgenic mice with overexpression of sphingosine kinase 1 (SphK1). The enzyme phosphorylates sphingosine, the only product of ceramide catabolism, to sphingosine-1-phosphate (S1P). The mice were fed either a standard chow or a high-fat diet for 6 weeks. Overexpression of SphK1 in chow-fed mice resulted in the reduction in the level of total ceramide in the quadriceps and soleus muscles but not in the white adipose tissue and the liver. The level of S1P increased insignificantly, and the level of sphingosine remained stable. This manipulation did not result in any changes in fasting plasma glucose, insulin, triglycerides, and glucose tolerance in mice fed a chow diet. This is very important data showing that the reduction in the level of ceramide in skeletal muscles does not affect the behavior of the examined variables in mice fed a chow diet. However, the role of SphK1 in the generation of insulin resistance evinced when the mice were fed a high-fat diet. Overexpression of the enzyme prevented ceramide—but not triglyceride—accumulation in the muscles seen in wild-type mice after the diet. The levels of other sphingolipid intermediates did not differ from the respective values in the wild-type mice fed a high-fat diet. Concomitantly, glucose tolerance was improved. Also ex vivo insulin-stimulated glucose uptake and Akt phosphorylation in skeletal muscles were enhanced. Finally, the data obtained in the hyperinsulinemic euglycemic clamp experiment showed improvement of insulin sensitivity after overexpression of SphK1 in the high-fat diet group. Taken together, Bruce et al. (15) provide convincing evidence for the role of SphK1 in the prevention of insulin resistance after a high-fat diet. The data resemble the data obtained after the inhibition of ceramide de novo synthesis (9,10) or after overexpression of acid ceramidase (11). However, since ceramide is a precursor of all complex sphingolipids, including sphingomyelin, a long-term reduction in its synthesis could have negative action on the balance of sphingolipids in the body. Bruce et al. (15) showed that similar effect may be achieved with overexpression of SphK1 and subsequent elevation in the rate of ceramide removal. This procedure seems to be much safer than the other ones since at least part of the newly synthetized caramide may be channeled into pathways of the complex sphingolipids synthesis. As mentioned before, the first step of ceramide catabolism is its deacylation by the enzyme ceramidase with formation of sphingosine. The results quoted above (11) concerned overexpression of acid ceramidase. Also, Bruce et al. (15) measured the expression of only acid ceramidase in the muscles and found it to remain stable. However, it should be kept in mind that there exists three isoforms of the enzyme: acid, neutral, and alkaline (6,7). According to Li et al. (16), the expression of acid ceramidase in the skeletal muscle of the mice is low. It cannot be, therefore, excluded that the expression of other isoforms of the enzyme increased thus leading to increased deacylation of ceramide. It should be added that physical exercise and a high-fat diet were shown to affect the activity of alkaline and neutral ceramidase in different types of the rat skeletal muscles (17,18).
To summarize, Bruce et al. (15) used a novel approach, namely overexpression of SphK1, to prevent accumulation of ceramide in skeletal muscles and the development of insulin resistance after a high-fat diet. As previously noted, this approach seems to be an efficient one and at the same time safer than the approaches used so far. It should also be stressed that sphingosine and especially S1P are biologically very active (19,20). Their levels in the muscles remained stable after overexpression of SphK1. It adds to the benefit of preventing the elevation in the level of ceramide in skeletal muscles via increasing the activity of the enzyme. It encourages further studies with the use of chemical compounds to increase the activity of the enzyme.
ACKNOWLEDGMENTS
No potential conflicts of interest relevant to this article were reported.
Footnotes
See accompanying original article, p. 3148.
REFERENCES
- 1.Lipina C, Hundal HS. Sphingolipids: agents provocateurs in the pathogenesis of insulin resistance. Diabetologia 2011;54:1596–1607 [DOI] [PubMed] [Google Scholar]
- 2.Straczkowski M, Kowalska I. The role of skeletal muscle sphingolipids in the development of insulin resistance. Rev Diabet Stud 2008;5:13–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Summers SA. Sphingolipids and insulin resistance: the five Ws. Curr Opin Lipidol 2010;21:128–135 [DOI] [PubMed] [Google Scholar]
- 4.Dobrzyń A, Górski J. Ceramides and sphingomyelins in skeletal muscles of the rat: content and composition. Effect of prolonged exercise. Am J Physiol Endocrinol Metab 2002;282:E277–E285 [DOI] [PubMed] [Google Scholar]
- 5.Straczkowski M, Kowalska I, Nikolajuk A, et al. Relationship between insulin sensitivity and sphingomyelin signaling pathway in human skeletal muscle. Diabetes 2004;53:1215–1221 [DOI] [PubMed] [Google Scholar]
- 6.Gault CR, Obeid LM, Hannun YA. An overview of sphingolipid metabolism: from synthesis to breakdown. Adv Exp Med Biol 2010;688:1–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Riboni L, Viani P, Bassi R, Prinetti A, Tettamanti G. The role of sphingolipids in the process of signal transduction. Prog Lipid Res 1997;36:153–195 [DOI] [PubMed] [Google Scholar]
- 8.Summers SA. Ceramides in insulin resistance and lipotoxicity. Prog Lipid Res 2006;45:42–72 [DOI] [PubMed] [Google Scholar]
- 9.Schmitz-Peiffer C. Targeting ceramide synthesis to reverse insulin resistance. Diabetes 2010;59:2351–2353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ussher JR, Koves TR, Cadete VJJ, et al. Inhibition of de novo ceramide synthesis reverses diet-induced insulin resistance and enhances whole-body oxygen consumption. Diabetes 2010;59:2453–2464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chavez JA, Holland WL, Bär J, Sandhoff K, Summers SA. Acid ceramidase overexpression prevents the inhibitory effects of saturated fatty acids on insulin signaling. J Biol Chem 2005;280:20148–20153 [DOI] [PubMed] [Google Scholar]
- 12.Chavez JA, Knotts TA, Wang LP, et al. A role for ceramide, but not diacylglycerol, in the antagonism of insulin signal transduction by saturated fatty acids. J Biol Chem 2003;278:10297–10303 [DOI] [PubMed] [Google Scholar]
- 13.Mitsutake S, Date T, Yokota H, Sugiura M, Kohama T, Igarashi Y. Ceramide kinase deficiency improves diet-induced obesity and insulin resistance. FEBS Lett 2012;586:1300–1305 [DOI] [PubMed] [Google Scholar]
- 14.Langeveld M, Aerts JMFG. Glycosphingolipids and insulin resistance. Prog Lipid Res 2009;48:196–205 [DOI] [PubMed] [Google Scholar]
- 15.Bruce CR, Risis S, Babb JR, et al. Overexpression of sphingosine kinase 1 prevents ceramide accumulation and ameliorates muscle insulin resistance in high-fat diet–fed mice. Diabetes 2012;61:3148–3155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li CM, Hong SB, Kopal G, et al. Cloning and characterization of the full-length cDNA and genomic sequences encoding murine acid ceramidase. Genomics 1998;50:267–274 [DOI] [PubMed] [Google Scholar]
- 17.Błachnio-Zabielska A, Baranowski M, Zabielski P, Górski J. Effect of exercise duration on the key pathways of ceramide metabolism in rat skeletal muscles. J Cell Biochem 2008;105:776–784 [DOI] [PubMed] [Google Scholar]
- 18.Błachnio-Zabielska A, Baranowski M, Zabielski P, Górski J. Effect of high fat diet enriched with unsaturated and diet rich in saturated fatty acids on sphingolipid metabolism in rat skeletal muscle. J Cell Physiol 2010;225:786–791 [DOI] [PubMed] [Google Scholar]
- 19.Bartke N, Hannun YA. Bioactive sphingolipids: metabolism and function. J Lipid Res 2009;50Suppl.S91–S96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takabe K, Paugh SW, Milstien S, Spiegel S. “Inside-out” signaling of sphingosine-1-phosphate: therapeutic targets. Pharmacol Rev 2008;60:181–195 [DOI] [PMC free article] [PubMed] [Google Scholar]