Abstract
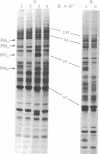
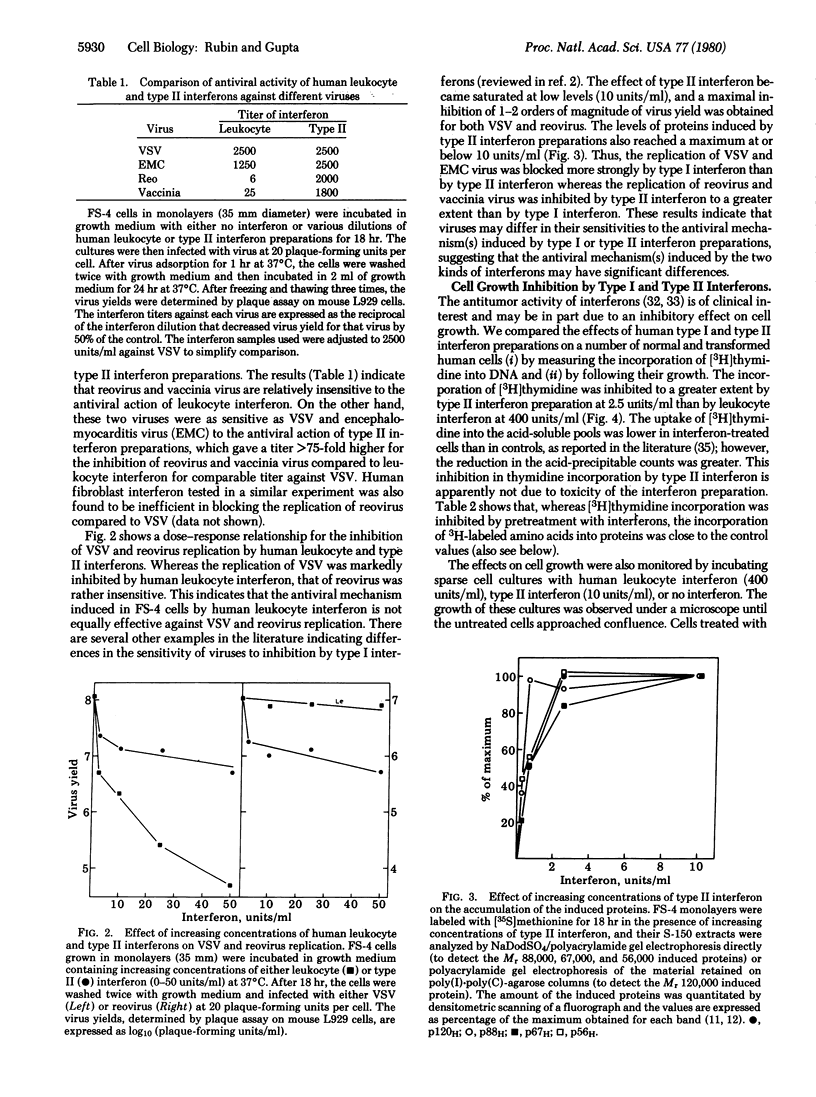
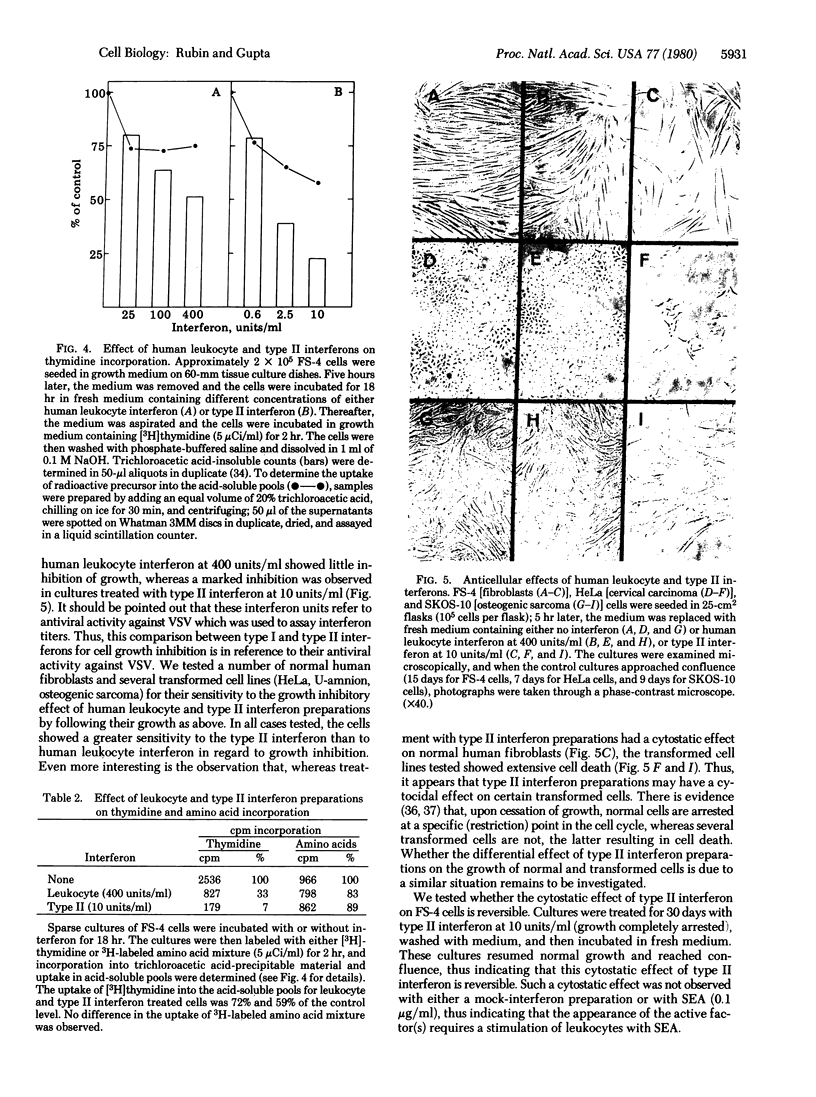
Treatment of human fibroblast FS-4 cultures with human type II interferon preparations induced the synthesis of at least four proteins that were similar in size to four of the five proteins induced by type I interferons (Mr 120,000, 88,000, 67,000, and 56,000). However, the Mr 67,000 and 56,000 proteins were induced more strongly by type II than by type I interferon, and a counterpart of a Mr 80,000 protein induced by type I interferons was not noticeably induced by type II interferon preparations. We therefore compared type I and type II interferons for relative antiviral activities against different viruses (vesicular stomatitis, encephalomyocarditis, and vaccinia viruses and reovirus) and for cell growth-inhibitory activities on various cell types. The replication of vesicular stomatitis and encephalomyocarditis viruses was inhibited more strongly by type I interferon, whereas reovirus and vaccinia virus showed greater sensitivity to type II interferon preparations. This indicates that viruses may differ in their sensitivity to human type I and type II interferons and that the antiviral mechanisms induced by type I and type II interferons may have significant differences. The type I and type II interferons may have significant differences. The type I and type II interferons may also differ in their efficacies as antiproliferative agents. Type II interferon preparations at 2.5 units/ml inhibited the incorporatin of [3H]thymidine to a greater extent than did type I interferon at 400 units/ml. (For both type I and type II interferons, the unit of interferon activity was defined as the concentration that decreased the yield of vesicular stomatitis virus by 50% in FS-4 cultures.) Furthermore, whereas type II interferon preparations had a reversible cytostatic effect on normal human fibroblasts at 10 units/ml, the transformed cells tested (HeLa, osteosarcoma, U-amnion) showed extensive cell death, thus indicating that it may have a cytocidal effect on certain tumor cells. It appears that human type II interferon (or a factor present in these preparations) may be a potent antitumor agent.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baglioni C. Interferon-induced enzymatic activities and their role in the antriviral state. Cell. 1979 Jun;17(2):255–264. doi: 10.1016/0092-8674(79)90151-x. [DOI] [PubMed] [Google Scholar]
- Ball L. A. Induction of 2'5'-oligoadenylate synthetase activity and a new protein by chick interferon. Virology. 1979 Apr 30;94(2):282–296. doi: 10.1016/0042-6822(79)90462-8. [DOI] [PubMed] [Google Scholar]
- Ball L. A., White C. N. Oligonucleotide inhibitor of protein synthesis made in extracts of interferon-treated chick embryo cells: comparison with the mouse low molecular weight inhibitor. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1167–1171. doi: 10.1073/pnas.75.3.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouty-Boyé D., Tovey M. G. Inhibition by interferon of thymidine uptake in chemostat cultures of L1210 cells. Intervirology. 1978;9(4):243–252. doi: 10.1159/000148942. [DOI] [PubMed] [Google Scholar]
- Crane J. L., Jr, Glasgow L. A., Kern E. R., Youngner J. S. Inhibition of murine osteogenic sarcomas by treatment with type I or type II interferon. J Natl Cancer Inst. 1978 Sep;61(3):871–874. [PubMed] [Google Scholar]
- De Ley M., Billiau A., De Somer P. Interferon-induced synthesis of a 63,000 dalton protein in mouse cells. Biochem Biophys Res Commun. 1979 Jul 27;89(2):701–705. doi: 10.1016/0006-291x(79)90686-7. [DOI] [PubMed] [Google Scholar]
- Farrell P. J., Broeze R. J., Lengyel P. Accumulation of an mRNA and protein in interferon-treated Ehrlich ascites tumour cells. Nature. 1979 Jun 7;279(5713):523–525. doi: 10.1038/279523a0. [DOI] [PubMed] [Google Scholar]
- Farrell P. J., Sen G. C., Dubois M. F., Ratner L., Slattery E., Lengyel P. Interferon action: two distinct pathways for inhibition of protein synthesis by double-stranded RNA. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5893–5897. doi: 10.1073/pnas.75.12.5893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman R. M., Sonnabend J. A. Inhibition of interferon action by puromycin. J Immunol. 1965 Oct;95(4):696–703. [PubMed] [Google Scholar]
- Gresser I., Tovey M. G. Antitumor effects of interferon. Biochim Biophys Acta. 1978 Oct 27;516(2):231–247. doi: 10.1016/0304-419x(78)90009-4. [DOI] [PubMed] [Google Scholar]
- Gupta S. L., Rubin B. Y., Holmes S. L. Interferon action: induction of specific proteins in mouse and human cells by homologous interferons. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4817–4821. doi: 10.1073/pnas.76.10.4817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson H. M., Stanton G. J., Baron S. Relative ability of mitogens to stimulate production of interferon by lymphoid cells and to induce suppression of the in vitro immune response. Proc Soc Exp Biol Med. 1977 Jan;154(1):138–141. [PubMed] [Google Scholar]
- Kerr I. M., Brown R. E. pppA2'p5'A2'p5'A: an inhibitor of protein synthesis synthesized with an enzyme fraction from interferon-treated cells. Proc Natl Acad Sci U S A. 1978 Jan;75(1):256–260. doi: 10.1073/pnas.75.1.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight E., Jr, Hunkapiller M. W., Korant B. D., Hardy R. W., Hood L. E. Human fibroblast interferon: amino acid analysis and amino terminal amino acid sequence. Science. 1980 Feb 1;207(4430):525–526. doi: 10.1126/science.7352259. [DOI] [PubMed] [Google Scholar]
- Knight E., Jr, Korant B. D. Fibroblast interferon induces synthesis of four proteins in human fibroblast cells. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1824–1827. doi: 10.1073/pnas.76.4.1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEVINE S. EFFECT OF ACTINOMYCIN D AND PUROMYCIN DIHYDROCHLORIDE ON ACTION OF INTERFERON. Virology. 1964 Dec;24:586–588. doi: 10.1016/0042-6822(64)90211-9. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Langford M. P., Georgiades J. A., Stanton G. J., Dianzani F., Johnson H. M. Large-scale production and physicochemical characterization of human immune interferon. Infect Immun. 1979 Oct;26(1):36–41. doi: 10.1128/iai.26.1.36-41.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Lebleu B., Sen G. C., Shaila S., Cabrer B., Lengyel P. Interferon, double-stranded RNA, and protein phosphorylation. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3107–3111. doi: 10.1073/pnas.73.9.3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardee A. B. A restriction point for control of normal animal cell proliferation. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1286–1290. doi: 10.1073/pnas.71.4.1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardee A. B., Dubrow R., Hamlin J. L., Kletzien R. F. Animal cell cycle. Annu Rev Biochem. 1978;47:715–750. doi: 10.1146/annurev.bi.47.070178.003435. [DOI] [PubMed] [Google Scholar]
- Radke K. L., Colby C., Kates J. R., Krider H. M., Prescott D. M. Establishment and maintenance of the interferon-induced antiviral state: studies in enucleated cells. J Virol. 1974 Mar;13(3):623–630. doi: 10.1128/jvi.13.3.623-630.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts W. K., Hovanessian A., Brown R. E., Clemens M. J., Kerr I. M. Interferon-mediated protein kinase and low-molecular-weight inhibitor of protein synthesis. Nature. 1976 Dec 2;264(5585):477–480. doi: 10.1038/264477a0. [DOI] [PubMed] [Google Scholar]
- Rubin B. Y., Gupta S. L. Interferon-induced proteins in human fibroblasts and development of the antiviral state. J Virol. 1980 May;34(2):446–454. doi: 10.1128/jvi.34.2.446-454.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel C. E., Joklik W. K. A protein synthesizing system from interferon-treated cells that discriminates between cellular and viral messenger RNAs. Virology. 1974 Apr;58(2):476–491. doi: 10.1016/0042-6822(74)90082-8. [DOI] [PubMed] [Google Scholar]
- Strander H. Interferons: anti-neoplastic drugs? Blut. 1977 Sep 18;35(4):277–288. doi: 10.1007/BF00996140. [DOI] [PubMed] [Google Scholar]
- Taylor J. Inhibition of interferon action by actinomycin. Biochem Biophys Res Commun. 1964;14:447–451. doi: 10.1016/0006-291x(64)90084-1. [DOI] [PubMed] [Google Scholar]
- Valle M. J., Jordan G. W., Haahr S., Merigan T. C. Characteristics of immune interferon produced by human lymphocyte cultures compared to other human interferons. J Immunol. 1975 Jul;115(1):230–233. [PubMed] [Google Scholar]
- Wiranowska-Stewart M., Lin L. S., Braude I. A., Stewart W. E., 2nd Production, partial purification and characterization of human and murine interferons--type II. Mol Immunol. 1980 May;17(5):625–633. doi: 10.1016/0161-5890(80)90160-1. [DOI] [PubMed] [Google Scholar]
- Young C. S., Pringle C. R., Follett E. A. Action of interferon in enucleated cells. J Virol. 1975 Feb;15(2):428–429. doi: 10.1128/jvi.15.2.428-429.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngner J. S. Properties of interferon induced by specific antigens. Tex Rep Biol Med. 1977;35:17–22. [PubMed] [Google Scholar]
- Zilberstein A., Federman P., Shulman L., Revel M. Specific phosphorylation in vitro of a protein associated with ribosomes of interferon-treated mouse L cells. FEBS Lett. 1976 Sep 15;68(1):119–124. doi: 10.1016/0014-5793(76)80418-8. [DOI] [PubMed] [Google Scholar]
- Zilberstein A., Kimchi A., Schmidt A., Revel M. Isolation of two interferon-induced translational inhibitors: a protein kinase and an oligo-isoadenylate synthetase. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4734–4738. doi: 10.1073/pnas.75.10.4734. [DOI] [PMC free article] [PubMed] [Google Scholar]