Abstract
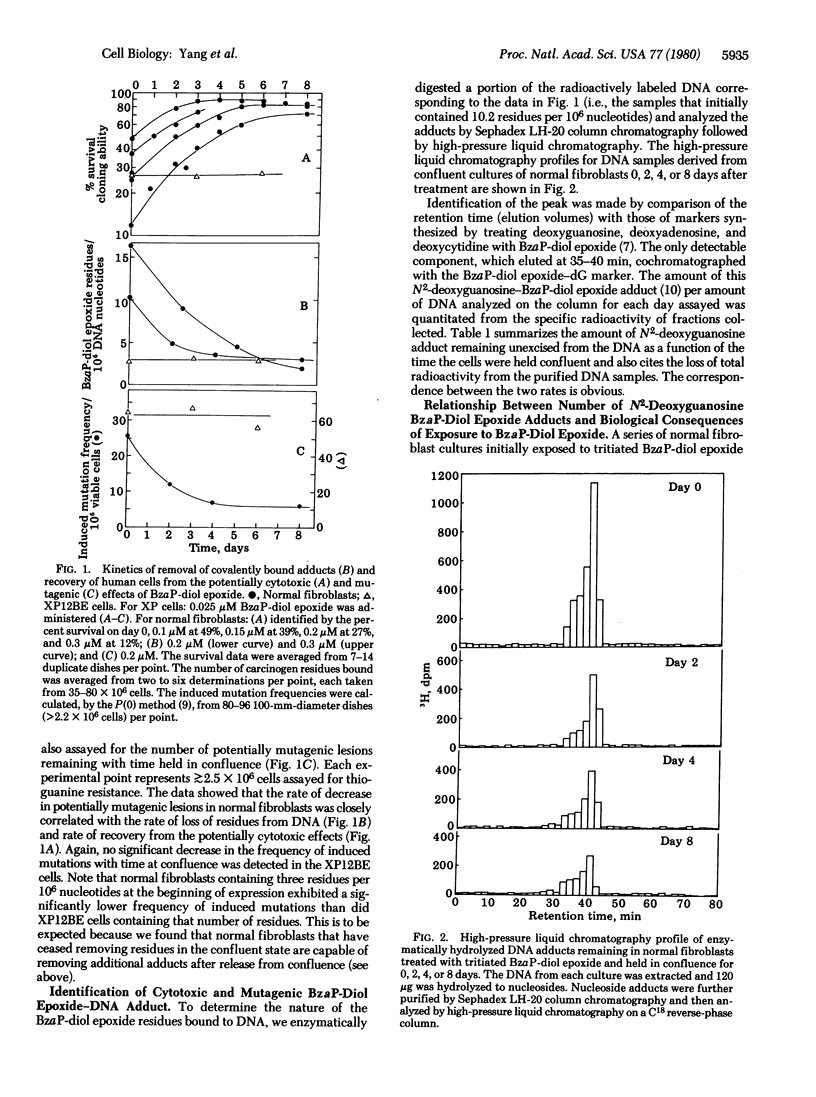
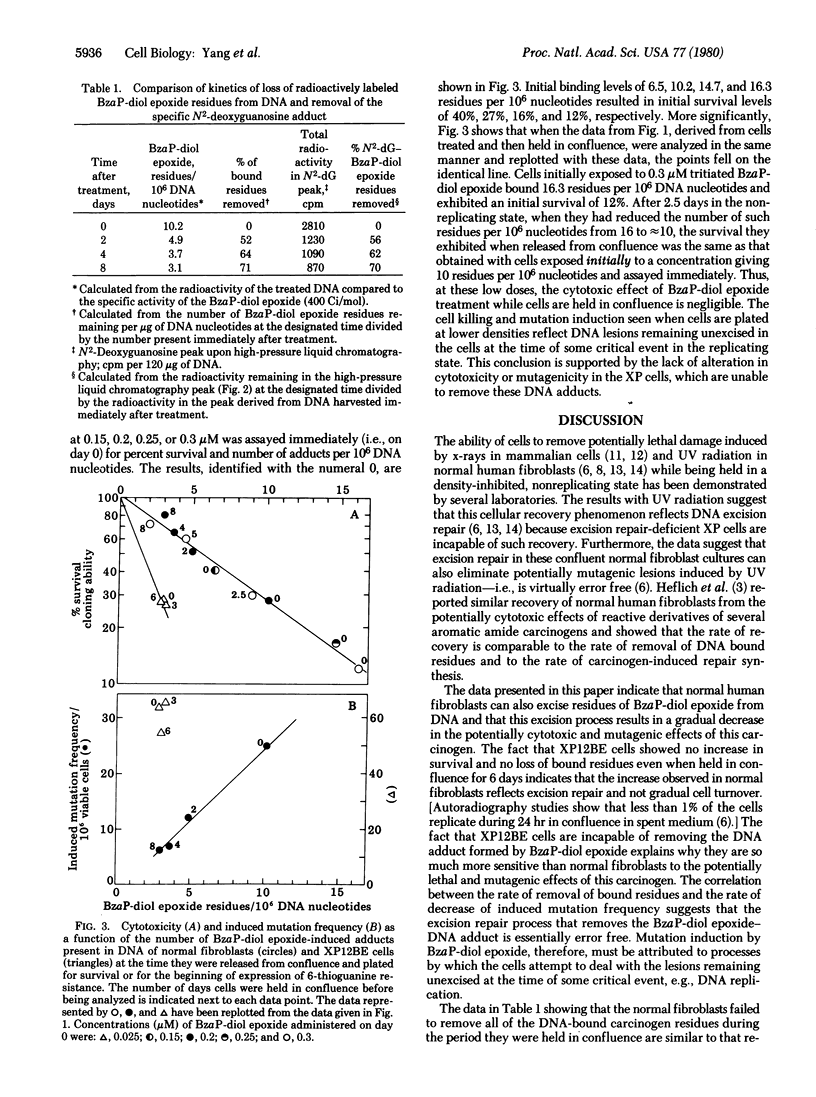
The ability of normal diploid human fibroblasts and excision repair-deficient xeroderma pigmentosum cells (XP12BE, complementation group A) to excise potentially cytotoxic or mutagenic lesions induced in DNA by (+/-)-7 beta, 8 alpha-dihydroxy-9 alpha, 10 alpha-epoxy-7,8,9,10-tetrahydrobenzo[a]pyrene (BzaP-diol epoxide) was determined. Large populations of cells were prevented from replicating by being grown to confluence; after 3 days they were exposed to tritiated BzaP-diol epoxide for 2 hr. One set of cultures was immediately released and assayed for the number of residues covalently bound to DNA, percent survival of colony-forming ability, and frequency of induced mutations. After various periods of time in confluence, other sets were similarly released and assayed. The normal cells exhibited a gradual increase in survival with time held in confluence (recovery from potentially cytotoxic lesions) which was directly correlated with a gradual loss of radioactivity from their DNA and a gradual decrease in the frequency of induced mutations. In contrast, no loss of radioactively labeled carcinogen from the DNA of the XP12BE cells could be detected during a 6-day period and their percent survival and frequency of induced mutations did not change. DNA from normal cells harvested immediately after treatment or after 2, 4, or 8 days in confluence was enzymatically hydrolyzed and analyzed by high-pressure liquid chromatograhy. Only a single peak was detected that cochromatographed with a standard prepared from deoxyguanosine treated with BzaP-diol epoxide. The kinetics of decrease of tritium label in this specific peak corresponded to the decrease in radioactivity of the total DNA with time and with the kinetics of recovery of the cells from the potentially cytotoxic and mutagenic effects of BzaP-diol epoxide. These results suggest that the N2-deoxyguanosine adduct is responsible for these biological effects and indicate that excision repair of this lesion by the normal human cells is "error free."
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baird W. M., Diamond L. The nature of benzo(a)pyrene-DNA adducts formed in hamster embryo cells depends on the length of time of exposure to benzo(a)pyrene. Biochem Biophys Res Commun. 1977 Jul 11;77(1):162–167. doi: 10.1016/s0006-291x(77)80178-2. [DOI] [PubMed] [Google Scholar]
- Brown H. S., Jeffrey A. M., Weinstein I. B. Formation of DNA adducts in 10T1/2 mouse embryo fibroblasts incubated with benzo(a)pyrene or dihydrodiol oxide derivatives. Cancer Res. 1979 May;39(5):1673–1677. [PubMed] [Google Scholar]
- Day R. S., 3rd, Scudiero D., Dimattina M. Excision repair by human fibroblasts of DNA damaged by r-7, t-8-dihyroxy-t-9,10-oxy-7,8,9,10- tetrahydrobenzo(a)pyrene. Mutat Res. 1978 Jun;50(3):383–394. doi: 10.1016/0027-5107(78)90043-x. [DOI] [PubMed] [Google Scholar]
- Feldman G., Remsen J., Wang T. V., Cerutti P. Formation and excision of covalent deoxyribonucleic acid adducts of benzo[a]pyrene 4,5-epoxide and benzo[a]pyrenediol epoxide I in human lung cells A549. Biochemistry. 1980 Mar 18;19(6):1095–1101. doi: 10.1021/bi00547a008. [DOI] [PubMed] [Google Scholar]
- Hahn G. M., Bagshaw M. A., Evans R. G., Gordon L. F. Repair of potentially lethal lesions in x-irradiated, density-inhibited Chinese hamster cells: metabolic effects and hypoxia. Radiat Res. 1973 Aug;55(2):280–290. [PubMed] [Google Scholar]
- Heflich R. H., Dorney D. J., Maher V. M., McCormick J. J. Reactive derivatives of benzo(a)pyrene and 7,12-dimethylbenz(a)anthracene cause S1 nuclease sensitive sites in DNA and "UV-like" repair. Biochem Biophys Res Commun. 1977 Jul 25;77(2):634–641. doi: 10.1016/s0006-291x(77)80026-0. [DOI] [PubMed] [Google Scholar]
- Heflich R. H., Hazard R. M., Lommel L., Scribner J. D., Maher V. M., McCormick J. J. A comparison of the DNA binding, cytotoxicity and repair synthesis induced in human fibroblasts by reactive derivatives of aromatic amide carcinogens. Chem Biol Interact. 1980 Jan;29(1):43–56. doi: 10.1016/0009-2797(80)90085-x. [DOI] [PubMed] [Google Scholar]
- Jennette K. W., Jeffrey A. M., Blobstein S. H., Beland F. A., Harvey R. G., Weinstein I. B. Nucleoside adducts from the in vitro reaction of benzo[a]pyrene-7,8-dihydrodiol 9,10-oxide or benzo[a]pyrene 4,5-oxide with nucleic acids. Biochemistry. 1977 Mar 8;16(5):932–938. doi: 10.1021/bi00624a019. [DOI] [PubMed] [Google Scholar]
- Kakefuda T., Yamamoto H. Modification of DNA by the benzo[a]pyrene metabolite diol-epoxide r-7,t-8-dihydroxy-t-9,10-oxy-7,8,9,10-tetrahydrobenzo[a]pyrene. Proc Natl Acad Sci U S A. 1978 Jan;75(1):415–419. doi: 10.1073/pnas.75.1.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konze-Thomas B., Levinson J. W., Maher V. M., McCormick J. J. Correlation among the rates of dimer excision, DNA repair replication, and recovery of human cells from potentially lethal damage induced by ultraviolet radiation. Biophys J. 1979 Nov;28(2):315–325. doi: 10.1016/S0006-3495(79)85179-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little J. B., Hahn G. M., Frindel E., Tubiana M. Repair of potentially lethal radiation damage in vitro and in vivo. Radiology. 1973 Mar;106(3):689–694. doi: 10.1148/106.3.689. [DOI] [PubMed] [Google Scholar]
- Maher V. M., Dorney D. J., Mendrala A. L., Konze-Thomas B., McCormick J. J. DNA excision-repair processes in human cells can eliminate the cytotoxic and mutagenic consequences of ultraviolet irradiation. Mutat Res. 1979 Sep;62(2):311–323. doi: 10.1016/0027-5107(79)90087-3. [DOI] [PubMed] [Google Scholar]
- Osborne M. R., Harvey R. G., Brookes P. The reaction of trans-7,8-dihydroxy-anti-9,10-epoxy-7,8,9,10-tetrahydrobenzo(a)pyrene with DNA involves attack at the N7-position of guanine moieties. Chem Biol Interact. 1978 Jan;20(1):123–130. doi: 10.1016/0009-2797(78)90087-x. [DOI] [PubMed] [Google Scholar]
- Simons J. W. Development of a liquid-holding technique for the study of DNA-repair in human diploid fibroblasts. Mutat Res. 1979 Feb;59(2):273–283. doi: 10.1016/0027-5107(79)90165-9. [DOI] [PubMed] [Google Scholar]
- Straub K. M., Meehan T., Burlingame A. L., Calvin M. Identification of the major adducts formed by reaction of benzo(a)pyrene diol epoxide with DNA in vitro. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5285–5289. doi: 10.1073/pnas.74.12.5285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weichselbaum R. R., Nove J., Little J. B. Deficient recovery from potentially lethal radiation damage in ataxia telengiectasia and xeroderma pigmentosum. Nature. 1978 Jan 19;271(5642):261–262. doi: 10.1038/271261a0. [DOI] [PubMed] [Google Scholar]
- Weinstein I. B., Jeffrey A. M., Jennette K. W., Blobstein S. H., Harvey R. G., Harris C., Autrup H., Kasai H., Nakanishi K. Benzo(a)pyrene diol epoxides as intermediates in nucleic acid binding in vitro and in vivo. Science. 1976 Aug 13;193(4253):592–595. doi: 10.1126/science.959820. [DOI] [PubMed] [Google Scholar]



