Abstract
Smoking is the most common cause of preventable morbidity and mortality in the United States, in part because it is an independent risk factor for the development of insulin resistance and type 2 diabetes. However, mechanisms responsible for smoking-induced insulin resistance are unclear. In this study, we found smokers were less insulin sensitive compared with controls, which increased after either 1 or 2 weeks of smoking cessation. Improvements in insulin sensitivity after smoking cessation occurred with normalization of IRS-1ser636 phosphorylation. In muscle cell culture, nicotine exposure significantly increased IRS-1ser636 phosphorylation and decreased insulin sensitivity, recapitulating the phenotype of smoking-induced insulin resistance in humans. The two pathways known to stimulate IRS-1ser636 phosphorylation (p44/42 mitogen-activated protein kinase [MAPK] and mammalian target of rapamycin [mTOR]) were both stimulated by nicotine in culture. Inhibition of mTOR, but not p44/42 MAPK, during nicotine exposure prevented IRS-1ser636 phosphorylation and normalized insulin sensitivity. These data indicate nicotine induces insulin resistance in skeletal muscle by activating mTOR. Therapeutic agents designed to oppose skeletal muscle mTOR activation may prevent insulin resistance in humans who are unable to stop smoking or are chronically exposed to secondhand smoke.
Although the prevalence of cigarette smoking has fallen dramatically since the mid-1950s, it is still widespread in the United States, with ∼20% prevalence reported by the Center for Disease Control (1). Smoking is the most common cause of preventable morbidity and mortality in the United States (2), and is an independent risk factor for the development of cardiovascular disease (3) and type 2 diabetes (4,5). Carrying an almost equal risk as active smoking for the development of cardiovascular disease is exposure to secondhand smoke (6). Data from National Health and Nutrition Examination Survey III for 1988 indicated that 88% of the population had detectable serum cotinine (7), a by-product of nicotine metabolism, indicating an almost nationwide exposure to cigarette smoke and the increase disease burden it brings. In 2002, still as much as 43% of the U.S. population had serum cotinine concentrations indicating secondhand smoke exposure (8). Therefore, despite efforts to decrease smoking and related diseases in the United States, we are still a nation with tobacco-related morbidity and mortality affecting almost half of the population. These epidemiologic observations underscore the importance of understanding how smoking impacts cardiovascular disease and diabetes risk. Unfortunately, the molecular link among smoking, secondhand smoke, and insulin resistance is unknown.
Nicotine, one of many components of tobacco smoke, is associated with decreased insulin sensitivity in humans and therefore may link smoking with insulin resistance (9,10). Nicotine binds to nicotinic acetylcholine α1 receptors in human skeletal muscle and muscle cell cultures (11,12). However, molecular mechanisms explaining nicotine- and smoking-induced insulin resistance (13–15) and the improvement after smoking cessation (15,16) are still unclear. Previous data from our laboratory found young chronic smokers were insulin-resistant compared with nonsmokers (17) with increased basal inhibition of insulin signaling and saturation of intramuscular lipids. Therefore, we combined a smoking-cessation intervention with nicotine exposure in muscle cell culture to elucidate mechanisms explaining smoking-induced insulin resistance. Our efforts focused on determining how nicotine increases basal inhibition of IRS-1 to lead the way for therapeutic strategies to increase insulin sensitivity in individuals who cannot stop smoking or are habitually exposed to secondhand smoke.
RESEARCH DESIGN AND METHODS
Subjects.
Twelve healthy sedentary nonsmokers and 10 smokers completed this intervention study (Table 1). Two subjects had urinary cotinine incompatible with smoking cessation (>30 ng/mL) and were therefore excluded from the analysis, leaving eight smokers who completed the intervention (7). Subjects gave informed consent and were excluded if they: had diabetes, hyperlipidemia, liver, kidney, thyroid, or lung disease, a BMI <20 or >25 kg/m2, or were taking medications that can affect glucose or lipid metabolism. All subjects were sedentary and engaged in moderate to vigorous exercise <3 h/week. Subjects were weight stable in the 6 months prior to participation in this research study. This study was approved by the Human Resources Committee at the University of Colorado at Boulder and the Colorado Multiple Institution Review Board at the University of Colorado at Anschutz Medical Campus.
TABLE 1.
Subject demographics
Preliminary testing.
Subjects reported to the General Clinical Research Center (GCRC) for screening procedures following a 12-h overnight fast, where they were given a health and physical exam followed by a fasting blood draw. Body composition was determined using dual energy X-ray absorptiometry analysis (Lunar DPX-IQ; Lunar Corporation, Madison, WI).
Diet and exercise control.
All subjects were given a prescribed diet for 3 days prior to both metabolic studies on the GCRC, with energy intake and composition as previously described (17). Subjects were asked to refrain from planned physical activity for 48 h before each metabolic study.
Baseline metabolic study.
Subjects arrived to the GCRC after an overnight fast at 7 a.m., when an antecubital vein in one arm was cannulated for isotope infusion, and a retrograde dorsal hand vein in the contralateral side was catheterized for blood sampling via the heated hand technique. To measure glucose turnover and intramuscular triglyceride (IMTG) fractional synthesis rate (FSR), background blood and breath samples were taken, followed by a primed (4.5 mg/kg) constant (0.03 mg/kg/min) infusion of [6,6-2H2]glucose, and a continuous infusion of [U-13C] palmitate (Isotec, Miamisburg, OH) (0.0174 µmol/kg/min). These infusions were continued for 4 h and throughout the muscle biopsy procedure. Subjects remained fasting during the tracer infusion. During the 4-h infusion, smokers smoked one cigarette (Camel Light; R.J. Reynolds, Winston-Salem, NC) every 30 min until the start of the final blood sampling for a total of eight cigarettes. Blood sampling was performed during minutes 210, 220, 230, and 240 of infusion. A percutaneous needle biopsy was performed after 240 min of isotope infusion for determination of IMTG and diacylglycerol (DAG) concentration, composition, and IMTG FSR (18). Muscle biopsies (∼150 mg) were taken from midway between the greater trochanter of the femur and the patella from similar depth to minimize variance in muscle fiber composition, which varies with depth and length in the vastus lateralis (19). Muscle was immediately flash frozen in liquid nitrogen and stored at −80°C until dissection and analysis.
After the tracer infusion, insulin sensitivity was determined via an insulin-modified frequently sampled intravenous glucose tolerance test using standard methods (20), as previously described (17).
Smoking-cessation intervention.
Ten days before the first tracer infusion study, smokers began taking buproprion (Zyban [GlaxoSmithKline]; 150 mg twice daily) to increase the compliance and success rate with smoking cessation. Smokers were taking buproprion during the metabolic studies both pre- and postcessation. Smokers stopped smoking for 1 (n = 4) or 2 (n = 4) weeks starting the day of the baseline metabolic study. The intervention was based on the American Cancer Society model and did not include the use of nicotine gum or patches. Subjects stopping smoking reported to the GCRC every 2 to 3 days to measure breath carbon monoxide (CO) and urine cotinine concentration to document smoking cessation. During the intervention, control subjects maintained their normal lifestyle.
Follow-up metabolic study.
Subjects returned to the GCRC after 1 or 2 weeks of smoking cessation or normal living to repeat the metabolic study. During this follow-up study, smokers who stopped smoking did not smoke cigarettes during the study, unlike their first visit. All other procedures were identical between the two visits.
Metabolite and hormone analyses.
Standard enzymatic assays were used to measure glucose (Olympus AU400e Chemistry analyzer; Olympus America Inc., Center Valley, PA), lactate (Sigma Kit #826; Sigma-Aldrich, St. Louis, MO), and free fatty acids (FFA) (NEFA Kit; Wako, Richmond, VA). Insulin, glucagon, and adiponectin were measured using a radioimmunoassay (Diagnostic Systems Laboratories, Inc., Webster, TX), and catecholamines were measured using high-performance liquid chromatography. Plasma interleukin-6 and tumor necrosis factor-α (TNF-α) were measured using an enzyme-linked immunosorbent assay kit (R&D Systems HS600B and HSTA00C, respectively; R&D Systems, Minneapolis, MN). Breath CO was measured using an EC50-Micro Smokerlyzer (Bedfont Scientific, Medford, NJ). Urine cotinine was measured using an enzyme-linked immunosorbent assay (Calbiotech, Inc., Spring Valley, CA).
Muscle lipid analysis.
Skeletal muscle samples were dissected free of extramuscular fat on ice, lyophilized, and processed as previously described to isolate intramuscular FFA, IMTG, and DAG concentration, enrichment, and composition (17).
Western blotting.
Frozen skeletal muscle samples were prepared for Western blot analysis and run on SDS-PAGE gels using standard techniques as described previously (17).
Plasma palmitate and glucose isotope analysis.
Plasma glucose derivatization and analysis was determined as previously described (21). Methylation and extraction of plasma palmitate was performed as previously described (22). Samples were run on an HP 6890 GC with a 30 m DB-23 capillary column (Hewlett Packard, Palo Alto, CA) connected to an HP 5973 MS (Hewlett Packard). Enrichments were calculated based on a standard curve of known enrichments and corrected for variations in abundance (23). Peak identities were determined by retention time and mass spectra compared with standards of known composition.
Isotope ratio mass spectrometry.
To measure whole-body palmitate oxidation rates, 2 mL of breath CO2 was transferred into a 20-mL exetainer for the measurement of 13CO2/12CO2 with continuous flow isotope ratio mass spectrometry (Δ V; Thermo Electron, Bremen, Germany). Each sample was injected (1.2 μL/injection) in duplicate for isotope ratio analyses, with an average SD for all injections of 0.0001 atom percent.
Cell culture.
The rat myotube L6 cell line was purchased (American Type Culture Collection, Manassas, VA) and grown to 60% confluence on six-well plates using Dulbecco’s modified Eagle’s medium containing 10% FBS, 0.5% fungizone, 200 units/mL penicillin, and 200 μg/mL streptomycin at 37°C under and atmosphere of 5% CO2 and 95% air. Differentiation was initiated by switching medium to skeletal muscle differentiation medium containing 2% horse serum, 0.5% fungizone, 200 units/mL penicillin, and 200 μg/mL streptomycin. Cells from passages 2–4 were used for experiments. Experiments were performed between 8 and 10 days of differentiation. Nicotine was added at 200 μmol final concentration for all experiments, which is roughly two times higher than a high plasma concentration for an active smoker (24,25). Inhibitors of p44/p42 mitogen-activated protein kinase (MAPK)/extracellular signal-related kinase (ERK) 1/2 (MEK; UO126) and mammalian target of rapamycin (mTOR; rapamycin) were each dissolved in dimethyl sulfoxide (DMSO) at 10 μmol final concentration for all experiments (both from Cell Signaling Technology, Inc., Danvers, MA).
Time-course experiments.
Cells were serum starved for 3.5 h and then preincubated with DMSO control and inhibitors for 30 min. After 0, 5, 10, 30, 60, and 120 min exposure to nicotine, cells were harvested in homogenization buffer previously described (17). After protein extraction and determination of protein concentration, cells were run on an SDS-PAGE Western blot using standard methods previously described (17). Antibodies against IRS-1ser636, IRS-1 total, phospho- and total p44/42 MAPK, phospho-p70s6k, and p70s6k total were purchased from Cell Signaling Technology.
Insulin-stimulated glucose uptake.
Cells were serum-starved for a total of 4 h prior to determination of insulin sensitivity using 3H-2-deoxyglucose uptake as previously described (26). DMSO vehicle and inhibitors were added after 1.5 h of serum starving and nicotine or nicotine vehicle added after 2 h of serum starving. Concentrations of vehicle, inhibitors, and nicotine were maintained during preincubation of cells with insulin and measurement of glucose uptake. Measures were performed in duplicate per assay.
Basal IRS-1ser636 phosphorylation.
Vehicle and inhibitors were added after 1.5 h of serum starving and nicotine or nicotine vehicle control added after 2 h of serum starving. After 4 h of serum starving and treatments, cells were harvested on ice, protein run on SDS-PAGE Western blots, and probed as described above.
Calculations.
IMTG FSR and IMTG and DAG saturation were calculated as previously described (17). Palmitate and glucose rate of disappearance and palmitate rate of oxidation were calculated using steady-state kinetics and a whole-body estimate of carbon label retention as previously described (27).
Statistics.
Data are presented as mean ± SEM. Differences in normally distributed data between smokers and nonsmokers, before and after the smoking-cessation intervention, were analyzed using a two-way repeated-measures ANOVA (JMP, Cary, NC). Nonnormally distributed data were log transformed prior to analysis. When the repeated-measures ANOVA indicated a significant interaction, changes before and after smoking cessation were determined using a paired t test. Differences in muscle cell culture were determined using unpaired t tests. An α level of 0.05 was used throughout.
RESULTS
All subjects were college-aged men and women with similar BMI, percent body fat, blood metabolites, and metabolic rate (Table 1). On average, smokers reported smoking almost one pack of cigarettes/day. The period of smoking cessation was only 1 to 2 weeks in order to minimize changes in body weight that could have confounded our results. We were successful, as there was no significant change in body weight during the intervention (Table 1). There were no differences in 1 to 2 weeks of smoking cessation, so data were combined to gain power for planned analyses.
Smoking-cessation intervention.
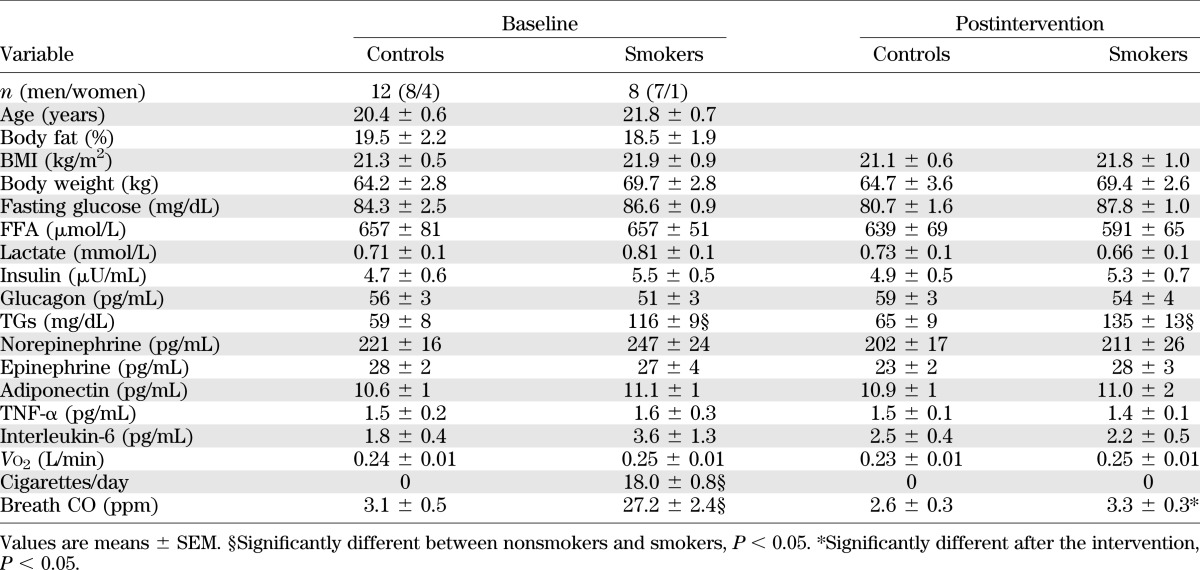
Fasting blood triglyceride (TG) concentration was roughly twice as high in smokers compared with nonsmokers (P < 0.0001), which did not change after smoking cessation (Table 1). As expected, smokers had greater breath CO measured during the first metabolic study, which decreased significantly and was not different from nonsmokers after smoking cessation (Fig. 1A). Urinary cotinine is a better measure of smoking cessation compliance due to a longer half-life. Baseline cotinine was significantly elevated compared with nonsmokers and decreased progressively to values not significantly different than nonsmokers after 7 and 14 days of smoking cessation (Fig. 1B).
FIG. 1.
Time-course breath CO (A) and urinary cotinine (B) concentration at baseline and during smoking cessation. Values are means ± SEM.
Smoking cessation increases insulin sensitivity.
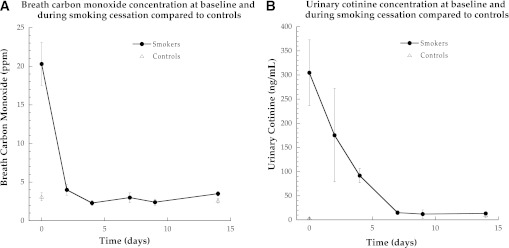
Insulin sensitivity was significantly lower in smokers compared with nonsmokers (Fig. 2, P = 0.03) and increased significantly in smokers after smoking cessation (P = 0.006). Insulin sensitivity was not different in nonsmokers after the intervention (P = 0.24). There were no significant differences in glucose effectiveness (the ability of glucose to promote its own transport) or the disposition index (insulin secretion adjusted for insulin resistance) between smokers and nonsmokers before or after the smoking cessation intervention.
FIG. 2.
Insulin sensitivity measured using the Bergman minimal model in nonsmokers and smokers. Data for the change in each individual are shown. Values are means ± SEM. §Significantly different than nonsmokers, P < 0.05; *significantly different after smoking cessation, P < 0.05. Si, insulin sensitivity index.
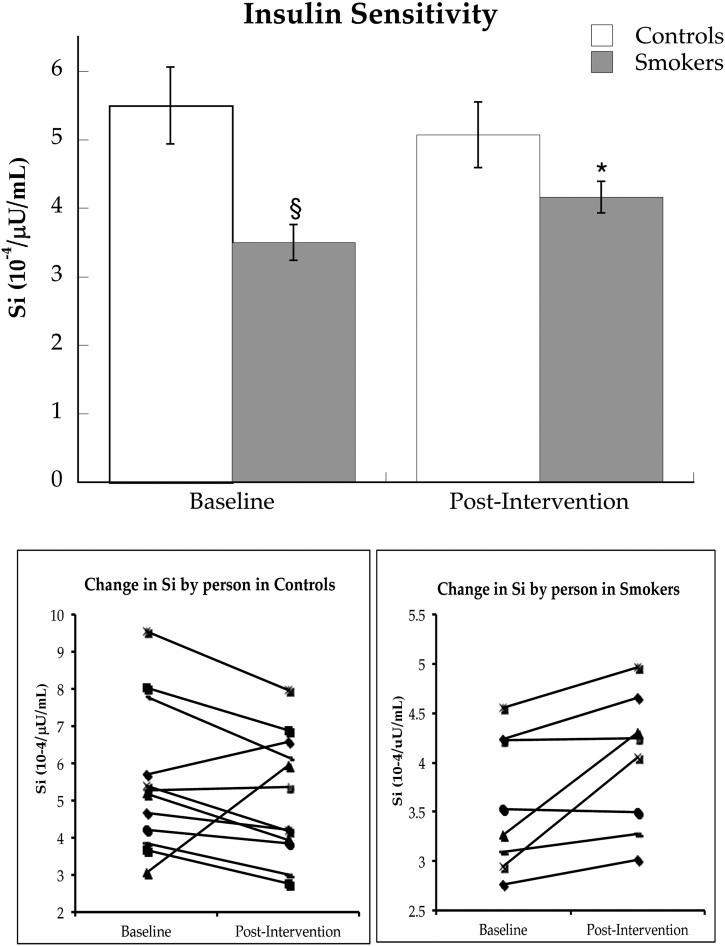
Similar to metabolic rate, whole-body resting fat oxidation was not significantly different between groups (control subjects = 1.4 ± 0.1, smokers = 1.3 ± 0.1 µmol/kg/min; P = 0.84) and did not change significantly after the intervention. Palmitate rate of appearance (Ra) was significantly higher in smokers compared with nonsmokers and remained higher after smoking cessation (Fig. 3A, P = 0.004). The rate of oxidation of plasma palmitate was not significantly different in smokers compared with nonsmokers (Fig. 3B, P = 0.23) and did not change after smoking cessation. Glucose Ra was also not different between smokers and controls at baseline (Fig. 3C, P = 0.18) and did not change significantly after the intervention (P = 0.18).
FIG. 3.
Palmitate Ra (A), oxidation (B), and glucose Ra (C) before and after smoking cessation intervention in control subjects and cigarette smokers. Values are means ± SEM. §Significantly different than control subjects, P < 0.05.
Muscle lipid metabolism after smoking cessation.
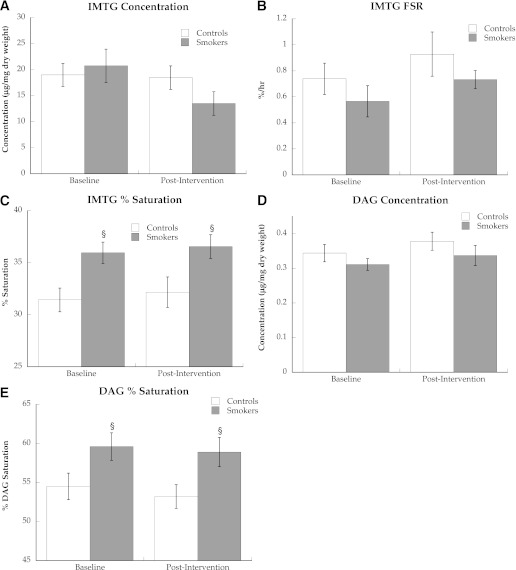
We did not find a difference in the resting concentration of IMTG or DAG (Fig. 4A and D) or FSR of IMTG (Fig. 4B) in smokers compared with nonsmokers before or after smoking cessation. However, the composition of IMTG and DAG differed between groups, with significantly greater proportion of saturated acyl side chains in smokers compared with nonsmokers before and after smoking cessation (Fig. 4C, P = 0.02, and Fig. 4E, P = 0.04).
FIG. 4.
IMTG concentration (A), FSR (B), and saturation (C) and intramuscular DAG concentration (D) and saturation (E) in nonsmokers and smokers. Values are means ± SEM. §Significantly different than nonsmokers, P < 0.05.
Alterations in protein content after smoking cessation.
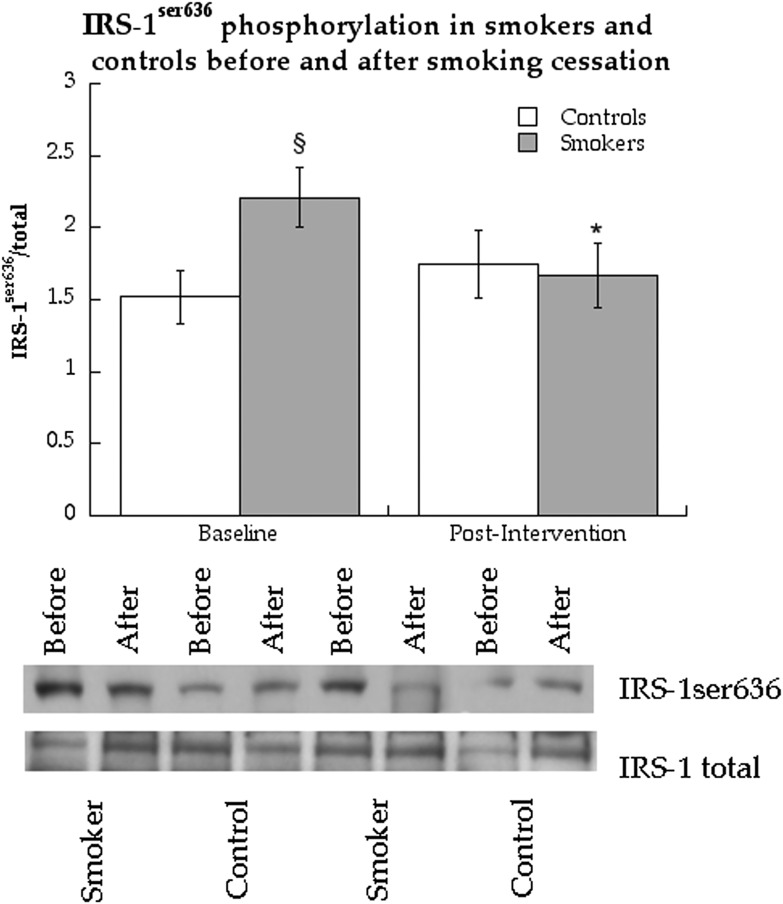
Western blot analyses were performed to measure potential mechanisms promoting the increase in insulin action after smoking cessation. Table 2 shows a summary of these analyses that were not significantly different between groups or following smoking cessation. The only difference in protein expression was significantly greater content of IRS-1ser636 phosphorylation in smokers compared with nonsmokers (Fig. 5, P = 0.02), which decreased in smokers (P = 0.047) to values that were not significantly different than nonsmokers after the intervention.
TABLE 2.
Summary of Western blot data from human studies
FIG. 5.
IRS-1 serine636 phosphorylation before and after smoking cessation in control subjects and cigarette smokers. Values are means ± SEM. §Significantly different than control subjects, P < 0.05; *significantly different after smoking cessation, P < 0.05.
Nicotine-induced insulin resistance in muscle cell culture.
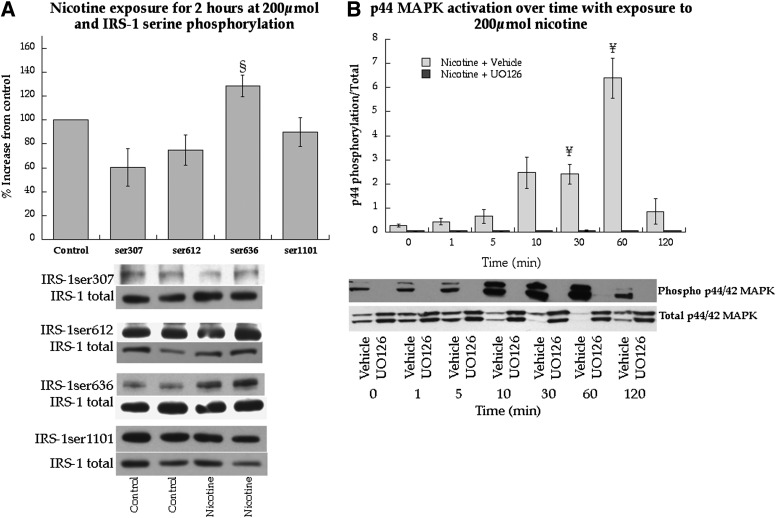
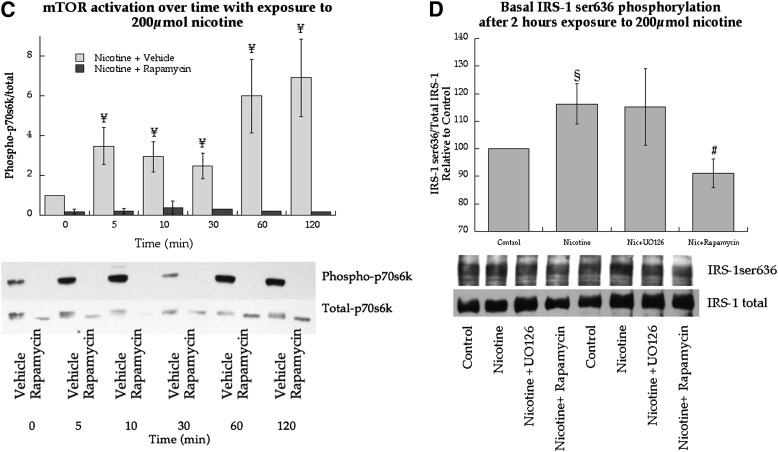
Cell-culture experiments were performed in L6 myotubes using nicotine exposure to determine mechanisms for smoking-induced IRS-1ser636 phosphorylation. We recapitulated human studies and found 2 h of nicotine exposure significantly increased IRS-1ser636 phosphorylation (P = 0.03, Fig. 6A). Nicotine exposure only increased IRS-1ser636 phosphorylation, whereas serine sites 307 (P = 0.25), 612 (P = 0.29), and 1,101 (P = 0.72) were not significantly changed (Fig. 6A). Serine636 phosphorylation is promoted by p44/p42 MAPK and mTOR activation. Therefore, we determined if nicotine stimulated these pathways in cell culture. Nicotine exposure significantly increased phosphorylation of p44/p42 MAPK after 30 and 60 min, but returned to baseline after 120 min (Fig. 6B). This effect could be blocked by the MEK inhibitor UO126. Nicotine exposure also increased mTOR activation from 5–120 min, which could be blocked using the mTOR inhibitor rapamycin (Fig. 6C).
FIG. 6.
Effects of nicotine on cell signaling and insulin sensitivity in L6 skeletal muscle myotubes. A: Serine phosphorylation of IRS-1 in response to nicotine exposure at 200 μmol. B: Stimulation of p44/p42 MAPK over time with continuous nicotine exposure at 200 μmol with and without MEK inhibitor UO126, the upstream kinase of p44/p42 activation. C: Stimulation of p70s6k over time with continuous nicotine exposure at 200 μmol with and without the mTOR inhibitor rapamycin. D: Effects of nicotine at 200 μmol on IRS-1ser636 phosphorylation in L6 muscle cell culture with and without inhibitors against p44/p42 MAPK and mTOR. Values are mean ± SEM. §Significantly different than control, P < 0.05; ¥significantly different than time 0, P < 0.05; #significantly different than nicotine, P < 0.05.
Fig. 6D shows the nicotine-induced increase in IRS-1ser636 phosphorylation compared with control (P = 0.04), which was not altered by UO126 (P = 0.98) during nicotine exposure. However, rapamycin during nicotine exposure significantly decreased IRS-1ser636 phosphorylation compared with nicotine treatment alone (P = 0.03).
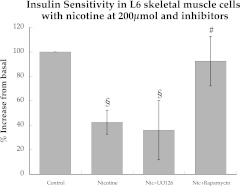
Nicotine administration recapitulated insulin resistance in L6 cells, as insulin-stimulated glucose uptake decreased 57% with 2 h of nicotine exposure (Fig. 7). UO126 during nicotine exposure did not improve insulin sensitivity. However, rapamycin administration during nicotine exposure significantly increased insulin sensitivity (P = 0.02) compared with nicotine alone to values not significantly different than control levels.
FIG. 7.
Effects of nicotine, with and without inhibitors against p44/p42 MAPK and mTOR, on insulin-stimulated glucose uptake in L6 muscle cell culture. Values are mean ± SEM. §Significantly different than control, P < 0.05; #significantly different than nicotine, P < 0.05.
DISCUSSION
The detrimental effect of cigarette smoking on insulin sensitivity is well documented. Smoking-induced insulin resistance has been reported in epidemiologic (28) as well as cross-sectional studies in middle-aged people (13,14). We previously reported insulin resistance in young, otherwise healthy college-aged men and women through inhibition of basal insulin signaling in skeletal muscle (17). This study reports on a cohort of subjects studied after 1 to 2 weeks of verified smoking cessation. Insulin sensitivity increased significantly after smoking cessation. The concentration of plasma TGs and saturation of intracellular lipids remained higher in ex-smokers compared with nonsmokers, but basal inhibition of insulin signaling reverted to normal. Cell-culture methods recapitulated insulin resistance with nicotine exposure and showed nicotine stimulated p44/p42 MAPK and mTOR. Only inhibition of mTOR activation was able to prevent nicotine-induced insulin resistance. Therefore, individuals who cannot stop smoking or who are exposed to second hand smoke may benefit from therapeutic agents designed to prevent mTOR activation to reduce their risk of type 2 diabetes.
The current data show 1 to 2 weeks of smoking cessation, without changes in body weight or adiposity, resulted in increased insulin sensitivity in a young population. These data are similar to others showing increased insulin sensitivity after short (13) and long-term (16) smoking cessation in older individuals. However, these data contrast reports that smoking cessation did not change glucose tolerance after 4 months (29) or increased risk of type 2 diabetes over 3 years, both of which were largely driven by weight gain (30). Insulin secretion was appropriate for the insulin resistance before and after smoking cessation, as revealed by unchanged disposition index scores. Combined, these data indicate that smoking cessation, without a change in body weight, may decrease diabetes risk. However, short-term smoking cessation did not completely normalize insulin sensitivity, indicating persistent metabolic alterations in ex-smokers.
Several metabolic variables remained significantly different in ex-smokers compared with nonsmokers, including increased plasma TG concentration and palmitate turnover, which may reflect or result in residual insulin resistance. Plasma TG concentration is likely only a marker of insulin resistance, as it is not thought to directly affect insulin sensitivity (31). Increased palmitate Ra, without differences in fasting catecholamines, insulin, or glucagon between groups, suggests adipocyte insulin resistance in smokers before and after smoking cessation. In steady state, palmitate appearance is matched by palmitate disappearance into peripheral tissues. Higher rates of FFA disappearance and nonadipocyte re-esterification have been reported in smokers, consistent with increased hepatic FFA uptake and VLDL-TG production, or increased storage of FFA as TG in muscle (15). Our data eliminate muscle as a site of increased FFA re-esterification before and after smoking cessation and point to increased hepatic FFA uptake and VLDL-TG production. These data suggest that future studies investigating differences in hepatic lipid content in smokers versus nonsmokers may be warranted. Short-term smoking cessation did not decrease basal palmitate turnover, indicating prolonged smoking cessation is required to normalize adipocyte insulin resistance. Increased systemic FFA release and VLDL-TG production may play an important role linking cigarette smoking and increased risk of cardiovascular disease (3).
In addition to increases in FFA turnover, cigarette smoking has been reported to increase plasma FFA concentration and skeletal muscle lipoprotein lipase without changing whole-body fat oxidation (15). This metabolic milieu could lead to alterations in intramuscular lipid metabolism promoting insulin resistance in smokers, which could normalize after smoking cessation. However, our data suggest alterations in IMTG and DAG concentration and IMTG synthesis rates are not responsible for the increase in insulin sensitivity after short-term smoking cessation. Interestingly, saturation of IMTG and DAG remained significantly increased in smokers after smoking cessation. Although the implications of increased IMTG saturation are currently unknown, increased DAG saturation is related to insulin resistance even without a change in total concentration (32–34), possibly by altering protein kinase C activation (35,36). Increased muscle lipid saturation may also reflect increased saturation of other intracellular lipids, such as ceramide (37) and long chain acyl-CoA (38), which may also promote insulin resistance. Therefore, persistently greater saturation of muscle lipids after smoking cessation may help explain why insulin sensitivity did not completely normalize in ex-smokers.
No differences were observed between smokers and nonsmokers, and no changes were found following smoking cessation in protein abundance associated with inflammation, lipid peroxidation, and endoplasmic reticulum stress. As previously reported (17), smokers had greater IRS-1ser636 phosphorylation compared with nonsmokers when they were actively smoking. Serine phosphorylation of many sites on IRS-1, including ser636, decreases IRS-1 tyrosine phosphorylation, phosphatidylinositol-3-kinase binding, and insulin-stimulated glucose uptake (39). Following smoking cessation, IRS-1ser636 phosphorylation decreased in smokers such that there were no differences between groups after the intervention. Basal inhibition of IRS-1 by phosphorylation of ser636 has been reported in other insulin-resistant states such as type 2 diabetes (40) and in insulin-resistant offspring of patients with type 2 diabetes (41). These data suggest that basal inhibition of insulin signaling may be an important mechanism responsible for smoking-induced insulin resistance and could also play a role in insulin resistance of secondhand smoke exposure.
Several pathways promote increased IRS-1ser636 phosphorylation that may be implicated in the insulin resistance of smoking. This specific serine site on IRS-1 can be phosphorylated by p44/p42 MAPK (40) and mTOR (42). Neither of these pathways was significantly altered in biopsies taken from smokers 45–60 min after smoking their last cigarette or after smoking cessation. These data suggest smoking may result in transitory stimulation of these pathways, which results in downstream serine phosphorylation of IRS-1 despite a return of the pathway initiating the change to control levels.
Based on this idea of transitory stimulation of insulin desensitizing pathways from smoking, we sought to evaluate mechanisms by which nicotine may promote IRS-1ser636 phosphorylation in L6 muscle culture. Nicotine was used because the majority of smoking-induced insulin resistance can be reproduced with transdermal nicotine administration in humans and thus is thought to be an important component of tobacco smoke promoting insulin resistance (10). However, nicotine infusion or transdermal administration does not acutely change insulin sensitivity in healthy individuals (43,44), whereas chronic use of nicotine gum appears to promote insulin resistance (9). The acute effects of nicotine diverge in insulin-resistant individuals, in whom nicotine infusion or transdermal administration decreases insulin sensitivity (10,43). Therefore, the literature suggests nicotine administration in nicotine naive individuals may require chronic exposure to impact insulin sensitivity, whereas an acute exposure is enough to decrease insulin sensitivity in individuals with underlying insulin resistance. In muscle cell culture, nicotine transiently stimulated p44/p42 MAPK and could be prevented by inhibition with UO126. However, preventing activation of p44/p42 MAPK by nicotine did not increase insulin sensitivity or alter IRS-1ser636 phosphorylation. Therefore, nicotine-induced insulin resistance is not likely to result from p44/p42 MAPK activation.
Our cell-culture approach revealed mTOR as the most likely candidate to explain increased basal inhibition of insulin signaling in smokers. This idea is supported by cancer literature showing mTOR activation in lung lesions from heavy smokers and clinical trials evaluating mTOR inhibitors for the treatment of tobacco-induced lung tumorigenesis (45). Activation of mTOR in humans has also been implicated in insulin resistance of nutritional abundance (46), which can be prevented with rapamycin (47). Similarly, in culture, rapamycin prevented nicotine-induced mTOR activation, prevented nicotine-induced IRS-1ser636 phosphorylation, and normalized insulin sensitivity during nicotine exposure. Therefore, mTOR plays an important role in the insulin resistance of smoking. These data agree with a recent cell-culture study reporting nicotine increased TNF-α expression through increased oxidative stress in the presence of palmitate, which lead to insulin resistance (48). Interestingly, TNF-α activates mTOR (42) and may be one mechanism explaining reversible IRS-1ser636 phosphorylation and insulin resistance. We did not find changes in plasma TNF-α after smoking cessation in the current study, suggesting nicotine may promote local increases in muscle TNF-α. In individuals who smoke or are exposed to secondhand smoke, preventing nicotine-induced mTOR activation in skeletal muscle could be a novel target to decrease diabetes risk.
There are several important limitations with this study. The nicotine dose used in these studies of 200 μmol was chosen in order to maximize our ability to see the influence of nicotine on insulin resistance. This concentration is approximately double the highest dose found in the plasma of smokers (24,25). Smoking-induced vascular dysfunction has been reported in some (49), but not all studies (50), and therefore may have influenced insulin sensitivity after smoking cessation. Although not measured in this study, smoking may increase sympathetic outflow in humans (51), which could reduce muscle blood flow and insulin sensitivity (52). Buproprion is not known to alter insulin sensitivity or muscle lipid metabolism, but theoretically could have influenced the results in smokers before or after smoking cessation. Cell culture showed persistent activation of mTOR with continuous nicotine exposure, which does not agree with muscle biopsy data showing no change in phosphorylation of p70s6k, the downstream kinase for mTOR, compared with controls or after smoking cessation. Therefore, the increase in mTOR activity from smoking may be transitory in humans as compared with cell culture and decrease to normal values 45–60 min after smoking a cigarette. Differences in nicotine clearance may change results of studies in culture compared with in vivo. Nicotine is metabolized by the liver with a half-life of 2 h (53), therefore without hepatic clearance conditions in culture will not completely mimic the in vivo condition. Our cell-culture work used an acute administration of a constant dose of nicotine on cell signaling. Chronic exposure, in addition to cyclical increases and decreases in nicotine from cigarette smoking or secondhand smoke exposure, may elicit different responses.
In conclusion, chronic cigarette smokers had lower insulin sensitivity compared with nonsmokers, which improved but did not normalize after 1 to 2 weeks of smoking cessation. Greater saturation of skeletal muscle lipids was maintained after smoking cessation and may partially explain the residual insulin resistance observed. Increased IRS-1ser636 phosphorylation in smokers normalized after smoking cessation, which may explain the increase in insulin sensitivity. Nicotine in muscle cell culture recapitulated smoking-induced insulin resistance and stimulated major pathways promoting IRS-1ser636 phosphorylation (p44/p42 MAPK and mTOR). However, only preventing mTOR activation mitigated IRS-1ser636 phosphorylation and nicotine-induced insulin resistance in culture. Therapeutic agents designed to oppose skeletal muscle mTOR activation may prove a novel strategy for individuals who are at increased risk of diabetes and cardiovascular disease because they cannot stop smoking or are exposed to secondhand smoke.
ACKNOWLEDGMENTS
This work was partially supported by the National Institutes of Health General Clinical Research Center Grant RR-00036, National Institute of Diabetes and Digestive and Kidney Diseases grants (DK-064811 to L.P., DK-26356 to R.H.E., and DK-059739 to B.C.B.), and a Colorado Tobacco Research Program grant (3K-027 to B.C.B.).
No potential conflicts of interest relevant to this article were reported.
B.C.B. designed the study, performed subject testing, performed cell culture experiments, analyzed data, and wrote the manuscript. L.P. helped design the study, provided medical oversight, performed all biopsies, and helped write the manuscript. D.H. and M.P. performed subject testing, analyzed samples, and edited the manuscript. A.K. performed subject testing, analyzed samples, helped with cell culture experiments, and edited the manuscript. A.M.S. performed subject testing and edited the manuscript. R.H.E. helped design the study and edited the manuscript. B.C.B. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Data from this study were presented in abstract form at the 71st Scientific Sessions of the American Diabetes Association, San Diego, California, 24–28 June 2011.
The authors thank Dr. Dennis Peterson at the University of Colorado Health Sciences Center for the generous donation of the 4-HNE antibody.
Footnotes
See accompanying commentary, p. 3078.
REFERENCES
- 1.Centers for Disease Control and Prevention (CDC) Cigarette smoking among adults—United States, 2007. MMWR Morb Mortal Wkly Rep 2008;57:1221–1226 [PubMed] [Google Scholar]
- 2.Schroeder SA, Warner KE. Don’t forget tobacco. N Engl J Med 2010;363:201–204 [DOI] [PubMed] [Google Scholar]
- 3.Kannel WB, D’Agostino RB, Belanger AJ. Fibrinogen, cigarette smoking, and risk of cardiovascular disease: insights from the Framingham Study. Am Heart J 1987;113:1006–1010 [DOI] [PubMed] [Google Scholar]
- 4.Feskens EJ, Kromhout D. Cardiovascular risk factors and the 25-year incidence of diabetes mellitus in middle-aged men. The Zutphen Study. Am J Epidemiol 1989;130:1101–1108 [DOI] [PubMed] [Google Scholar]
- 5.Perry IJ, Wannamethee SG, Walker MK, Thomson AG, Whincup PH, Shaper AG. Prospective study of risk factors for development of non-insulin dependent diabetes in middle aged British men. BMJ 1995;310:560–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barnoya J, Glantz SA. Cardiovascular effects of secondhand smoke: nearly as large as smoking. Circulation 2005;111:2684–2698 [DOI] [PubMed] [Google Scholar]
- 7.Pirkle JL, Flegal KM, Bernert JT, Brody DJ, Etzel RA, Maurer KR. Exposure of the US population to environmental tobacco smoke: the Third National Health and Nutrition Examination Survey, 1988 to 1991. JAMA 1996;275:1233–1240 [PubMed] [Google Scholar]
- 8.Pirkle JL, Bernert JT, Caudill SP, Sosnoff CS, Pechacek TF. Trends in the exposure of nonsmokers in the U.S. population to secondhand smoke: 1988-2002. Environ Health Perspect 2006;114:853–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eliasson B, Taskinen MR, Smith U. Long-term use of nicotine gum is associated with hyperinsulinemia and insulin resistance. Circulation 1996;94:878–881 [DOI] [PubMed] [Google Scholar]
- 10.Epifano L, Di Vincenzo A, Fanelli C, et al. Effect of cigarette smoking and of a transdermal nicotine delivery system on glucoregulation in type 2 diabetes mellitus. Eur J Clin Pharmacol 1992;43:257–263 [DOI] [PubMed] [Google Scholar]
- 11.Albuquerque EX, Pereira EF, Alkondon M, Rogers SW. Mammalian nicotinic acetylcholine receptors: from structure to function. Physiol Rev 2009;89:73–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kelić S, Olsson T, Kristensson K. Interferon-gamma promotes proliferation of rat skeletal muscle cells in vitro and alters their AChR distribution. J Neurol Sci 1993;114:62–67 [DOI] [PubMed] [Google Scholar]
- 13.Attvall S, Fowelin J, Lager I, Von Schenck H, Smith U. Smoking induces insulin resistance—a potential link with the insulin resistance syndrome. J Intern Med 1993;233:327–332 [DOI] [PubMed] [Google Scholar]
- 14.Eliasson B, Attvall S, Taskinen MR, Smith U. The insulin resistance syndrome in smokers is related to smoking habits. Arterioscler Thromb 1994;14:1946–1950 [DOI] [PubMed] [Google Scholar]
- 15.Hellerstein MK, Benowitz NL, Neese RA, et al. Effects of cigarette smoking and its cessation on lipid metabolism and energy expenditure in heavy smokers. J Clin Invest 1994;93:265–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eliasson B, Attvall S, Taskinen MR, Smith U. Smoking cessation improves insulin sensitivity in healthy middle-aged men. Eur J Clin Invest 1997;27:450–456 [DOI] [PubMed] [Google Scholar]
- 17.Bergman BC, Perreault L, Hunerdosse DM, Koehler MC, Samek AM, Eckel RH. Intramuscular lipid metabolism in the insulin resistance of smoking. Diabetes 2009;58:2220–2227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bergström J, Hermansen L, Hultman E, Saltin B. Diet, muscle glycogen and physical performance. Acta Physiol Scand 1967;71:140–150 [DOI] [PubMed] [Google Scholar]
- 19.Lexell J, Henriksson-Larsén K, Winblad B, Sjöström M. Distribution of different fiber types in human skeletal muscles: effects of aging studied in whole muscle cross sections. Muscle Nerve 1983;6:588–595 [DOI] [PubMed] [Google Scholar]
- 20.Boston RC, Stefanovski D, Moate PJ, Sumner AE, Watanabe RM, Bergman RN. MINMOD Millennium: a computer program to calculate glucose effectiveness and insulin sensitivity from the frequently sampled intravenous glucose tolerance test. Diabetes Technol Ther 2003;5:1003–1015 [DOI] [PubMed] [Google Scholar]
- 21.Bergman BC, Cornier MA, Horton TJ, Bessesen DH. Effects of fasting on insulin action and glucose kinetics in lean and obese men and women. Am J Physiol Endocrinol Metab 2007;293:E1103–E1111 [DOI] [PubMed] [Google Scholar]
- 22.Patterson BW, Zhao G, Elias N, Hachey DL, Klein S. Validation of a new procedure to determine plasma fatty acid concentration and isotopic enrichment. J Lipid Res 1999;40:2118–2124 [PubMed] [Google Scholar]
- 23.Patterson BW, Zhao G, Klein S. Improved accuracy and precision of gas chromatography/mass spectrometry measurements for metabolic tracers. Metabolism 1998;47:706–712 [DOI] [PubMed] [Google Scholar]
- 24.Kilaru S, Frangos SG, Chen AH, et al. Nicotine: a review of its role in atherosclerosis. J Am Coll Surg 2001;193:538–546 [DOI] [PubMed] [Google Scholar]
- 25.Schievelbein H, Eberhardt R, Löschenkohl K, Rahlfs V, Bedall FK. Absorption of nicotine through the oral mucosa. I. Measurement of nicotine concentration in the blood after application of nicotine and total particulate matter. Agents Actions 1973;3:254–258 [DOI] [PubMed] [Google Scholar]
- 26.Klip A, Gumà A, Ramlal T, Bilan PJ, Lam L, Leiter LA. Stimulation of hexose transport by metformin in L6 muscle cells in culture. Endocrinology 1992;130:2535–2544 [DOI] [PubMed] [Google Scholar]
- 27.Wolfe R. Radioactive and Stable Isotope Tracers in Biomedicine: Principles and Practice of Kinetic Analysis. New York, Wiley-Liss, 1992 [Google Scholar]
- 28.Manson JE, Ajani UA, Liu S, Nathan DM, Hennekens CH. A prospective study of cigarette smoking and the incidence of diabetes mellitus among US male physicians. Am J Med 2000;109:538–542 [DOI] [PubMed] [Google Scholar]
- 29.Nilsson P, Lundgren H, Söderström M, Fagerström KO, Nilsson-Ehle P. Effects of smoking cessation on insulin and cardiovascular risk factors—a controlled study of 4 months’ duration. J Intern Med 1996;240:189–194 [DOI] [PubMed] [Google Scholar]
- 30.Yeh HC, Duncan BB, Schmidt MI, Wang NY, Brancati FL. Smoking, smoking cessation, and risk for type 2 diabetes mellitus: a cohort study. Ann Intern Med 2010;152:10–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shulman GI. Cellular mechanisms of insulin resistance. J Clin Invest 2000;106:171–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bergman BC, Perreault L, Hunerdosse DM, Koehler MC, Samek AM, Eckel RH. Increased intramuscular lipid synthesis and low saturation relate to insulin sensitivity in endurance-trained athletes. J Appl Physiol 2010;108:1134–1141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Hees AM, Jans A, Hul GB, Roche HM, Saris WH, Blaak EE. Skeletal muscle fatty acid handling in insulin resistant men. Obesity (Silver Spring) 2011;19:1350–1359 [DOI] [PubMed] [Google Scholar]
- 34.Bergman BC, Hunerdosse DM, Kerege A, Playdon MC, Perreault L. Localisation and composition of skeletal muscle diacylglycerol predicts insulin resistance in humans. Diabetologia 2012;55:1140–1150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hinderliter AK, Dibble AR, Biltonen RL, Sando JJ. Activation of protein kinase C by coexisting diacylglycerol-enriched and diacylglycerol-poor lipid domains. Biochemistry 1997;36:6141–6148 [DOI] [PubMed] [Google Scholar]
- 36.Montell E, Turini M, Marotta M, et al. DAG accumulation from saturated fatty acids desensitizes insulin stimulation of glucose uptake in muscle cells. Am J Physiol Endocrinol Metab 2001;280:E229–E237 [DOI] [PubMed] [Google Scholar]
- 37.Holland WL, Brozinick JT, Wang LP, et al. Inhibition of ceramide synthesis ameliorates glucocorticoid-, saturated-fat-, and obesity-induced insulin resistance. Cell Metab 2007;5:167–179 [DOI] [PubMed] [Google Scholar]
- 38.Houmard JA, Tanner CJ, Yu C, et al. Effect of weight loss on insulin sensitivity and intramuscular long-chain fatty acyl-CoAs in morbidly obese subjects. Diabetes 2002;51:2959–2963 [DOI] [PubMed] [Google Scholar]
- 39.Gual P, Le Marchand-Brustel Y, Tanti JF. Positive and negative regulation of insulin signaling through IRS-1 phosphorylation. Biochimie 2005;87:99–109 [DOI] [PubMed] [Google Scholar]
- 40.Bouzakri K, Roques M, Gual P, et al. Reduced activation of phosphatidylinositol-3 kinase and increased serine 636 phosphorylation of insulin receptor substrate-1 in primary culture of skeletal muscle cells from patients with type 2 diabetes. Diabetes 2003;52:1319–1325 [DOI] [PubMed] [Google Scholar]
- 41.Morino K, Petersen KF, Dufour S, et al. Reduced mitochondrial density and increased IRS-1 serine phosphorylation in muscle of insulin-resistant offspring of type 2 diabetic parents. J Clin Invest 2005;115:3587–3593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ozes ON, Akca H, Mayo LD, et al. A phosphatidylinositol 3-kinase/Akt/mTOR pathway mediates and PTEN antagonizes tumor necrosis factor inhibition of insulin signaling through insulin receptor substrate-1. Proc Natl Acad Sci USA 2001;98:4640–4645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Axelsson T, Jansson PA, Smith U, Eliasson B. Nicotine infusion acutely impairs insulin sensitivity in type 2 diabetic patients but not in healthy subjects. J Intern Med 2001;249:539–544 [DOI] [PubMed] [Google Scholar]
- 44.Mora-Martínez JM, González-Ortiz M, Balcázar-Muñoz BR, Martínez-Abundis E. Acute effect of the transdermal administration of nicotine on insulin sensitivity in healthy individuals with and without a family history of type 2 diabetes mellitus in the first branch. Metab Syndr Relat Disord 2004;2:227–233 [DOI] [PubMed] [Google Scholar]
- 45.Memmott RM, Dennis PA. The role of the Akt/mTOR pathway in tobacco carcinogen-induced lung tumorigenesis. Clin Cancer Res 2010;16:4–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Newgard CB, An J, Bain JR, et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab 2009;9:311–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Krebs M, Brunmair B, Brehm A, et al. The Mammalian target of rapamycin pathway regulates nutrient-sensitive glucose uptake in man. Diabetes 2007;56:1600–1607 [DOI] [PubMed] [Google Scholar]
- 48.Tatebe J, Morita T. Enhancement of TNF-α expression and inhibition of glucose uptake by nicotine in the presence of a free fatty acid in C2C12 skeletal myocytes. Horm Metab Res 2011;43:11–16 [DOI] [PubMed] [Google Scholar]
- 49.Ijzerman RG, Serne EH, van Weissenbruch MM, de Jongh RT, Stehouwer CD. Cigarette smoking is associated with an acute impairment of microvascular function in humans. Clin Sci (Lond) 2003;104:247–252 [DOI] [PubMed] [Google Scholar]
- 50.Rönnemaa EM, Rönnemaa T, Utriainen T, et al. Decreased blood flow but unaltered insulin sensitivity of glucose uptake in skeletal muscle of chronic smokers. Metabolism 1999;48:239–244 [DOI] [PubMed] [Google Scholar]
- 51.Narkiewicz K, van de Borne PJ, Hausberg M, et al. Cigarette smoking increases sympathetic outflow in humans. Circulation 1998;98:528–534 [DOI] [PubMed] [Google Scholar]
- 52.Jamerson KA, Julius S, Gudbrandsson T, Andersson O, Brant DO. Reflex sympathetic activation induces acute insulin resistance in the human forearm. Hypertension 1993;21:618–623 [DOI] [PubMed] [Google Scholar]
- 53.Benowitz NL, Jacob P, 3rd, Jones RT, Rosenberg J. Interindividual variability in the metabolism and cardiovascular effects of nicotine in man. J Pharmacol Exp Ther 1982;221:368–372 [PubMed] [Google Scholar]