Abstract
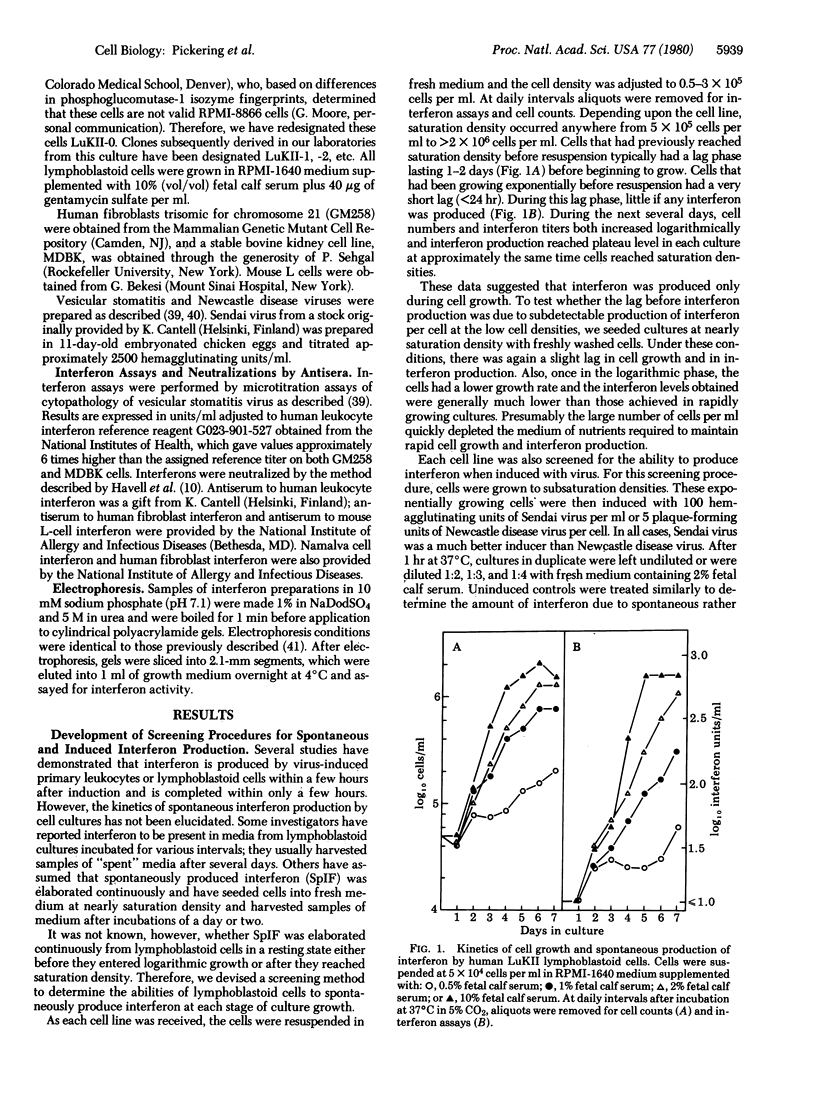
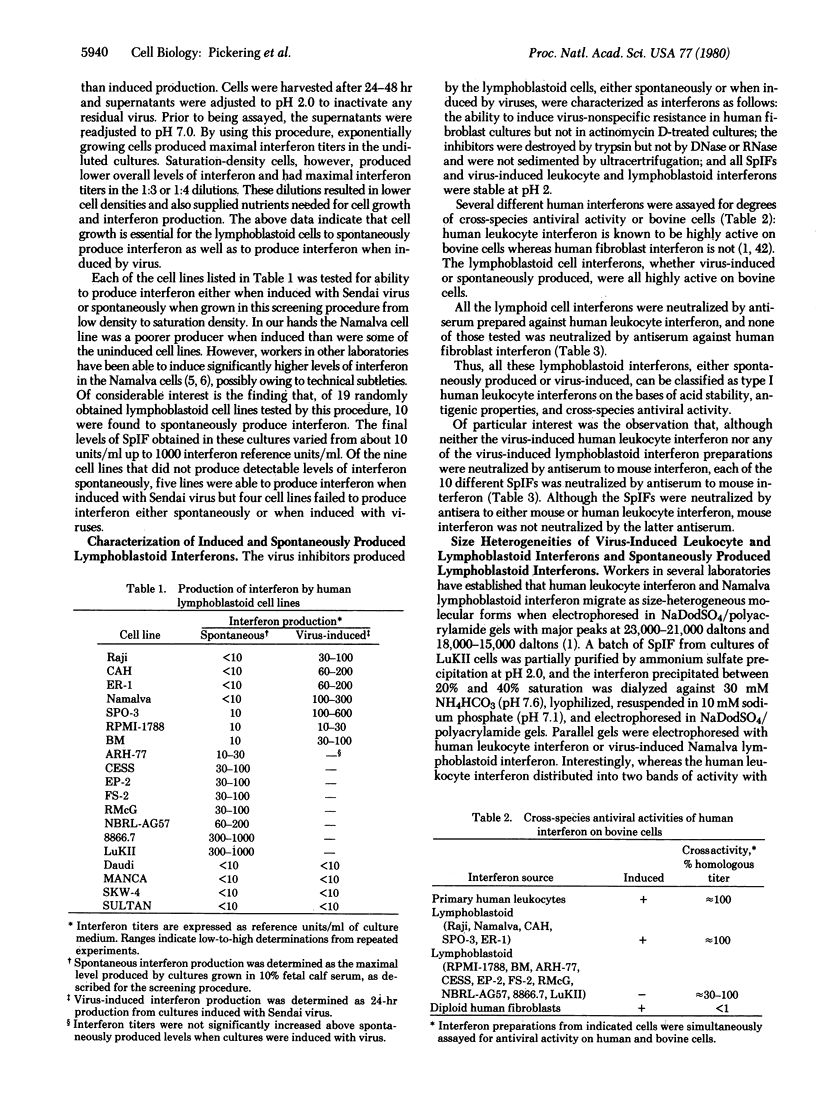
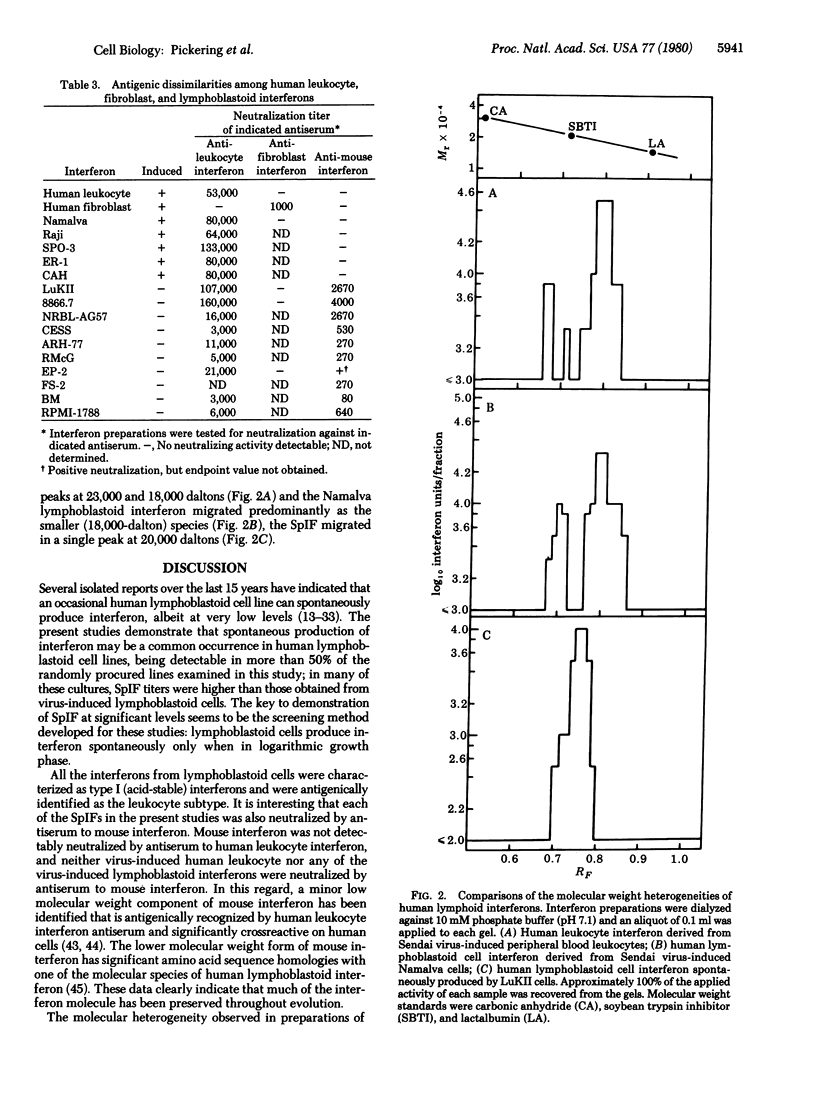
Several established lines of human lymphoblastoid cells were evaluated for abilities to produce interferons. Some cell lines were able to produce interferon when induced with either Newcastle disease virus or Sendai virus, whereas others failed to produce detectable interferon when so induced. However, several cell lines were able to spontaneously produce interferon without induction. Spontaneously produced interferon was liberated by cells only during logarithmic growth phase, reaching levels ranging from about 10 reference units/ml of growth medium for some cell lines to 1000 reference units/ml for others. The interferons produced by induced lymphoblastoid cells and the spontaneously produced interferons were all characterized as type I human leukocyte interferon by high levels of cross-species antiviral activities on bovine cells and by neutralizations by antiserum to human leukocyte interferon but not by antiserum to human fibroblast interferon. However, analysis by electrophoresis in sodium dodecyl sulfate/polyacrylamide gels revealed that spontaneously produced interferon was less size heterogeneous than human leukocyte interferon, migrating as a single band of activity with a peak at 20,000 daltons, whereas human leukocyte interferon contained peaks of major activity at 23,000 and 18,000 daltons and virus-induced Namalva lymphoblastoid cell interferon migrated predominantly as the 18,000-dalton form. Also, although neither virus-induced primary leukocyte interferon nor any of the virus-induced lymphoblastoid cell interferons were neutralized by antiserum to mouse interferon, all of the spontaneously produced interferons were neutralized by antiserum to mouse interferons. These data suggest significant structural similarities between the active cores of certain interferons from phylogenetically diverse animal species.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams A., Lidin B., Strander H., Cantell K. Spontaneous interferon production and Epstein-Barr virus antigen expression in human lymphoid cell lines. J Gen Virol. 1975 Aug;28(2):219–223. doi: 10.1099/0022-1317-28-2-219. [DOI] [PubMed] [Google Scholar]
- Adams A., Strander H., Cantell K. Sensitivity of the Epstein-Barr virus transformed human lymphoid cell lines to interferon. J Gen Virol. 1975 Aug;28(2):207–217. doi: 10.1099/0022-1317-28-2-207. [DOI] [PubMed] [Google Scholar]
- Billiau A., De Somers P., Edy V. G., De Clercq E., Heremans H. Human fibroblast interferon for clinical trials: pharmacokinetics and tolerability in experimental animals and humans. Antimicrob Agents Chemother. 1979 Jul;16(1):56–63. doi: 10.1128/aac.16.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantell K., Hirvonen S. Large-scale production of human leukocyte interferon containing 10(8) units per ml. J Gen Virol. 1978 Jun;39(3):541–543. doi: 10.1099/0022-1317-39-3-541. [DOI] [PubMed] [Google Scholar]
- Cavalieri R. L., Havell E. A., Vilcek J., Pestka S. Synthesis of human interferon by Xenopus laevis oocytes: two structural genes for interferons in human cells. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3287–3291. doi: 10.1073/pnas.74.8.3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton B. J., Paucker K. Antigenic properties of human lymphoblastoid interferons. Infect Immun. 1979 Feb;23(2):244–248. doi: 10.1128/iai.23.2.244-248.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edy V. G., Billiau A., De Somer P. Non-appearance of injected fibroblast interferon in circulation. Lancet. 1978 Feb 25;1(8061):451–452. doi: 10.1016/s0140-6736(78)91251-5. [DOI] [PubMed] [Google Scholar]
- Epstein L. B., Salmon S. E. The production of interferon by malignant plasma cells from patients with multiple myeloma. J Immunol. 1974 Mar;112(3):1131–1138. [PubMed] [Google Scholar]
- Gresser I., Bandu M. T., Brouty-boye D., Tovey M. Pronounced antiviral activity of human interferon on bovine and porcine cells. Nature. 1974 Oct 11;251(5475):543–545. doi: 10.1038/251543a0. [DOI] [PubMed] [Google Scholar]
- HENLEGHENLE W. EVIDENCE FOR A PERSISTENT VIRAL INFECTION IN A CELL LINE DERIVED FROM BURKITT'S LYMPHOMA. J Bacteriol. 1965 Jan;89:252–258. doi: 10.1128/jb.89.1.252-258.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haase A. T., Johnson J. S., Kasel J. A., Margolis S., Levy H. B. Induction of interferon in lymphoblastoid cell lines. Proc Soc Exp Biol Med. 1970 Mar;133(3):1076–1083. doi: 10.3181/00379727-133-34628. [DOI] [PubMed] [Google Scholar]
- Hanley D. F., Wiranowska-Stewart M., Stewart W. E., 2nd Pharmacology of interferons I. Pharmacologic distinctions between human leukocyte and fibroblast interferons. Int J Immunopharmacol. 1979;1(3):219–226. doi: 10.1016/0192-0561(79)90045-6. [DOI] [PubMed] [Google Scholar]
- Havell E. A. Isolation of a subspecies of murine interferon antigenically related to human leukocyte interferon. Virology. 1979 Jan 30;92(2):324–330. doi: 10.1016/0042-6822(79)90137-5. [DOI] [PubMed] [Google Scholar]
- Havell E. A., Vilcek J. Production of high-titered interferon in cultures of human diploid cells. Antimicrob Agents Chemother. 1972 Dec;2(6):476–484. doi: 10.1128/aac.2.6.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havell E. A., Yip Y. K., Vilcek J. Characteristics of human lymphoblastoid (Namalva) interferon. J Gen Virol. 1978 Jan;38(1):51–59. doi: 10.1099/0022-1317-38-1-51. [DOI] [PubMed] [Google Scholar]
- Jarvis A. P., Colby C. Murine interferon system regulation: isolation and characterization of a mutant 3T6 cell engaged in the semiconstitutive synthesis of interferon. Cell. 1978 Jun;14(2):355–363. doi: 10.1016/0092-8674(78)90121-6. [DOI] [PubMed] [Google Scholar]
- Johnston M. D., Christofinis G., Ball G. D., Fantes K. H., Finter N. B. A culture system for producing large amounts of human lymphoblastoid interferon. Dev Biol Stand. 1979;42:189–192. [PubMed] [Google Scholar]
- Klein E., Klein G., Nadkarni J. S., Nadkarni J. J., Wigzell H., Clifford P. Surface IgM-kappa specificity on a Burkitt lymphoma cell in vivo and in derived culture lines. Cancer Res. 1968 Jul;28(7):1300–1310. [PubMed] [Google Scholar]
- Klein G., Dombos L., Gothoskar B. Sensitivity of Epstein-Barr virus (EBV) producer and non-producer human lymphoblastoid cell lines to superinfection with EB-virus. Int J Cancer. 1972 Jul 15;10(1):44–57. doi: 10.1002/ijc.2910100108. [DOI] [PubMed] [Google Scholar]
- Klein G., Vilcek J. Attempts to induce interferon production by IdUrd induction and EBV superinfection in human lymphoma lines and their hybrids. J Gen Virol. 1980 Jan;46(1):111–117. doi: 10.1099/0022-1317-46-1-111. [DOI] [PubMed] [Google Scholar]
- Lidin B., Adams A. Spontaneous interferon production and Epstein-barr virus antigen expression in two human lymphoid cell lines and their somatic cell hybrids. Intervirology. 1975;5(3-4):205–215. doi: 10.1159/000149897. [DOI] [PubMed] [Google Scholar]
- Minnefor A. B., Halsted C. C., Seto D. S., Glade P. R., Moore G. E., Carver D. H. Production of interferon by long-term suspension cultures of leukocytes derived from patients with viral and nonviral diseases. J Infect Dis. 1970 Apr;121(4):442–444. doi: 10.1093/infdis/121.4.442. [DOI] [PubMed] [Google Scholar]
- Morgan M. J., Colby C., Jr, Hulse J. L. Isolation and characterization of virus-resistant mouse embryo fibroblasts. J Gen Virol. 1973 Sep;20(3):377–385. doi: 10.1099/0022-1317-20-3-377. [DOI] [PubMed] [Google Scholar]
- PULVERTAFT J. V. A STUDY OF MALIGNANT TUMOURS IN NIGERIA BY SHORT-TERM TISSUE CULTURE. J Clin Pathol. 1965 May;18:261–273. doi: 10.1136/jcp.18.3.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith T. J., Wagner R. R. Rabbit macrophage interferons. I. Conditions for biosynthesis by virus-infected and uninfected cells. J Exp Med. 1967 Apr 1;125(4):559–577. doi: 10.1084/jem.125.4.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith T. J., Wagner R. R. Rabbit macrophage interferons. II. Some physicochemical properties and estimations of molecular weights. J Exp Med. 1967 Apr 1;125(4):579–593. doi: 10.1084/jem.125.4.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinitz M., Klein G., Koskimies S., Makel O. EB virus-induced B lymphocyte cell lines producing specific antibody. Nature. 1977 Sep 29;269(5627):420–422. doi: 10.1038/269420a0. [DOI] [PubMed] [Google Scholar]
- Stewart W. E., 2nd, Chudzio T., Lin L. S., Wiranowska-Stewart M. Interferoids: in vitro and in vivo conversion of native interferons to lower molecular weight forms. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4814–4818. doi: 10.1073/pnas.75.10.4814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart W. E., 2nd Distinct molecular species of interferons. Virology. 1974 Sep;61(1):80–86. doi: 10.1016/0042-6822(74)90243-8. [DOI] [PubMed] [Google Scholar]
- Stewart W. E., 2nd, Havell E. A. Characterization of a subspecies of mouse interferon cross-reactive on human cells and antigenically related to human leukocyte interferon. Virology. 1980 Feb;101(1):315–318. doi: 10.1016/0042-6822(80)90512-7. [DOI] [PubMed] [Google Scholar]
- Stewart W. E., 2nd, Lin L. S., Wiranowska-Stewart M., Cantell K. Elimination of size and charge heterogeneities of human leukocyte interferons by chemical cleavage. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4200–4204. doi: 10.1073/pnas.74.10.4200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart W. E., 2nd, Wiranowska-Stewart M., Koistinen V., Cantell K. Effect of glycosylation inhibitors on the production and properties of human leukocyte interferon. Virology. 1979 Sep;97(2):473–476. doi: 10.1016/0042-6822(79)90359-3. [DOI] [PubMed] [Google Scholar]
- Strander H., Mogensen K. E., Cantell K. Production of human lymphoblastoid interferon. J Clin Microbiol. 1975 Jan;1(1):116–117. doi: 10.1128/jcm.1.1.116-117.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swart B. E., Young B. G. Inverse relationship of interferon production and virus content in cell lines from Burkitt's lymphoma and acute leukemias. J Natl Cancer Inst. 1969 Jun;42(6):941–944. [PubMed] [Google Scholar]
- Taira H., Broeze R. J., Jayaram B. M., Lengyel P., Hunkapiller M. W., Hood L. E. Mouse interferons: amino terminal amino acid sequences of various species. Science. 1980 Feb 1;207(4430):528–530. doi: 10.1126/science.7352261. [DOI] [PubMed] [Google Scholar]
- Tovey M. G., Begon-Lours J., Gresser I., Morris A. G. Marked enhancement of interferon production in 5-bromodeoxyuridine treated human lymphoblastoid cells. Nature. 1977 Jun 2;267(5610):455–457. doi: 10.1038/267455a0. [DOI] [PubMed] [Google Scholar]
- Tálas M., Szolgay E., Rózsa K. S. Further study of spontaneous interferon produced by hamster peritoneal cells. Arch Gesamte Virusforsch. 1972;38(2):149–158. doi: 10.1007/BF01249665. [DOI] [PubMed] [Google Scholar]
- Tálas M., Weiszfeiler G., Bátkai L. Hamster interferons induced by polyoma virus. Acta Virol. 1968 Jul;12(4):378–380. [PubMed] [Google Scholar]
- Zajac B. A., Henle W., Henle G. Autogenous and virus-induced interferons from lines of lymphoblastoid cells. Cancer Res. 1969 Aug;29(8):1467–1475. [PubMed] [Google Scholar]
- Zoon K. C., Buckler C. E., Bridgen P. J., Gurari-Rotman D. Production of human lymphoblastoid interferon by Namalva cells. J Clin Microbiol. 1978 Jan;7(1):44–51. doi: 10.1128/jcm.7.1.44-51.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoon K. C., Smith M. E., Bridgen P. J., zur Nedden D., Anfinsen C. B. Purification and partial characterization of human lymphoblast interferon. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5601–5605. doi: 10.1073/pnas.76.11.5601. [DOI] [PMC free article] [PubMed] [Google Scholar]



