Abstract
Brown rice is known to improve glucose intolerance and prevent the onset of diabetes. However, the underlying mechanisms remain obscure. In the current study, we investigated the effect of brown rice and its major component, γ-oryzanol (Orz), on feeding behavior and fuel homeostasis in mice. When mice were allowed free access to a brown rice–containing chow diet (CD) and a high-fat diet (HFD), they significantly preferred CD to HFD. To reduce hypothalamic endoplasmic reticulum (ER) stress on an HFD, mice were administered with 4-phenylbutyric acid, a chemical chaperone, which caused them to prefer the CD. Notably, oral administration of Orz, a mixture of major bioactive components in brown rice, also improved glucose intolerance and attenuated hypothalamic ER stress in mice fed the HFD. In murine primary neuronal cells, Orz attenuated the tunicamycin-induced ER stress. In luciferase reporter assays in human embryonic kidney 293 cells, Orz suppressed the activation of ER stress–responsive cis-acting elements and unfolded protein response element, suggesting that Orz acts as a chemical chaperone in viable cells. Collectively, the current study is the first demonstration that brown rice and Orz improve glucose metabolism, reduce hypothalamic ER stress, and, consequently, attenuate the preference for dietary fat in mice fed an HFD.
The number of people who are obese or have type 2 diabetes is increasing dramatically worldwide, and this is being accompanied by drastic changes in dietary habits (1,2). To date, numerous studies have provided insights into the pathophysiology of the obesity–diabetes syndrome as well as numerous potential drug targets (3). However, to date, these works have failed to prevent the increase in both obesity and type 2 diabetes, suggesting that modification of lifestyle may be the most effective approach (3).
The endoplasmic reticulum (ER) is the main site of protein synthesis, folding, and trafficking within cells. The accumulation of newly synthesized “unfolding” proteins creates a pathologic condition defined as ER stress, which results in the unfolding protein response. The accumulation of unfolding proteins is toxic to cells and induces apoptotic cell death. It also causes several ER stress–related diseases, including neurodegenerative disorders, inflammation, and arteriosclerosis (4). Recent studies have given rise to a concept that pathophysiologies of obesity and diabetes are closely related to exaggerated ER stress in a variety of target organs (5–7).
Studies have shown that brain ER stress is related to glucose intolerance, hypertension, and amyotrophic lateral sclerosis in mice (8–11). Notably in the hypothalamus, exaggerated ER stress and inflammation in obese mice is linked to the suppression of leptin receptor signaling, which is involved in regulating appetite and energy expenditure (7,11). Inhibitor of nuclear factor κB (NF-κB) kinase subunit-β (IKKβ)/NF-κB is known to play a crucial role in inflammation. Importantly, Zhang et al. (11) reported in 2008 that NF-κB was activated in the hypothalamus in mice by a high-fat diet (HFD), whereas infusion of an ER stress inhibitor, tauroursodeoxycholic acid, into the intrathird ventricle rescued the activation of NF-κB in the hypothalamus. They also showed that HFD-induced ER stress-augmented IKKβ-mediated elevation of the protein suppressor of cytokine signaling 3 (SOCS3), leading to insulin and leptin resistance in the hypothalamus (11). In 2011, Meng et al. (12) reported that HFD-induced oxidative stress and ER stress attenuated hypothalamic autophagy, resulting in IKKβ/NF-κB activation.
Grains, such as rice, wheat, and corn, comprise a considerable part of the human diet. The outer parts of grains contain multiple nutrients, but most of these parts are removed during the refining process. Recent studies have shown that whole-grain diets improve insulin sensitivity and prevent the occurrence of diabetes compared with refined-grain diets (13,14). In our ongoing studies, brown rice (BR) decreased postprandial blood glucose and insulin levels compared with white rice (WR) in humans (M.S. and C.K. manuscript in preparation). Interestingly, switching the staple food from WR to BR was associated with a significant loss of body weight in subjects with metabolic syndrome. However, the underlying molecular mechanisms of these phenomena are unclear.
One of the major bioactive components in BR is γ-oryzanol (Orz), a mixture of ferulic acid esters with phytosterols (15). Orz possesses a variety of biologic properties, including cholesterol-lowering (16–19), anti-inflammatory (20,21), anticancer (22), antidiabetic (23), and antioxidant activities (16,24,25). Notably, ferulic acid (a metabolite of Orz) alone produces an intrinsic lipid-lowering effect (17) and prevents ER stress-induced cell death in a human neuronal cell line (26). However, the potential effects of Orz on glucose metabolism and body weight regulation remained unclear.
In the current study, we demonstrated that BR and its major component, Orz, improve glucose metabolism in mice fed an HFD. Our data provide novel evidence that BR and Orz reduce hypothalamic ER stress and attenuate the preference for dietary fat in mice.
RESEARCH DESIGN AND METHODS
Animals.
Male C57BL/6J mice (Oriental BioService, Inc., Kyoto, Japan) were housed with a 12-h light–dark cycle at 24°C. Animals were allowed free access to food and water. At age 8 weeks, mice were randomly divided into six groups 1): control chow diet (CD; 10% of energy as fat), 2) CD plus 30% (w/w) BR, 3) CD plus 30% (w/w) WR, 4), control HFD (45% of energy as fat), 5) HFD plus BR, and 6) HFD plus WR (Table 1). All diets were manufactured as pellets by Research Diets (New Brunswick, NJ). Body weights and food intake were measured weekly. All animal experiments were approved by the animal experiment ethics committee of the University of the Ryukyus.
TABLE 1.
Compositions of CD and HFD
Dietary fat preferences.
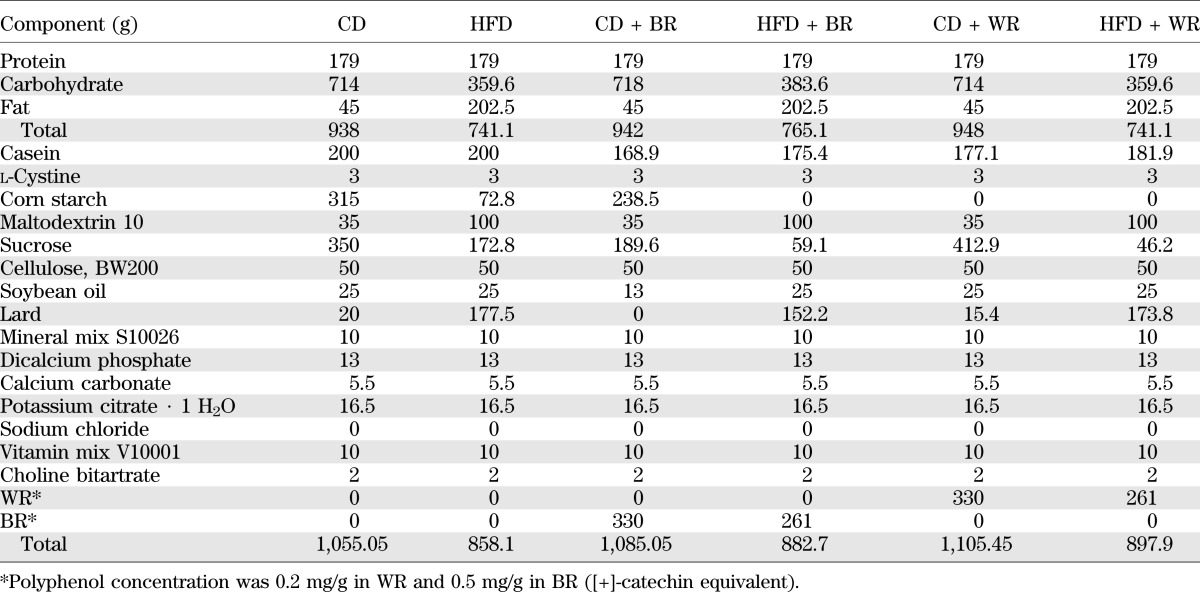
Dietary fat preferences were evaluated in the two-food CD versus HFD choice tests. The mice were allowed free access to the CD and the HFD. At age 8 weeks, mice were randomly divided into three groups: the control (CD vs. HFD), BR-containing diet (CD + BR vs. HFD + BR), and the WR-containing diet (CD + WR vs. HFD + WR) groups (Fig. 4A). Intake of the CDs and HFDs was measured weekly and analyzed for changes in the preference for dietary fat. HFD preference was calculated according to the formula: HFD preference = [(HFD intake/total food intake) × 100].
FIG. 4.
Impact of BR on dietary preference in mice. A: Dietary fat preferences were evaluated in the two-food CD vs. HFD choice tests. Mice were allowed free access to CD and HFD. Mice were randomly divided into three groups: the control (CD vs. HFD), BR-containing diet (CD + BR vs. HFD + BR), and the WR-containing diet (CD + WR vs. HFD + WR) groups. HFD preference in mice fed BR (B) and 4-PBA–treated mice (D). C: Body weight of mice fed BR during two-food CD vs. HFD choice tests (n = 8–12; 4 mice per cage). Data are expressed as mean ± SEM. *P < 0.05, **P < 0.01 compared with control or vehicle mice; †P < 0.05, ††P < 0.01 compared with WR-fed mice. (A high-quality digital representation of this figure is available in the online issue.)
Administration of Orz and 4-phenylbutyric acid.
Orz (Wako Pure Chemical Industries, Osaka, Japan) was dissolved in 0.5% methyl cellulose solution. Orz (20, 80, or 320 μg/g body weight) was delivered into the stomach by a gavage needle every day for 13 weeks. A solution of 4-phenylbutyric acid (4-PBA; Wako Pure Chemical Industries) was prepared by titrating equimolar amounts of 4-PBA and sodium hydroxide to pH 7.4. A dose of 4-PBA (120 μg/g body weight) was injected intraperitoneally daily for 8 weeks.
Analyses of metabolic parameters.
Blood glucose levels were determined from whole venous blood taken from mouse tails using a Medisafe Mini automatic glucometer (Terumo, Tokyo, Japan) at 9:00 a.m. Blood samples were taken from the retro-orbital venous plexuses. Concentrations of plasma insulin and leptin were measured using an enzyme-linked immunosorbent assay (ELISA) kit (Shibayagi Co., Ltd., Gunma, Japan, and Morinaga Institute of Biological Science, Inc., Tokyo, Japan).
Fecal triglyceride contents.
The feces from each group were collected weekly for 2 weeks. After drying, total lipids were extracted from the feces according to the procedure of Folch et al. (27). Fecal triglyceride concentrations were measured using the triglyceride E-test kit (Wako Pure Chemical Industries).
Glucose tolerance test and insulin tolerance test.
The glucose tolerance test (GTT) and insulin tolerance test (ITT) were performed after 8 weeks of diet feeding. For the GTT, mice received glucose (2.0 g/kg body weight) delivered into the stomach by a gavage needle or injected into the intraperitoneal cavity after 20 h of fasting. Blood glucose levels were measured at the indicated time points. For the ITT, mice were injected with insulin (0.5 units/kg body weight; Novo Nordisk, Bagsvaerd, Denmark) into the intraperitoneal cavity after 4 h of fasting.
Luciferase reporter assay.
The reporter plasmids, pGL4-luc2P Luciferase Reporter Vector (Promega, Madison, WI), contain the luciferase gene under ER stress–responsive cis-acting elements such as the ER stress response element (ERSE-I, CCTTCACCAATCGGCGGCCTCCACGACGG; ERSE-II, GGACGCCGATTGGGCCACGTTGGGAGAGTGCCT), and unfolded protein response element (UPRE; CTCGAGACAGGTGCTGACGTGGCATTC). Reporter plasmid-transfected human embryonic kidney (HEK) 293 cells were cotreated with tunicamycin (2 μg/mL) and Orz (10 μmol/L) or 4-PBA (1 mmol/L). Luciferase activities were assessed by an EnVision reader (PerkinElmer, Waltham, MA).
Primary neuronal culture.
Neuronal cells were isolated from cortices of fetal C57BL/6J mice and cultured as previously described (28). After 1 week of culture, Orz (0.1, 1 μmol/L) was added into media for 2 h. After the pretreatment period, cells were stimulated with tunicamycin (0.1 μg/mL) for 5 h, washed with PBS, and total RNA was extracted.
Quantitative real-time PCR.
Total RNA was extracted using Trizol reagent (Invitrogen, Carlsbad, CA), and cDNA was synthesized using an iScript cDNA Synthesis Kit (Bio-Rad, Hercules, CA) according to the manufacturers’ instructions. Quantitative real-time PCR was performed using StepOneplus Real-Time PCR systems, TaqMan Gene Expression Master Mix (Applied Biosystems, Foster City, CA), and SYBR Green (Takara Bio, Shiga, Japan), following the manufacturers’ instructions. mRNA levels were normalized to those of 18S rRNA. The primer and probe sets used for quantitative real-time PCR analysis are shown in Supplementary Table 1.
Western blots.
Mouse recombinant leptin (1 μg; Wako Pure Chemical Industries) was dissolved in 2 μL PBS. Mice were anesthetized with pentobarbital (50 mg/kg body weight i.p.), and administrated leptin intracerebroventricularly with the use of a Hamilton microsyringe. At 30 min after leptin administration, the hypothalamus was sampled and homogenized as described (29). Western blots were performed according to the Invitrogen NuPage protocol (Invitrogen). The antibodies used were anti-phospho-signal transducer and activator of transcription 3 (STAT3; Tyr705) and anti-STAT3 antibody (Cell Signaling Technology, Inc., Danvers, MA). ECL Plus Western Blotting Detection System (GE Healthcare U.K. Ltd., Little Chalfont, U.K.) and LAS-4000 mini image analyzer (Fuji Film, Tokyo, Japan) were used for detection.
Statistical analysis.
Data are expressed as the mean ± SEM of triplicate experiments. Data were analyzed using one-way and repeated-measures ANOVA, followed by the multiple comparison test (Ryan’s method). For all other measurements, the Student t test was used to analyze differences between groups. Differences were considered significant at P < 0.05.
RESULTS
Body weight, food intake, and fecal triglyceride contents.
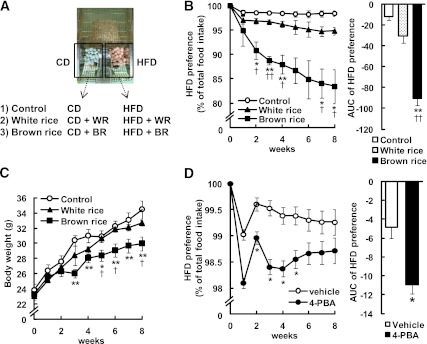
C57BL/6J mice were fed BR or WR for 10 weeks, and their body weight and food intake were monitored. Neither BR nor WR affected body weight gain in mice fed the CDs or HFDs (Fig. 1A and B). There was no difference in daily food intake between mice fed BR and WR for 10 weeks (CD, 27.3 ± 2.3 g/day; CD + WR, 26.1 ± 0.1 g/day; CD + BR, 28.5 ± 2.5 g/day; HFD, 32.5 ± 0.8 g/day; HFD + WR, 34.5 ± 1.5 g/day; HFD + BR, 31.7 ± 0.4 g/day). Feces were collected during 13 to 15 weeks of BR feeding. BR was significantly increased fecal triglyceride extracts on both the CDs and HFDs (CD, 0.09 ± 0.05 mg/g feces; CD + WR, 0.15 ± 0.01 mg/g feces; CD + BR, 0.23 ± 0.05 mg/g feces; HFD, 0.17 ± 0.08 mg/g feces; HFD + WR, 0.35 ± 0.06 mg/g feces; CD + BR, 0.65 ± 0.16 mg/g feces; Fig. 1E and F).
FIG. 1.
Effects of BR on body weight in mice fed CD (A) and HFD (B). Effects of BR on blood glucose levels in mice fed CD (C) and HFD (D) in the fed state. Fecal triglyceride (TG) contents of mice fed CD (E) and HFDs (F). Data are expressed as mean ± SEM (n = 10–12). *P < 0.05, **P < 0.01 compared with control mice; †P < 0.05, ††P < 0.01 compared with WR-fed mice.
Glucose homeostasis.
In mice fed CDs for 10 weeks, neither BR nor WR affected blood glucose levels (CD, 133 ± 4 mg/dL; CD + WR, 135 ± 3 mg/dL; CD ± BR, 130 ± 3 mg/dL; Fig. 1C). In mice fed the HFDs, however, blood glucose levels were significantly lower in the BR group than in the control and WR groups (HFD, 156 ± 4 mg/dL; HFD + WR, 156 ± 4 mg/dL; HFD + BR, 132 ± 3 mg/dL; Fig. 1D). Neither BR nor WR added to the CDs or HFDs affected plasma insulin levels (CD, 722 ± 75 pg/mL; CD + WR, 775 ± 78 pg/mL; CD + BR, 747 ± 156 pg/mL; HFD, 1,055 ± 102 pg/mL; HFD + WR, 1,100 ± 104 pg/mL; HFD + BR, 991 ± 143 pg/mL).
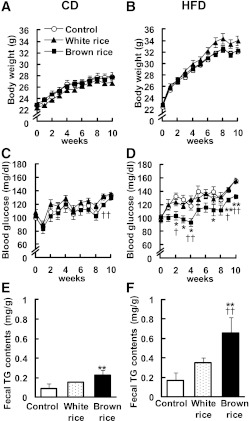
GTT and ITT were performed to further evaluate the effect of BR on glucose metabolism. When glucose was delivered into the stomach, blood glucose levels tended to decrease in the BR-fed groups compared with the WR-fed groups fed the CDs and HFDs (Fig. 2A and B). We observed a significant reduction in the area under the curve (AUC) for glucose during the GTT in the HFD + BR group (HFD, 28,610 ± 797 mg⋅dL−1⋅min−1; HFD + WR, 27,400 ± 1172 mg⋅dL−1⋅min−1; HFD + BR, 23,383 ± 538 mg⋅dL−1⋅min−1; Fig. 2B). When insulin was injected into the intraperitoneal cavity, blood glucose levels were significantly lower in the mice fed BR than the mice fed WR only in the HFD-fed groups (Fig. 2C and D). We also observed a significant reduction in the AUC for glucose during the ITT in the HFD + BR group (HFD, 28,610 ± 797 mg⋅dL−1⋅min−1; HFD + WR, 12,835 ± 581 mg⋅dL−1⋅min−1; HFD + BR, 10,116 ± 454 mg⋅dL−1⋅min−1; Fig. 2D).
FIG. 2.
Effects of BR on glucose homeostasis in mice. Blood glucose levels and AUC during GTT in mice fed CD (A) and HFD (B) for 10 weeks. Blood glucose levels and the AUC during ITT after 10 weeks of BR feeding in mice fed the CD (C) and the HFD (D). Data are expressed as mean ± SEM (n = 3–6). *P < 0.05, **P < 0.01 compared with control mice; †P < 0.05, ††P < 0.01 compared with WR-fed mice.
BR decreased hypothalamic ER stress in HFD-fed mice.
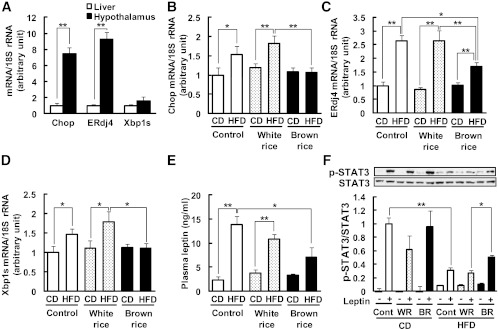
To investigate the effect of BR on hypothalamic ER stress, we analyzed the mRNA levels of ER stress–responsive genes such as CCAAT/enhancer-binding protein-homologous protein (Chop), endoplasmic reticulum resident DNAJ 4 (ERdj4), and the spliced form of X-box binding protein 1 (Xbp1s). In response to ER stress, Xbp1 mRNA is spliced into its active form by inositol-requiring protein 1 (IRE1) (30). The mRNAs of ER stress–responsive genes were highly expressed in the hypothalamus compared with the liver (Chop, 7.5-fold; ERdj4, 9.3-fold; Xbp1s, 1.6-fold; Fig. 3A). The mRNA levels of ER stress–responsive genes were significantly upregulated in the hypothalami of the mice fed the HFDs compared with those fed the CDs (Chop, 1.5-fold; ERdj4, 2.6-fold; Xbp1s, 1.5-fold; Fig. 3B–D). BR did not affect the mRNA levels of Chop, ERdj4, or Xbp1s in the hypothalami of the mice fed the CDs. However, BR significantly decreased the mRNA levels of Chop, ERdj4, and Xbp1s in the hypothalami of the mice fed the HFDs (42, 36, and 38% reduction vs. HFD + WR, respectively; Fig. 3B–D).
FIG. 3.
Effects of BR on hypothalamic ER stress in mice. A: Comparison of Chop, ERdj4, and Xbp1s mRNA levels in the hypothalamus and liver. The tissues were dissected from mice fed CD. Mice were fed BR or WR for 10 weeks. Levels of mRNA in the hypothalamus are shown for Chop (B), ERdj4 (C), and Xbp1s (D). The mRNA levels were determined using real-time PCR. Values were normalized to that of 18S rRNA. E: Plasma leptin concentrations were measured by ELISA. F: Leptin-induced (1 μg, i.c.v.) STAT3 phosphorylation. Blots show phospho-STAT3 (p-STAT3) and STAT3, and graph shows the p-STAT3-to-STAT3 ratio. Data are expressed as mean ± SEM (n = 3–9). *P < 0.05, **P < 0.01.
Previous reports indicated that ER stress contributes, at least in part, to the development of leptin resistance through inflammatory pathways (7,11). Thus, the mRNA levels of inflammatory cytokines in the hypothalamus were investigated. The results showed a similar trend to ER stress–responsive genes (Supplementary Fig. 1). The plasma leptin levels of the mice fed the HFDs were significantly greater than those of the mice fed the CDs (13.8 ± 1.8 vs. 2.3 ± 0.7 ng/mL; Fig. 3E). BR significantly decreased plasma leptin levels in mice the HFD (7.1 ± 2.0 vs. 13.8 ± 1.8 ng/ mL; Fig. 3E). To assess hypothalamic leptin sensitivity, phosphorylation of STAT3 was measured. The intracerebroventricular administration of leptin substantially increased STAT3 phosphorylation in hypothalami from mice fed the CDs (Fig. 3F). The levels of STAT3 phosphorylation were significantly decreased in mice fed the HFDs (31 ± 0.1% vs. CD; Fig. 3F), indicating insensitivity to leptin. However in mice fed the HFDs, BR significantly increased leptin-induced STAT3 phosphorylation compared with WR (1.9-fold vs. HFD + WR; Fig. 3F), indicating that BR ameliorated hypothalamic leptin resistance.
BR attenuated the preference for dietary fat.
To investigate the effect of BR on feeding behavior, we measured food consumption for 8 weeks when mice were allowed to choose freely between the CDs and HFDs. Total food intake did not differ among control, WR, and BR groups (data not shown). The control group strongly preferred the HFD (Fig. 4B). In contrast, in the group fed the diet containing BR, the HFD preference was decreased significantly (∼15.0% reduction vs. control, ∼11.4% reduction vs. WR diet group; Fig. 4B), leading to decreased body weight gain (control, 32.94 ± 1.33 g; WR, 31.69 ± 0.92 g; BR, 30.28 ± 0.44 g; Fig. 4C).
To test whether hypothalamic ER stress causes the attenuation of the preference for dietary fat due to BR feeding, mice were treated with PBS (vehicle) or 4-PBA (120 μg⋅g body weight−1⋅day−1). Food consumption and the preference for the HFD were measured. Although total food intake did not differ (data not shown), HFD preference was decreased mildly but significantly in the group treated with 4-PBA compared with the vehicle-treated group (HFD preference, ∼1.1% reduction vs. vehicle; AUC for HFD preference, 2.2-fold decrease vs. vehicle; Fig. 4D).
Effects of long-term Orz administration on body weight and glucose metabolism in HFD-fed mice.
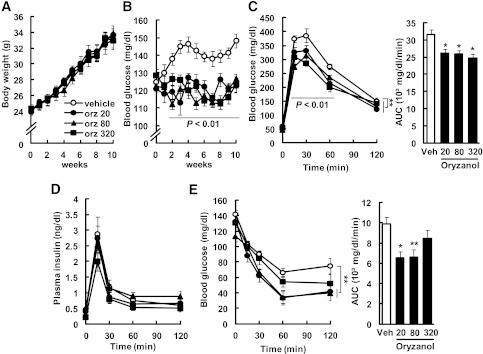
Oral Orz administration did not affect the body weights or plasma insulin levels of the mice fed the HFDs (plasma insulin: control, 451 ± 88 pg/mL; Orz 20, 405 ± 61 pg/mL; Orz 80, 495 ± 115 pg/mL; Orz 320, 218 ± 27 pg/mL; Fig. 5A and D); however, Orz significantly decreased blood glucose levels of these mice (vehicle, 157 ± 8 mg/mL; Orz 20, 131 ± 5 mg/mL; Orz 80, 127 ± 4 mg/mL; Orz 320, 128 ± 5 mg/mL; Fig. 5B). The GTT revealed that the increase in blood glucose was significantly blunted in the Orz-treated groups compared with the vehicle-treated group (AUC: vehicle, 31,673 ± 1,290 mg⋅dL−1⋅min−1; Orz 20, 26,120 ± 1,173 mg⋅dL−1⋅min−1; Orz 80, 26,016 ± 894 mg⋅dL−1⋅min−1; Orz 320, 24,815 ± 1,095 mg⋅dL−1⋅min−1; Fig. 5C). The blood glucose levels 15, 30, and 60 min after the injection of glucose decreased significantly in the Orz-treated groups compared with the vehicle-treated group (Fig. 5C). Between the Orz-treated and the vehicle-treated group, there was no difference in plasma insulin levels during the GTT (Fig. 5D). The AUCs for glucose during the ITTs of Orz-treated groups were significantly smaller than those of the vehicle-treated group (vehicle, 9,906 ± 649 mg⋅dL−1⋅min−1; Orz 20, 6,524 ± 643 mg⋅dL−1⋅min−1; Orz 80, 6,596 ± 786 mg⋅dL−1⋅min−1; Orz 320, 8,451 ± 812 mg⋅dL−1⋅min−1; Fig. 5E). When mice were injected with insulin, the blood glucose levels 60 min after the injection decreased markedly in Orz-treated groups compared with the vehicle-treated group (Fig. 5E).
FIG. 5.
Effects of long-term administration of Orz on body weight (A) and blood glucose (B) were examined in mice fed the HFD ad libitum. Blood glucose levels and the AUC for glucose during the GTT (C) and the ITT (E) and plasma insulin levels (D) during the GTT after 10 weeks of Orz treatment at doses of 20, 80, and 320 μg⋅g body weight−1⋅day−1. Data are expressed as mean ± SEM (n = 3–6). *P < 0.05, **P < 0.01 compared with vehicle-treated mice.
Orz suppressed the activation of ER stress–responsive cis-acting elements in HEK293 cells.
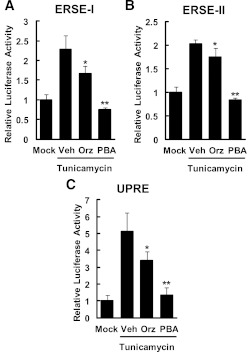
We examined the effects of Orz on the activities of the reporter genes carrying cis-acting element such as ERSEs and UPRE upstream of the luciferase gene. HEK293 cells transfected with reporter genes were cotreated with tunicamycin (2 μg/mL) and Orz (1 μmol/L) for 15 h (ERSEs) and 24 h (UPRE). Orz significantly suppressed the tunicamycin-induced activation of cis-acting elements (ERSE-I, 27%; ERSE-II, 64%; UPRE, 34% reduction vs. vehicle; Fig. 6).
FIG. 6.
Effects of Orz on the activities of ER stress–responsive cis-acting elements in HEK293 cells. Reporter genes-transfected HEK293 cells were cotreated with tunicamycin (2 μg/mL) and Orz (1 μmol/L) or 4-PBA (1 mmol/L) for 15 h (ERSEs) or 24 h (UPRE). The activities of ERSE-I (A), ERSE-II (B), and UPRE (C) were measured. Data are expressed as mean ± SEM (n = 4). *P < 0.05, **P < 0.01 compared with vehicle (Veh)-treated cells.
Orz decreased tunicamycin-induced ER stress in murine primary neuronal cells.
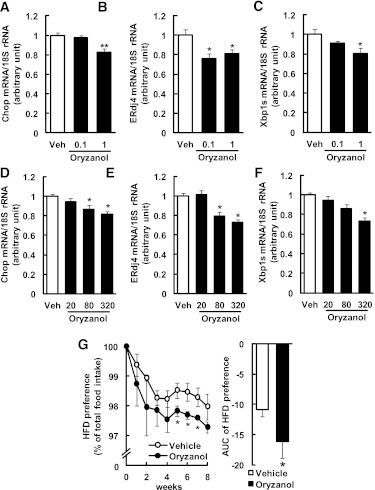
To investigate the effect of Orz on ER stress in primary neuronal cells, cells were pretreated with Orz (0.1, 1 μmol/L) for 2 h and then stimulated with tunicamycin (0.1 μg/mL). Pretreatement of cells with Orz (1 μmol/L) significantly suppressed the expression of ER stress–responsive genes (Chop, 83%; ERdj4, 81%; and Xbp1s, 90% of vehicle; Fig. 7A–C).
FIG. 7.
Effects of Orz on hypothalamic ER stress and dietary preference in mice. Murine primary neuronal cells were pretreated with Orz (0.1, 1 μmol/L) and then stimulated with tunicamycin (0.1 μg/mL). The mRNA levels are shown for Chop (A), ERdj4 (B), and Xbp1s (C). Values were normalized to that of 18S rRNA and are expressed as levels relative to that of vehicle (Veh)-pretreated cells (n = 4–8). Mice were treated with Orz at doses of 20, 80 and 320 μg⋅g body weight−1⋅day−1 for 13 weeks, and mRNA levels were measured in the hypothalamus for Chop (D), ERdj4 (E), and Xbp1s (F). Values were normalized to that of 18S rRNA and are expressed as levels relative to that of vehicle-treated mice (n = 8–11). The mRNA levels were determined using real-time PCR. G: HFD preference in Orz-treated mice (80 μg⋅g body weight−1⋅day−1). Mice were allowed free access to CD and HFD (n = 6; 2 mice per cage). Data are expressed as mean ± SEM. *P < 0.05, **P < 0.01 compared with vehicle-treated cells or mice.
Orz decreased hypothalamic ER stress in HFD-fed mice.
To investigate whether Orz treatment reduces hypothalamic ER stress, we analyzed the mRNA levels of Chop, ERdj4, and Xbp1s in the hypothalami of mice fed the HFDs and treated with vehicle or Orz at various dosages (20, 80, or 320 μg⋅g body weight−1⋅day−1) for 13 weeks. Orz decreased Chop, ERdj4, and Xbp1s mRNA levels in the hypothalamus (∼19%, ∼27%, and ∼27% reduction vs. vehicle, respectively; Fig. 7D–F). However, the mRNA levels of these genes in the liver did not change significantly (Chop, 101–115%; ERdj4, 89–118%; and Xbp1s, 65–87% of vehicle).
Orz attenuated the preference for dietary fat.
To test whether Orz attenuates the preference for dietary fat, mice were treated with vehicle or Orz (80 μg⋅g body weight−1⋅day−1). Food consumption and the preference for the HFD were assessed. HFD preference was significantly decreased in the Orz-treated group compared with that of the vehicle-treated group (HFD preference, ∼1.0% reduction vs. vehicle; AUC for HFD preference, 1.5-fold decrease vs. vehicle; Fig. 7G).
DISCUSSION
The major finding of the current study is that BR attenuates preference for an HFD via the inhibition of hypothalamic ER stress. Notably, we demonstrated that Orz—one of the major bioactive components in rice bran—exerts a similar effect to BR. Previous studies show that exposure to high-fat high-sugar foods alters sensitivity of the central reward pathway and amplifies the preference for such foods (31,32). The current study is the first to highlight the effects of hypothalamic ER stress on feeding behavior. BR significantly decreased ER stress–responsive genes in the hypothalamus of mice fed the HFD. This notion is confirmed by the finding that the reduction of ER stress by a chemical chaperone attenuated the preference for the HFD.
We also found that mice fed BR preferred the CD to the HFD and had lighter body weights than mice fed the control and WR diets (Fig. 4B and C). Neither BR nor WR affected plasma insulin levels; therefore, BR attenuates the preference for dietary fat in an insulin-independent manner. Furthermore, BR decreased the expression of ER stress–responsive genes (a proapoptotic gene, Chop, and genes related with protein folding such as Xbp1s and ERdj4) in the mice fed the HFDs (Fig. 3B–D). It is noteworthy that the expression levels of ER stress–responsive genes were much higher in the hypothalamus than in the liver (Fig. 3A), which raises the possibility that ER stress plays a crucial role in hypothalamic function. ER stress provokes leptin resistance in the hypothalamus, which leads to hyperphagia and obesity (7). The notion that hypothalamic ER stress is linked with a preference for dietary fat is supported by results of the administration of the chemical chaperone 4-PBA (Fig. 4D). Previous reports show that 4-PBA exerts chaperone activity within the central nervous system (7,33). Of note, we demonstrated that administrating an artificial sweetener, aspartame, to early-weaned mice increased hypothalamic ER stress and the resultant preference for dietary fat (K.Y. and C. Kozuka, manuscript in preparation). Taken together, the results of our study raise the possibility that hypothalamic ER stress influences the tone of preference for dietary fat.
Recent reports showed that lateral hypothalamic neurons expressing orexin and other transmitters provide modulatory input to midbrain dopamine neurons, consequently modulating the brain reward function (34,35). Studies have also shown that leptin decreases the brain reward function in the ventral tegmental area (36,37). In the current study, BR decreased plasma leptin concentrations and ameliorated hypothalamic leptin resistance in mice fed the HFDs (Fig. 3E and F). Thus, it is also tempting to speculate that BR may attenuate the preference for dietary fat through the brain reward function. The underlying mechanisms require further investigation.
Previous studies have shown that a high intake of BR is associated with a lower risk of type 2 diabetes in humans (14,38,39), although the underlying mechanism remains unknown. The results of the current study indicate that BR significantly decreased blood glucose levels (Fig. 1D) and improved glucose metabolism (Fig. 2B and D) in the mice fed the HFDs. Notably, BR did not affect body weight (Fig. 1A and B) or plasma insulin levels. Moreover, BR decreased the expressions of the ER stress–responsive genes in the hypothalamus of the mice fed the HFDs (Fig. 3B–D). Hypothalamic ER stress is known to induce ER stress in the liver and adipose tissue, contributing to systemic glucose intolerance (7). Treatment with 4-PBA can improve glucose metabolism in type 2 diabetic ob/ob mice (40).
BR decreased intestinal absorption of triglyceride in mice fed the CDs and HFDs (Fig. 1E and F). Previous studies showed that a couple of lipase inhibitors extracted from BR inhibited pancreatic lipase activity, leading to a decrease in intestinal lipid absorption (41–43). Evidence has accrued that lipid accumulation increases ER stress in adipose tissue and liver (44). On the basis of these results, we speculate that BR improves glucose metabolism through central as well as peripheral mechanisms.
Similar to BR, the results in the current study demonstrate that long-term oral administration of Orz improves glucose intolerance in mice (Fig. 5), accompanied by a significant reduction in hypothalamic ER stress and resultant attenuation in preference for dietary fat (Fig. 7D–G). This finding was reinforced by the data that Orz reduced significantly tunicamycin-induced ER stress in murine primary neuronal cells (Fig. 7A–C). Together with a previous report that orally administrated Orz to rabbit was considerably distributed in the brain (45), our finding shows a potential of Orz to act directly on the hypothalamus. Importantly, Orz suppressed the tunicamycin-induced activation of ER stress responsive cis-acting elements in HEK293 cells (Fig. 6), suggesting that Orz act as a chaperone in viable cells. Rice bran contains a large amount of Orz, and BR contains about 30–60 mg/100 g Orz (46,47). On the basis of these data, the mice fed BR in our experiments consumed ∼10–20 μg⋅g body weight−1⋅day−1 Orz. Consequently, we administered Orz (20, 80, or 320 μg⋅g body weight−1⋅day−1) within the physiologic range. The results of the current study suggest that Orz plays a crucial role in metabolically beneficial effects of BR on glucose homeostasis and food preference.
It is noteworthy that gastric administration of Orz for 13 weeks improved glucose intolerance and insulin sensitivity without increasing body weight or plasma insulin levels (Fig. 5), highlighting the metabolically beneficial impacts of Orz without increasing body weight. The metabolic impact of Orz on whole-body glucose homeostasis is currently under investigation in our laboratory.
In summary, the results of the current study show for the first time that BR and one of its components, Orz, improve HFD-induced glucose dysmetabolism and attenuate the preference for dietary fat by decreasing hypothalamic ER stress. These findings suggest that BR and its extracts may be useful tools for ameliorating obesity and metabolic syndrome.
ACKNOWLEDGMENTS
This work was supported in part by Special Account Budget for Education and Research granted by the Japan Ministry of Education, the Takeda Science Foundation (Specified Research Grant), Grants-in-Aid from MEXT, Japan (Category C) (Nos. 22591014, 22592105), the Okinawa Medical Science Research Foundation (Research promotion), the University of the Ryukyus Foundation, and the project “Establishing a research hub toward the development of an intellectual cluster in Okinawa Prefecture, Japan.”
No potential conflicts of interest relevant to this article were reported.
C.K. researched data and wrote the manuscript. Ko.Y., S.S., R.U., S.T., H.O., T.I., Ke.Y., M.H., H.T., C.T., M.M., S.O., and M.S. contributed to discussion. H.M. wrote the manuscript. H.M. is the guarantor of this work, had full access to all the data, and takes full responsibility for the integrity of data and the accuracy of data analysis.
The authors thank Toshihiko Ogawa, Keiko Nakamoto, Keiko Iwabuchi, Megumi Yonaha, Asako Oshiro, Mamiko Hirata, Maki Katayanagi, Nanae Yamamoto, and Chikako Noguchi (all of Graduate School of Medicine, University of the Ryukyus) for their assistance.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db11-1767/-/DC1.
REFERENCES
- 1.Hossain P, Kawar B, El Nahas M. Obesity and diabetes in the developing world--a growing challenge. N Engl J Med 2007;356:213–215 [DOI] [PubMed] [Google Scholar]
- 2.Zimmet P, Alberti KGMM, Shaw J. Global and societal implications of the diabetes epidemic. Nature 2001;414:782–787 [DOI] [PubMed] [Google Scholar]
- 3.Freedman DH. How to fix the obesity crisis. Sci Am 2011;304:40–47 [DOI] [PubMed] [Google Scholar]
- 4.Yoshida H. ER stress and diseases. FEBS J 2007;274:630–658 [DOI] [PubMed] [Google Scholar]
- 5.Hotamisligil GS. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell 2010;140:900–917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ozcan U, Ozcan L, Yilmaz E, et al. Loss of the tuberous sclerosis complex tumor suppressors triggers the unfolded protein response to regulate insulin signaling and apoptosis. Mol Cell 2008;29:541–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ozcan L, Ergin AS, Lu A, et al. Endoplasmic reticulum stress plays a central role in development of leptin resistance. Cell Metab 2009;9:35–51 [DOI] [PubMed] [Google Scholar]
- 8.Purkayastha S, Zhang G, Cai D. Uncoupling the mechanisms of obesity and hypertension by targeting hypothalamic IKK-β and NF-κB. Nat Med 2011;17:883–887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Purkayastha S, Zhang H, Zhang G, Ahmed Z, Wang Y, Cai D. Neural dysregulation of peripheral insulin action and blood pressure by brain endoplasmic reticulum stress. Proc Natl Acad Sci U S A 2011;108:2939–2944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hetz C, Thielen P, Matus S, et al. XBP-1 deficiency in the nervous system protects against amyotrophic lateral sclerosis by increasing autophagy. Genes Dev 2009;23:2294–2306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang X, Zhang G, Zhang H, Karin M, Bai H, Cai D. Hypothalamic IKKbeta/NF-kappaB and ER stress link overnutrition to energy imbalance and obesity. Cell 2008;135:61–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meng Q, Cai D. Defective hypothalamic autophagy directs the central pathogenesis of obesity via the IkappaB kinase beta (IKKbeta)/NF-kappaB pathway. J Biol Chem 2011;286:32324–32332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pereira MA, Jacobs DR, Jr, Pins JJ, et al. Effect of whole grains on insulin sensitivity in overweight hyperinsulinemic adults. Am J Clin Nutr 2002;75:848–855 [DOI] [PubMed] [Google Scholar]
- 14.Sun Q, Spiegelman D, van Dam RM, et al. White rice, brown rice, and risk of type 2 diabetes in US men and women. Arch Intern Med 2010;170:961–969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lerma-Garcia MJ, Herrero-Martinez JM, Simo-Alfonso EF, Mendonca CRB, Ramis-Ramos G. Composition, industrial processing and applications of rice bran gamma-oryzanol. Food Chem 2009;115:389–404 [Google Scholar]
- 16.Jin Son M, W Rico C, Hyun Nam S, Young Kang M. Influence of oryzanol and ferulic acid on the lipid metabolism and antioxidative status in high fat-fed mice. J Clin Biochem Nutr 2010;46:150–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cicero AFG, Gaddi A. Rice bran oil and gamma-oryzanol in the treatment of hyperlipoproteinaemias and other conditions. Phytother Res 2001;15:277–289 [DOI] [PubMed] [Google Scholar]
- 18.Rong N, Ausman LM, Nicolosi RJ. Oryzanol decreases cholesterol absorption and aortic fatty streaks in hamsters. Lipids 1997;32:303–309 [DOI] [PubMed] [Google Scholar]
- 19.Wilson TA, Nicolosi RJ, Woolfrey B, Kritchevsky D. Rice bran oil and oryzanol reduce plasma lipid and lipoprotein cholesterol concentrations and aortic cholesterol ester accumulation to a greater extent than ferulic acid in hypercholesterolemic hamsters. J Nutr Biochem 2007;18:105–112 [DOI] [PubMed] [Google Scholar]
- 20.Islam MS, Murata T, Fujisawa M, et al. Anti-inflammatory effects of phytosteryl ferulates in colitis induced by dextran sulphate sodium in mice. Br J Pharmacol 2008;154:812–824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Akihisa T, Yasukawa K, Yamaura M, et al. Triterpene alcohol and sterol ferulates from rice bran and their anti-inflammatory effects. J Agric Food Chem 2000;48:2313–2319 [DOI] [PubMed] [Google Scholar]
- 22.Yasukawa K, Akihisa T, Kimura Y, Tamura T, Takido M. Inhibitory effect of cycloartenol ferulate, a component of rice bran, on tumor promotion in two-stage carcinogenesis in mouse skin. Biol Pharm Bull 1998;21:1072–1076 [DOI] [PubMed] [Google Scholar]
- 23.Son MJ, Rico CW, Nam SH, Kang MY. Effect of oryzanol and ferulic acid on the glucose metabolism of mice fed with a high-fat diet. J Food Sci 2011;76:H7–H10 [DOI] [PubMed] [Google Scholar]
- 24.Xu ZM, Hua N, Godber JS. Antioxidant activity of tocopherols, tocotrienols, and gamma-oryzanol components from rice bran against cholesterol oxidation accelerated by 2,2′-azobis(2-methylpropionamidine) dihydrochloride. J Agric Food Chem 2001;49:2077–2081 [DOI] [PubMed] [Google Scholar]
- 25.Wang T, Hicks KB, Moreau R. Antioxidant activity of phytosterols, oryzanol, and other phytosterol conjugates. J Am Oil Chem Soc 2002;79:1201–1206 [Google Scholar]
- 26.Hiratsuka T, Matsuzaki S, Miyata S, et al. Yokukansan inhibits neuronal death during ER stress by regulating the unfolded protein response. PLoS ONE 2010;5:e13280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Folch J, Lebaron FN. The isolation from brain tissue of a trypsin-resistant protein fraction containing combined inositol, and its relation to neurokeratin. J Neurochem 1956;1:101–108 [DOI] [PubMed] [Google Scholar]
- 28.Saito K, Kondo E, Matsushita M. MicroRNA 130 family regulates the hypoxia response signal through the P-body protein DDX6. Nucleic Acids Res 2011;39:6086–6099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miyamoto L, Toyoda T, Hayashi T, et al. Effect of acute activation of 5′-AMP-activated protein kinase on glycogen regulation in isolated rat skeletal muscle. J Appl Physiol 2007;102:1007–1013 [DOI] [PubMed] [Google Scholar]
- 30.Yoshida H, Matsui T, Yamamoto A, Okada T, Mori K. XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell 2001;107:881–891 [DOI] [PubMed] [Google Scholar]
- 31.Ong ZY, Muhlhausler BS. Maternal “junk-food” feeding of rat dams alters food choices and development of the mesolimbic reward pathway in the offspring. FASEB J 2011;25:2167–2179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Avena NM, Rada P, Hoebel BG. Evidence for sugar addiction: behavioral and neurochemical effects of intermittent, excessive sugar intake. Neurosci Biobehav Rev 2008;32:20–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Inden M, Kitamura Y, Takeuchi H, et al. Neurodegeneration of mouse nigrostriatal dopaminergic system induced by repeated oral administration of rotenone is prevented by 4-phenylbutyrate, a chemical chaperone. J Neurochem 2007;101:1491–1504 [DOI] [PubMed] [Google Scholar]
- 34.Berthoud HR, Lenard NR, Shin AC. Food reward, hyperphagia, and obesity. Am J Physiol Regul Integr Comp Physiol 2011;300:R1266–R1277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fulton S. Appetite and reward. Front Neuroendocrinol 2010;31:85–103 [DOI] [PubMed] [Google Scholar]
- 36.Bruijnzeel AW, Corrie LW, Rogers JA, Yamada H. Effects of insulin and leptin in the ventral tegmental area and arcuate hypothalamic nucleus on food intake and brain reward function in female rats. Behav Brain Res 2011;219:254–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hommel JD, Trinko R, Sears RM, et al. Leptin receptor signaling in midbrain dopamine neurons regulates feeding. Neuron 2006;51:801–810 [DOI] [PubMed] [Google Scholar]
- 38.Karupaiah T, Aik CK, Heen TC, et al. A transgressive brown rice mediates favourable glycaemic and insulin responses. J Sci Food Agric 2011;91:1951–1956 [DOI] [PubMed] [Google Scholar]
- 39.Kim TH, Kim EK, Lee MS, et al. Intake of brown rice lees reduces waist circumference and improves metabolic parameters in type 2 diabetes. Nutr Res 2011;31:131–138 [DOI] [PubMed] [Google Scholar]
- 40.Ozcan U, Yilmaz E, Ozcan L, et al. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science 2006;313:1137–1140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Raghavendra MP, Prakash V. Phenylboronic acid—a potent inhibitor of lipase from Oryza sativa. J Agric Food Chem 2002;50:6037–6041 [DOI] [PubMed] [Google Scholar]
- 42.Takahashi H, inventor; Yakurigaku Chuo Kenkyusho, assignee. Lipase inhibitor derived from a defatted rice germ U.S. patent 5,503,831. 2 April 1996, p. 347 [Google Scholar]
- 43.Tsutsumi K, Kawauchi Y, Kondo Y, Inoue Y, Koshitani O, Kohri H. Water extract of defatted rice bran suppresses visceral fat accumulation in rats. J Agric Food Chem 2000;48:1653–1656 [DOI] [PubMed] [Google Scholar]
- 44.Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest 2006;116:1793–1801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fujiwara H, Hiraoka R, Kawashima Y. The absorption, elimination, distribution and metabolism of triterpene alcohol ferulate (y-Oryzanol). Yakubutsu Ryoho 1972;5:123–130 [Google Scholar]
- 46.Miller A, Engel KH. Content of gamma-oryzanol and composition of steryl ferulates in brown rice (Oryza sativa L.) of European origin. J Agric Food Chem 2006;54:8127–8133 [DOI] [PubMed] [Google Scholar]
- 47.Ohtsubo K, Suzuki K, Yasui Y, Kasumi T. Bio-functional components in the processed pre-germinated brown rice by a twin-screw extruder. J Food Compost Anal 2005;18:303–316 [Google Scholar]