Abstract
Maternal obesity increases offspring birth weight and susceptibility to obesity. Adiponectin is an adipocyte-secreted hormone with a prominent function in maintaining energy homeostasis. In contrast to adults, neonatal blood adiponectin levels are positively correlated with anthropometric parameters of adiposity. This study was designed to investigate the role of adiponectin in maternal obesityenhanced fetal fat deposition. By using high-fat diet–induced obese mouse models, our study showed that maternal obesity increased fetal fat tissue mass, with a significant elevation in fetal blood adiponectin. However, adiponectin gene knockout (Adipoq−/−) attenuated maternal obesity-induced high fetal fat tissue mass. We further studied the effects of fetal adiponectin on fetal fat deposition by using a cross breeding approach to create Adipoq−/+ and Adipoq−/− offspring, whereas maternal adiponectin was null. Adipoq−/+ offspring had more fat tissue mass at both birth and adulthood. Significantly high levels of lipogenic genes, such as sterol regulatory element–binding protein 1c and fatty acid synthase, were detected in the livers of Adipoq−/+ fetuses. In addition, expression of genes for placental fatty acid transport was significantly increased in Adipoq−/+ fetuses. Together, our study indicates that adiponectin enhances fetal fat deposition and plays an important role in maternal obesity-induced high birth weight.
Obesity impairs glucose and lipid metabolism and is a risk factor for many diseases. In the United States, adult obesity has reached epidemic levels. Most strikingly, the prevalence of obesity in children (aged 6–19 years) has tripled to 17% since 1980 (1). Recent studies have clearly demonstrated that maternal obesity increases not only offspring birth weight, but also offspring susceptibility to obesity during their lifetime (2–4). Therefore, elucidating the underlying mechanism of maternal obesity-induced high birth weight and offspring adiposity will be important to curbing the soaring rate of obesity.
Adiponectin is an adipocyte-derived hormone that enhances insulin sensitivity. In contrast to adults, in which adiponectin expression is inversely associated with adiposity, infant blood adiponectin concentrations are positively correlated with body weight and body length (5–9). During late pregnancy, opposite changes in adiponectin gene expression in maternal and fetal compartments create a significant difference in adiponectin concentration between maternal and fetal circulations. At delivery, neonatal blood adiponectin levels are four- to sevenfold higher than those in maternal circulation (10). During the same period, fetal fat is exponentially deposited in humans (11). Adiponectin inhibits energy expenditure (12,13) and enhances lipid accumulation in adipocytes by increasing adipocyte differentiation and suppressing lipolysis (14–16). Overexpression of adiponectin increases adipose tissue mass in adult mice (12,17). These studies prompted us to hypothesize that changes in adiponectin levels in maternal and fetal compartments during gestation coordinately regulates fetal fat deposition and plays an important role in maternal obesity-induced offspring adiposity.
By using genetic approaches to manipulate adiponectin gene expression in maternal and fetal tissues and by using high-fat (HF) feeding to induce maternal obesity, our study showed that adiponectin gene knockout attenuated maternal obesity-induced high birth weight in mice. The results indicate that adiponectin increases fetal fat deposition and has a significant impact on fat tissue mass from the neonatal stage to adulthood. Furthermore, our study also suggests that increased placental fatty acid transport and fetal hepatic de novo lipogenesis might contribute to adiponectin-enhanced fetal fat deposition.
RESEARCH DESIGN AND METHODS
Materials.
Anti–phospho-acetyl-CoA carboxylase 1 (ACC1) (S79), ACC1, phospho–AMP-activated protein kinase (T172), AMP-activated protein kinase α, and phospho-glycogen synthase kinase 3β (GSK3β) (S9) were ordered from Cell Signaling Technology (Danvers, MA). Antibodies against GSK3α/β, fatty acid synthase (FASN), and glyceraldehyde-3-phosphate dehydrogenase were from Santa Cruz Biotechnology (Santa Cruz, CA). HF diet (60 kcal% fat; D12492) and low-fat (LF) diet (10 kcal% fat; D12450B) were produced by Research Diet (New Brunswick, NJ).
Animal models.
C57BL/6 mice were ordered from The Jackson Laboratory (Bar Harbor, ME). Adiponectin gene knockout (Adipoq−/−) mice were created in Dr. Philipp Scherer’s laboratory. Wild-type (WT) littermates were used as controls for Adipoq−/− mice. The mice were randomly grouped. The experiments using mouse models were carried out under the Association for Assessment and Accreditation of Laboratory Animal Care guidelines with approval from the University of California San Diego Animal Care and Use Committees.
Obese dams were created by feeding 8-week-old female mice with HF diet for 6 weeks and during mating and gestation. LF diet was provided to female mice of the same age, which served as lean control dams. Without further specific indication, the rest of the mice, including sires, were fed with regular chow diet.
To study the effect of adiponectin on fetal fat deposition, two breeding schemes were used. Scheme 1 was designed for studying the role of adiponectin in maternal obesity-induced high fetal and birth weight (Supplementary Fig. 1A): Adipoq−/− or WT females were mated with the same genotype sires. Scheme 2 was designed to study the effect of fetal adiponectin on fetal fat deposition and to eliminate the effects from maternal adiponectin: Adipoq−/− females were cross-mated with WT or Adipoq−/− sires to produce Adipoq−/+ or Adipoq−/− offspring (Supplementary Fig. 1B). Pregnancy was determined as the presence of a vaginal plug and was assigned the title e0.5. Fetuses were collected by Caesarean section at indicated ages. Body composition was measured using EchoMRI (EchoMRI, Houston, TX) with pooled fetuses.
In situ hybridization.
The studies were provided by the service of Phylogeny (Columbus, OH). To detect adiponectin gene expression during mouse development, a series of tissue samples from fetuses, newborns, and adult C57BL/6 mice were probed with adiponectin riboprobes. Gene expression patterns were analyzed by both X-ray file autoradiography and emulsion autoradiography. Cellular level results were revealed at low magnification as bright labeling on a dark background. Anatomical data were shown at high magnification as black labeling by silver grains on hematoxylin-stained background. Oil Red O staining was performed for comparison with in situ hybridization labeling.
Cell culture.
Mouse embryonic fibroblasts (MEFs) from WT and GSK3β gene knockout (GSK3β−/−) mice were created by the laboratory of Dr. James R. Woodgett (Mount Sinai Hospital, Toronto, Ontario, Canada). A coculture system was used for the adiponectin treatment (15,18). Confluent MEFs were cocultured overnight in an insert well with Ad-Acrp30– or Ad-green fluorescent protein–transduced FAO cells using Dulbecco’s modified Eagle’s medium without FBS. After overnight coculture, adiponectin in the medium reached a level of 40–50% of that in C57BL/6 mouse serum (15,18).
Western blot and real-time PCR assays.
Protein samples were resolved using NuPAGE gels (Invitrogen). Protein was blotted with indicated antibodies (see details in figure legends). Protein levels were semiquantified by image density scanning using Quantity One software (Bio-Rad) with an internal reference for assays of multiple gels.
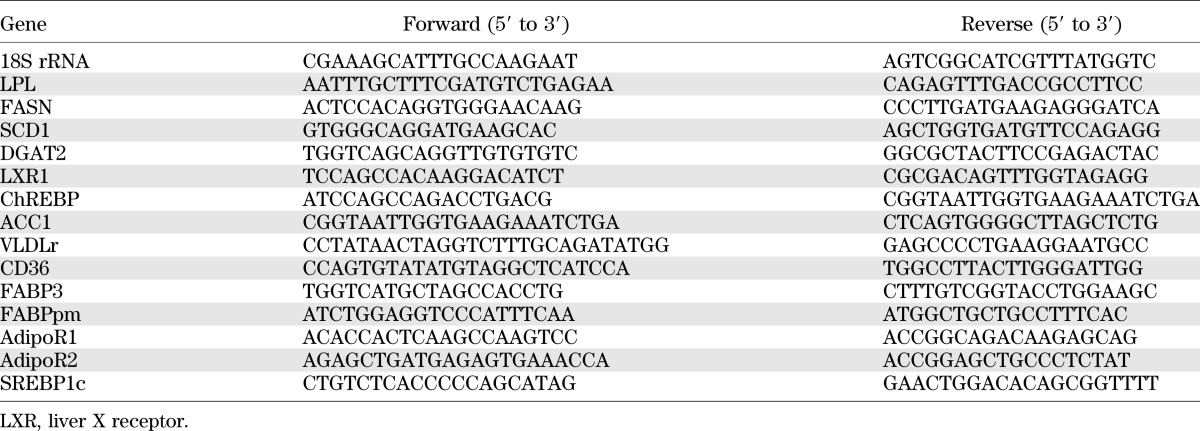
Total RNA was prepared from liver and placenta tissues using TRIzol reagent (Invitrogen). Real-time PCR was performed using an mx3000p Real-Time PCR system (Stratagene) and SYBR Green dye (Molecular Probes, Eugene, OR). The sequences for the PCR primers are in Table 1.
TABLE 1.
Sequences for real-time PCR primers
Statistical analysis.
Data are expressed as mean ± SEM. Statistical analysis was performed using the Student t test or ANOVA, followed by Bonferroni posttests using Prism software (GraphPad). Differences were considered significant at P < 0.05.
RESULTS
Mouse adiponectin gene expression during fetal development.
Adiponectin is a 30-kDa protein. During the secretion process, adiponectin self-associates to form multimeric structures that increase its molecular weight up to ∼360 kDa. Theoretically, any protein with a molecular weight >0.5 kDa cannot pass the placental barrier. By detecting adiponectin protein in the blood of Adipoq−/− dams and their Adipoq−/+ fetuses or vice versa, our studies clearly indicate that adiponectin cannot be transported through the placenta (Supplementary Fig. 2), which is consistent with previous reports (19,20). Therefore, maternal and fetal adiponectin are not interchangeable.
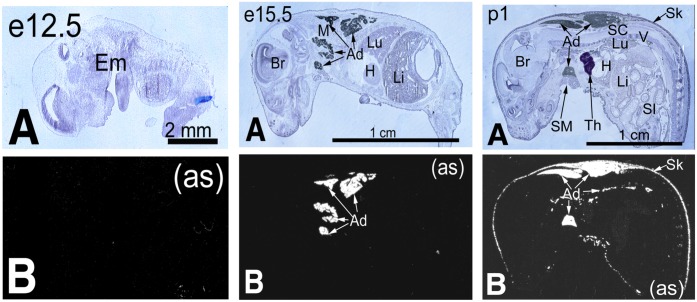
We studied the expression of fetal adiponectin during mouse development by using in situ hybridization, real-time PCR, and C57BL/6 fetuses. Our results showed that there was no fetal adiponectin gene expression during early pregnancy until embryonic day (e)15.5 (Fig. 1, real-time PCR results were not shown). The in situ hybridization results also showed that fetal adiponectin gene expression was initially from brown adipose-like tissues in the intrascapular, dorsal, and ventral axillae regions. Interestingly, to further characterize these adiponectin-expressing tissues using Oil Red O staining, we found that there was almost no lipid accumulation in these tissues at e15.5, but significant lipid droplets were detected in the same region at birth (Supplementary Fig. 3). These results indicate that during late gestation, mouse fetal adiponectin gene expression is turned on in brown adipose-like tissues even before apparent lipid droplet formation.
FIG. 1.
Fetal adiponectin gene expression. A: Anatomical view of C57BL/6 fetuses and newborn mice. Ad, adipose; Br, brain; Em, embryo; H, heart; Li, liver; Lu, lung; M, skeletal muscle; SI, small intestine; Sk, skin; SM, submaxillary gland; Th, thymus; V, vertebrae. B: Adiponectin mRNA was detected by in situ hybridization with labeled adiponectin antisense (as) riboprobe. Gene expression patterns were analyzed by both X-ray file autoradiography and emulsion autoradiography. Cellular level results are shown at low magnification as bright labeling on a dark background. Anatomical data are revealed at high magnification as black labeling by silver grains on hematoxylin-stained background. (A high-quality digital representation of this figure is available in the online issue.)
Adiponectin gene knockout attenuated maternal obesity-induced high fetal body weight and fat tissue mass.
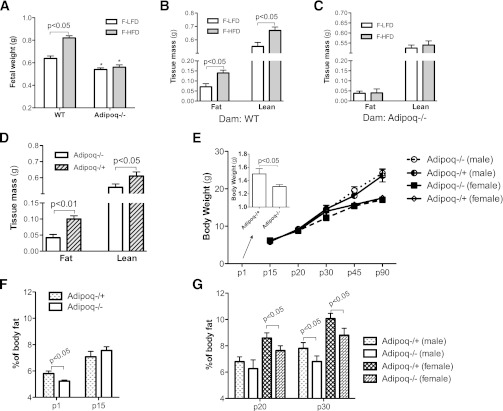
Maternal obesity increases offspring birth weight in humans (3,21). The same phenomenon has been reported in both mice and rats (2,22). To study the role of adiponectin in maternal obesity-induced high birth weight, Adipoq−/− and WT mice were used (breeding scheme 1, Supplementary Fig. 1A). Maternal obesity was induced by HF feeding 6 weeks before and during pregnancy. Body weight and body composition of 17.5-day-old fetuses (e17.5) were compared. Consistent with previous reports, the body weight of fetuses from obese WT dams was significantly higher than that of fetuses from lean WT dams (Fig. 2A). Both fat and lean body mass of fetuses from obese WT dams were significantly higher than that of fetuses from lean WT dams (Fig. 2B). However, the differences in fetal body weight and fat tissue mass disappeared when the same HF or LF feeding regimes were applied to Adipoq−/− dams (Fig. 2A and C). These results clearly show that adiponectin gene deletion attenuated maternal obesity-induced high fetal body weight and fat tissue mass. Interestingly, fetal serum adiponectin protein levels and adiponectin mRNA levels in intrascapular fat were remarkably elevated in fetuses from obese WT dams (Supplementary Fig. 2). Although these observations of increased adiponectin gene expression, blood protein level, and fat tissue mass agree with human studies that found that neonatal adiposity positively correlates with circulating adiponectin concentrations, our current study does not provide further evidence to explain why maternal obesity increases fetal adiponectin gene expression.
FIG. 2.
Adiponectin increased fetal fat tissue mass. Two breeding schemes were used for these studies. Adipoq−/− or WT dams were mated with the same genotype sires (scheme 1, Supplementary Fig. 1A), whereas maternal obesity was induced by HF feeding (A–C). Body weights of e17.5-old fetuses from obese and lean dams were compared (A). Body composition of fetuses from WT (B) or Adipoq−/− (C) obese dams were scanned using magnetic resonance imaging and pooled fetal samples; n = 6. Adipoq−/+ and Adipoq−/− offspring were produced by using scheme 2 (Supplementary Fig. 1B), and all dams were Adipoq−/− (D and E). All offspring and dams were fed with regular chow (D–G). Litter size was adjusted to five to six (E–G). Male and female offspring were separated after weaning (p20). Fetal fat and lean tissue mass were compared between Adipoq−/+ and Adipoq−/− fetuses (D, e17.5; n = 6). Body weight and body composition of Adipoq−/+ and Adipoq−/− offspring were monitored from p1–p90 (E–G; n = 21–24). Data of p1 and p15 mice represent both sexes (F). *P < 0.05 vs. Adipoq−/− offspring. F-HFD, fetus from HF diet–fed dams; F-LFD, fetus of LF diet–fed dams.
Comparing body weight and body composition of fetuses from LF-fed lean WT and Adipoq−/− dams, our study showed that adiponectin knockout fetuses have significantly less body weight (Fig. 2A) and fat content (0.071 ± 0.02 vs. 0.042 ± 0.013 g).
Adiponectin increased fetal fat deposition and body fat in adults.
To further study the effect of adiponectin on fetal fat deposition and body fat after birth, Adipoq−/− dams were crossed with Adipoq−/− or WT sires to produce Adipoq−/+ and Adipoq−/− offspring (breeding scheme 2, Supplementary Fig. 1B). Although blood adiponectin levels in Adipoq−/+ mice are ∼50% of Adipoq+/+ mice (23), a comparison of adiposity between Adipoq−/− and Adipoq−/+ offspring should provide important information about the role of fetal adiponectin on fat deposition. Furthermore, in this setting, all dams were Adipoq−/−, which eliminates the influence of maternal adiponectin during gestation and lactation.
Our results showed that body weight of e17.5-old Adipoq−/+ fetuses was significantly higher than that of Adipoq−/− mice (0.641 ± 0.021 vs. 0.581 ± 0.032 g; P = 0.027). When comparing body composition, we found that Adipoq−/+ fetuses displayed an increase in both lean and fat tissue mass (Fig. 2D). However, the magnitude of increase in fat mass was almost twofold, whereas lean tissue mass was increased ∼20% compared with Adipoq−/− fetuses (Fig. 2D). These results indicate that fetal adiponectin increases fetal growth and enhances fat deposition. The underlying mechanisms for adiponectin-enhanced lean tissue growth are pursued through a separate project.
We also studied offspring body weight and composition from delivery to adulthood. At birth, Adipoq−/+ mice were heavier than Adipoq−/− mice (Fig. 2E, inset bar graph). However, the difference quickly disappeared, and no statistical difference in body weight between Adipoq−/+ and Adipoq−/− offspring was detected for the rest of the study (Fig. 2E, mice were grouped by sex after day 20 postdelivery [p20]). Interestingly, when comparing body fat, our results showed that Adipoq−/− offspring displayed a quick catchup in body fat immediately after birth at p15 (Fig. 2F). However, this trend was reversed at later times, and Adipoq−/+ mice exhibited significantly higher body fat tissue mass during the rest of the study period (Fig. 2G), which is consistent with previous studies using adult Adipoq−/− and WT mice (13,15).
Adiponectin increased fetal blood free fatty acid levels.
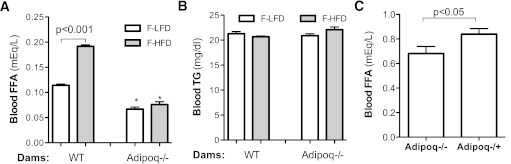
Fatty acid (FA) supply plays an important role in fetal fat deposition. Measuring serum free FA (FFA) levels revealed that maternal HF feeding increased fetal blood FFA levels nearly twofold (Fig. 3A) without a significant change in triglyceride (TG) levels (Fig. 3B). In comparing Adipoq−/− and WT fetuses, FFA concentrations were significantly lower in Adipoq−/− fetuses, regardless of the adiposity status of dams (Fig. 3A). Furthermore, there was no difference of FFA levels between Adipoq−/− fetuses from lean and obese dams (Fig. 3A). These results indicate that adiponectin gene deletion diminishes maternal obesity-induced elevation of fetal FFA levels.
FIG. 3.
Adiponectin and maternal obesity increased fetal blood FFA. Fetuses were generated using the same breeding scheme 1 (A and B) or 2 (C), as in Supplementary Fig. 1. For the maternal obesity study (A and B), dams were fed with HF or LF diet for 6 weeks before and during gestation. Regular chow was provided to the dams described in C. Fetal blood was collected by heart puncture. Maternal obesity increased blood FFA levels of fetuses from WT dams but not in fetuses from Adipoq−/− dams (A). Maternal HF feeding did not alter fetal TG levels of both WT and Adipoq−/− dams (B). Blood FFA concentrations of Adipoq−/+ fetuses were significantly higher than that of Adipoq−/− fetuses (C). *P < 0.05 vs. F-LF diet or F-HF diet fetuses of WT dams. F-HFD, fetuses from HF diet–fed dams; F-LFD, fetus from LF diet–fed dams. n = 21–24.
Adiponectin regulates lipid metabolism in both maternal and fetal compartments. To specifically look at the effect of fetal adiponectin on fetal blood FFA levels and to eliminate the effect of maternal adiponectin, we compared fetal serum lipid profiles between Adipoq−/+ and Adipoq−/− fetuses from chow-fed Adipoq−/− dams (breeding scheme 2, Supplementary Fig. 1B). As shown in Fig. 3C, FFA levels of Adipoq−/+ fetuses were significantly higher than that of Adipoq−/− fetuses. Together, these studies suggest that fetal adiponectin increases FFA levels in fetal circulation.
Adiponectin and maternal obesity increased placental lipoprotein lipase gene expression.
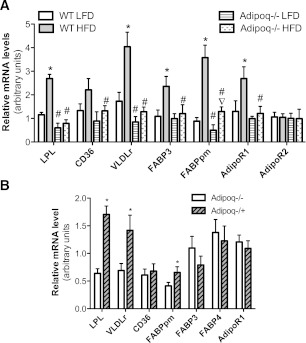
The placenta plays a pivotal role in fetal nutrition supply. It has been reported that placenta size is positively correlated with birth weight. To our surprise, there was no significant difference in placenta weight between Adipoq−/+ and Adipoq−/− fetuses or fetuses from obese and control dams in either genotype (Supplementary Fig. 3). FA can be transported through the placenta by diffusion and FA carrier proteins. Furthermore, we studied the expression levels of genes involved in VLDL-TG catabolism and fatty acid transport. As shown in Fig. 4A, mRNA levels of lipoprotein lipase (LPL), VLDLr, FA-binding protein (FABP) 3, and FABPpm were significantly increased in placentas from obese WT dams compared with placentas from control dams. These results strongly suggest that there is enhanced FA transport in the placentas of obese WT dams. This is further supported by the study, which showed a remarkable increase of blood FFA in fetuses from obese dams (Fig. 3A). It is worth mentioning that adiponectin receptors AdipoR1 and AdipoR2 are expressed in mouse placenta. Similar to human placenta, AdipoR1 expression levels in mouse placenta are much higher than that of AdipoR2 (data not shown). Increased AdipoR1 gene expression in the placentas of obese dams further supports the importance of adiponectin in maternal obesity-enhanced placental FA transport (Fig. 4A).
FIG. 4.
Adiponectin and maternal obesity enhanced expression of genes that facilitate placental FA transport. Placentas were collected by Cesarean section at e17.5. mRNA levels were determined by real-time PCR. Maternal obesity was induced by HF-diet feeding using WT and Adipoq−/− dams and breeding scheme 1 (A) (see detail in the Research Design and Methods section and Supplementary Fig. 1A). The breeding scheme 2 was used to produce Adipoq−/+ or Adipoq−/− fetuses, whereas maternal adiponectin was null (B) (Supplementary Fig. 1B). These dams were fed with chow (B). Elevated mRNA levels of placental LPL, VLDLr, FABP3, and FABPpm and AdipoR1 were detected in placentas from obese WT dams (A, designated as WT HFD). However, the differences were diminished, except for FABPpm, in placentas of HFD- and LFD-fed Adipoq−/− dams (A, designated as Adipoq−/− HFD and Adipoq−/− LFD). Elevated mRNA levels of LPL, VLDLr, and FABPpm were observed in placentas of Adipoq−/+ fetuses, whereas the dams were Adipoq−/− (B). *P < 0.05 vs. WT LFD (A) or Adipoq−/− fetuses (B); #P < 0.05 vs. WT LFD or WT HFD (A); ▽P < 0.05 vs. Adipoq−/− LFD. HFD, HF diet; LFD, LF diet; n = 12.
In line with the observation that adiponectin gene knockout abolishes maternal obesity-induced high fetal body fat and fetal serum FFA, the expression levels of LPL, VLDLr, and FABP3 were similar between placentas from HF-fed and LF-fed Adipoq−/− dams (Fig. 4B). These results imply that adiponectin gene deletion abolishes maternal obesity-enhanced placental FA transport.
Our previous studies have demonstrated that adiponectin enhances LPL gene expression in mouse skeletal muscle (24). Similarly, placenta LPL mRNA levels were significantly decreased in chow-fed Adipoq−/− dams when compared with chow-fed WT dams (Fig. 4C). Furthermore, in comparing placentas between Adipoq−/+ and Adipoq−/− fetuses, significantly increased LPL, VLDLr, and FABPpm mRNA levels were detected in placentas of Adipoq−/+ fetus (Fig. 4D).
Adiponectin and maternal obesity increased lipogenic gene expression in fetal livers.
FA is a main component of lipids. Studies indicate that besides placenta-transported FAs, de novo lipogenesis also plays an important role in fetal development and fetal fat deposition (25,26). The liver is the main organ for de novo lipogenesis in both fetuses and adults. With the development of the fetal liver, hepatic lipogenesis provides a significant amount of FA for fetal tissue growth and fat deposition (27,28).
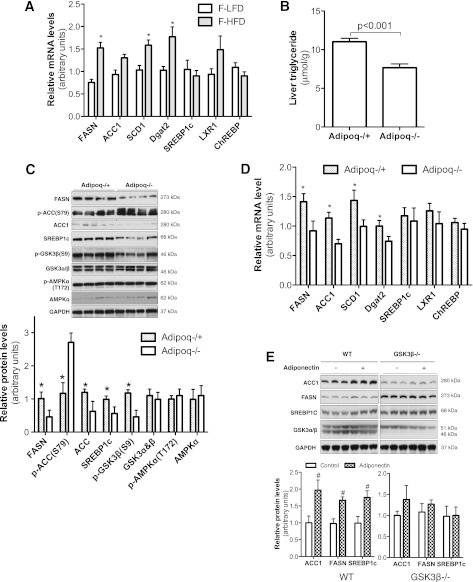
To study the effects of maternal obesity on de novo lipogenesis, we compared the expression levels of key lipogenic genes in fetal livers between fetuses from lean and obese WT dams. Significantly elevated mRNA levels of several key lipogenic genes, such FASN, stearoyl CoA desaturase 1 (SCD1), and Dgat2 were observed in the fetal livers from obese dams (Fig. 5A), suggesting that maternal obesity increases fetal liver de novo lipogenesis. Interestingly, similar to primates (29), maternal high-fat feeding in mice also robustly increased expression of gluconeogenesis genes, including PEPCK and G6Pase (Supplementary Fig. 4).
FIG. 5.
Adiponectin and maternal obesity increased lipogenic gene expression and activation in fetal livers. WT fetuses were produced by crossing HF diet–induced obese WT dams with WT sires (Scheme 1, Supplementary Fig. 1A) (A). Adipoq−/+ and Adipoq−/− fetuses (B–D) were generated by breeding Adipoq−/− dams with Adipoq−/− or Adipoq+/+ sires, which were fed with chow (Scheme 2, Supplementary Fig. 1B). Liver samples were collected from e17.5-old fetuses, whereas dams were at fed state. The mRNA levels of the main lipogenic genes in fetal livers was measured by real-time PCR (A and D); liver tissue TG levels were compared between Adipoq−/+ and Adipoq−/− fetuses (B). Protein or phospho-protein levels were detected by Western blotting using livers from Adipoq−/+ and Adipoq−/− fetuses (C). Confluent WT and GSK3β−/− MEFs were treated with adiponectin overnight. Increased ACC1, FASN, and SREBP1c were observed in adiponectin-treated WT MEFs, but not in GSK3β−/− MEFs (E). Quantified data are presented in the bottom graph with arbitrary units (C and E). n = 8–12. *P < 0.05 vs. fetuses from lean WT dams (A), or Adipoq−/− fetuses (C and D); #P < 0.05 vs. control MEFs (E). LXR, liver X receptor.
To investigate whether adiponectin enhances fetal fat deposition through de novo lipogenesis, hepatic TG content and lipogenic gene expression were studied using e17.5-old fetuses from the cross breeding scheme in which maternal adiponectin was null. We found that Adipoq−/+ fetal liver TG levels were significantly higher compared with Adipoq−/− fetuses (Fig. 5B). Protein levels of lipogenic transcription factor sterol regulatory element–binding protein 1c (SREBP1c) and the rate-limiting lipogenic enzymes FASN and acetyl-CoA carboxylase 1 (ACC1) were significantly higher in the livers of Adipoq−/+ fetuses (Fig. 5C). Phosphorylation of ACC1 at Ser79 inhibits ACC1 activity. Opposite of protein levels, phosphorylation of ACC1 at Ser79 was remarkably lower in the livers of Adipoq−/+ fetuses (Fig. 5C). Therefore, the livers from Adipoq−/+ fetuses exhibited increased ACC1 expression as well as activity. Consistent with protein levels, mRNA levels of the rate-limiting enzymes of lipogenesis, such as ACC1, FASN, SCD1, and Dgat2, were significantly higher in livers of Adipoq−/+ fetuses (Fig. 5D). Together, our studies indicate that fetal liver lipogenesis was significantly enhanced in Adipoq−/+ fetuses. These results lead us to propose that fetal adiponectin enhances hepatic de novo lipogenesis.
SREBP1c plays a key role in hepatic de novo lipogenesis. Our results showed that SREBP1c protein levels, but not mRNA levels, were significantly higher in the livers of Adipoq−/+ fetuses (Fig. 5C and D), which prompted us to test whether adiponectin increases SREBP1c protein through a posttranslational mechanism. Our study also showed that the phosphorylation levels of GSK3β, which deactivates its kinase activity, were also significantly increased in the livers of Adipoq−/+ fetuses (Fig. 5C). Previous studies have reported that activation of GSK3β reduces SREBP1c protein levels by increasing its protein degradation (30–32). We used GSK3β gene knockout MEFs and a coculture system to verify if adiponectin increases SREBP1c protein levels via GSK3β (18). Consistent with the results from our in vivo study (Fig. 5C), adiponectin treatment increased protein levels of SREBP1c, ACC1, and FASN in WT MEFs (Fig. 5E). However, these stimulative effects were not observed in GSK3β−/− MEFs (Fig. 5E). Together, these results indicate that inhibition of GSK3β might mediate the adiponectin-induced increase of SREBP1c protein levels in fetal livers.
DISCUSSION
Fetal programming has been proposed as maternal malnutrition during pregnancy causes lifelong adverse effects on offspring (33). Recent studies about maternal obesity-induced offspring adiposity have further reinforced this idea and indicate that intrauterine metabolic environment plays a key role in fetal programming. Human and animal studies have revealed that maternal obesity or excessive weight gain during gestation strongly increases offspring birth weight and the risk of obesity during the offspring’s life-course (4). Presumably, obese female offspring will further impose the adverse intrauterine environment to their offspring. Therefore, there is a vicious cycle of maternal and offspring obesity, which should contribute to the soaring obese population. Elucidating the underlying mechanisms of maternal obesity-associated high birth weight and offspring obesity will generate critical information for solving the obesity epidemic. In this article, our study provides strong evidence indicating that adiponectin plays an important role in mediating maternal obesity-enhanced fetal fat deposition.
In contrast to leptin, adiponectin displays characteristics of starvation hormones, which enhance energy conservation by reducing energy expenditure (12,13). Emerging evidence has indicated that adiponectin plays an important role in regulating lipid metabolism. For example, our recent study and another group’s work demonstrated that adiponectin suppresses lipolysis (15,16). Transgenic overexpression of adiponectin increases adipose tissue mass in mice (12,17). A decrease in adipose tissue mass was also reported in Adipoq−/− and adiponectin antisense transgenic mice (13,15). Our current study indicates that maternal obesity increases fetal adiponectin levels and fetal fat tissue mass, whereas adiponectin gene knockout diminishes maternal obesity-induced fetal fat tissue mass (Fig. 2). Most importantly, Adipoq−/+ offspring exhibit more fat tissue mass at birth and even later life (Fig. 2D–F). These results strongly support the hypothesis that adiponectin enhances fetal fat deposition. It is worth pointing out that our study indicates that adiponectin also increases fetal lean tissue mass, but to a lesser extent when compared with fat. Although this study did not provide the underlying mechanism, these observations warrant further investigation to clarify whether they are the secondary effects of increased placental nutrient supply or a direct effect of adiponectin on fetal cell proliferation.
During human pregnancy, maternal blood adiponectin levels increase before the second trimester, and then steadily decline to the lowest level at term (34–8). Reduction of maternal adiponectin during this time point has also been observed in mice (39,40). Expression of adiponectin in human fetuses has been detected not only in adipose tissue, but also in several nonadipose tissues during mid and late gestation (5,41). Our studies demonstrate that mouse fetal adiponectin gene expression is turned on during late gestation and starts in brown adipose-like tissue even before lipid droplet formation (Fig. 1 and Supplementary Fig. 1). The results of human placental adiponectin gene expression studies were contradictory. By using more sensitive and accurate techniques, recent studies have excluded the contribution of the placenta to the high level of adiponectin in fetal blood (5,19,41,42). Our study agrees with the later studies and confirms that there was no adiponectin mRNA in mouse placentas (data not shown). Regardless of the source, fetal blood adiponectin steadily elevates, whereas maternal adiponectin levels decrease during late gestation. Therefore, at birth, a four- to sevenfold difference in blood adiponectin levels between offspring and mother is established (5,7,8).
There is a significant adjustment of maternal lipid metabolism during pregnancy (43). From mid- to the third trimester of pregnancy, due to the exponential fetal tissue growth and intensified nutrient demands, maternal lipid metabolism switches from anabolic to catabolic conditions (43). Increased lipolysis provides FA and glycerol for maternal hepatic VLDL synthesis, which creates a hypertriglyceridemia in maternal circulation (44). Adiponectin inhibits adipocyte lipolysis (15,16), whereas maternal adiponectin levels steadily decrease during the late gestation (34–38). Thus, reduced adiponectin levels may contribute to increased maternal lipolysis and fetal FA supply.
Maternal TG-enriched VLDL is the main FA supplier for the fetus (11). Intact VLDL-TG particles cannot be transported through the placenta. Only nonesterified FAs can be taken up by the microvillous membrane and transported to the fetus. Therefore, the FA of VLDL-TGs should be released by lipase at the placenta. LPL gene expression dramatically increases during the last trimester of pregnancy, correlating with the increased placental FA transport (45–47). Placental LPL expression and activity positively correlate with fetal size and fat mass (46). Our study showed that maternal obesity increases the expression of placenta LPL and several genes that facilitate FA transport (Fig. 4A). Similar to the stimulative effect of adiponectin on LPL gene expression in skeletal muscle (24), significantly elevated placental LPL gene expression was observed in Adipoq−/+ fetuses compared with Adipoq−/− fetuses (Fig. 4D). In addition, fetal blood adiponectin levels were remarkably increased by maternal HF feeding (Supplementary Fig. 2). Therefore, these results indicate that adiponectin may enhance placental FA transport by increasing the expression of LPL and the genes that transport FAs.
It has been proposed that during early gestation, embryonic lipids are derived from maternal FA crossing the placenta, whereas in advanced gestation, there is a gradual shift to de novo synthesis in fetal tissue (48). However, recent studies using genetic mouse models demonstrated that de novo FA synthesis is important for fetal development (25). Dietary supplement of saturated FA of dams did not rescue embryonic death of FASN gene knockout fetuses, which suggests that maternal FA supply is largely insufficient to meet the requirements of embryo growth (25,49). In addition, during late gestation of rats, de novo fetal FA synthesis supplies about half of the amount of FA that is accumulated in fetal fat tissue (26). With the development of the fetal liver, hepatic lipogenesis provides a significant amount of FA for fetal tissue growth and fat deposition (27,28). Interestingly, the rate of fetal liver de novo lipogenesis using glucose as a substrate is ∼15 times higher than that in maternal liver (27,28). By comparing key lipogenic gene expression and activation status between fetuses with or without adiponectin, our study indicates that adiponectin enhances fetal liver lipogenic gene expression and activity that might lead to higher de novo lipogenesis. Further studies are undertaken to investigate the role of adiponectin-enhanced hepatic de novo lipogenesis in maternal obesity-induced fetal fat deposition. It should be pointed out that the stimulative effects of adiponectin on lipogenic gene expression and ACC1 phosphorylation were almost identically observed in adult mice when comparing the livers of adult WT and Adipoq−/− mice under the refed state, which turns on de novo lipogenesis (data not shown).
SREBP1c is one of the key transcription factors that regulates lipogenic enzyme gene transcription in response to nutritional intake and hormonal stimulation. A prominent function of adiponectin is to increase insulin sensitivity, whereas insulin is well-known for stimulating expression of lipogenic genes including SREBP1c (50). It is logical to postulate that adiponectin enhances lipogenesis through SREBP1c. Some studies indeed found that adiponectin stimulates SREBP1c gene expression and lipogenic processes in hepatic stellate cells and 3T3-L1 preadipocytes (14,51). Our results show that SREBP1c protein levels were remarkably higher in the livers of Adipoq−/+ fetuses than in Adipoq−/− fetuses. In line with this observation, SREBP1c expression is decreased in the livers of adult Adipoq−/− mice (52). In contrast, the same group later reported that adiponectin decreases SREBP1c gene transcription in db/db mouse livers (53). However, our study showed no significant difference in SREBP1c mRNA levels between Adipoq−/+ and Adipoq−/− fetuses (Fig. 5D). Our study also demonstrated that GSK3β gene knockout attenuates adiponectin-induced increase of SREBP1c protein in cultured cells (Fig. 5E). Therefore, our studies suggest that adiponectin increases SREBP1c protein levels by inhibiting GSK3β-mediated protein degradation, which has been observed in other studies (30–32).
In summary, our current study suggests that opposite changes in levels of maternal and fetal circulating adiponectin coordinates lipid metabolism in maternal and fetal compartments and favors fetal fat deposition. Further studies are required to elucidate whether and how maternal and fetal adiponectin regulates placental FA transport and fetal liver de novo lipogenesis.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health Grants DK-080418 and RHD069634A (to J.S.) and American Diabetes Association Grant 7-07-CD23 (to J.S.).
No other potential conflicts of interest relevant to this article were reported.
L.Q., H.s.Y., A.M., and B.K. contributed research data. W.W.H. contributed to research design and discussion. J.S. designed the study and wrote the manuscript. J.S. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors thank Dr. Philipp Scherer (University of Texas Southwestern Medical Center) for providing adiponectin gene knockout mice.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db12-0055/-/DC1.
REFERENCES
- 1.Ogden CL, Carroll MD, Curtin LR, Lamb MM, Flegal KM. Prevalence of high body mass index in US children and adolescents, 2007-2008. JAMA 2010-2008;303:242–249 [DOI] [PubMed] [Google Scholar]
- 2.Jones HN, Woollett LA, Barbour N, Prasad PD, Powell TL, Jansson T. High-fat diet before and during pregnancy causes marked up-regulation of placental nutrient transport and fetal overgrowth in C57/BL6 mice. FASEB J 2009;23:271–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Catalano PM, Farrell K, Thomas A, et al. Perinatal risk factors for childhood obesity and metabolic dysregulation. Am J Clin Nutr 2009;90:1303–1313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dabelea D, Crume T. Maternal environment and the transgenerational cycle of obesity and diabetes. Diabetes 2011;60:1849–1855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Corbetta S, Bulfamante G, Cortelazzi D, et al. Adiponectin expression in human fetal tissues during mid- and late gestation. J Clin Endocrinol Metab 2005;90:2397–2402 [DOI] [PubMed] [Google Scholar]
- 6.Schmelzle HR, Fusch C. Body fat in neonates and young infants: validation of skinfold thickness versus dual-energy X-ray absorptiometry. Am J Clin Nutr 2002;76:1096–1100 [DOI] [PubMed] [Google Scholar]
- 7.Pardo IM, Geloneze B, Tambascia MA, Barros-Filho AA. Hyperadiponectinemia in newborns: relationship with leptin levels and birth weight. Obes Res 2004;12:521–524 [DOI] [PubMed] [Google Scholar]
- 8.Chan TF, Yuan SS, Chen HS, et al. Correlations between umbilical and maternal serum adiponectin levels and neonatal birthweights. Acta Obstet Gynecol Scand 2004;83:165–169 [DOI] [PubMed] [Google Scholar]
- 9.Kotani Y, Yokota I, Kitamura S, Matsuda J, Naito E, Kuroda Y. Plasma adiponectin levels in newborns are higher than those in adults and positively correlated with birth weight. Clin Endocrinol (Oxf) 2004;61:418–423 [DOI] [PubMed] [Google Scholar]
- 10.Sivan E, Mazaki-Tovi S, Pariente C, et al. Adiponectin in human cord blood: relation to fetal birth weight and gender. J Clin Endocrinol Metab 2003;88:5656–5660 [DOI] [PubMed] [Google Scholar]
- 11.Haggarty P. Placental regulation of fatty acid delivery and its effect on fetal growth–a review. Placenta 2002;23(Suppl. A):S28–S38 [DOI] [PubMed] [Google Scholar]
- 12.Kim JY, van de Wall E, Laplante M, et al. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J Clin Invest 2007;117:2621–2637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kubota N, Yano W, Kubota T, et al. Adiponectin stimulates AMP-activated protein kinase in the hypothalamus and increases food intake. Cell Metab 2007;6:55–68 [DOI] [PubMed] [Google Scholar]
- 14.Fu Y, Luo N, Klein RL, Garvey WT. Adiponectin promotes adipocyte differentiation, insulin sensitivity, and lipid accumulation. J Lipid Res 2005;46:1369–1379 [DOI] [PubMed] [Google Scholar]
- 15.Qiao L, Kinney B, Schaack J, Shao J. Adiponectin inhibits lipolysis in mouse adipocytes. Diabetes 2011;60:1519–1527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wedellová Z, Dietrich J, Siklová-Vítková M, et al. Adiponectin inhibits spontaneous and catecholamine-induced lipolysis in human adipocytes of non-obese subjects through AMPK-dependent mechanisms. Physiol Res 2011;60:139–148 [DOI] [PubMed] [Google Scholar]
- 17.Combs TP, Pajvani UB, Berg AH, et al. A transgenic mouse with a deletion in the collagenous domain of adiponectin displays elevated circulating adiponectin and improved insulin sensitivity. Endocrinology 2004;145:367–383 [DOI] [PubMed] [Google Scholar]
- 18.Qiao L, Kinney B, Yoo HS, Lee B, Schaack J, Shao J. Adiponectin increases skeletal muscle mitochondrial biogenesis by suppressing mitogen-activated protein kinase phosphatase-1. Diabetes 2012;61:1463–1470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mazaki-Tovi S, Kanety H, Pariente C, et al. Determining the source of fetal adiponectin. J Reprod Med 2007;52:774–778 [PubMed] [Google Scholar]
- 20.Bouchard L, Hivert MF, Guay SP, St-Pierre J, Perron P, Brisson D. Placental adiponectin gene DNA methylation levels are associated with mothers' blood glucose concentration. Diabetes 2012;61:1272–1280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mamun AA, O’Callaghan M, Callaway L, Williams G, Najman J, Lawlor DA. Associations of gestational weight gain with offspring body mass index and blood pressure at 21 years of age: evidence from a birth cohort study. Circulation 2009;119:1720–1727 [DOI] [PubMed] [Google Scholar]
- 22.Samuelsson AM, Matthews PA, Argenton M, et al. Diet-induced obesity in female mice leads to offspring hyperphagia, adiposity, hypertension, and insulin resistance: a novel murine model of developmental programming. Hypertension 2008;51:383–392 [DOI] [PubMed] [Google Scholar]
- 23.Nawrocki AR, Rajala MW, Tomas E, et al. Mice lacking adiponectin show decreased hepatic insulin sensitivity and reduced responsiveness to peroxisome proliferator-activated receptor gamma agonists. J Biol Chem 2006;281:2654–2660 [DOI] [PubMed] [Google Scholar]
- 24.Qiao L, Zou C, van der Westhuyzen DR, Shao J. Adiponectin reduces plasma triglyceride by increasing VLDL triglyceride catabolism. Diabetes 2008;57:1824–1833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chirala SS, Chang H, Matzuk M, et al. Fatty acid synthesis is essential in embryonic development: fatty acid synthase null mutants and most of the heterozygotes die in utero. Proc Natl Acad Sci USA 2003;100:6358–6363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hummel L, Zimmermann T, Wagner H. Quantitative evaluation of the fetal fatty acid synthesis in the rat. Acta Biol Med Ger 1978;37:229–232 [PubMed] [Google Scholar]
- 27.Ballard FJ, Hanson RW. Changes in lipid synthesis in rat liver during development. Biochem J 1967;102:952–958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lorenzo M, Caldés T, Benito M, Medina JM. Lipogenesis in vivo in maternal and foetal tissues during late gestation in the rat. Biochem J 1981;198:425–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McCurdy CE, Bishop JM, Williams SM, et al. Maternal high-fat diet triggers lipotoxicity in the fetal livers of nonhuman primates. J Clin Invest 2009;119:323–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bengoechea-Alonso MT, Ericsson J. A phosphorylation cascade controls the degradation of active SREBP1. J Biol Chem 2009;284:5885–5895 [DOI] [PubMed] [Google Scholar]
- 31.Kim KH, Song MJ, Yoo EJ, Choe SS, Park SD, Kim JB. Regulatory role of glycogen synthase kinase 3 for transcriptional activity of ADD1/SREBP1c. J Biol Chem 2004;279:51999–52006 [DOI] [PubMed] [Google Scholar]
- 32.Sundqvist A, Bengoechea-Alonso MT, Ye X, et al. Control of lipid metabolism by phosphorylation-dependent degradation of the SREBP family of transcription factors by SCF(Fbw7). Cell Metab 2005;1:379–391 [DOI] [PubMed] [Google Scholar]
- 33.Barker DJ, Winter PD, Osmond C, Margetts B, Simmonds SJ. Weight in infancy and death from ischaemic heart disease. Lancet 1989;2:577–580 [DOI] [PubMed] [Google Scholar]
- 34.Fuglsang J, Skjaerbaek C, Frystyk J, Flyvbjerg A, Ovesen P. A longitudinal study of serum adiponectin during normal pregnancy. BJOG 2006;113:110–113 [DOI] [PubMed] [Google Scholar]
- 35.Mazaki-Tovi S, Kanety H, Pariente C, et al. Maternal serum adiponectin levels during human pregnancy. J Perinatol 2007;27:77–81 [DOI] [PubMed] [Google Scholar]
- 36.Eriksson B, Löf M, Olausson H, Forsum E. Body fat, insulin resistance, energy expenditure and serum concentrations of leptin, adiponectin and resistin before, during and after pregnancy in healthy Swedish women. Br J Nutr 2010;103:50–57 [DOI] [PubMed] [Google Scholar]
- 37.Ritterath C, Rad NT, Siegmund T, Heinze T, Siebert G, Buhling KJ. Adiponectin during pregnancy: correlation with fat metabolism, but not with carbohydrate metabolism. Arch Gynecol Obstet 2010;281:91–96 [DOI] [PubMed] [Google Scholar]
- 38.Catalano PM, Hoegh M, Minium J, et al. Adiponectin in human pregnancy: implications for regulation of glucose and lipid metabolism. Diabetologia 2006;49:1677–1685 [DOI] [PubMed] [Google Scholar]
- 39.Kondo E, Sugiyama T, Kusaka H, Toyoda N. Adiponectin mRNA Levels in Parametrial Adipose Tissue and Serum Adiponectin Levels are Reduced in Mice During Late Pregnancy. Horm Metab Res 2004;36:465–469 [DOI] [PubMed] [Google Scholar]
- 40.Combs TP, Berg AH, Rajala MW, et al. Sexual differentiation, pregnancy, calorie restriction, and aging affect the adipocyte-specific secretory protein adiponectin. Diabetes 2003;52:268–276 [DOI] [PubMed] [Google Scholar]
- 41.Pinar H, Basu S, Hotmire K, et al. High molecular mass multimer complexes and vascular expression contribute to high adiponectin in the fetus. J Clin Endocrinol Metab 2008;93:2885–2890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Haugen F, Ranheim T, Harsem NK, Lips E, Staff AC, Drevon CA. Increased plasma levels of adipokines in preeclampsia: relationship to placenta and adipose tissue gene expression. Am J Physiol Endocrinol Metab 2006;290:E326–E333 [DOI] [PubMed] [Google Scholar]
- 43.Cetin I, Alvino G, Cardellicchio M. Long chain fatty acids and dietary fats in fetal nutrition. J Physiol 2009;587:3441–3451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bansal N, Cruickshank JK, McElduff P, Durrington PN. Cord blood lipoproteins and prenatal influences. Curr Opin Lipidol 2005;16:400–408 [DOI] [PubMed] [Google Scholar]
- 45.Bonet B, Brunzell JD, Gown AM, Knopp RH. Metabolism of very-low-density lipoprotein triglyceride by human placental cells: the role of lipoprotein lipase. Metabolism 1992;41:596–603 [DOI] [PubMed] [Google Scholar]
- 46.Magnusson-Olsson AL, Hamark B, Ericsson A, Wennergren M, Jansson T, Powell TL. Gestational and hormonal regulation of human placental lipoprotein lipase. J Lipid Res 2006;47:2551–2561 [DOI] [PubMed] [Google Scholar]
- 47.Gauster M, Hiden U, Blaschitz A, et al. Dysregulation of placental endothelial lipase and lipoprotein lipase in intrauterine growth-restricted pregnancies. J Clin Endocrinol Metab 2007;92:2256–2263 [DOI] [PubMed] [Google Scholar]
- 48.Herrera E, Amusquivar E. Lipid metabolism in the fetus and the newborn. Diabetes Metab Res Rev 2000;16:202–210 [DOI] [PubMed] [Google Scholar]
- 49.Strable MS, Ntambi JM. Genetic control of de novo lipogenesis: role in diet-induced obesity. Crit Rev Biochem Mol Biol 2010;45:199–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kersten S. Mechanisms of nutritional and hormonal regulation of lipogenesis. EMBO Rep 2001;2:282–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shafiei MS, Shetty S, Scherer PE, Rockey DC. Adiponectin regulation of stellate cell activation via PPARγ-dependent and -independent mechanisms. Am J Pathol 2011;178:2690–2699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yano W, Kubota N, Itoh S, et al. Molecular mechanism of moderate insulin resistance in adiponectin-knockout mice. Endocr J 2008;55:515–522 [DOI] [PubMed] [Google Scholar]
- 53.Awazawa M, Ueki K, Inabe K, et al. Adiponectin suppresses hepatic SREBP1c expression in an AdipoR1/LKB1/AMPK dependent pathway. Biochem Biophys Res Commun 2009;382:51–56 [DOI] [PubMed] [Google Scholar]