Abstract
The hypothalamus is critically involved in the regulation of feeding. Previous studies have shown that glucose ingestion inhibits hypothalamic neuronal activity. However, this was not observed in patients with type 2 diabetes. Restoring energy balance by reducing caloric intake and losing weight are important therapeutic strategies in patients with type 2 diabetes. We hypothesized that caloric restriction would have beneficial effects on the hypothalamic neuronal response to glucose ingestion. Functional magnetic resonance imaging was performed in 10 male type 2 diabetic patients before and after a 4-day very-low-calorie diet (VLCD) at a 3.0 Tesla scanner using a blood oxygen level–dependent technique for measuring neuronal activity in the hypothalamus in response to an oral glucose load. Hypothalamic signals were normalized to baseline value, and differences between the pre- and postdiet condition were tested using paired t tests. Pre-VLCD scans showed no response of the hypothalamus to glucose intake (i.e., no signal decrease after glucose intake was observed). Post-VLCD scans showed a prolonged signal decrease after glucose ingestion. The results of the current study demonstrate that short-term caloric restriction readily normalizes hypothalamic responsiveness to glucose ingestion in patients with type 2 diabetes.
The hypothalamus plays a key role in the regulation of feeding. It contains glucose-sensitive neurons that are stimulated by falling blood glucose levels and implicated in hypoglycemia-induced feeding (1). Moreover, various hypothalamic neuronal circuits are involved in the control of glucose metabolism (2).
Blood oxygen level–dependent (BOLD) functional magnetic resonance imaging (fMRI) has been widely applied in spatiotemporal mapping of the human brain function and measuring neuronal activity. MRI contrast in BOLD measurements is based on the fact that the BOLD signal arises from local field inhomogeneities, caused by magnetic susceptibility differences between deoxyhemoglobin levels in the blood in capillaries and venous vessels and the surrounding tissue (3). This phenomenon enables the possibility to determine local parts in the brain that are activated by an external trigger. The main advantage of BOLD fMRI is its noninvasive nature and local sensitivity combined with a good spatial resolution. Its main disadvantage refers to the nature of the BOLD signal: it is only an indirect measure of neural activity. Nevertheless, over the years, BOLD fMRI has shown to be a sensitive marker of brain activation. In this respect, it was shown that healthy lean volunteers demonstrated a significant dose-dependent decrease of the BOLD signal in the hypothalamus after glucose ingestion (4).
Type 2 diabetes is a disease of impaired glucose homeostasis and insulin action, with energy imbalance and anomalous fuel flux as metabolic hallmarks. It has been shown that the hypothalamic neuronal activity is altered in patients with type 2 diabetes, demonstrated by the absence of a BOLD signal decrease after glucose ingestion (5). This finding may suggest that the hypothalamus in patients with type 2 diabetes inappropriately perceives and/or processes signals in response to a nutrient load, reflecting an abnormal perception of the current metabolic status.
Restoration of the energy balance by reduction of the caloric intake and subsequent weight loss are important therapeutic strategies in type 2 diabetes. In the current study, we hypothesized that caloric restriction normalizes the hypothalamic response to a nutrient load. Therefore, the purpose of the current study was to determine the effect of a very-low-calorie diet (VLCD) on hypothalamic neuronal activity after glucose ingestion measured by BOLD fMRI.
RESEARCH DESIGN AND METHODS
Subjects.
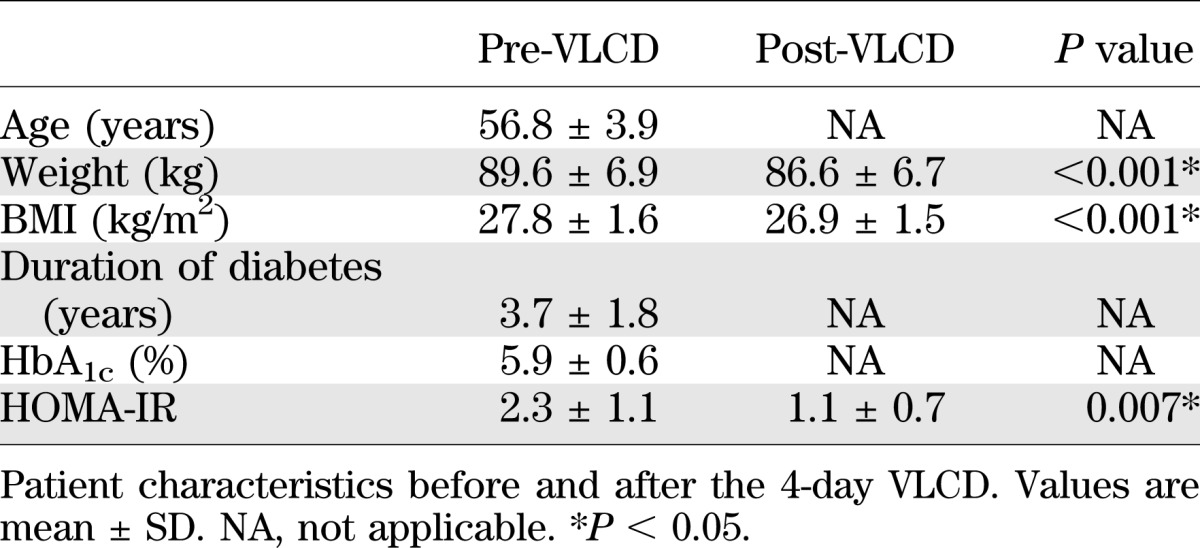
We recruited 10 Caucasian men diagnosed with type 2 diabetes according to World Health Organization criteria. At intake, mean age was 56.8 ± 3.9 years, weight 89.6 ± 6.9 kg, and BMI 27.9 ± 1.6 kg/m2. Diabetes duration was 3.7 ± 1.8 years, and HbA1c was 5.9 ± 0.6%. Subjects’ type 2 diabetes treatment consisted of metformin and/or a diet.
Main exclusion criteria were treatment with insulin or sulfonylurea derivatives, being on a weight-reducing diet already, weight changes of >3 kg in the last 3 months, any type of chronic disease, smoking, and contraindications for MRI. The study protocol was approved by the local institutional review board, and written informed consent was obtained from every subject.
Design.
Functional MRI was performed before and following a VLCD of 4 days. The VLCD comprised three liquid food shakes per day, containing a total of 450 kcal (Modifast Intensive; Nutrition & Santé Benelux n.v., Brussels, Belgium). The subjects were advised to drink at least 1.5 L of water per day.
To assure a craving status, subjects were asked to fast from 10 p.m. the night preceding each scan the next early morning. During fasting, no food or drinks were allowed, except water. Fasting glucose and insulin levels were determined to calculate insulin sensitivity according to the homeostasis model assessment parameter of insulin resistance (HOMA-IR), which is the product of the fasting serum insulin level (mU/L) and the plasma glucose level (mmol/L) divided by 22.5 (6).
Data acquisition.
MRI was performed at our institution using a 3.0 Tesla Achieva clinical scanner (Philips Healthcare, Best, the Netherlands). The protocol comprised a scout view for planning two single-slice, midsagittal scans: a T1-weighted Turbo Spin Echo sequence for imaging anatomical structures (repetition time 550 ms, echo time 10 ms, field of view 208 × 208 mm, voxelsize = 0.52 × 0.52 × 14 mm, scan time 1.15 min) and a T2*-weighted, gradient echo echo-planar imaging sequence that renders BOLD contrast, which is related to neuronal activity (repetition time 120 ms, echo time 30 ms, flip angle 30°, field of view 208 × 208 mm, voxelsize = 0.81 × 0.90 × 14 mm, scan time 38.10 min, 900 time points). During this sequence, 300 mL water containing 75 g of glucose (standard glucose tolerance test solution) was ingested through a tube after 8.5 min. Scanning was continued after complete ingestion of the glucose solution. Slice thickness of 14 mm was chosen to encompass the hypothalamus in the left to right direction, the single-slice technique for sufficient signal-to-noise ratio.
Functional MRI data analysis.
Each of the 900 modulus images was registered to the image that was acquired halfway the scan by means of Multimodality Image Registration using Information Theory by maximization of mutual information (7). The resulting registration matrix was then applied to the real and imaginary images that were calculated from the original modulus and phase images. To reduce phase artifacts caused by swallowing or head motion, complex data were averaged for each set of four subsequent volumes, and finally modulus images were recalculated, rendering 225 images that were corrected for movement and phase errors. The T1 scan was also registered to the functional scan, using the same algorithm and reference image.
The hypothalamus was segmented manually, using the anatomical image as an aid to delineate anatomical borders. Subdivision of the hypothalamus into four regions of interest (ROIs) was performed according to Matsuda et al. (8). As a reference, an ROI was drawn in gray matter, superior of the genu of the corpus callosum. For each ROI, the mean signal for each time point was established, and its baseline signal was calculated (i.e., the signal averaged over all measurements up to 0.5 min before drinking of the glucose solution started). Subsequently, measurements were then normalized to the baseline value, yielding the signal change relative to baseline. To correct for scanner drift, the signal in the reference ROI was subtracted from that in the hypothalamus ROIs.
Statistical analysis.
For each time point, normalized hypothalamic signal was averaged for all subjects, and data were pooled in 2-min time slots. Subsequently, differences between pre- and postdiet condition were tested by a paired t test for each time slot after baseline (i.e., for all 15 time slots from the moment drinking was started). Because we performed 15 t tests, a Bonferroni-corrected threshold of P < 0.0033 (= 0.05/15) was applied to correct for multiple comparisons. This method is comparable to differential regression analysis (9).
Data are expressed as mean ± SD for demographic and biochemical characteristics and as mean percentage ± SEM for BOLD signal change.
RESULTS
The VLCD was generally well tolerated and induced a mean body weight loss of 3.0 ± 1.4 kg. Consequently, weight and BMI decreased to 86.6 ± 6.7 kg (P < 0.001) and 26.9 ± 1.5 kg/m2 (P < 0.001), respectively, after the VLCD. HOMA-IR decreased from 2.3 ± 1.1 to 1.1 ± 0.7 (P = 0.007) (Table 1).
TABLE 1.
Patient characteristics
After the VLCD, fasting serum insulin decreased from 8.8 ± 4.4 to 4.8 ± 3.2 mU/L (P = 0.013) and fasting plasma glucose decreased from 6.4 ± 1.3 to 5.3 ± 1.0 mmol/L (P = 0.010). Triglycerides, total cholesterol, HDL cholesterol, and the total cholesterol/HDL ratio did not change (Table 2). Glucose drinking duration was 3.26 ± 1.20 and 3.00 ± 0.53 min for the first and second visit, respectively (P = 0.387).
TABLE 2.
Biochemical characteristics
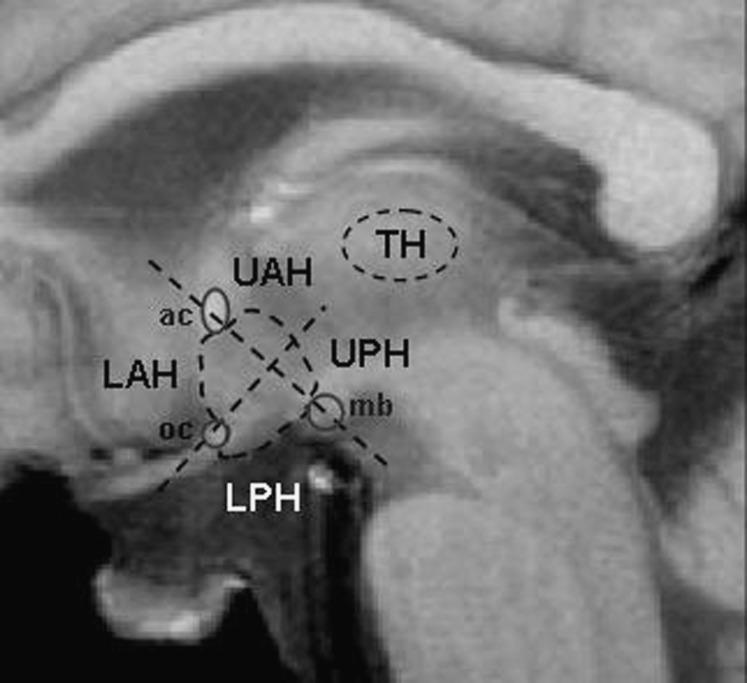
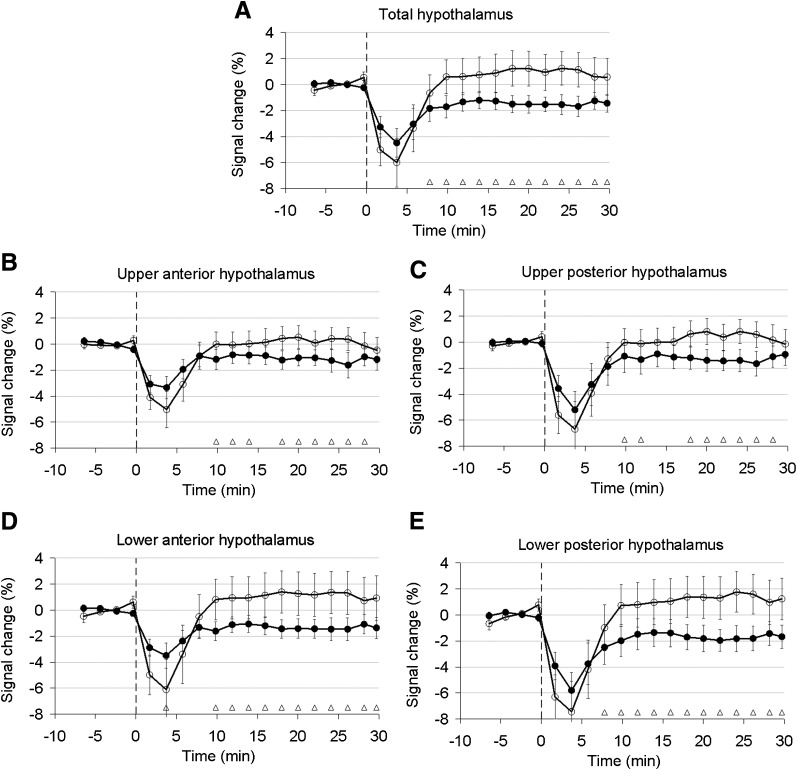
Figure 1 shows which anatomical landmarks were used for drawing ROIs and division of the hypothalamus into four subregions. Figure 2 shows the percentage signal change from baseline value averaged for all subjects in the total hypothalamus and its quadrants for measurements before and after the VLCD. In all graphs, a signal drop was observed that was associated with movement of the head during drinking. Pre-VLCD scans showed no response of the hypothalamus to glucose intake (i.e., no signal decrease after glucose intake was observed). Post-VLCD scans showed a prolonged signal decrease after glucose ingestion. This effect was observed in all quadrants, but was most pronounced in the lower quadrants. A significant prolonged decrease in BOLD signal of 2 to 3% after glucose administration between pre- and post-VLCD in the total hypothalamus was observed from t = 8 min onwards (P < 0.0033) (Fig. 2).
FIG. 1.
Anatomical landmarks used for drawing ROIs and division of the hypothalamus into subregions. ac, anterior commissure; LAH, lower anterior hypothalamus; LPH, lower posterior hypothalamus; mb, mammillary body; oc, optic chiasm; TH, thalamus; UAH, upper anterior hypothalamus; UPH, upper posterior hypothalamus.
FIG. 2.
Relative fMRI signal in the total (A), upper anterior (B), upper posterior (C), lower anterior (D), and lower posterior (E) hypothalamus before and after VLCD. Signal is normalized to the preprandial signal, which is calculated as average over the first 8 min. Each circle corresponds with the average signal for 2 min in all subjects. The vertical dashed lines indicate the start of glucose ingestion. Error bars indicate ± 1 SEM. White circles, pre-VLCD; black circles, post-VLCD; white triangle, P < 0.0033.
Glucose concentrations were lower after the VLCD and may have resulted in the observed BOLD signal decrease. Therefore, the relation between fasting blood glucose level and the BOLD effect was tested in both the pre-VLCD as well as in the post-VLCD condition using a multivariate linear model adjusted for age. No significant correlations were found (P = 0.36 and P = 0.70, respectively). In addition, no correlation was found between the difference in fasting blood glucose levels between the pre- and post-VLCD condition with the difference in corresponding BOLD signals (P = 0.89).
DISCUSSION
We previously showed that glucose ingestion fails to inhibit hypothalamic neuronal activity in type 2 diabetic patients (5). In this study, we demonstrate that after following a VLCD of 4 days, the hypothalamus responds to glucose ingestion with an order of magnitude similar to that in healthy subjects (4,5). We therefore suggest that short-term caloric restriction normalizes hypothalamic responsiveness to glucose ingestion in patients with type 2 diabetes.
The hypothalamus is critically involved in the feeding and metabolic regulatory system. Several hypothalamic nuclei, including the lateral hypothalamic area and the ventromedial hypothalamus, contain glucose-sensing neurons. These neurons communicate extensively with other appetite-regulating neuronal systems. A rise in glucose levels causes glucose-responsive neurons to increase their firing rate, whereas glucose-sensitive neurons decrease their firing rate (10,11). In addition, the hypothalamus contains myriad receptors responsive to neurotransmitters including dopamine, serotonin, histamine, γ-aminobutyric acid, and estrogen, which are involved in feeding behavior (11). Furthermore, insulin and the adipocyte-derived hormone leptin signal the hypothalamus, resulting in reduced food intake (12).
Previous studies have shown a diminished hypothalamic neuronal activity after glucose ingestion in healthy subjects (4,5,13,14). High preprandial signal in the hypothalamus may be connected with a state of craving that subdues when the need for energy is met. Smeets et al. (14) reported that glucose ingestion more effectively inhibited hypothalamic neuronal activity compared with intravenous glucose administration. Gastrointestinal signals and/or insulin are therefore very likely involved in the hypothalamic response to glucose intake (14). Glucose ingestion triggers intestinal cells to release several hormones, including peptide YY, glucagon-like peptide-1, and oxyntomodulin into the circulation (15,16). These gut hormones signal food intake to the appetite-regulating circuits of the brain and act in the hypothalamus to induce satiety (17–19). A functional neuroimaging study in rats showed that oxyntomodulin and glucagon-like peptide-1 inhibit neuronal activity in hypothalamic nuclei (20). Furthermore, it has been shown that peptide YY facilitates insulin action (21).
In accordance with the findings of Vidarsdottir et al. (5), glucose ingestion initially failed to reduce hypothalamic BOLD signals in our type 2 diabetic patients. Therefore, in subjects with type 2 diabetes, the hypothalamus appears to inappropriately perceive and/or process signals in response to a nutrient load. In addition, Matsuda et al. (8) found attenuated hypothalamic fMRI signal inhibition in response to glucose ingestion in obese subjects with normal glucose tolerance compared with lean subjects, which was correlated with fasting plasma glucose and insulin concentration, and was independent of BMI.
Remarkably, after type 2 diabetic patients followed a 4-day VLCD, a normal BOLD signal pattern was observed after glucose ingestion (i.e., prolonged inhibition of neuronal activity). This BOLD signal pattern was comparable to those described in healthy male subjects (4,5). Apparently, the hypothalamus is capable to return to a normal responsive pattern following a glucose load. It may be hypothesized that a caloric restriction recovers the sensitivity of glucose-sensitive neurons in type 2 diabetes. However, it must be noted that the magnitude of an increase in serum glucose does not increase the magnitude or duration of the decrease in hypothalamic activity (14). Furthermore, fasting blood glucose levels were lower after the VLCD. Because no correlation was found between these glucose levels and the BOLD effect in both pre- and post-VLCD condition, it is unlikely that the lower fasting blood glucose level after the VLCD has resulted in the observed BOLD signal decrease.
More likely, insulin sensitivity may play a role. Caloric restriction has proven to be beneficial in type 2 diabetes for obtaining metabolic control (22) independent of body weight reduction (23). Besides lowering the BMI, a 6-day VLCD improved peripheral insulin sensitivity in type 2 diabetic patients, measured by glucose disposal rate and HOMA-IR (24). Our data also show that after a short-term VLCD, insulin sensitivity was improved, measured by HOMA-IR. Insulin signals the brain about the status of body fat stores. The arcuate nucleus, a key hypothalamic region involved in energy homeostasis, contains populations of neuropeptide Y and proopiomelanocortin neurons, which express insulin receptors. Insulin stimulates proopiomelanocortin resulting in reduced food intake and increased energy expenditure. Conversely, neuropeptide Y is inhibited by insulin, again resulting in reduced food intake (12). Considering the afferent signaling of insulin to the arcuate nucleus, it may be possible that an improvement in insulin sensitivity after a VLCD is in part responsible for the normalization of hypothalamic responsiveness to glucose ingestion. Moreover, improved insulin sensitivity may also favor the hypothalamic response to gut hormones, because these hormones support insulin action (21).
Caloric restriction may also be a way to sensitize the hypothalamus to glucose ingestion in nondiabetic subjects. However, the intrinsic limitations of the BOLD phenomenon restrict this application. In the current study, we determined a VLCD BOLD effect up to 3% in patients with type 2 diabetes. Our current glucose-triggered BOLD response reaches levels that are comparable with healthy control subjects, also measured at a magnetic field strength of 3.0 Tesla (5). At a field strength of 3.0 Tesla, a 3% change is about the theoretical maximal BOLD effect that can be determined using an echo time of 30 ms (25). Although it cannot be excluded that a VLCD will have effect on the hypothalamus in nondiabetic subjects, an additional BOLD effect can hardly be measured because the normal response is already up to 3%.
We have chosen not to use water as an additional control reference because the intrinsic reference, or calibration, of our experiments is the BOLD signal before glucose administration (i.e., the BOLD signal in the first 8 min). Many experiments in both control subjects and subjects with diabetic type 2 have clearly shown that water on itself (no glucose) does not alter the BOLD response (4,5,8,26). For studying the effect of a VLCD in type 2 diabetes, the addition of water as control condition would only have very limited additional value.
Lifestyle intervention, including body weight reduction and reduced intake of total and saturated fat, can reduce the incidence of type 2 diabetes in people at risk and improve health in type 2 diabetic patients (27,28). This study shows that the absence of a hypothalamic response to glucose intake in patients with type 2 diabetes can be reversed by a 4-day VLCD. As hypothalamic neuronal activity contributes to the control of postprandial metabolism, this may be one of the key factors that explain the strong metabolic improvement that can be observed in type 2 diabetic patients on caloric restriction. It must be noted that the fundamental mechanism behind the hypothalamic response to glucose ingestion is yet unclear, as is the effect of VLCD in this study, and therefore requires further research.
ACKNOWLEDGMENTS
This research was performed within the framework of the Center for Translational Molecular Medicine (www.ctmm.nl), project PREDICCt (Grant 01C-104), and supported by the Netherlands Heart Foundation, the Dutch Diabetes Research Foundation, and the Dutch Kidney Foundation.
No potential conflicts of interest relevant to this article were reported.
W.M.T. performed MRI scanning and researched data. R.L.W. researched data and wrote the first and successive drafts of the manuscript. M.P. included patients and researched data. H.J.L., J.W.A.S., A.d.R., M.A.v.B., and H.P. revised the manuscript critically for important intellectual content. J.v.d.G. researched data and revised the manuscript critically for important intellectual content. J.v.d.G. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
REFERENCES
- 1.Liu XH, Morris R, Spiller D, White M, Williams G. Orexin a preferentially excites glucose-sensitive neurons in the lateral hypothalamus of the rat in vitro. Diabetes 2001;50:2431–2437 [DOI] [PubMed] [Google Scholar]
- 2.van den Hoek AM, van Heijningen C, Schröder-van der Elst JP, et al. Intracerebroventricular administration of neuropeptide Y induces hepatic insulin resistance via sympathetic innervation. Diabetes 2008;57:2304–2310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ogawa S, Lee TM, Kay AR, Tank DW. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc Natl Acad Sci USA 1990;87:9868–9872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smeets PA, de Graaf C, Stafleu A, van Osch MJ, van der Grond J. Functional MRI of human hypothalamic responses following glucose ingestion. Neuroimage 2005;24:363–368 [DOI] [PubMed] [Google Scholar]
- 5.Vidarsdottir S, Smeets PA, Eichelsheim DL, et al. Glucose ingestion fails to inhibit hypothalamic neuronal activity in patients with type 2 diabetes. Diabetes 2007;56:2547–2550 [DOI] [PubMed] [Google Scholar]
- 6.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–419 [DOI] [PubMed] [Google Scholar]
- 7.Maes F, Collignon A, Vandermeulen D, Marchal G, Suetens P. Multimodality image registration by maximization of mutual information. IEEE Trans Med Imaging 1997;16:187–198 [DOI] [PubMed] [Google Scholar]
- 8.Matsuda M, Liu Y, Mahankali S, et al. Altered hypothalamic function in response to glucose ingestion in obese humans. Diabetes 1999;48:1801–1806 [DOI] [PubMed] [Google Scholar]
- 9.Cho ZH, Son YD, Kang CK, Han JY, Wong EK, Bai SJ. Pain dynamics observed by functional magnetic resonance imaging: differential regression analysis technique. J Magn Reson Imaging 2003;18:273–283 [DOI] [PubMed] [Google Scholar]
- 10.Williams G, Bing C, Cai XJ, Harrold JA, King PJ, Liu XH. The hypothalamus and the control of energy homeostasis: different circuits, different purposes. Physiol Behav 2001;74:683–701 [DOI] [PubMed] [Google Scholar]
- 11.King BM. The rise, fall, and resurrection of the ventromedial hypothalamus in the regulation of feeding behavior and body weight. Physiol Behav 2006;87:221–244 [DOI] [PubMed] [Google Scholar]
- 12.Niswender KD, Schwartz MW. Insulin and leptin revisited: adiposity signals with overlapping physiological and intracellular signaling capabilities. Front Neuroendocrinol 2003;24:1–10 [DOI] [PubMed] [Google Scholar]
- 13.Liu Y, Gao JH, Liu HL, Fox PT. The temporal response of the brain after eating revealed by functional MRI. Nature 2000;405:1058–1062 [DOI] [PubMed] [Google Scholar]
- 14.Smeets PA, Vidarsdottir S, de Graaf C, et al. Oral glucose intake inhibits hypothalamic neuronal activity more effectively than glucose infusion. Am J Physiol Endocrinol Metab 2007;293:E754–E758 [DOI] [PubMed] [Google Scholar]
- 15.Kim BJ, Carlson OD, Jang HJ, Elahi D, Berry C, Egan JM. Peptide YY is secreted after oral glucose administration in a gender-specific manner. J Clin Endocrinol Metab 2005;90:6665–6671 [DOI] [PubMed] [Google Scholar]
- 16.Meier JJ, Nauck MA, Kranz D, et al. Secretion, degradation, and elimination of glucagon-like peptide 1 and gastric inhibitory polypeptide in patients with chronic renal insufficiency and healthy control subjects. Diabetes 2004;53:654–662 [DOI] [PubMed] [Google Scholar]
- 17.Batterham RL, Cowley MA, Small CJ, et al. Gut hormone PYY(3-36) physiologically inhibits food intake. Nature 2002;418:650–654 [DOI] [PubMed] [Google Scholar]
- 18.Flint A, Raben A, Astrup A, Holst JJ. Glucagon-like peptide 1 promotes satiety and suppresses energy intake in humans. J Clin Invest 1998;101:515–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cohen MA, Ellis SM, Le Roux CW, et al. Oxyntomodulin suppresses appetite and reduces food intake in humans. J Clin Endocrinol Metab 2003;88:4696–4701 [DOI] [PubMed] [Google Scholar]
- 20.Chaudhri OB, Parkinson JR, Kuo YT, et al. Differential hypothalamic neuronal activation following peripheral injection of GLP-1 and oxyntomodulin in mice detected by manganese-enhanced magnetic resonance imaging. Biochem Biophys Res Commun 2006;350:298–306 [DOI] [PubMed] [Google Scholar]
- 21.van den Hoek AM, Heijboer AC, Corssmit EP, et al. PYY3-36 reinforces insulin action on glucose disposal in mice fed a high-fat diet. Diabetes 2004;53:1949–1952 [DOI] [PubMed] [Google Scholar]
- 22.Henry RR, Gumbiner B. Benefits and limitations of very-low-calorie diet therapy in obese NIDDM. Diabetes Care 1991;14:802–823 [DOI] [PubMed] [Google Scholar]
- 23.Williams KV, Mullen ML, Kelley DE, Wing RR. The effect of short periods of caloric restriction on weight loss and glycemic control in type 2 diabetes. Diabetes Care 1998;21:2–8 [DOI] [PubMed] [Google Scholar]
- 24.Lara-Castro C, Newcomer BR, Rowell J, et al. Effects of short-term very low-calorie diet on intramyocellular lipid and insulin sensitivity in nondiabetic and type 2 diabetic subjects. Metabolism 2008;57:1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van der Zwaag W, Francis S, Head K, et al. fMRI at 1.5, 3 and 7 T: characterising BOLD signal changes. Neuroimage 2009;47:1425–1434 [DOI] [PubMed] [Google Scholar]
- 26.Smeets PA, de Graaf C, Stafleu A, van Osch MJ, van der Grond J. Functional magnetic resonance imaging of human hypothalamic responses to sweet taste and calories. Am J Clin Nutr 2005;82:1011–1016 [DOI] [PubMed] [Google Scholar]
- 27.Lindström J, Ilanne-Parikka P, Peltonen M, et al. Finnish Diabetes Prevention Study Group Sustained reduction in the incidence of type 2 diabetes by lifestyle intervention: follow-up of the Finnish Diabetes Prevention Study. Lancet 2006;368:1673–1679 [DOI] [PubMed] [Google Scholar]
- 28.Uusitupa M, Lindi V, Louheranta A, Salopuro T, Lindström J, Tuomilehto J, Finnish Diabetes Prevention Study Group Long-term improvement in insulin sensitivity by changing lifestyles of people with impaired glucose tolerance: 4-year results from the Finnish Diabetes Prevention Study. Diabetes 2003;52:2532–2538 [DOI] [PubMed] [Google Scholar]