Abstract
Brown adipose tissue (BAT) is currently considered as a target to combat obesity and diabetes in humans. BAT is densely innervated by the sympathetic nervous system (SNS) and can be stimulated by β-adrenergic agonists, at least in animals. However, the exact role of the β-adrenergic part of the SNS in BAT activation in humans is not known yet. In this study, we measured BAT activity by 2-deoxy-2-[18F]fluoro-d-glucose ([18F]FDG) positron emission tomography/computed tomography imaging in 10 lean men during systemic infusion of the nonselective β-agonist isoprenaline (ISO) and compared this with cold-activated BAT activity. ISO successfully mimicked sympathetic stimulation as shown by increased cardiovascular and metabolic activity. Energy expenditure increased to similar levels as during cold exposure. Surprisingly, BAT was not activated during β-adrenergic stimulation. We next examined whether the high plasma free fatty acid (FFA) levels induced by ISO competed with glucose ([18F]FDG) uptake in BAT locations by blocking lipolysis with acipimox (ACI). ACI successfully lowered plasma FFA, but did not increase [18F]FDG-uptake in BAT. We therefore conclude that systemic nonselective β-adrenergic stimulation by ISO at concentrations that increase energy expenditure to the same extent as cold exposure does not activate BAT in humans, indicating that other tissues are responsible for the increased β-adrenergic thermogenesis.
Brown adipose tissue (BAT) is present and functional in human adults, as was shown recently (1–3). Due to its high metabolic capacity, this organ is of great interest in the current worldwide obesity problem and its related metabolic diseases such as type 2 diabetes. In support, several studies in humans have shown that adiposity is negatively correlated with BAT presence and activity (1,2,4,5). Moreover, a study by Bartelt et al. (6) showed in mice that BAT is involved in triglyceride clearance and thus could improve blood lipid profile. Furthermore, transplantation of BAT in streptozotocin-diabetic mice improved glucose homeostasis and reversed type 1 diabetes (7). In humans, it has been shown that glucose uptake in BAT can increase 12-fold during cold exposure (8). A recent study demonstrated cold-induced fatty acid oxidation in BAT (9). This study also showed that oxidative metabolism in BAT contributed to whole-body energy expenditure during acute cold exposure. Therefore, BAT is currently regarded as an organ with preventive and therapeutic potential for diseases related to the metabolic syndrome.
From animal studies, it is clear that BAT is under hypothalamic control and is densely innervated by the sympathetic nervous system (SNS) (10). When thermogenesis is needed (e.g., in the cold), postganglionic sympathetic neurons release norepinephrine (NE) acting on (mainly) the β-adrenergic receptors present on the cell surface, leading to the activation of uncoupling protein-1. This protein is located in the inner mitochondrial membrane and is responsible for the generation of heat by uncoupling respiration from ATP synthesis (11).
In humans, several studies indicate the role of the SNS in cold-induced thermogenesis and BAT activation. Cold-induced thermogenesis in humans correlates significantly with fasting NE plasma concentrations (12). In support, Zingaretti et al. (13) showed that human brown adipocytes are innervated by the SNS, and Virtanen et al. (3) demonstrated the presence of the β3-adrenergic receptor (β3-AR) mRNA in these cells. Finally, blocking the β1- and β2-AR receptor through oral administration of propranolol decreased [18F]FDG uptake in BAT in cancer patients at room temperature (14,15), indicating a role for these receptors in activating BAT under these conditions. Although the role of β-AR stimulation on BAT has not been directly examined in humans, its role in stimulating thermogenesis in humans is well-established (16), making it likely that β-AR receptor stimulation could be an alternative way to activate BAT.
In this study, we use the nonselective β-AR agonist isoprenaline (ISO) to mimic β-adrenergic SNS activity and investigate its effect on BAT activity and performed cold exposure as a positive control. We show that ISO stimulated thermogenesis to similar levels as cold exposure; however, BAT was not involved in the thermogenic effect of ISO.
RESEARCH DESIGN AND METHODS
The ethics committee of Maastricht University Medical Centre approved the study protocol. All study participants provided written informed consent, and all procedures conformed the standards of the Declaration of Helsinki.
Subjects.
A total of 10 healthy male subjects (age 22.5 ± 2.5 years, BMI 21.6 ± 1.6 kg/m2, fat percentage 15.6 ± 2.9%) were enrolled in the study. All subjects were screened for medical history and status. Cardiovascular status was screened by means of an electrocardiogram and blood pressure measurement. All subjects had normal blood glucose levels. Body composition was determined by means of dual X-ray absorptiometry (type Discovery A; Hologic).
Study design.
First, the subjects underwent a mild cold experiment in order to define BAT presence and activity, followed by the ISO experiment. Subsequently, 5 of 10 subjects underwent a second ISO experiment in combination with ACI. A minimal washout period of 5 days was taken between the experiments.
Subjects were measured in the morning after overnight fasting and asked to refrain from heavy exercise 24 h before the measurements. After arrival, subjects swallowed a telemetric pill (CoreTemp HT150002; HQ, Inc.) for core temperature measurement and iButtons (Maxim Integrated Products) were placed on 14 ISO-defined sites to measure skin temperature (17). A chest strap (Polar T31; Polar USA) and a pressure cuff (Cresta) were attached to measure heart rate and blood pressure, respectively. Laser Doppler probes were attached for skin perfusion measurements at the ventral side of the hand at the base of the thumb, at the ventral side of the hallux (Perimed PF4000; Perimed), at the ventral side of the forearm halfway between the elbow and the wrist, and at the abdomen halfway between the umbilicus and the left lateral side of the body (Perimed PF5000; Perimed). Finally, a cannula was inserted in the left antecubital vein for blood sampling during cold exposure. During the ISO experiments, a second cannula in the antecubital vein of the contralateral arm was placed for ISO infusion. The measurements were performed in a specially equipped air-permeable tent (Colorado Altitude Training), in which ambient temperature could be tightly controlled. Subjects were measured in semisupine position in a nephrodialysis chair in order to lie comfortably. During the measurements, subjects wore standardized clothing (0.49 clo).
Mild cold experiment.
The protocol started with a baseline period of 45 min in thermoneutral conditions (24 to 25°C), followed by 2 h of mild cold exposure in which an individualized protocol was used. Each subject was cooled down until shivering occurred. After this, air temperature was slightly increased until shivering stopped. Mild cold was applied by means of air-conditioning. After 1 h of cold exposure, the [18F]FDG tracer was injected intravenously. Subjects were asked to lie still during the experiment in order to prevent artifact by muscle activity. Shivering was detected visually, and subjects were asked to report shivering on a self-created visual analog scale every 10 min. The cooling protocol has been validated previously (5). After the cooling protocol, subjects were transferred to the positron emission tomography (PET) and computed tomography (CT) scanner (Gemini TF PET-CT; Philips).
ISO experiment.
After the baseline period of 45 min, ISO (ISO sulfate) was infused intravenously in the antecubital vein in incremental dosages (6, 12, and 24 ng [kg fat-free mass−1 min−1]) for 30 min (doses 1 and 2) and 55 min (dose 3). A previous study has shown that it takes about 8 min for each ISO dose to reach a steady-state concentration in the body (18). The extended duration of each dose was necessary to obtain a stable period for energy expenditure. The [18F]FDG tracer was injected intravenously 10 min after the start of dose 3. Venous blood samples were taken during baseline and at the end of each dose. Cardiovascular function was continuously monitored by means of electrocardiography (Cardiolife TEC 7100K; Nihon Kohden) and blood pressure.
ISO experiment in combination with ACI.
Subsequently, five subjects underwent a second ISO experiment, in which two times 250 mg of ACI was given orally (2 h before and at the start of baseline) to lower circulating fatty acids. ACI is a nicotinic acid derivative and blocks lipolyis in fat tissue by decreasing cyclic AMP, and therefore decreasing the lipolytic effects of hormone-sensitive lipase (19). The experimental protocol was similar to the ISO experiment as explained before. One subject did not ingest the second capsule due to adverse effects. However, plasma FFA levels during the tracer injection were still lower compared with ISO (ISO + ACIdose3: 171 vs. ISOdose3: 631 μmol/L).
PET scanning protocol.
One hour before the PET-CT scan, subjects were intravenously injected with 50 MBq (1.35 mCi) of [18F]FDG. Imaging started with a low-dose CT scan (120 kV, 30 mAs), immediately followed by a PET scan. A total of six to seven bed positions (6 min per bed position) were necessary to cover the area where BAT is usually found (i.e., abdominal, thoracic, and neck region). The PET image was used to determine the [18F]FDG uptake, and the CT image was used for PET attenuation correction and localization of the [18F]FDG uptake sites. The voxel size of both reconstructed image sets was 4 × 4 × 4 mm3.
PET analysis.
We used PMOD software, version 3.0 (PMOD Technologies), for the analysis of BAT activity. Both the researcher and a nuclear medicine physician interpreted the PET-CT image. The regions of interest were manually outlined in each slice (4 mm) in the fusion (PET and CT) image. We handled two criteria to qualify [18F]FDG uptake as BAT activity in these regions. First, the region drawn should be localized in fat tissue as determined by the CT scan (Hounsfield units: −10 to −180). Second, the standardized uptake value (SUV) in each region should be at least 1.5 (approximately six times higher than in white adipose tissue [WAT]). BAT activity of each region is determined by the average SUV uptake (SUV mean) times the volume of the region (cm3), expressed as SUV total. To compare SUV mean in BAT, WAT, and skeletal muscle, we drew regions of interest in the supraclavicular BAT region, subcutaneous abdominal adipose tissue, and in both arms on the deltoid muscle. In order to locate the supraclavicular BAT region during the two ISO experiments, we used the PET-CT fusion image during cold exposure as a reference.
Statistical analysis.
Statistical analysis was performed with PASW Statistics 18.0 for Mac (SPSS). Reported data are expressed as means ± SD. Paired sample t tests were used to compare findings between baseline and cold and one-way ANOVA for repeated measurements with post hoc analysis, including Bonferroni correction, to compare baseline with the three ISO doses. Wilcoxon test was used measure differences within the ISO and ACI experiment and between ISO and the ISO and ACI experiment. Two-way ANOVA for repeated measurements was used to compare cold exposure with ISOdose3. Both study phase (baseline versus intervention) and study type (cold versus ISOdose3) were taken as independent variables in the model, and the interaction effect between these two variables was analyzed. Spearman rank correlation was used to identify correlations between variables. P values of <0.05 were considered as statistically significant.
Blood analysis.
Venous blood was collected for analysis of several blood parameters. The supernatant (plasma) was used for analysis of FFA (NEFA-HR set; Wako Chemicals, Neuss, Germany), free glycerol (Glycerol kit; R-Biopharm, Darmstadt, Germany), total glycerol (ABX Triglyceriden CP, Radiometer; Horiba ABX, Montpellier, France), and glucose (ABX Glucose HK CP, Radiometer; Horiba ABX, Montpellier, France) on a COBAS FARA centrifugal spectrophotometer (Roche Diagnostica, Basel, Switzerland). Triglyceride levels were calculated by using the difference in total and free glycerol. Serum insulin was analyzed with a Human Insulin Specific RIA kit (Millipore) on a γ Counter (2470 Automatic γ Counter Wizard2; Wallac, PerkinElmer). Thyroid-related hormones were determined from the supernatant of the serum. Plasma thyroid-stimulating hormone was measured by an electrochemiluminescence immunoassay kit on a COBAS 6000 system (Roche Diagnostica). Total thyroxine was determined by a solid-phase, competitive chemiluminescent enzyme immunoassay on an Immulite 2000 system (Siemens). Free thyroxine was analyzed by a solid-phase time-resolved fluoroimmunoassay FT4 kit on an AutoDELFIA system (PerkinElmer).
RESULTS
ISO and cold-induced thermogenesis.
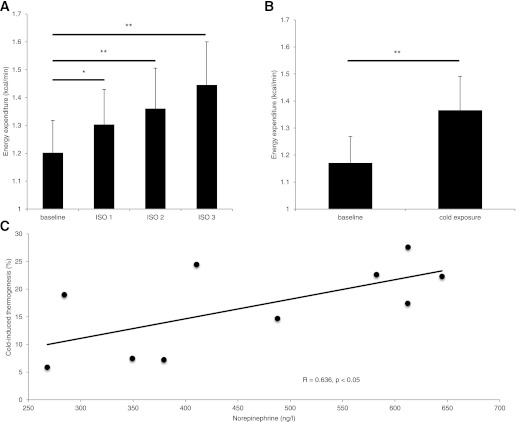
We first examined the effect of increasing doses of ISO in lean healthy subjects on thermogenesis. ISO significantly increased energy expenditure (P < 0.001, one-way ANOVA), with the highest increase of 19.7 ± 7.2% during 24 ng/kg fat-free mass−1 min−1 (Fig. 1A). We then compared this increase in energy expenditure with the increase observed during cold exposure. Cold exposure increased energy expenditure to similar levels (baseline: 1.17 ± 0.10; mild cold: 1.37 ± 0.13 kcal/min; P < 0.001), with an average cold-induced thermogenesis of 16.9 ± 7.8% (Fig. 1B). There was no interaction effect between cold exposure and ISO on energy expenditure (P > 0.05, two-way ANOVA), confirming a similar increase in energy expenditure. Both cold exposure and ISO significantly decreased the respiratory exchange ratio (Table 1). There was no difference between cold exposure and ISO (P > 0.05, two-way ANOVA).
FIG. 1.
ISO- and cold-induced thermogenesis. A: Energy expenditure during baseline and the three ISO doses. B: Energy expenditure during baseline and cold exposure. C: Relationship between the plasma NE level and cold-induced thermogenesis. Values are expressed as means ± SD. *P < 0.05, **P < 0.001, ISO (n = 10) and cold exposure (n = 10).
TABLE 1.
Cardiovascular effects, skin perfusion, and body temperatures during cold exposure and ISO
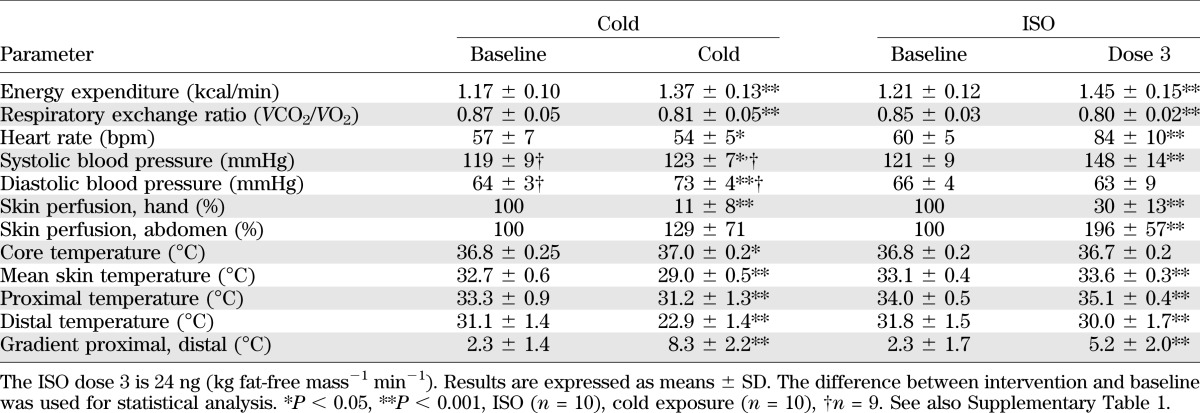
Stimulation of the β-AR receptors leads to an increase in heart rate (mainly β-1) and vasodilatation of blood vessels (16). We found that ISO increased heart rate (baseline 60 ± 5 vs. ISOdose3: 84 ± 10 bpm; P < 0.001) and systolic blood pressure (baseline 121 ± 9 vs. ISOdose3: 148 ± 14 mmHg; P < 0.001) (Table1), due to its inotropic and chronotropic effects on the heart. Diastolic blood pressure was unchanged during ISO.
With respect to body temperatures (Table 1), ISO significantly increased skin temperature in the proximal area, whereas it dropped in the distal areas. Skin perfusion corresponded to these temperature changes, demonstrating decreased perfusion in the hand and increased perfusion in the abdominal region. Cold exposure increased both systolic and diastolic blood pressure, whereas it decreased heart rate. As expected, cold exposure decreased skin temperatures and skin perfusion in the hand, but slightly increased core temperature. The effects during ISO doses 1 and 2 are presented in Supplementary Table 1.
We measured several blood metabolites and hormones, as demonstrated in Supplemental Tables 2 and 3. Plasma NE levels were increased during cold exposure (244 ± 72 versus 463 ± 143 ng/L; P < 0.001), as shown previously (8,12); however, not during ISO. A significant relation was found between NE levels and cold-induced thermogenesis (R = 0.64; P < 0.05) (Fig. 1C); however, not with BAT activity during cold (R = −0.09; P = 0.80). Cold exposure significantly increased plasma FFA (baseline: 324 ± 84 vs. cold: 637 ± 398 μmol/L; P < 0.05), free glycerol (baseline: 56.6 ± 14.4 vs. cold: 106.7 ± 59.6 μmol/L; P < 0.05), and triglycerides (baseline: 638 ± 171 vs. cold: 685 ± 200 μmol/L; P < 0.05), whereas plasma glucose decreased (baseline: 4.9 ± 0.4 vs. cold: 4.7 ± 0.4 mmol/L; P < 0.05). As expected, serum insulin levels were significantly increased by ISO.
BAT activity during ISO and cold exposure.
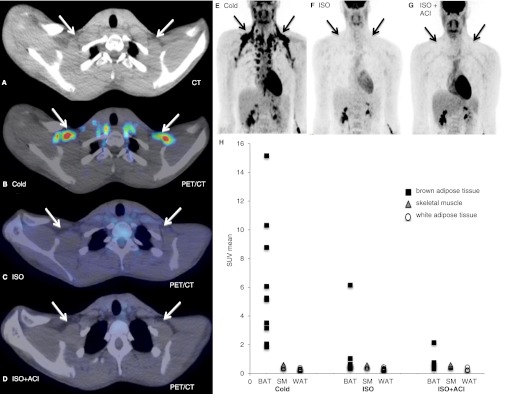
We next studied if ISO activates BAT at a dose that increases thermogenesis to a similar extent as cold exposure. As expected, cold exposure led to substantial [18F]FDG uptake in the neck, supraclavicular, paraspinal, para-aortic, axillary, mediastinal, and perirenal regions (891 ± 1027 SUV total; Fig. 2B and E). The anatomical location of BAT was comparable to previous findings, with the largest depot in the supraclavicular region (Fig. 2E). Surprisingly, after ISO in 9 of 10 subjects, no detectable [18F]FDG uptake in BAT locations could be observed (Fig. 2C and F) despite the thermogenic effect of ISO. In only one subject, BAT activity was observed (SUV total = 838).
FIG. 2.
BAT activity during ISO infusion and cold exposure. A: CT image of the supraclavicular area. B: Cold exposure [18F]FDG PET/CT image showing cold-activated BAT. C: ISO [18F]FDG PET/CT image showing no BAT activity during ISO. D: ISO + ACI [18F]FDG PET/CT image showing no effect on [18F]FDG uptake in supraclavicular BAT. E: Cold exposure [18F]FDG PET/CT image of the upper body showing BAT activity in the neck, supraclavicular, paraspinal, para-aortic, axillary, mediastinal, and perirenal regions. F: ISO [18F]FDG PET/CT image showing no BAT activity during ISO. G: ISO + ACI [18F]FDG PET/CT image showing no effect on [18F]FDG uptake in BAT locations. H: SUV mean values for BAT, SM, and WAT during cold exposure, ISO, and ISO + ACI; ISO (n = 10), ISO + ACI (n = 5). (A high-quality digital representation of this figure is available in the online issue.)
In addition to BAT, other tissues may contribute to cold- or ISO-induced thermogenesis. Therefore, we examined the average uptake of [18F]FDG into skeletal muscle and WAT (Fig. 2H). Interestingly, the average SUV uptake (SUV mean) in skeletal muscle was similar during ISO and cold exposure (ISO: 0.53 ± 0.07 vs. cold: 0.50 ± 0.08 SUV mean; P = 0.26). Similarly, [18F]FDG uptake in WAT was also comparable during ISO and cold exposure (ISO: 0.28 ± 0.10 vs. cold: 0.27 ± 0.07 SUV mean; P = 0.90).
ISO and ACI.
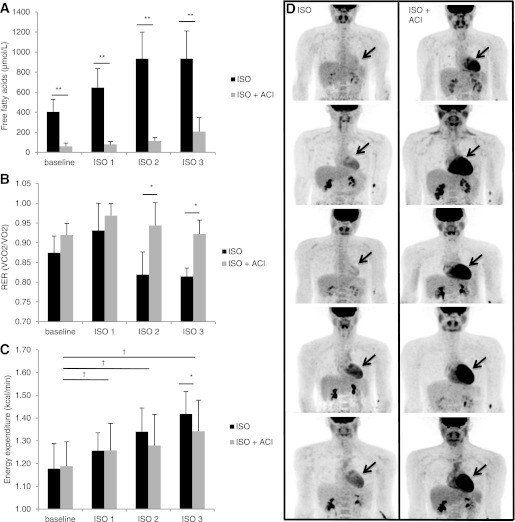
The current method to detect BAT activity is by measuring radiolabeled glucose uptake ([18F]FDG). It is known that ISO stimulates lipolysis in WAT and that high fatty acid levels may compete with glucose for uptake in multiple tissues (20). We therefore next examined if the ISO-induced increase in plasma FFA might compete with glucose for uptake into BAT. Indeed, ISO led to an increase in plasma concentration of FFA to very high levels (Fig. 3A and Supplementary Table 2). The nicotinic acid derivative ACI was used to block lipolysis in WAT (19). Indeed, ACI significantly reduced the concentration of FFA by ∼78% in the plasma (ISO: 934 ± 276 vs. ISO + ACI: 207 ± 140 μmol/L; P < 0.05) (Supplementary Table 2) and resulted in an increased whole-body glucose oxidation as demonstrated by the increase in the respiratory exchange ratio (ISO: 0.81 ± 0.02 vs. ISO + ACI: 0.92 ± 0.04; P < 0.001) (Fig. 3B). Lowering circulating plasma FFA levels by ACI very clearly increased the uptake of [18F]FDG into the heart; indeed, demonstrating that substrate competition can affect [18F]FDG uptake (Fig. 3D). Next, we examined if ISO would lead to [18F]FDG uptake in BAT when plasma FFA were reduced by ACI. The reduction of plasma FFA levels did not lead to enhanced [18F]FDG uptake in BAT in four of five subjects. One person showed a slight increase in BAT activity; however, this BAT activity was minimal compared with the activity during mild cold exposure (ISO + ACI: 27 vs. cold: 3132 SUV total). Furthermore, ACI did not lead to enhanced [18F]FDG uptake in skeletal muscle. The combination of ISO with ACI significantly increased energy expenditure by 12.8% (P < 0.05). This was, however, significantly lower compared with ISO infusion alone (P < 0.05) (Fig. 3C). The effects of ACI in combination with ISO on cardiovascular effects, skin perfusion, and body temperatures are presented in Supplementary Table 1.
FIG. 3.
The effects of ACI on FFA levels, substrate use, energy expenditure, and [18F]FDG uptake. A: Plasma FFA levels during ISO and ISO + ACI. B: Respiratory exchange ratio during ISO and ISO + ACI. C: Energy expenditure during ISO and ISO + ACI. D: The left panel shows the three-dimensional reconstructed PET images in five subjects during ISO infusion indicating no brown fat activity and a low [18F]FDG uptake in the heart. The right panel shows the same individuals during ISO in combination with ACI. ACI increases [18F]FDG uptake in the heart as indicated by the black arrows; however, not in BAT locations. Values are expressed as means ± SD. *Significant difference between ISO and ISO + ACI (P < 0.05), **P < 0.001, †significant difference (P < 0.05) between ISO + ACI dose and baseline, ISO (n = 10) ISO + ACI (n = 5).
DISCUSSION
There is compelling evidence that BAT in animals is under sympathetic control and can be activated through β-AR stimulation (11). However, the exact role of the SNS in activating BAT in humans is less clear. The main objective of this study was to examine whether nonselective β-AR stimulation through ISO activates BAT in humans. Contrary to our expectations, the results show that ISO does not activate BAT in humans, despite the observed increase in thermogenesis.
Cold exposure increased energy expenditure by ∼17%, comparable to cold-induced thermogenesis levels reported earlier (1). This increased thermogenesis upon cold exposure was accompanied by increased BAT activity in anatomical locations comparable to previous findings, with the largest depot in the supraclavicular region. Interestingly, cold exposure increased plasma triglycerides in the current study, which seems to be in contrast to the triglyceride clearance by BAT occurring in mice during cold exposure (6). However, it has already been previously shown by Vallerand et al. (21) that the FFAs needed for enhanced lipid oxidation during cold exposure do not originate from plasma triglycerides, but rather from intracellular lipolysis. More research is needed to investigate if BAT can affect triglyceride clearance in humans.
Furthermore, we confirm that short-term cold exposure increased plasma NE levels as previously found by Orava et al. (8) and that these levels were related to cold-induced thermogenesis. Based on these results and evidence obtained from rodent BAT studies, we hypothesized that mimicking β-adrenergic SNS activation by infusion of the β-AR agonist ISO would activate BAT in humans.
ISO indeed mimicked β-adrenergic sympathetic activity as shown by the increased heart rate and whole-body energy expenditure. The increase of ∼20% in energy expenditure during ISO was comparable to that during previous studies with similar dosages (22–24). Contrary to our expectations, however, ISO did not activate BAT in 9 of 10 subjects. In rodents, brown adipose tissue has been shown to have a dense β-AR receptor population. Both in vitro (25,26) and in vivo (27,28) experiments in animals have shown that stimulating these receptors leads to BAT activation. Also, in humans, the β-AR receptor family is likely to be involved in BAT thermogenesis, as demonstrated by the presence of β3-AR mRNA in human supraclavicular BAT (3) and the inhibition of BAT activity by β-AR receptor antagonism (14,15). Moreover, a recent study by Mattson et al. (27) showed the presence of mRNA of all β-AR subtypes in human multipotent adipocyte-derived stem cells differentiated into brown adipocytes. Stimulating these cells by ISO and CL-316243 (β3-AR agonist) led to increased uncoupling protein-1 mRNA and protein levels, indicating β-AR receptor involvement in cell activation. We were therefore surprised not to find BAT activity upon β-AR stimulation.
In the current study, we measured BAT activity in humans by means of radioactive [18F]FDG uptake. Theoretically, however, high plasma fatty acid levels may compete with glucose for cellular uptake, thereby underestimating true BAT activity. Therefore, due to the lipolytic effects of ISO, we hypothesized that the high plasma FFA levels might have competed with glucose ([18F]FDG) uptake. We showed that the simultaneous administration of ISO and ACI, which inhibits lipolysis, was able to lower fatty acids and that this resulted in elevated glucose uptake in the heart, illustrating that, indeed, substrate competition may be a factor determining [18F]FDG uptake. However, lowering circulating fatty acid levels did not lead to increased tracer uptake in BAT locations. One could argue that BAT thermogenesis was blunted by ACI through the inhibition of lipolysis by lowering cAMP levels as seen in WAT. However, two animal studies showed no effect of nicotinic acid (ACI is a derivative of nicotinic acid) on FFA and cAMP levels in BAT (29,30), which demonstrates no inhibiting effects of ACI on BAT activation. The fact that the increase in energy expenditure upon ISO with ACI was 8% lower compared with ISO alone can most likely be explained by the reduction in plasma FFA levels, as previously shown (31).
Based on these findings, we can conclude that systemic nonselective β-AR stimulation, at concentrations that increase energy expenditure to the same extent as cold exposure, does not activate BAT in humans. It is surprising that blocking the β1- and β2-receptors by propranolol decreases BAT activity (14,15), whereas stimulating these receptors by ISO does not activate human BAT. A plausible explanation is that the systemic concentration of ISO was not sufficient to trigger the β-receptors on brown adipocytes. The concentrations of NE that occur at the postsynaptic areas are high (∼100 nmol/L) during central sympathetic stimulation (e.g., cold), which cannot be reached in plasma during physiological conditions (25). We cannot exclude the possibility that higher doses of ISO are able to stimulate BAT in humans. However, these high doses would lead to unwanted side effects such as tachycardia, arrhythmias, and pronounced systolic blood pressure elevations. Central stimulation, therefore, remains an interesting option for future studies, although achieving such in human studies will be difficult.
Interestingly, these findings thus show that metabolic processes in tissues other than BAT cause the ISO-induced thermogenesis. β-AR receptors are expressed in various tissues, such as the heart, skeletal muscle, adipose tissue, liver, bronchi, blood vessels, and pancreas (16). A small part of the increased thermogenesis can be explained by the energy cost of cardiac and respiratory work upon sympathetic stimulation (32). However, skeletal muscle likely explains a major part. The contribution of skeletal muscle to epinephrine-induced thermogenesis has been estimated at 40% (33). Moreover, a study by Astrup et al. (34) showed that ephedrine-induced thermogenesis is located for 50% in skeletal muscle. In our study, the average tracer uptake in skeletal muscle was similar during ISO and cold exposure, indicating similar activity in both situations. However, additional studies focused on skeletal muscle are needed to measure its contribution during cold-induced and adrenergic thermogenesis.
The mechanisms behind skeletal muscle thermogenesis are not fully known. Interestingly, Wijers et al. (35) demonstrated, with high-resolution respirometry in permeabilized human skeletal muscle biopsies, that skeletal muscle mitochondrial uncoupling correlated significantly to the increase in whole-body energy expenditure after 3 days of cold exposure. This finding indicates that mitochondrial uncoupling could be such a mechanism. Other possible mechanisms involved are futile calcium cycling, protein turnover, and substrate cycling (36).
It should be noted that although [18F]FDG uptake shows whether BAT is active, it provides limited information on the actual thermogenic activity of the tissues, as fatty acids are an important substrate during cold and ISO infusion. Furthermore, the uptake of [18F]FDG in BAT can occur without simultaneous increased blood perfusion as shown during insulin infusion (8). This could imply that BAT glucose uptake takes place without concurrent thermogenesis. However, due to practical limitations, we were not able to use these techniques in the current study.
From the present results, it can be concluded that systemic nonselective β-AR stimulation increased thermogenesis without concomitant BAT activation, indicating that other tissues are involved.
ACKNOWLEDGMENTS
This work is partly financed by the Netherlands Organization for Scientific Research (TOP 91209037 to W.D.v.M.L.), and by the EU FP7 project DIABAT (HEALTH-F2-2011-278373).
No potential conflicts of interest relevant to this article were reported.
M.J.V. designed and carried out the experiments, analyzed data, and wrote the manuscript. A.A.J.J.v.d.L. assisted during the experiments, contributed to the discussion, and reviewed and edited the manuscript. B.B., M.A.v.B., and P.S. contributed to the design of the study and discussion and reviewed and edited the manuscript. R.W. contributed to the discussion and reviewed and edited the manuscript. W.D.v.M.L. designed the study, contributed to the discussion, and reviewed and edited the manuscript. M.J.V. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
This study was presented at the 19th European Congress on Obesity, Lyon, France, 9–12 May 2012.
The authors thank Ivo Pooters (Maastricht University Medical Centre) for assistance during the experiments and Jos Stegen (Maastricht University Medical Centre), Paul Menheere, and Nancy Hendrix (Maastricht University Medical Centre) for assistance with the biochemical analysis. The authors also thank Mariëlle Visser, Christian Urbach, Emiel Beijer, Loek Wouters, Paul Schoffelen, and Paul Bergs (Maastricht University Medical Centre) for technical support, Boris Kingma and Guy Vijgen for helpful suggestions, and the Literature Club for all of the fruitful discussions.
Footnotes
Clinical trial reg. no. ISRCTN21413505, http://isrctn.org.
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db12-0288/-/DC1.
REFERENCES
- 1.van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, et al. Cold-activated brown adipose tissue in healthy men. N Engl J Med 2009;360:1500–1508 [DOI] [PubMed] [Google Scholar]
- 2.Saito M, Okamatsu-Ogura Y, Matsushita M, et al. High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes 2009;58:1526–1531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Virtanen KA, Lidell ME, Orava J, et al. Functional brown adipose tissue in healthy adults. N Engl J Med 2009;360:1518–1525 [DOI] [PubMed] [Google Scholar]
- 4.Cypess AM, Lehman S, Williams G, et al. Identification and importance of brown adipose tissue in adult humans. N Engl J Med 2009;360:1509–1517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vijgen GH, Bouvy ND, Teule GJ, Brans B, Schrauwen P, van Marken Lichtenbelt WD. Brown adipose tissue in morbidly obese subjects. PLoS ONE 2011;6:e17247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bartelt A, Bruns OT, Reimer R, et al. Brown adipose tissue activity controls triglyceride clearance. Nat Med 2011;17:200–205 [DOI] [PubMed] [Google Scholar]
- 7.Gunawardana SC, Piston DW. Reversal of type 1 diabetes in mice by brown adipose tissue transplant. Diabetes 2012;61:674–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Orava J, Nuutila P, Lidell ME, et al. Different metabolic responses of human brown adipose tissue to activation by cold and insulin. Cell Metab 2011;14:272–279 [DOI] [PubMed] [Google Scholar]
- 9.Ouellet V, Labbé SM, Blondin DP, et al. Brown adipose tissue oxidative metabolism contributes to energy expenditure during acute cold exposure in humans. J Clin Invest 2012;122:545–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakamura K, Morrison SF. Central efferent pathways mediating skin cooling-evoked sympathetic thermogenesis in brown adipose tissue. Am J Physiol Regul Integr Comp Physiol 2007;292:R127–R136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev 2004;84:277–359 [DOI] [PubMed] [Google Scholar]
- 12.Wijers SL, Saris WH, van Marken Lichtenbelt WD. Individual thermogenic responses to mild cold and overfeeding are closely related. J Clin Endocrinol Metab 2007;92:4299–4305 [DOI] [PubMed] [Google Scholar]
- 13.Zingaretti MC, Crosta F, Vitali A, et al. The presence of UCP1 demonstrates that metabolically active adipose tissue in the neck of adult humans truly represents brown adipose tissue. FASEB J 2009;23:3113–3120 [DOI] [PubMed] [Google Scholar]
- 14.Parysow O, Mollerach AM, Jager V, Racioppi S, San Roman J, Gerbaudo VH. Low-dose oral propranolol could reduce brown adipose tissue F-18 FDG uptake in patients undergoing PET scans. Clin Nucl Med 2007;32:351–357 [DOI] [PubMed] [Google Scholar]
- 15.Söderlund V, Larsson SA, Jacobsson H. Reduction of FDG uptake in brown adipose tissue in clinical patients by a single dose of propranolol. Eur J Nucl Med Mol Imaging 2007;34:1018–1022 [DOI] [PubMed] [Google Scholar]
- 16.van Baak MA. The peripheral sympathetic nervous system in human obesity. Obes Rev 2001;2:3–14 [DOI] [PubMed] [Google Scholar]
- 17.van Marken Lichtenbelt WD, Daanen HA, Wouters L, et al. Evaluation of wireless determination of skin temperature using iButtons. Physiol Behav 2006;88:489–497 [DOI] [PubMed] [Google Scholar]
- 18.Martinsson A, Lindvall K, Melcher A, Hjemdahl P. Beta-adrenergic receptor responsiveness to isoprenaline in humans: concentration-effect, as compared with dose-effect evaluation and influence of autonomic reflexes. Br J Clin Pharmacol 1989;28:83–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Christie AW, McCormick DK, Emmison N, Kraemer FB, Alberti KG, Yeaman SJ. Mechanism of anti-lipolytic action of acipimox in isolated rat adipocytes. Diabetologia 1996;39:45–53 [DOI] [PubMed] [Google Scholar]
- 20.Randle PJ, Garland PB, Hales CN, Newsholme EA. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet 1963;1:785–789 [DOI] [PubMed] [Google Scholar]
- 21.Vallerand AL, Jacobs I. Energy metabolism during cold exposure. Int J Sports Med 1992;13(Suppl. 1):S191–S193 [DOI] [PubMed] [Google Scholar]
- 22.Bell C, Stob NR, Seals DR. Thermogenic responsiveness to beta-adrenergic stimulation is augmented in exercising versus sedentary adults: role of oxidative stress. J Physiol 2006;570:629–635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schiffelers SL, Blaak EE, Baarends EM, et al. beta-Adrenoceptor-mediated thermogenesis and lipolysis in patients with chronic obstructive pulmonary disease. Am J Physiol Endocrinol Metab 2001;280:E357–E364 [DOI] [PubMed] [Google Scholar]
- 24.Stob NR, Seals DR, Jørgen J, et al. Increased thermogenic responsiveness to intravenous beta-adrenergic stimulation in habitually exercising humans is not related to skeletal muscle beta2-adrenergic receptor density. Exp Physiol 2007;92:823–830 [DOI] [PubMed] [Google Scholar]
- 25.Atgié C, D’Allaire F, Bukowiecki LJ. Role of beta1- and beta3-adrenoceptors in the regulation of lipolysis and thermogenesis in rat brown adipocytes. Am J Physiol 1997;273:C1136–C1142 [DOI] [PubMed] [Google Scholar]
- 26.Mohell N, Nedergaard J, Cannon B. Quantitative differentiation of alpha- and beta-adrenergic respiratory responses in isolated hamster brown fat cells: evidence for the presence of an alpha 1-adrenergic component. Eur J Pharmacol 1983;93:183–193 [DOI] [PubMed] [Google Scholar]
- 27.Mattsson CL, Csikasz RI, Chernogubova E, et al. β₁-Adrenergic receptors increase UCP1 in human MADS brown adipocytes and rescue cold-acclimated β₃-adrenergic receptor-knockout mice via nonshivering thermogenesis. Am J Physiol Endocrinol Metab 2011;301:E1108–E1118 [DOI] [PubMed] [Google Scholar]
- 28.Shih MF, Taberner PV. Selective activation of brown adipocyte hormone-sensitive lipase and cAMP production in the mouse by beta 3-adrenoceptor agonists. Biochem Pharmacol 1995;50:601–608 [DOI] [PubMed] [Google Scholar]
- 29.Bizzi A, Codegoni AM, Lietti A, Garattini S. Different responses of white and brown adipose tissue to drugs affecting lipolysis. Biochem Pharmacol 1968;17:2407–2412 [DOI] [PubMed] [Google Scholar]
- 30.Bertin R, Portet R. Effects of lipolytic and antilipolytic drugs on metabolism of adenosine 3′:5′-monophosphate in brown adipose tissue of cold acclimated rats. Eur J Biochem 1976;69:177–183 [DOI] [PubMed] [Google Scholar]
- 31.Schiffelers SL, Brouwer EM, Saris WH, van Baak MA. Inhibition of lipolysis reduces beta1-adrenoceptor-mediated thermogenesis in man. Metabolism 1998;47:1462–1467 [DOI] [PubMed] [Google Scholar]
- 32.Sjöström L, Schutz Y, Gudinchet F, Hegnell L, Pittet PG, Jéquier E. Epinephrine sensitivity with respect to metabolic rate and other variables in women. Am J Physiol 1983;245:E431–E442 [DOI] [PubMed] [Google Scholar]
- 33.Simonsen L, Bülow J, Madsen J, Christensen NJ. Thermogenic response to epinephrine in the forearm and abdominal subcutaneous adipose tissue. Am J Physiol 1992;263:E850–E855 [DOI] [PubMed] [Google Scholar]
- 34.Astrup A, Bülow J, Madsen J, Christensen NJ. Contribution of BAT and skeletal muscle to thermogenesis induced by ephedrine in man. Am J Physiol 1985;248:E507–E515 [DOI] [PubMed] [Google Scholar]
- 35.Wijers SL, Schrauwen P, Saris WH, van Marken Lichtenbelt WD. Human skeletal muscle mitochondrial uncoupling is associated with cold induced adaptive thermogenesis. PLoS ONE 2008;3:e1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wijers SL, Saris WH, van Marken Lichtenbelt WD. Recent advances in adaptive thermogenesis: potential implications for the treatment of obesity. Obes Rev 2009;10:218–226 [DOI] [PubMed] [Google Scholar]