Engagement of EP2 receptors dampens mast cell IgE-dependent signaling causing suppression of FcεRI-mediated allergic responses; thus agonism of EP2 may be beneficial in allergic diseases.
Keywords: Butaprost calcium, FcεI, Fyn, PGE2
Abstract
The experimental administration of PGE2 for the treatment of asthma dampens clinical symptoms, and similar efficacy has been found in dust mite-induced hypersensitivity reactions in animal models. Here, we investigate the mechanism by which PGE2 mediates suppression of MC degranulation. We find that the effect of PGE2 on FcεRI-dependent MC degranulation varies from activating to suppressing, depending on the relative ratio of EP2 to EP3 expression on these cells with suppression evident only in cells having increased EP2 to EP3 expression. Consistent with a role for EP2 in suppressing MC responses in vitro, we found that a selective EP2 agonist, Butaprost, inhibited MC-mediated FcεRI-induced immediate hypersensitivity in a model of PCA. EP2 engagement on MCs increased cAMP production and inhibited FcεRI-mediated calcium influx. In addition, it also decreased the extent of FcεRI-induced Fyn kinase activity, leading to decreased phosphorylation of key signaling molecules such as Gab2 and Akt. Treatment with an antagonist of cAMP or shRNA down-regulation of PKA (the principal intracellular target of cAMP) reversed the EP2-mediated inhibitory effect on MC degranulation and restored calcium influx and phosphorylation of Akt. Collectively, the findings demonstrate that EP2 suppresses the Fyn-mediated signals that are central to FcεRI-dependent MC degranulation, suggesting that engagement of the EP2 on MCs may be beneficial in dampening allergic responses.
Introduction
The prevalence of allergic diseases has almost doubled over the past 25 years, particularly in Western society. Current treatments are still limited, particularly for complex diseases such as asthma, which present multiple phenotypes and varied features that can respond differently to therapies. Approved drugs are effective in relieving the symptoms of certain allergic diseases; however, in some patients, they provide limited or no relief.
Clinical studies have shown that experimental treatment with PGE2, a product of the COX-2, prevented airway inflammation and airway hyper-reactivity in allergic asthma patients or in exercise- and aspirin-induced bronchoconstriction [1, 2]. Conversely, several studies have shown a link between asthmatic patients and low levels of PGE2 in isolated airway cells [3–5], suggesting a homeostatic role for PGE2 in the control of airway reactivity. In previous work, we found that s.c. injection of PGE2 reduced airway hyper-responsiveness in the HDM mouse model of allergic airway hypersensitivity [6]. Additional mouse studies have shown a potential anti-inflammatory role for PGE2 by demonstrating that inhibition of COX-2, which leads to the decrease of PGE2 levels, results in increased airway hyper-reactivity [7–11]. The pre-emptive effect of PGE2 in blocking airway inflammation has been associated with its ability to cause production of anti-inflammatory cytokines such as IL-10 in DCs [12]. However, other results point toward an effect of PGE2 in directly dampening MC responses [13–15]. Regardless of the mode of action, the collective findings provide strong evidence in favor of a negative regulatory role for PGE2 in certain allergic responses.
MCs are key players in IgE-mediated allergic inflammation (type I hypersensitivity reaction) [16]. FcεRI-mediated activation of MCs by the interaction of cell surface-bound allergen-specific IgE with the allergen leads to the release of a wide variety of autacoids such as histamine, eicosanoids, cytokines, and enzymes that contribute to the initiation and regulation of the allergic response [17]. Thus, exploring the mechanisms underlying the suppressive effects of PGE2 on MC activation may uncover novel therapeutic strategies in allergic disease.
PGE2 acts on four distinct GPCRs—EP1, EP2, EP3, and EP4 (reviewed in ref. [18])—which differ in their signaling pathways and can trigger diverse cellular responses. PGE2Rs are expressed in cultured MCs [15, 19, 20]. As EP1–4 may mediate opposing responses depending on cell type or condition [13–15, 19, 20], it is possible that the expression pattern of these receptors on MCs may determine whether PGE2 elicits a stimulatory or inhibitory effect on MC responses.
Based on previous reports [13–15] suggesting that EP2 could be key in mediating the suppression of MC responses (such as degranulation, cytokine, and PG production), we first set out to confirm whether this receptor is responsible for suppression of MC function. In addition, we explored herein whether the expression of EP2 relative to other family members might determine the previously reported stimulatory or inhibitory effects of PGE2 [7–11, 13–15]. Given the lack of knowledge on the molecular mechanisms downstream of EP2 that could contribute to the suppression of IgE/antigen-dependent MC degranulation, we also investigated how EP2 agonism impinges on FcεRI-mediated signaling. Our findings show that the inhibitory effect of PGE2 on MC degranulation is mediated by EP2; however, this effect may be masked by the expression of other PGE2Rs, particularly EP3, in various MC types. This argues that a selective EP2 agonist could be acting through MCs and might be a good choice for potential therapeutic intervention in allergic disease. Consistent with this view, we found that use of Butaprost, a selective EP2 agonist, dampens the allergic response in vivo (PCA) and that this is dependent on the presence of MCs in the skin. Mechanistically, we demonstrate that engagement of EP2 results in cAMP/PKA-dependent interference with FcεRI-induced signals and calcium responses, which are essential for MC responses. This delineates a novel mode by which MC responsiveness might be modulated.
MATERIALS AND METHODS
Reagents and antibodies
DNP-specific mouse IgE was produced as described previously [21]. Biotinylated anti-hIgE was from Abbiotec (San Diego, CA, USA) and streptavidin antigen was purchased from BD Biosciences (San Jose, CA, USA). DNP-HSA antigen, forskolin, IBMX, DMSO, formamide, formaline, and Evans blue were from Sigma-Aldrich (St. Louis, MO, USA). The methyl ester form of Butaprost (EP2 agonist) [22] and PGE2 were purchased from Cayman Chemicals (Ann Arbor, MI, USA). The Rp-isomer adenosine 3′,5′-cyclic monophosphorothioate triethylammonium salt (a PKA antagonist) was obtained from Calbiochem (San Diego, CA, USA). Rabbit anti-EP2 antibody was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA); rabbit antiphospo-Akt (S473), antiphospho-SrcY416 (pY416), antiphospho-Gab2 (pY452), and anti-PKA (C-α) antibodies were from Cell Signaling Technology (Beverly, MA, USA). Mouse anti-Akt and mouse anti-β-actin antibodies were from BD Biosciences and mouse antiphosphotyrosine antibody (4G10) from Millipore (Billerica, MA, USA).
Cells and mice
All mice were maintained and used in accordance with U.S. NIH guidelines and NIAMS-approved animal study proposal A010-04-03 (NIAMS, NIH, Bethesda, MD, USA). Mice used for PCA were 6- to 8-week-old female BALB/c or C57BL/6 KitW-sh/W-sh from The Jackson Laboratory (Bar Harbor, ME, USA). WT and lyn−/− mice (C57BL/6) [23] used for generating BMMC cultures and PDMCs were from The Jackson Laboratory and were bred at Taconic Farms (Germantown, NY, USA). PDMCs and BMMCs from EP2-deficient [24] and matched WT mice were kindly provided by Drs. M. Kovarova and B. H. Koller (Department of Medicine, University of North Carolina, Chapel Hill, NC, USA). Bone marrow progenitors were isolated from the tibias of 5- to 6-week-old C57BL/6 mice and grown in culture for 4–8 weeks, as described previously [21, 25]. For PDMC cultures, the peritoneal lavage from 5- to 6-week-old mice was cultured in RPMI media supplemented with 20% FBS, 20 ng/ml SCF, and 20 ng/ml IL-3 for a minimum of 12 days. BMMCs were grown in RPMI media supplemented with 10% FBS, 20 ng/ml SCF, and 20 ng/ml IL-3 as described previously [26]. FcεRI and c-Kit receptor expression was monitored by FACS analysis as described previously [25], and cells were used for experiments when 95% of the population was double-positive for these receptors. The C57.1 MC clone, originally derived from a BALB/c mouse [27], was kindly provided by Dr. Stephen J. Galli (Department of Pathology, Stanford University Medical Center, Stanford, CA, USA). C57.1 cells were grown in DMEM supplemented with 10% FCS, L-glutamine, and 2-ME. LAD2 HuMCs, a MC line derived from a patient with untreated MC sarcoma [28], and primary human peripheral blood-derived CD34+ cells [29] were kindly provided by Dr. A. Gilfillan (NIAMS, NIH). LAD2 cells were cultured in serum-free media (StemPro-34 serum-free medium, Invitrogen, Grand Island, NY, USA), supplemented with 2 mM L-glutamine, 100 U/ml penicillin, 50 μg/ml streptomycin, and 100 ng/ml SCF. CD34+-derived HuMCs were cultured in the same media as LAD2 cells supplemented with 100 ng/ml rhIL-6 (PeproTech, Rocky Hill, NJ, USA), and rhIL-3 (30 ng/ml) was added for the first week. One-half of the culture medium was replaced every 7 days. Cultures at 8–10 weeks consisted of >99% HuMC. 293LTV cells used for viral production (Cell Biolabs, San Diego, CA, USA) were cultured in DMEM media supplemented with 10% FCS (Invitrogen, Grand Island, NY, USA).
PCA
Mice were passively sensitized with an i.d. injection of 75 ng DNP-specific mouse IgE (20 μl) in the right ear, whereas the contralateral ear was injected with 20 μl PBS as a negative control. After 24 h, mice were treated with an i.v. injection of 0.3 mg/kg EP2 agonist (Butaprost) or vehicle (100 μl PBS with 0.1% DMSO). Thirty minutes later, mice were challenged i.v. with 250 μg antigen (DNP-HSA) in PBS containing 1% Evans blue (100 μl). Mice were then killed with CO2, 30 min postantigen injection. The right and left ears were removed and minced and the Evans blue dye extracted with 700 μl formamide at 55°C for 2 h, and the absorbance of Evans blue was determined at 620 nm. Some of the mice used for these experiments were MC-deficient mice (KitW-sh/W-sh), reconstituted or not i.d. with PDMC. In brief, the left and the right ears of 5-week-old KitW-sh/W-sh mice were injected with 5 × 106 PDMC (>98% positive for FcεRI and cKit receptor expression), which had been washed and resuspended in PBS (maximum volume of 100 μl/ear). After 6 weeks for dermal engraftment of MCs [30], mice were subjected to PCA.
Quantitative real-time PCR for expression of the EP2
RNA was extracted from the various types of murine MC and HuMC lines (2×106 cells) using the RNeasy Mini Kit with on-column DNAse treatment (Qiagen, Valencia, CA, USA), and the expression of EP1–4 mRNA in these samples was determined by real-time PCR using TaqMan gene expression assays (Applied Biosystems, Carlsbad, CA, USA). The gene expression assays for EP1–4 in the mouse were, respectively, Mm00443097_m1, Mm00436051_m1, Mm0.1316856_m1, and Mm00436053_m1, whereas those for the human were Hs00168752_m1, Hs00168754_m1, Hs00168755_m1, and Hs00168761_m1. As an endogenous control, we used the expression of mouse GAPDH and hGAPDH, respectively. Expression was calculated according to the comparative threshold method normalized to GAPDH expression.
β-Hexosaminidase release
Murine cells were sensitized with 1 μg/ml DNP-specific IgE and HuMCs with 100 ng/ml biotinylated hIgE for 2 h in SCF-free media. After sensitization, cells were washed and resuspended in HEPES buffer [10 mM HEPES (pH 7.4), 137 mM NaCl, 2.7 mM KCl, 0.4 mM Na2HPO4-7 H2O, 5.6 mM glucose, 1.8 mM CaCl2, and 1.3 mM MgSO4] with 0.04% BSA (Sigma-Aldrich). Cells were distributed in the wells of a V-bottom 96-well plate (50,000 murine MCs/well or 30,000 HuMCs/well) and treated with 10−5 M Butaprost, 10−5 M PGE2, or vehicle (PBS with 0.1% DMSO) for 15 min at 37°C. The concentration of Butaprost chosen was based on the previously reported [13, 15] inhibitory concentration for MCs and from our analysis of the effectiveness of various concentrations in inhibiting degranulation of various types of MCs expressing detectable levels of EP2. Where indicated, cells were pretreated at 37°C with 1 mM Rp-cAMP (a PKA antagonist) or 30 μm forskolin as positive control for 1 h prior to the addition of Butaprost. Murine or HuMCs were then stimulated, respectively, with 50 ng/ml antigen (DNP-HSA) or 10 ng/ml antigen (strepavidin) for 30 min at 37°C. The enzymatic activity of the granule marker β-hexosaminidase, released to the extracellular media, was measured as described [25] from the supernatants and is expressed as a percent of the total activity present in the cells.
Immunoprecitipitation and immunoblots
To determine expression of EP2, murine MCs (2×106 cells) were lysed in lysis buffer (borate-buffered saline containing 60 mM octyglucoside, 1% v/v Triton X-100, 1% v/v Thermo Halt protease, and phosphatase inhibitor cocktail, 100×, 5 mg/ml Pepstatin A, and 2 mM PMSF) and incubated on ice for 20 min. Lysates were cleared by centrifugation at 14,000 g for 20 min at 4°C, and the protein concentration was determined by the BCA protein assay (Thermo Fisher, Waltham, MA, USA). Tris-glycine sample buffer (Invitrogen, Grand Island, NY, USA) was added 1:1 to lysates (which contained equal protein amounts), and they were boiled for 3 min. Lysates from HuMCs (1×106 cells) were prepared as described [31]. Proteins were resolved by electrophoresis on 12% NuPAGE Tris-glycine gels (Invitrogen) and transferred to nitrocellulose membranes. In experiments testing the effects of Butaprost on total tyrosine or Akt phosphorylation, MCs were first placed in SCF-free media overnight and then sensitized with 1 μg/ml DNP-specific mouse IgE in HEPES buffer containing 0.04% BSA for 2 h. Cells were subsequently washed and treated with 10−5 M Butaprost or vehicle (PBS with 0.1% DMSO) for 15 min a 37°C and then stimulated with 50 ng/ml antigen (DNP-HSA) for 1, 3, and 9 min. The reaction was stopped by placing the tubes on ice. Cell lysates were prepared, and proteins were resolved in Trys-glycine gels and transferred to nitrocellulose membranes as described above. The membranes were probed with the corresponding primary antibodies or with antibody to Akt as a loading control and then washed, and the proteins were visualized with corresponding infrared-labeled secondary antibodies. Detected proteins were visualized with the use of an infrared imaging system (Odyssey, LI-COR Biosciences, Lincoln, NE, USA).
For Fyn immunoprecipitations, 2.5 × 107 PDMCs were sensitized with IgE and challenged with antigen and the cell pellets lysed as described above. Equal amounts of cell lysate protein were incubated with antibody to Fyn (8 μg/sample), prebound overnight to protein G-sepharose beads (Amersham Biosciences, Piscatawy, NJ, USA). After a 3-h incubation at 4°C, immunocomplexes were washed five times with lysis buffer, and protein was recovered by boiling in Tris-glycine SDS sample buffer that contained 1% 2-ME and 1 mM orthovanadate. Proteins were then resolved by SDS-PAGE and analyzed by Western blot.
Calcium mobilization measurements
For cytosolic calcium measurements, cells were sensitized overnight with IgE and loaded with 1 μM FURA-2 AM (Invitrogen) for 30 min. After washing, cells were resuspended in Tyrodes buffer [10 mM HEPES (pH 7.4), 137 mM NaCl, 2.7 mM KCl, 0.4 mM Na2HPO4, 5.6 mM glucose, 1.8 mM CaCl2, and 1.3 mM MgSO4 with 0.04% BSA] and divided into 96-well plates (30,000 cells/well). Cells were treated for 15 min with 10−5 M Butaprost, 10−5 M PGE2, or vehicle (0.1% DMSO+PBS) and challenged with antigen (25 ng/ml). Changes in intracellular calcium concentrations were monitored with a microplate fluorescence reader Wallac Victor 2 1420 multilabel counter (Perkin Elmer Life Sciences, Waltham, MA, USA) as emission of FURA-2 at 510 nm during fast excitation between 340 and 380 nm at 37°C. In indicated experiments, calcium was omitted from the incubation buffer, and following challenge with antigen (5 min), 1.8 mM CaCl2 was added to incubation buffer to assess the rate of extracellular calcium influx. Background fluorescence was determined in unlabeled cells. The values for background fluorescence at 340 nm and 380 nm were subtracted from the experimental fluorescence values at the respective wavelengths. The ratio (340/380) of the net fluorescence values of each measurement was determined and is reported.
Measurement of cAMP levels
PDMCs were resuspended in HEPES buffer at the concentration of 106 cells/ml and were pretreated with an inhibitor of the cAMP phosphodiesterase (IBMX; 1 mM) for 1 h to prevent degradation of cAMP. Afterward, cells were treated with 10−5 M Butaprost or vehicle (0.1% DMSO + PBS) for 15 min. As a positive control, cells were incubated for 1 h with 30 μm forskolin. Cells were then washed, and cell pellets were lysed by resuspension in 0.1 M HCl/0.1% Triton X-100 (107 cells/ml) plus two freeze-thaw cycles. cAMP in the cleared lysates was measured by ELISA (Sigma-Aldrich).
PKA shRNA construction and gene transduction
A lentiviral-based transduction system was used for shRNA-mediated gene silencing of the C-α catalytic subunit of PKA in PDMCs. Bacterial glycerol stocks of a shRNA clone for mouse PKA C-α were purchased from Sigma-Aldrich. The shRNA sequence used was TRCN0000012460. Nontarget shRNA (SHC002) was used as a negative control. 293LTV cells (Cell Biolabs) were cotransfected with 3.9 μg PKA shRNA vector and 39 μl lentiviral packaging mix (Sigma-Aldrich) using Lipofectamine 2000 (Invitrogen). The viral supernatants were collected 72 h post-transfection and concentrated by centrifugation at 20,000 g for 2 h. The viral pellet was resuspended in 1 ml culture medium and used to transduce 1 × 107 PDMCs. Two days post-transduction, cells were changed to virus-free medium and allowed to recover for 2 days. Subsequently, selection was started using 1.5 μg/ml puromycin (Sigma-Aldrich). Cells were kept in selection media for 1 week, at the end of which >98% of nontransduced cells were not viable. After a 2-day recovery in media without puromycin, cells were used for the experiments. Silencing of PKA C-α expression was demonstrated by Western blot.
Statistics
Statistical significance was determined using a two-tailed Student's t test, and statistical differences for different cell treatments during measurement of calcium responses were determined by two-way ANOVA, as indicated in the figure legends. A P value of <0.05 was considered significant. Data are shown as mean ± sem.
RESULTS
Effect of PGE2 and of a selective EP2 agonist on MC degranulation
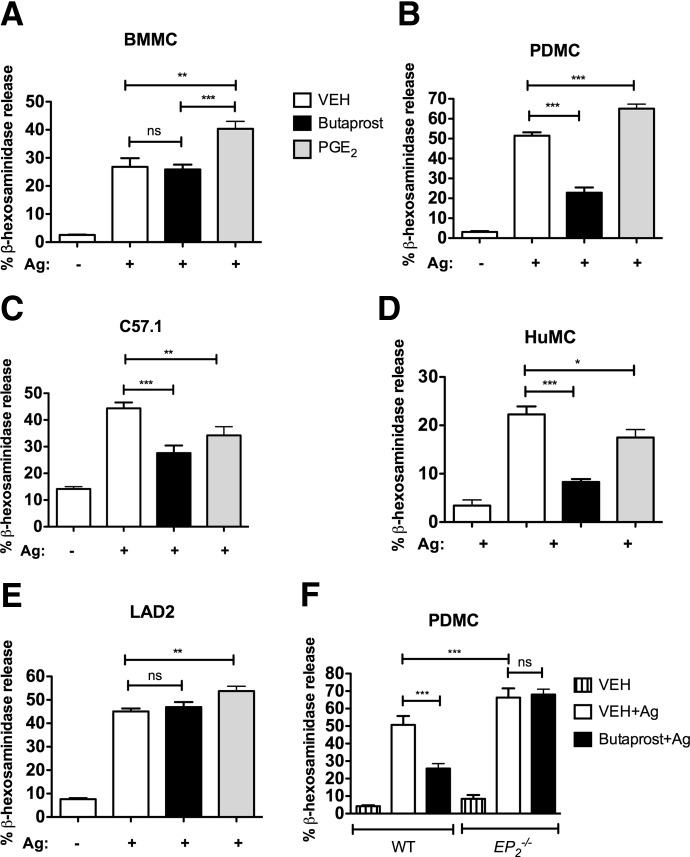
Stimulation of EPRs by PGE2 has been described to enhance FcεRI–mediated MC degranulation via EP3 [14]. However, the positive effects of PGE2 seem to vary depending on the MC type [14, 15, 19, 20]. In our hands, PGE2 caused a >30% enhancement in β-hexosaminidase release from BMMC (Fig. 1A), an ∼25% enhancement from PDMC (Fig. 1B), and 16% for LAD2 cells (Fig. 1E). In contrast, PGE2 dampened β-hexosaminidase release by >25% in the murine MC line C57.1 (Fig. 1C) and in CD34+-derived HuMCs (Fig. 1D). Previous studies have shown that PGE2-binding EP2 inhibited FcεRI-mediated PGD2 and TNF production, decreased COX expression [14], and reduced lung HuMC activity and migration [13, 15]. Thus, we first confirmed the described inhibitory effect of EP2 engagement on MC degranulation [15]. Selective activation of EP2 by the agonist Butaprost in PDMC and in the MC line C57.1 reduced FcεRI-induced MC degranulation by 60% and 30%, respectively (Fig. 1B and C). Similarly, degranulation of CD34+-derived HuMC was reduced by Butaprost treatment by >50% (Fig. 1D). However, Butaprost had no effect on BMMC (Fig. 1A) and LAD2 cells (Fig. 1E). As shown in Fig. 1F, the inhibitory effect of Butaprost in PDMC was not observed in cells obtained from EP2-null mice, demonstrating that Butaprost inhibits FcεRI-mediated degranulation via the selective activation of EP2 and not another unknown target. Our results, together with results reported previously [13–15, 19, 20, 32], indicate that PGE2 can potentiate (likely through EP3) or suppress MC degranulation, and selective activation of EP2 can mediate this latter response.
Figure 1. The effects of PGE2 and the selective EP2 agonist Butaprost on FcεRI-induced MC degranulation vary depending on the MC type.
Murine cells (A–C and F) were sensitized with 1 μg/ml DNP-specific IgE and HuMCs (D and E) with 100 ng/ml biotinylated hIgE. Cells were treated with 10−5 M Butaprost, 10−5 M PGE2, or vehicle (VEH; PBS with 0.1% DMSO) for 15 min and stimulated or not with 50 ng/ml antigen (DNP-HSA; murine cells) or 10 ng/ml antigen (strepavidin; human cells), as described in Materials and Methods. β-Hexosaminidase activity released to the extracellular media after 30 min was measured using a colorimetric assay. PGE2 enhanced MC degranulation in BMMC (A), PDMC (B and F), and LAD2 (E) and inhibited MC degranulation in C57.1 cells (C) and HuMCs (D). The inhibitory effect of Butaprost in PDMC was not observed in PDMCs derived from the EP2-deficient mouse (F). Results are representative of at least four independent experiments done in triplicates and reported as mean ± sem (*P<0.05; **P<0.01; ***P<0.001).
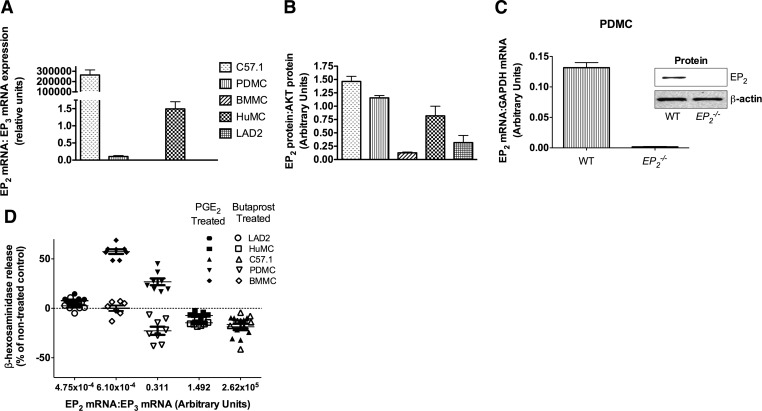
The ratio of EP2 to EP3 expression determines the positive or negative effect of PGE2 on MC degranulation
In agreement with the conclusion that EP2 dampens MC degranulation, we found that the relative expression of EP2 mRNA to EP3 mRNA markedly differed (Fig. 2A) in the various MC types and was to some extent predictive of the effects of Butaprost. BMMC and LAD2 cells had low expression of EP2, correlating with the lack of an effect of Butaprost on degranulation (Figs. 2A and B and 1A and E). C57.1 showed the highest ratio of EP2:EP3 mRNA levels followed by HuMCs and PDMCs (Fig. 2A). The protein expression of EP2 of C57.1 cells was also high relative to BMMCs or LAD2 cells but relatively similar to that of PDMCs or HuMCs (Fig. 2B). PGE2 treatment of IgE/antigen-stimulated C57.1 or HuMCs (which had the highest ratio of EP2:EP3 mRNA levels) caused some inhibition of degranulation (Fig. 1C and D), whereas cells having a lower ratio (BMMC, LAD2, and PDMC) showed some enhancement in degranulation (Fig. 1A, B, and E). Treatment with Butaprost showed a more pronounced inhibitory effect on HuMC (64%; Fig. 1D) and PDMC (49%; Fig. 1B) than in C57.1 cells (39%; Fig. 1C). As expected, EP2-null MCs had no detectable EP2 mRNA or protein, indicating the suitability of the probes and antibody to detect EP2 mRNA and protein expression (Fig. 2C), and showed no inhibitory effect on FcεRI-induced degranulation (Fig. 1F) upon Butaprost treatment.
Figure 2. Expression of EP2 in murine MCs and HuMCs and relationship between EP2:EP3 expression ratio and the effects of PGE2 and Butaprost on degranulation.
EP2 expression in murine MCs and HuMCs was measured at the mRNA level by quantitative RT-PCR (A) and at the protein level by Western blot (B). (A) Results are an average of four independent experiments done in duplicate. (B) Represents quantification of EP2 by Western blot of three independent experiments (mean±sem) normalized to total AKT protein as a loading control. mRNA (C) or protein (inset) for EP2 was not detected in the EP2−/− PDMCs. (D) Ratios of EP2:EP3 mRNA expression (x-axis) of various types of MCs with the corresponding effect of PGE2 (filled symbols) and Butaprost (open symbols) on degranulation. Data are reported as the percent change relative to the nontreated control response (all responses are shown as a baseline of “0”) for each cell type upon IgE/antigen stimulation. Data are shown for eight individual experiments with mean and sem.
Analysis of the relative expression of all of the other PGE2Rs in the different cell types revealed varied expression of these receptors depending on the cell type (Supplemental Fig. 1). In all MC types, no mRNA for EP1 was found, and mRNA for EP2, EP3, and EP4 was abundant, but the relative abundance of EP2 to EP3 was most consistent with the variable effect of PGE2 (negative or positive, respectively) on MC degranulation in the various types of MCs studied (Fig. 2D). Thus, in MCs that had a low EP2:EP3 ratio (i.e., LAD2 and BMMC), Butaprost was ineffective, whereas PGE2 had minimal effect or enhanced degranulation (Fig. 2D). In contrast, in MCs where the EP2:EP3 ratio was higher (i.e., C57.1 cells and HuMCs), Butaprost and PGE2 showed an inhibitory effect on degranulation (Fig. 2D). In PDMCs, which express similar mRNA levels of EP3 as BMMCs but unlike BMMCs, also express EP2, PGE2 and Butaprost showed opposing effects on degranulation (i.e., stimulatory and inhibitory, respectively; Fig. 2D). This provided the opportunity to test whether antagonism of EP3 in these cells would shift the stimulatory effect of PGE2 on IgE/antigen-dependent PDMC degranulation to an inhibitory effect. As shown in Supplemental Fig. 2, antagonism of EP3 on PDMCs with the selective inhibitor L826266 resulted in the inhibition of the enhancing effect of PGE2 at optimal concentrations. This reversal of the stimulatory effect of PGE2 on IgE/antigen-dependent degranulation to an inhibitory one was observed at concentrations of PGE2 that induced partial or optimal enhancement of degranulation when EP3 was not antagonized. Interestingly, EP2-null PDMCs, which expressed ∼1.5-fold more EP3 mRNA than their WT counterparts (Supplemental Fig. 1F), showed an approximate increase of 25% in their degranulation relative to WT PDMCs (Fig. 1F), consistent with a role for EP3 in stimulating MC degranulation. This suggests a possible autocrine role for PGE2Rs in FcεRI-induced MC degranulation. Our findings show that varying ratios of the functionally opposing EP2 to EP3 appear to influence the effect of PGE2 on MC degranulation. This may explain the PGE2-suppressive [1, 2] or activating [33] effects reported during inflammation of the lung or skin, respectively.
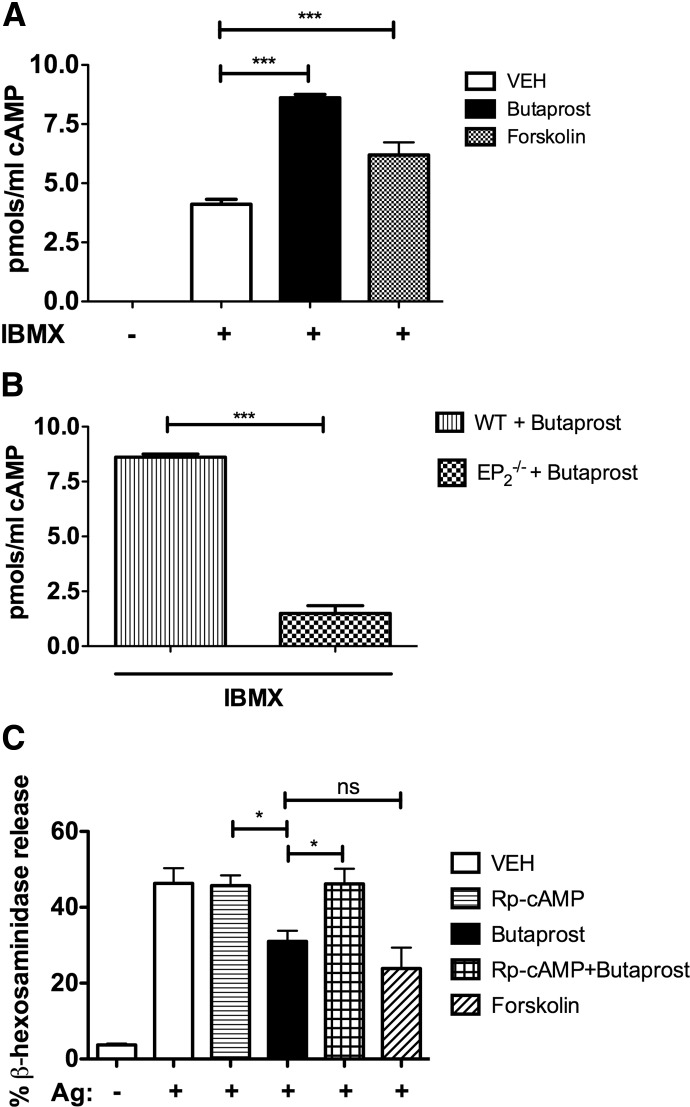
cAMP production by EP2 agonism suppresses MC degranulation
EP2 is known to couple to Gαs subunits and activate adenylate cyclase [31, 33, 34]. In agreement, Butaprost induced a twofold increase in the levels of intracellular cAMP in PDMC when compared with cells treated with vehicle alone (Fig. 3A). The effect of Butaprost was EP2-specific, as this treatment failed to induce cAMP production in EP2-null PDMCs (Fig. 3B). Forskolin, a direct activator of adenylate cyclase in MCs [35], caused a 1.5-fold increase in intracellular cAMP (Fig. 3A). Increased cAMP production has long been recognized to inhibit MC responses [36]; thus, we compared the effects of Butaprost-induced (EP2-mediated) with that of forskolin-induced (receptor-independent) cAMP production. As shown in Fig. 3C, forskolin inhibited FcεRI-induced MC degranulation by 50%, which was similar to the effect of Butaprost on MC degranulation (no significant difference was found). This indicated that increased levels of cAMP were key to the inhibitory effect, independent of how cAMP was generated. To explore whether this inhibition was mediated through cAMP-induced activation of cAMP-dependent kinases, PDMCs were pretreated with an antagonist of cAMP (Rp-cAMP), which is an effective, competitive inhibitor of cAMP-dependent PKA in MCs [35]. Whereas Rp-cAMP pretreatment of FcεRI-stimulated PDMCs had no marked effect, this compound completely reversed the inhibitory effect of Butaprost on MC degranulation (Fig. 3C). Collectively, the findings demonstrate that EP2-induced cAMP-dependent signaling is the basis for the observed suppression of MC degranulation.
Figure 3. Involvement of cAMP formation in the suppressive effect of Butaprost on MC degranulation.
PDMCs from WT (A and B) or EP2-deficient mice (B) were pretreated with 1 mM IBMX (a phosphodiesterase inhibitor) for 1 h and treated for 15 min with 10−5 M Butaprost (A and B) or 1 h with 30 μm forskolin (A). cAMP accumulated in the cells was measured by ELISA. cAMP levels were not detectable in the absence of IBMX. (C) Treatment with the cAMP antagonist (Rp-cAMP; 1 mM) for 1 h prevented the suppressive effects of Butaprost on MC degranulation. Results are an average of three independent experiments from at least two independent cell cultures performed in duplicate, and data are expressed as mean ± sem (*P<0.05; ***P<0.001).
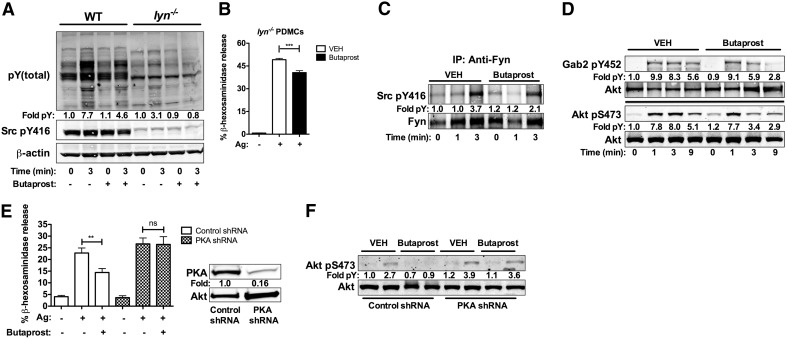
Butaprost suppresses Fyn-dependent signaling, and silencing of PKA restores signals and MC degranulation
We sought to uncover the mechanisms by which cAMP causes the inhibition of MC degranulation. The Src family tyrosine kinases, Fyn and Lyn, are activated rapidly upon FcεRI engagement in MCs and can phosphorylate multiple targets that propagate signals and mediate MC responses [26]. Butaprost treatment of PDMCs caused a modest reduction in pY proteins following FcεRI engagement and in the level of Y416 phosphorylation, which is associated with Src family kinase activity (Fig. 4A). Fyn kinase is an important regulator of MC degranulation [26] and contributes to the phosphotyrosine cascade; thus, we tested if the suppressive effect of Butaprost would be evident in PDMC from Lyn-null mice (cells in which Fyn was shown to drive degranulation dominantly) [26, 37]. Whereas pY was markedly reduced in Lyn-null PDMCs relative to WT cells (Fig. 4A), Butaprost further reduced the extent of phosphorylation, albeit without these differences achieving significance. Importantly, Butaprost still mediated considerable suppression of MC degranulation in Lyn-null PDMCs (Fig. 4B). This suggested that suppression of FcεRI-dependent MC degranulation by EP2 engagement was, at least in part, independent of Lyn. To explore if EP2 agonism could impinge on Fyn-dependent signaling, we first immunoprecipitated Fyn kinase from Butaprost-pretreated or untreated PDMCs following FcεRI-stimulation for 1 or 3 min. Butaprost treatment significantly inhibited (P<0.05) Fyn phosphorylation on the activation loop Y416 at 3 min poststimulation (Fig. 4C) but had no effect on the phosphorylation of the inhibitory Y508 (data not shown). Consistent with a dampening of Fyn activity, Butaprost pretreatment also caused a reduction in the phosphorylation of other signaling proteins downstream of Fyn, namely, the adaptor Gab2 and the survival/proliferation-promoting kinase Akt (Fig. 4D). These findings demonstrate that EP2 agonism causes a reduction in Fyn-dependent signaling in PDMCs. This is consistent with the previous findings of an essential role for Fyn-dependent signals in MC degranulation [26].
Figure 4. Fyn kinase signaling by IgE/antigen is inhibited by Butaprost, and silencing PKA restores signals and MC degranulation.
(A) Butaprost treatment reduced the general pY induced by FcεRI as well as phosphorylation of Src kinases at Y416 for WT and lyn−/− PDMCs. Numbers indicate the fold pY induction relative to WT PDMCs at 0 time. Arrowheads indicated pY bands. (B) Degranulation of lyn−/− PDMCs in response to IgE/antigen in the presence or absence of Butaprost was measured as in Fig. 1. Butaprost inhibited the degranulation of lyn−/− PDMCs. (C) Fyn kinase was immunoprecipitated (IP) from IgE/antigen-stimulated PDMCs, treated or not with 10−5 M Butaprost. Immunoprecipitates were probed with antiphosphoY416 to determine potential changes in Fyn activity. Fold induction (pY) relative to vehicle-only treatment at Time 0 is shown. (D) Phosphorylation of Gab2 and AKT in PDMC, treated or not with 10−5 M Butaprost at the indicated times after stimulation with IgE/antigen. Fold induction relative to vehicle treatment at Time 0 is shown. (E) PKA C-α expression was silenced by lentiviral shRNA expression, and effect on degranulation was determined. Degranulation of PKA-silenced and nontarget control cells in the presence or absence of Butaprost following IgE/antigen stimulation. Inset shows a representative immunoblot demonstrating the extent of PKA silencing. (F) Silencing of PKA by lentiviral shRNA restores Akt phosphorylation following IgE/antigen stimulation in Butaprost-treated cells. Cells transduced with control or PKA shRNA were treated with Butaprost or vehicle and stimulated by IgE/antigen. Cell lysates were prepared, and the phosphorylation status of Akt (S473) was determined as a measure of Fyn pathway activity. Akt phosphorylation was restored when PKA was silenced (see E). Fold induction relative to vehicle nontarget-treated cells (0 min) is shown. All experiments used at least two independent cell cultures and were conducted at least three times. Western blot data are representative of a minimum of two individual experiments.
Recently, it has been reported that cAMP can negatively regulate Fyn activity through PKA-dependent regulation of Csk in T cells [38]. To test if the inhibitory effect of Butaprost on IgE/antigen-dependent signaling could be mediated through the cAMP-dependent PKA, we silenced its C-α catalytic subunit using lentiviral-based shRNA. Silencing of PKA C-α was efficient with a 84% reduction in protein expression (Fig. 4E). Importantly, silencing of PKA caused a complete reversal of the inhibitory effect of Butaprost on β-hexosaminidase release (Fig. 4E), similar to our findings of cAMP antagonism (Fig. 3C). To assess if IgE/antigen-dependent signaling was no longer dampened upon silencing of PKA, we explored the phosphorylation state of Akt, which we previously showed to be a highly sensitive measure for Fyn-dependent signaling [26]. As shown in Fig. 4F, silencing of PKA C-α reversed the inhibitory effect of Butaprost on Akt phosphorylation. Collectively, the findings demonstrate that EP2 engagement mediates PKA activation and suppression of FcεRI-induced IgE/antigen-dependent signals and degranulation.
Butaprost induces a reduction in extracellular calcium influx
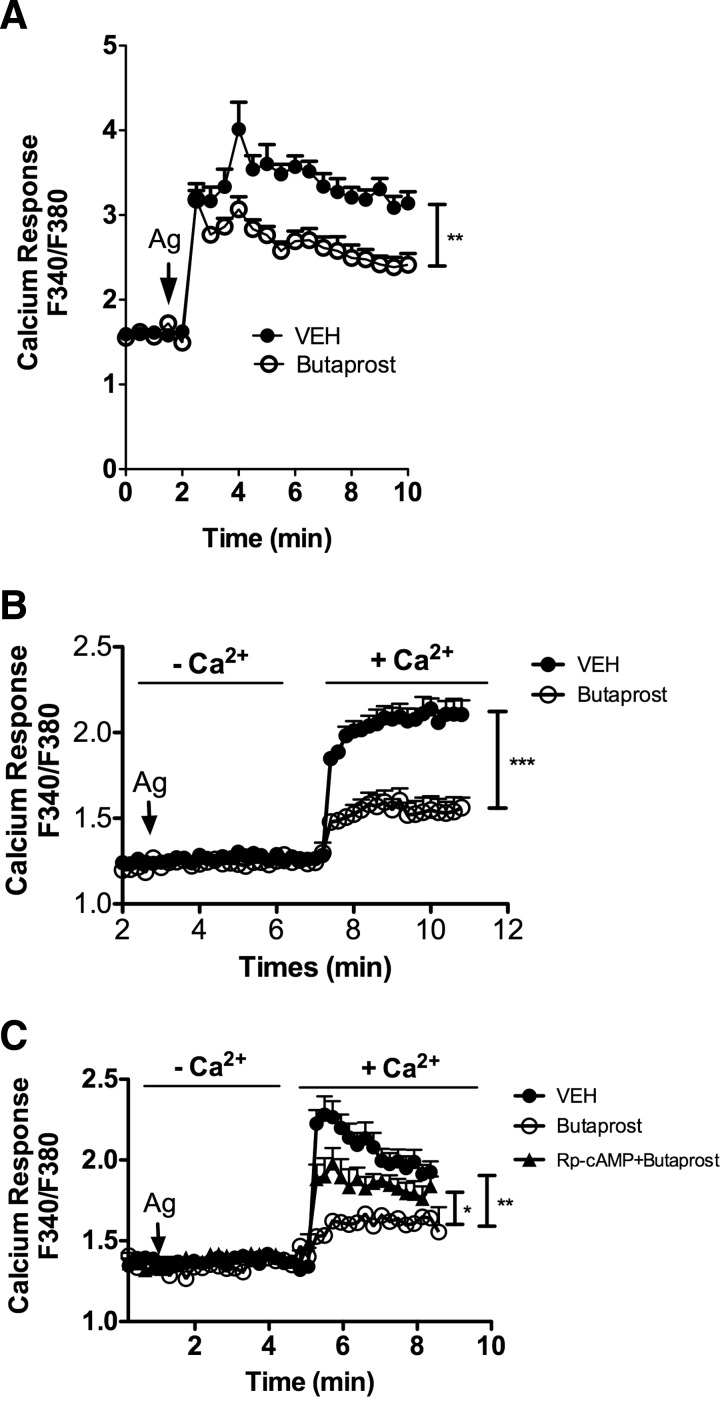
Given our prior findings [39] of a role for Fyn kinase in controlling calcium influx, we explored the effects of Butaprost on calcium responses, which are known to be important in promoting FcεRI-mediated MC degranulation [40]. Correlating with the inhibitory effect of Butaprost on degranulation, EP2 agonism considerably inhibited the intracytosolic FcεRI-induced calcium response in PDMCs (Fig. 5A) but not in BMMCs (Supplemental Fig. 2A), which do not express EP2 (Fig. 2A). To assess if the reduction in calcium mobilization was a consequence of impaired fluxes from intracellular stores or whether this resulted from a diminished influx of calcium from the extracellular medium, cells were first activated in calcium-free media, and then calcium was replenished in the extracellular medium. This distinguishes the contributions of the intracellular stores to the rise in intracellular calcium levels from the extracellular influx of calcium. As shown in Fig. 5B, FcεRI stimulation in the absence of extracellular calcium did not elicit a detectable rise in the intracellular calcium concentration, a characteristic of PDMCs relative to other MC types. However, replenishment of the extracellular medium with calcium caused a marked elevation in the intracellular calcium concentration, and this was reduced markedly if the cells were pretreated with Butaprost (Fig. 5B). Similar results, albeit not as pronounced, were obtained in HuMCs (Supplemental Fig. 2B). To determine if the reduced calcium influx seen in Butaprost-treated MCs was a result of increased cAMP, we first treated PDMCs with Rp-cAMP, the antagonist of cAMP, followed by Butaprost treatment and subsequent FcεRI stimulation. The use of Rp-cAMP, which prevented the Butaprost-dependent suppression of PDMC degranulation (Fig. 3C), caused considerable restoration of calcium influx (Fig. 5C). This suggests that partial restoration of calcium influx may be sufficient for complete restoration of MC degranulation.
Figure 5. EP2 mediates inhibition of extracellular calcium influx.
(A) Intracytosolic calcium changes were monitored after IgE/antigen stimulation of FURA-2-loaded PDMCs. Changes are reported as the ratio of flouresence emitted upon excitation at 340 or 380 nm with emission captured at 510 nm for each time-point. Butaprost (10−5 M) or vehicle was added to cells 15 min before challenge with antigen. (B) Cells were stimulated with antigen in the absence of extracellular calcium. When indicated, CaCl2 was added to the media to promote calcium influx, and changes in intracytosolic calcium concentration were monitored as in A. (C) The cAMP antagonist Rp-cAMP partially restored the Butaprost-mediated inhibition of calcium influx. PDMCs were treated with 1 mM Rp-cAMP for 1 h prior to antigen stimulation, and experiment was performed as in B. Results are the average of six to eight independent experiments (*P<0.05; **P<0.01;***P<0.001).
Butaprost inhibits MC responses in vivo
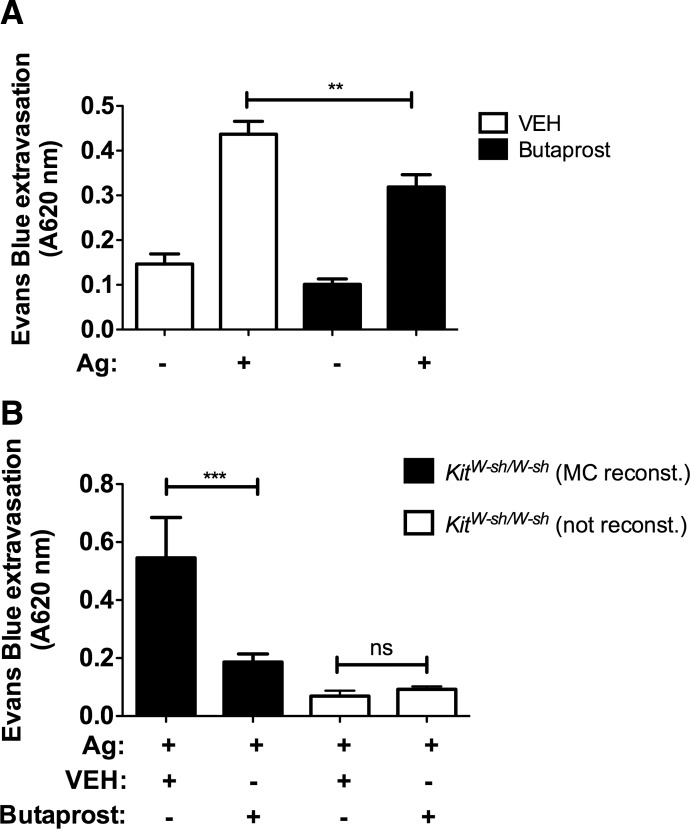
Given the in vitro inhibitory effects of Butaprost on MC degranulation, we set out to test the hypothesis that if manifested, the suppressive effects of EP2 in vivo required the presence of MCs. We chose a model of PCA, as it is a localized, MC-dependent response that is easily measurable by the increased vascular permeability at the site of challenge. WT mice, which were locally sensitized with DNP-specific IgE in the ear and subsequently challenged with DNP, showed a marked increase (approximately threefold) in vascular permeability (as measured by extravasation of Evans blue dye) as a result of MC-mediated release of vasoactive mediators (Fig. 6A). When mice were first treated with Butaprost (30 min prior to challenge) and were then subsequently challenged with DNP, vascular permeability was decreased by 30% (P<0.01; Fig. 6A). This demonstrated the suppressive effect of EP2 engagement in vivo. No significant effects were observed in the nonsensitized ears. MCs are required for the PCA response, as demonstrated by the lack of a response in the MC-deficient KitW-sh/W-sh mouse (Fig. 6B). Importantly, reconstitution of the skin of KitW-sh/W-sh with MCs and subsequent sensitization with DNP-specific IgE, followed by a DNP challenge, showed restoration of vascular permeability in the PCA model (Fig. 6B). Furthermore, treatment of MC-reconstituted KitW-sh/W-sh mice with Butaprost prior to the DNP challenge caused a marked reduction (>60%) in vascular permeability (Fig. 6B). These findings demonstrate that the selective agonism of EP2 in vivo (with Butaprost) required the presence of MCs and caused a dampening of vascular permeability, an important determinant of the severity of an allergic response.
Figure 6. Butaprost suppresses FcεRI-induced MC degranulation in vivo.
(A) An i.v. injection of Butaprost (0.3 mg/kg) prior to antigen (DNP-HSA) challenge (250 μg) prevented the skin hypersensitivity reaction induced by local sensitization (75 ng DNP-specific IgE/ear) and antigen activation of skin MCs, as measured by extravasation of Evans blue into the ears (n=10). (B) KitW-sh/ W-sh mice were reconstituted (reconst.) with PDMCs i.d. (ear), 6 weeks prior to the induction of PCA. Mice (n=4–5/condition) were then treated as in A (**P<0.01; ***P<0.001).
DISCUSSION
The multiple effects of PGE2 on allergic responses have long been enigmatic. It has been proposed that many cell types (DCs, eosinophils, regulatory T cells) may be targets for PGE2-driven effects. Nonetheless, PGE2-mediated modulation of MC function has been a focus of attention, and it was shown to promote MC degranulation and migration and induce leukotriene C4 production and cytokine secretion [19, 34]. On the other hand, other studies have demonstrated that PGE2 attenuates many of those MC responses [13–15]. There is increasing evidence [13–15, 19, 32] that this apparent dichotomy is related to the multiple EPRs expressed on MCs, although to date, this evidence has been incomplete. Herein, we present evidence demonstrating that the inhibitory versus stimulatory effects of PGE2 are related to the relative expression of EP2 to EP3 on the MC. Selective engagement of EP2 was found to dampen MC degranulation, and this effect was associated with its expression in a particular MC type. MCs, where EP2 expression was dominant (C57.1 cell and HuMC), showed an inhibition of FcεRI-induced degranulation to PGE2, whereas in those cells (PDMC, BMMC, and LAD2) where EP3 expression was more dominant, PGE2 enhanced this response. Selective engagement of EP2 by Butaprost also caused dampening of allergen-induced MC degranulation in vivo, and in the absence of EP2 expression (in MCs from EP2-null mice), suppression of MC degranulation by EP2 agonism was no longer observed. We also discovered that EP2-dependent activation of cAMP/PKA suppresses IgE/antigen-dependent signals and calcium influx, which is central for MC degranulation. These findings advance the understanding of how PGE2 can play a role in suppression and enhancement of allergic responses.
The beneficial effects of experimental administration of PGE2 in the relief of the symptoms of asthma in the human lung [1, 2] and in the reduction of HDM-induced airway hypersensitivity [6] in mice have been associated with decreased MC responsiveness. Although EP2-induced suppression appears to be part of the response to PGE2 in the lung, EP3-induced stimulation may predominate in other tissues such as the skin, where PGE2 mediates strong inflammatory responses [41]. Previous reports suggest that PGE2 can elicit different responses in MCs from different species [13, 14, 19], different sites (i.e., lung HuMCs vs. cord blood-derived [13–15], or even different donors [20]). Use of agonists or antagonists for the various PGE2Rs indicates that EP2 plays a role in suppression of lung HuMC degranulation [13, 15] and in dampening the production of TNF and eicosanoids in cord-derived HuMC [14]. In contrast, models in which the EP3 is genetically deleted link this receptor to the stimulation of MC early and late responses [14, 32]. Moreover, the competing signals of EP2 and EP3 also result in variable effects of PGE2 in MC migration depending on the MC type [13]. Thus, our formal demonstration that blocking of EP3 on PDMCs changes the effect of PGE2 treatment from stimulatory to inhibitory in an IgE/antigen-stimulated response and that the expression levels of EP2 and EP3 in distinct types of murine MCs and HuMCs are associated with the inhibitory or stimulatory effects of PGE2 in MC degranulation strongly supports the view that the relative levels of EP2:EP3 are central in determining the cells response to PGE2 treatment. Nonetheless, we cannot formally exclude the possibility that EP4, an inhibitory receptor in other immune cells [14, 42, 43], which is expressed to varying levels in MCs, may also contribute to the inhibitory effects of PGE2. However, this seems unlikely, as the levels of EP4 mRNA were found to be similar in BMMCs and PDMCs, and treatment of BMMCs with PGE2 elicited a greater enhancement of degranulation than observed for PDMCs (see Supplemental Fig. 1 and Fig. 1A and B).
Our findings contrast with a recent report suggesting that the heterogeneity of responses to PGE2 of CD34+-derived, cultured HuMCs from different donors results from differential coupling of PGE2Rs to signaling components rather than from differences in the ratio of EP2 to EP3 expression [20]. Although we agree that differences in signaling component coupling are likely to exist between different types of MCs, our findings suggest that the relative expression of these receptors is a key determinant in the observed differences in response. In our experiments, PGE2 and Butaprost inhibited allergen-induced degranulation in CD34+-derived, cultured HuMCs from at least three different donors, correlating with the higher expression of EP2 to EP3 at the mRNA level. In contrast, Kuehn et al. [20] found that PGE2 had a stimulating effect or no effect in MC responses, depending on the donor, and Butaprost showed no effect. The apparent discrepancy in these findings might result from the higher dose of PGE2 used in our study (10−5 M), a dose previously shown to elicit maximal inhibitory responses in lung HuMCs [15], which is in keeping with the lower affinity of EP2 (Kd>10 nM) when compared with EP3 (Kd<1 nM) [44]. The failure of Butaprost to cause inhibition of HuMC responses in the Kuehn et al. study [20] might also arise from the use of different chemical forms of Butaprost, which vary in their affinity for EP2 and in their biological activity [22]. We used a Butaprost with the stereochemical structure S from Cayman Chemicals that is known to be a more active form for EP2 than the stereochemical structure R from Sigma-Aldrich [22].
EP2 couples to Gαs, regulating intracellular cAMP production, whereas EP3 couples predominantly to Gαi, regulating PLCβ and PI3Kγ activities. EP2-mediated inhibition of MC [14], T cell, and other immune cell responses [45, 46] has been linked to cAMP production. Elevations in the intracellular levels of cAMP induced by other stimuli, including adenosine [45, 46], β-adrenoreceptor [47], and OX40-OX40L [35] have also been shown to dampen MC responses, although the mechanisms are not well understood. In agreement, we demonstrate that cAMP induced by EP2 activation is a negative regulator of allergen-induced MC immediate responses. cAMP exerts these effects via PKA, as antagonism of cAMP by an inactive cAMP analog or shRNA silencing of the catalytic subunit of PKA completely ablated the suppressive effects of Butaprost. In T cells, cAMP inhibits IL-2 production and T cell proliferation, and this negative regulation is also mediated by PKA, which is recruited to the immunological synapse in proximity with the TCR, where it phosphorylates Csk, which in turn, phosphorylates the C-terminal inhibitory tyrosine residue (Y517) in the Src kinases Lck and Fyn, preventing their activation and T cell responses [38]. In MCs, two Src family tyrosine kinases, Lyn and Fyn, associate with the FcεRI and are key for receptor phosphorylation (Lyn) and the initiation of phosphorylation cascades (Lyn and Fyn) that mediate MC responses [48]. Whereas we could partly eliminate a role for Lyn kinase in Butaprost-dependent suppression of MC degranulation, this treatment suppressed the pY of Fyn kinase activation loop Y396 (recognized by an antibody to Y416), induced upon FcεRI stimulation. Moreover, we observed that the phosphorylation of other molecular components requiring Fyn kinase activity, namely, the adaptor Gab2 and the survival/proliferation-inducing kinase Akt, was similarly reduced. This effect was PKA-dependent, as shRNA silencing of PKA prevented Butaprost inhibition of Akt phosphorylation, a sensitive readout for Fyn-dependent IgE/antigen-mediated signaling [26]. Unlike in T cells, we found no evidence for involvement of the negative regulator Csk, as treatment with Butaprost did not increase the phosphorylation of the C-terminal inhibitory tyrosine residue (Y508) of Fyn (data not shown), which reflects Csk activation. However, the negative effect of Butaprost on the Fyn activation loop Y396 and the restoration of FcεRI-dependent Akt phosphorylation (a Fyn-dependent event) in the presence of Butaprost by shRNA silencing of PKA indicate that IgE/antigen-mediated signals are a direct or indirect target of PKA. That Fyn itself may be a direct target for PKA is not surprising, as Fyn has multiple serine phosphorylation sites, and it has been reported that S21 phosphorylation of Fyn by PKA can enhance its activity [49]. Obviously, whether other S/T phosphorylation sites on Fyn may serve to dampen its activity and whether these may be targeted by PKA remain important questions to be addressed in the future. Nonetheless, recent studies [50] on the mechanisms by which OX40:OX40L interactions cause the suppression of IgE/antigen-dependent MC degranulation revealed that the increased cAMP/PKA activity initiated by such interactions also dampened responses through inhibiting Fyn/RhoA-dependent organization of microtubules in MCs.
An intriguing aspect of this study is the finding that EP2 activation reduced FcεRI-induced calcium influx without markedly affecting calcium mobilization from intracellular stores. Calcium is an important regulator of MC responses, and in particular, the absence of calcium influx ablates MC degranulation [39]. Our findings show that the suppression of calcium entry by Butaprost following FcεRI stimulation was at least in part through cAMP. This finding is consistent with the role of cAMP in reducing Fyn-dependent signals, as we previously found that calcium entry is markedly affected in Fyn-null MCs [39]. The findings of this previous work showed a role for Fyn kinase in the expression of the TRPC1. Interestingly, whereas reconstitution of Fyn-null MCs with Fyn completely restored calcium influx, reconstitution of TRPC1 channels in Fyn-null MCs caused only a partial restoration of calcium influx, indicating that Fyn kinase activity was important in promoting other modes of calcium influx. This view—of the existence of multiple modes that regulate calcium influx—is consistent with our findings that cAMP antagonism did not completely restore the dampened calcium influx caused by Butaprost engagement of EP2 (Fig. 5C). EP2 has been found to mediate the closure of the intermediate conductance calcium-activated K+ channel KCa3.1 in lung HuMCs [13] by a Gs-mediated mechanism that was cAMP-independent. Reduction in the activity of this channel inhibited FcεRI-dependent calcium influx and degranulation [51, 52]. Together, with the findings reported herein, one can conclude that EP2 can modulate calcium influx in a cAMP-dependent and independent manner.
In summary, our findings promote a further understanding of the mechanisms by which EP2 attenuates MC degranulation and allergic responses. The reproducibility of EP2-mediated suppression of lung HuMCs [13, 15] and of HuMC lines suggests that translational studies to explore polymorphisms or mutations associated with disease development or activity are warranted. The suppression of cutaneous anaphylaxis by EP2 agonism promotes its potential as a therapeutic target in allergic disease.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the Intramural Research Program of NIAMS of the U.S. National Institutes of Health and by a grant from Fondo de Investigación Sanitaria (Ref. PS09/00171) managed by the Instituto de Salud Carlos III of the Spanish Ministry of Health and by CIBERES. We are grateful for the support of the Flow Cytometry Section and the Laboratory Animal Care and Use Section of the Office of Science and Technology, NIAMS.
SEE CORRESPONDING EDITORIAL ON PAGE 1129
The online version of this paper, found at www.jleukbio.org, includes supplemental information.
- C57.1
- CI.MC/C57.1
- CSK
- C-terminal Src kinase
- EP1—4
- PGE1—4R
- HDM
- house dust mite
- HuMC
- human mast cell
- MC
- mast cell
- NIAMS
- National Institute of Arthritis and Musculoskeletal and Skin Diseases
- OX40L
- OX40 ligand
- PCA
- passive cutaneous anaphylaxis
- PDMC
- peritoneal-derived mast cell
- pY
- tyrosine phosphorylation
- rh
- recombinant human
- SCF
- stem cell factor
- shRNA
- small hairpin RNA
- TRPC1
- transient receptor potential channel type 1
AUTHORSHIP
All authors were involved in summarizing data and writing the manuscript, as well as the conceptual development of this work. M.S-P. and A.O. conducted experiments and generated necessary reagents and cells.
REFERENCES
- 1. Melillo E., Woolley K. L., Manning P. J., Watson R. M., O'Byrne P. M. (1994) Effects of inhaled PGE2 on exercise-induced bronchoconstriction and urinary LTC4 excretion in aspirin-sensitive asthmatics. Am. J. Respir. Crit. Care Med. 153, 572–575 [DOI] [PubMed] [Google Scholar]
- 2. Gauvreau G. M., Watson R. M., O'Byrne P. M. (1999) Protective effects of inhaled PGE2 on allergen-induced airway responses and airway inflammation. Am. J. Respir. Crit. Care Med. 159, 31–36 [DOI] [PubMed] [Google Scholar]
- 3. Pierzchalska M., Szabó Z., Sanak M., Soja J., Szczeklik A. (2003) Deficient prostaglandin E2 production by bronchial fibroblasts of asthmatic patients, with special reference to aspirin-induced asthma. J. Allergy Clin. Immunol. 111, 1041–1048 [DOI] [PubMed] [Google Scholar]
- 4. Perez-Novo C. A., Watelet J. B., Claeys C., Van Cauwenberge P., Bachert C. (2005) Prostaglandin, leukotriene, and lipoxin balance in chronic rhinosinusitis with and without nasal polyposis. J. Allergy Clin. Immunol. 115, 1189–1196 [DOI] [PubMed] [Google Scholar]
- 5. Roca-Ferrer J., Garcia-Garcia F. J., Pereda J., Perez-Gonzalez M., Pujols L., Alobid I., Mullol J., Picado C. (2011) Reduced expression of COXs and production of prostaglandin E(2) in patients with nasal polyps with or without aspirin-intolerant asthma. J. Allergy Clin. Immunol. 128, 66–72 [DOI] [PubMed] [Google Scholar]
- 6. Herrerias A., Torres R., Serra M., Marco A., Roca-Ferrer J., Picado C., de Mora F. (2009) Subcutaneous prostaglandin E(2) restrains airway mast cell activity in vivo and reduces lung eosinophilia and Th(2) cytokine overproduction in house dust mite-sensitive mice. Int. Arch. Allergy Immunol. 149, 323–332 [DOI] [PubMed] [Google Scholar]
- 7. Gavett S. H., Madison S. L., Chulada P. C., Scarborough P. E., Qu W., Boyle J. E., Tiano H. F., Lee C. A., Lagenbach R., Roggli V. L., Zeldin D. C. (1999) Allergic lung responses are increased in prostaglandin H synthase-deficient mice. J. Clin. Invest. 104, 721–732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Peebles R. S. J., Hashimoto K., Morrow J. D., Dworski R., Collins R. D., Hashimoto Y., Christman J. W., Kang K. H., Jarzecka K., Furlong J., Mitchell D. B., Talati M., Graham B. S., Sheller J. R. (2002) Selective cyclooxygenase-1 and -2 inhibitors each increase allergic inflammation and airway hyperresponsiveness in mice. Am. J. Respir. Crit. Care Med. 165, 1154–1160 [DOI] [PubMed] [Google Scholar]
- 9. Nakata J., Kondo M., Tamaoki J., Takemiya T., Nohara M., Yamagata K., Nagai A. (2005) Augmentation of allergic inflammation in the airways of cyclooxygenase-2 deficient mice. Respirology 10, 149–156 [DOI] [PubMed] [Google Scholar]
- 10. Torres R., Herrerias A., Serra M., Roca-Ferrer J., Pujols L., Marco A., Picado C., de Mora F. (2008) An intranasal selective antisense oligonucleotide impairs lung cyclooxygenase-2 production and improves inflammation, but worsens airway function, in house dust mite sensitive mice. Respir. Res. 9, 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Torres R., Pérez M., Marco A., Picado C., de Mora F. (2009) A cyclooxygenase-2 selective inhibitor worsens respiratory function and enhances mast cell activity in ovalbumin-sensitized mice. Arch. Bronconeumol. 45, 162–167 [DOI] [PubMed] [Google Scholar]
- 12. Harizi H., Juzan M., Pitard V., Moreau J. F., Gualde N. (2002) Cyclooxygenase-2-issued prostaglandin E(2) enhances the production of endogenous IL-10, which down-regulates dendritic cell functions. J. Immunol. 168, 2255–2263 [DOI] [PubMed] [Google Scholar]
- 13. Duffy S. M., Cruse G., Cockerill S. L., Brightling C. E., Bradding P. (2008) Engagement of the EP2 prostanoid receptor closes the K+ channel KCa3.1 in human lung mast cells and attenuates their migration. Eur. J. Immunol. 38, 2548–2556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Feng C., Beller E. M., Bagga S., Boyce J. A. (2006) Human mast cells express multiple EP receptors for prostaglandin E2 that differentially modulate activation responses. Blood 107, 3243–3250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kay L. J., Yeo W. W., Peachell P. T. (2006) Prostaglandin E2 activates EP2 receptors to inhibit human lung mast cell degranulation. Br. J. Pharmacol. 147, 707–713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Holgate S. T., Hardy C., Robinson C., Agius R. M., Howarth P. H. (1986) The mast cell as a primary effector cell in the pathogenesis of asthma. J. Allergy Clin. Immunol. 77, 274–282 [DOI] [PubMed] [Google Scholar]
- 17. Williams C. M., Galli S. J. (2000) The diverse potential effector and immunoregulatory roles of mast cells in allergic disease. J. Allergy Clin. Immunol. 105, 847–859 [DOI] [PubMed] [Google Scholar]
- 18. Chung K. F. (2005) Evaluation of selective prostaglandin E2 (PGE2) receptor agonists as therapeutic agents for the treatment of asthma. Sci. STKE 2005, pe47. [DOI] [PubMed] [Google Scholar]
- 19. Gomi K., Zhu F. G., Marshall J. S. (2000) Prostaglandin E2 selectively enhances the IgE-mediated production of IL-6 and granulocyte-macrophage colony-stimulating factor by mast cells through an EP1/EP3-dependent mechanism. J. Immunol. 165, 6545–6552 [DOI] [PubMed] [Google Scholar]
- 20. Kuehn H. S., Jung M. Y., Beaven M. A., Metcalfe D. D., Gilfillan A. M. (2011) Distinct PGE(2)-responder and non-responder phenotypes in human mast cell populations: “all or nothing” enhancement of antigen-dependent mediator release. Immunol. Lett. 141, 45–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Razin E. (1990) Culture of bone marrow-derived mast cells: a model for studying oxidative metabolism of arachidonic acid and synthesis of other molecules derived from membrane phospholipids. Methods Enzymol. 187, 514–520 [DOI] [PubMed] [Google Scholar]
- 22. Regan J. W., Bailey T. J., Pepperl D. J., Pierce K. L., Bogardus A. M., Donello J. E., Fairbairn C. E., Kedzie K. M., Woodward D. F., Gil D. W. (1994) Cloning of a novel human prostaglandin receptor with characteristics of the pharmacologically defined EP2 subtype. Mol. Pharmacol. 46, 213–220 [PubMed] [Google Scholar]
- 23. Alvarez-Errico D., Yamashita Y., Suzuki R., Odom S., Furumoto Y., Yamashita T., Rivera J. (2010) Functional analysis of Lyn kinase A and B isoforms reveals redundant and distinct roles in FcεRI-dependent mast cell activation. J. Immunol. 184, 5000–5008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tilley S. L., Audoly L. P., Hicks E. H., Kim H. S., Flannery P. J., Coffman T. M., Koller B. H. (1999) Reproductive failure and reduced blood pressure in mice lacking the EP2 prostaglandin E2 receptor. J. Clin. Invest. 103, 1539–1545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Saitoh S., Arudchandran R., Manetz T. S., Zhang W., Sommers C. L., Love P. E., Rivera J., Samelson L. E. (2000) LAT is essential for Fc(ε)RI-mediated mast cell activation. Immunity 12, 525–535 [DOI] [PubMed] [Google Scholar]
- 26. Parravicini V., Gadina M., Kovarova M., Odom S., Gonzalez-Espinosa C., Furumoto Y., Saitoh S., Samelson L. E., O'Shea J. J., Rivera J. (2002) Fyn kinase initiates complementary signals required for IgE-dependent mast cell degranulation. Nat. Immunol. 3, 741–748 [DOI] [PubMed] [Google Scholar]
- 27. Tsai M., Hunt J., Arm J. P., London C., Gurish M., Galli S. J. (1996) Cl.MC/C57.1 (C57) mouse mast cell line is of BALB/c origin and tumorigenic in BALB/c mice. FASEB J. 10, A1253 [Google Scholar]
- 28. Kirshenbaum A. S., Akin C., Wu Y., Rottem M., Goff J. P., Beaven M. A., Rao V. K., Metcalfe D. D. (2003) Characterization of novel stem cell factor responsive human mast cell lines LAD 1 and 2 established from a patient with mast cell sarcoma/leukemia; activation following aggregation of FcεRI or FcγRI. Leuk. Res. 27, 677–682 [DOI] [PubMed] [Google Scholar]
- 29. Kirshenbaum A. S., Kessler S. W., Goff J. P., Metcalfe D. D. (1991) Demonstration of the origin of human mast cells from CD34+ bone marrow progenitor cells. J. Immunol. 146, 1410–1415 [PubMed] [Google Scholar]
- 30. Hershko A. Y., Suzuki R., Charles N., Alvarez-Errico D., Sargent J. L., Laurence A., Rivera J. (2011) Mast cell interleukin-2 production contributes to suppression of chronic allergic dermatitis. Immunity 35, 562–571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kuehn H. S., Beaven M. A., Ma H. T., Kim M. S., Metcalfe D. D., Gilfillan A. M. (2008) Synergistic activation of phospholipases Cg and Cb: a novel mechanism for PI3K-independent enhancement of FcεRI-induced mast cell mediator release. Cell. Signal. 20, 625–636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nguyen M., Solle M., Audoly L. P., Tilley S. L., Stock J. L., McNeish J. D., Coffman T. M., Dombrowicz D., Koller B. H. (2002) Receptors and signaling mechanisms required for prostaglandin E2-mediated regulation of mast cell degranulation and IL-6 production. J. Immunol. 169, 4586–4593 [DOI] [PubMed] [Google Scholar]
- 33. Williams T. J., Lewis G. P. (1976) Proceedings: The pro-inflammatory activity of E-, A-, D- and F-type prostaglandins and analogues 16, 16-dimethyl-PGE2 and (15S)-15-methyl-PGE2 in rabbit skin; the relationship between potentiation of plasma exudation and local blood flow changes. Br. J. Pharmacol. 56, 341P–342P [PMC free article] [PubMed] [Google Scholar]
- 34. Kuehn H. S., Rådinger M., Brown J. M., Ali K., Vanhaesebroeck B., Beaven M. A., Metcalfe D. D., Gilfillan A. M. (2010) Btk-dependent Rac activation and actin rearrangement following FcεRI aggregation promotes enhanced chemotactic responses of mast cells. J. Cell Sci. 123, 2576–2585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gri G., Piconese S., Frossi B., Manfroi V., Merluzzi S., Tripodo C., Viola A., Odom S., Rivera J., Colombo M. P., Pucillo C. E. (2008) CD4+CD25+ regulatory T cells suppress mast cell degranulation and allergic responses through OX40-OX40L interaction. Immunity 29, 771–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Csaba G., Török O. (1977) The effect of cyclic AMP on the maturation and degranulation of mast cells. Acta Biol. Acad. Sci. Hung. 28, 153–156 [PubMed] [Google Scholar]
- 37. Odom S., Gomez G., Kovarova M., Furumoto Y., Ryan J. J., Wright H. V., Gonzalez-Espinosa C., Hibbs M. L., Harder K. W., Rivera J. (2004) Negative regulation of immunoglobulin E-dependent allergic responses by Lyn kinase. J. Exp. Med. 199, 1491–1502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mosenden R., Taskén K. (2011) Cyclic AMP-mediated immune regulation—overview of mechanisms of action in T cells. Cell. Signal. 23, 1009–1016 [DOI] [PubMed] [Google Scholar]
- 39. Suzuki R., Liu X., Olivera A., Aguiniga L., Yamashita Y., Blank U., Ambudkar I., Rivera J. (2010) Loss of TRPC1-mediated Ca2+ influx contributes to impaired degranulation in Fyn-deficient mouse bone marrow-derived mast cells. J. Leukoc. Biol. 88, 863–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Janiszewski J., Huizinga J. D., Blennerhassett M. G. (1992) Mast cell ionic channels: significance for stimulus-secretion coupling. Can. J. Physiol. Pharmacol. 70, 1–7 [DOI] [PubMed] [Google Scholar]
- 41. Goulet J. L., Pace A. J., Key M. L., Byrum R. S., Nguyen M., Tilley S. L., Morham S. G., Langenbach R., Stock J. L., McNeish J. D., Smithies O., Coffman T. M., Koller B. H. (2004) E-prostanoid-3 receptors mediate the proinflammatory actions of prostaglandin E2 in acute cutaneous inflammation. J. Immunol. 173, 1321–1326 [DOI] [PubMed] [Google Scholar]
- 42. Foudi N., Kotelevets L., Louedec L., Leséche G., Henin D., Chastre E., Norel X. (2008) Vasorelaxation induced by prostaglandin E2 in human pulmonary vein: role of the EP4 receptor subtype. Br. J. Pharmacol. 154, 1631–1639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Luschnig-Schratl P., Sturm E. M., Konya V., Philipose S., Marsche G., Fröhlich E., Samberger C., Lang-Loidolt D., Gattenlöhner S., Lippe I. T., Peskar B. A., Schuligoi R., Heinemann A. (2011) EP4 receptor stimulation down-regulates human eosinophil function. Cell. Mol. Life Sci. 68, 3573–3587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hata A. N., Breyer R. M. (2004) Pharmacology and signaling of prostaglandin receptors: multiple roles in inflammation and immune modulation. Pharmacol. Ther. 103, 147–166 [DOI] [PubMed] [Google Scholar]
- 45. Breyer M. D., Breyer R. M. (2001) G protein-coupled prostanoid receptors and the kidney. Annu. Rev. Physiol. 63, 579–605 [DOI] [PubMed] [Google Scholar]
- 46. Qian X., Zhang J., Liu J. (2011) Tumor-secreted PGE2 inhibits CCL5 production in activated macrophages through cAMP/PKA signaling pathway. J. Biol. Chem. 286, 2111–2120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Weston M. C., Peachell P. T. (1998) Regulation of human mast cell and basophil function by cAMP. Gen. Pharmacol. 31, 715–719 [DOI] [PubMed] [Google Scholar]
- 48. Gilfillan A. M., Rivera J. (2009) The tyrosine kinase network regulating mast cell activation. Immunol. Rev. 228, 149–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Yeo M. G., Oh H. J., Cho H. S., Chun J. S., Marcantonio E. E., Song W. K. (2011) Phosphorylation of Ser 21 in Fyn regulates its kinase activity, focal adhesion targeting, and is required for cell migration. J. Cell. Physiol. 226, 236–247 [DOI] [PubMed] [Google Scholar]
- 50. Sibilano R., Frossi B., Suzuki R., D'Inca F., Gri G., Piconese S., Colombo M. P., Rivera J., Pucillo C. E. (2012) Modulation of FcεRI-dependent mast cell response by OX40L via Fyn, PI3K, and RhoA. J. Allergy Clin. Immunol. doi: 10.1016/j.jaci. 2012.03.032 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 51. Duffy S. M., Lawley W. J., Conley E. C., Bradding P. (2001) Resting and activation-dependent ion channels in human mast cells. J. Immunol. 167, 4261–4270 [DOI] [PubMed] [Google Scholar]
- 52. Duffy S. M., Berger P., Cruse G., Yang W., Bolton S. J., Bradding P. (2004) The K1 channel IKCa1 potentiates Ca21 influx and degranulation in human lung mast cells. J. Allergy Clin. Immunol. 114, 66–72 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.