Systemic anti-Gr-1 antibody treatment fails to deplete hepatic MDSC.
Keywords: MDSC, liver, RB6-8C5, tolerance, inflammatory neutrophils
Abstract
Recent studies show that the liver is a preferred organ for the accumulation of MDSC. In this study, we examined the effect of systemic RB6-8C5 treatment on hepatic MDSC in tumor-bearing mice. EL4 tumor-bearing mice were injected i.p. with RB6-8C5, and hepatic, splenic, and blood MDSCs were analyzed by flow cytometry. Unexpectedly, hepatic MDSC remained in the liver, although RB6-8C5 completely eliminated them from the spleen and peripheral blood 24 h after treatment. Secondary antibody staining confirmed the presence of RB6-8C5-bound MDSC in the liver of mice with s.c. tumors. Similar observations were made in two other (colon and melanoma) tumor models. Whereas RB6-8C5 injection induced cell death of hepatic MDSC, as shown by Annexin V/7-AAD staining, these cells were replaced immediately, leading to a constant, increased frequency of hepatic MDSC. Adoptively transferred MDSC migrated preferentially to the liver after RB6-8C5 treatment, suggesting that hepatic MDSCs are reconstituted rapidly after depletion. Finally, hepatic MDSC remained immunosuppressive despite RB6-8C5 injection. Our study demonstrates that RB6-8C5 is not suitable for depletion of hepatic MDSCs and analysis of their function.
Introduction
MDSCs represent a heterogeneous group of immature myeloid cells comprising macrophages, monocytes, neutrophils, DCs, and myeloid progenitor cells at different developmental stages [1]. MDSCs were first described in tumor-bearing mice as CD11b+ and Gr-1+ cells with potent immune-suppressor function. MDSC can also be found in cancer-independent inflammatory settings such as murine models of chronic hepatitis B virus infection, autoimmune hepatitis, or colitis [2–4]. Murine MDSC can be divided into monocytic CD11b+Gr-1midLy6GnegLy6ChiCD49d+ MDSC and polymorphonuclear CD11b+Gr-1hiLy6G+Ly6Clow/midCD49dneg MDSC [5]. Human homologues have also been described [6].
Although an increase of MDSC in patients with tumor and infectious diseases has been known for many years [7–9], their potent immune-regulatory function has only been recognized recently. MDSCs have a strong, suppressive function on adaptive and innate immune responses. They block the proliferation and function of CD4+ and CD8+ T cells through several mechanisms, including the generation of ROS and NO, depletion of cystine or arginine from the microenvironment, and release of immune-suppressive cytokines [1, 10]. In addition to T cells, MDSCs also interact with other immune cells. MDSCs have been found to block NK cell cytotoxicity [11], induce the generation of Tregs [12], and modulate macrophages to immunosuppressive M2 phenotype [13]. MDSCs have been considered an important factor in helping tumors evade immune surveillance and promoting their progression. Therefore, strategies that target MDSCs are being tested for cancer treatment [14].
Increased numbers of MDSCs have been found in murine models of HCC and patients with primary HCC [12, 15, 16]. Furthermore, an increase in hepatic MDSC can be detected in mice with s.c. growing tumors of different origin as well as in mice with early preinvasive pancreatic neoplasias [17–19]. The accumulation occurs in the presence and absence of liver metastasis.
Hepatitis also triggers hepatic infiltration of CD11b+Gr-1+ cells. Increased frequencies of this cell population can be found in acute hepatitis induced by injection of an antibody specific for 4-1BB [20], cannabidiol, staphylococcal enterotoxin B [21], and Con A [22–24]. Anti-Gr-1 depletion was found to block liver protection of IL-6 in a 4-1BB-induced liver injury model [20], indicating an important role for CD11b+Gr-1+ cells in preventing excessive liver damage. However, it is not clear whether such cells are immunosuppressive MDSCs or represent inflammatory neutrophils, as both are Gr-1-positive.
Many approaches have been applied to eliminate MDSCs and to study their function. Different chemotherapeutic agents have been found to selectively decrease MDSCs and to restore CD8+ T cell function in tumor-bearing mice [25–27]. However, these drugs also target cells other than MDSCs that can potentially cause MDSC-independent effects. Antibody depletion represents an alternative method used by many investigators to specifically eliminate MDSCs from mice. RB6-8C5, an anti-Gr-1 mAb, is widely used for depletion of Gr-1+ MDSC, including hepatic MDSC by many investigators [16, 20, 22, 28, 29].
In this study, we examined the potential of RB6-8C5 to deplete hepatic MDSCs in tumor-bearing mice. Unexpectedly, our data showed that RB6-8C5 failed to deplete hepatic MDSC, whereas it efficiently eliminated CD11b+Gr-1+ cells from the spleen and peripheral blood. RB6-8C5 antibody injection increased the death rate of MDSCs, but these cells were substituted immediately with newly generated MDSCs, either from bone marrow or from peripheral hematopoesis. After injection of this antibody, very high numbers of hepatic RB6-8C5-bound MDSCs still remained in the liver and fully maintained their immunosuppressive function. Our results clearly indicate that RB6-8C5 cannot eliminate hepatic MDSC and therefore, should not be used to study the function of hepatic MDSC. Moreover, as this antibody can affect the cell viability, the results generated from studies using RB6-8C5 for the depletion of MDSCs have to be interpreted carefully.
MATERIALS AND METHODS
Tumor-bearing mouse model and anti-Gr-1 depletion
Female C57BL/6, B6-LY5.2/Cr, and BALB/c mice, 6–8 weeks of age, were obtained from NCI–Frederick (Frederick, MD, USA). Mice were injected s.c. with 1 × 106 EL4 lymphoma, B16-GM-CSF melanoma, or CT-26 colon carcinoma cells. Two to 3 weeks later, when tumors reached a maximum diameter of 15–20 mm, mice were injected i.p. with 200 μg RB6-8C5 antibody (Bio X Cell, West Lebanon, NH, USA) or a rat IgG2b isotype control (Bio X Cell). The endotoxin level in both antibodies was <0.5 European unit/mg using Limulus Amebocyte Lysate Chromogenic Endotoxin Quantitation Kit (Pierce, Thermo Fisher Scientific, Rockford, IL, USA). Twenty-four hours later or at experimentally designed time-points, mice were killed, and liver, spleen, and peripheral blood were harvested. All experiments were performed according to the institutional guidelines and approved by a NCI-Bethesda (Bethesda, MD, USA) Institutional Animal Care and Use protocol.
Cell preparation
Livers were removed immediately after mice were killed. After homogenization, debris was removed by passing through nylon mesh. Liver-infiltrating cells were isolated by isotonic Percoll centrifugation (850 g, 25 min). RBCs were lysed using ACK lysing buffer (Quality Biological, Gaithersburg, MD, USA). Splenocytes were isolated, as described previously. Peripheral blood was collected by heart puncture. Peripheral RBCs were removed by using ACK lysing buffer.
Flow cytometry
Cells were surface-labeled with indicated antibodies for 15 min at 4°C. Flow cytometry was performed on a BD FACSCalibur platform, and results were analyzed using FlowJo software, version 9.3.1.2 (Tree Star, Ashland, OR, USA). The following antibodies were used for flow cytometry analysis: anti-Gr-1-APC (clone RB6-8C5, BioLegend, San Diego, CA, USA), anti-Ly6C-APC (clone HK1.4, eBioscience, San Diego, CA, USA), anti-Ly6G-PE (clone 1A8, BD PharMingen, Franklin Lakes, NJ, USA), anti-CD11b-FITC (clone M1/70.15, ImmunoTools, Germany), goat anti-rat second antibody-biotin (BD PharMingen), streptavidin-PE (BD PharMingen), anti-CD45.1-biotin (clone A20, BD PharMingen), anti-CD45.2-PE (clone 104, BD Phar-Mingen), and anti-CD45-APC (clone 30-F11, eBioscience). Isotype controls were used as indicated. PI was used to exclude dead cells. Cell death and apoptosis were detected with Annexin V-PE (BD PharMingen) and 7-AAD (BD PharMingen) staining, according to the manufacturer's instructions. Cell proliferation was detected by Ki-67-PE (clone SolA15, eBioscience) staining.
In vitro suppression assay
The suppressive function of RB6-8C5-bound MDSCs was measured by their ability to inhibit the proliferation of antigen-specific CD8 T cells. CFSE-labeled splenocytes from OT-I mice were seeded at 2 × 105 cells/well in 96-well plates in the presence of 0.1 μg/ml OVA257–264 peptide. MDSCs were isolated by autoMACS using anti-CD11b magnetic beads (Miltenyi Biotec, Germany) from tumor-bearing mice treated with RB6-8C5 or isotype control. Secondary antibody staining confirmed that >90% of isolated hepatic MDSCs from RB6-8C5-treated mice were coated with RB6-8C5. MDSCs were added to CFSE-labeled OT-I splenocytes at indicated ratios. All experiments were performed in duplicates. Proliferation of CD8+ cells was analyzed after 48 h by flow cytometry.
Adoptive-transfer assay
Donor bone marrow-derived MDSCs were isolated from CD45.1 congenic C57BL/6 EL4 tumor-bearing mice by autoMACS using anti-CD11b magnetic beads. Flow cytometry confirmed that >85% of CD11b+ bone marrow cells were Gr1+. Two hundred micrograms of RB6-8C5 or isotype control antibody was injected into CD45.2 C57BL/6 EL4 tumor-bearing mice. After 24 h, 5 × 106 freshly isolated CD45.1 MDSCs were injected i.v. Three hours after cell transfer, mice were killed. The presence of CD45.1 donor cells was measured by flow cytometry.
Statistical analysis
Statistical analysis was performed with GraphPad Prism 5 (GraphPad Software, San Diego, CA, USA). Data are shown as the mean ± sem. One-tail Mann-Whitney test was used, and all P values <0.05 were considered to be significant.
RESULTS
Analysis of RB6-8C5 staining of murine MDSC
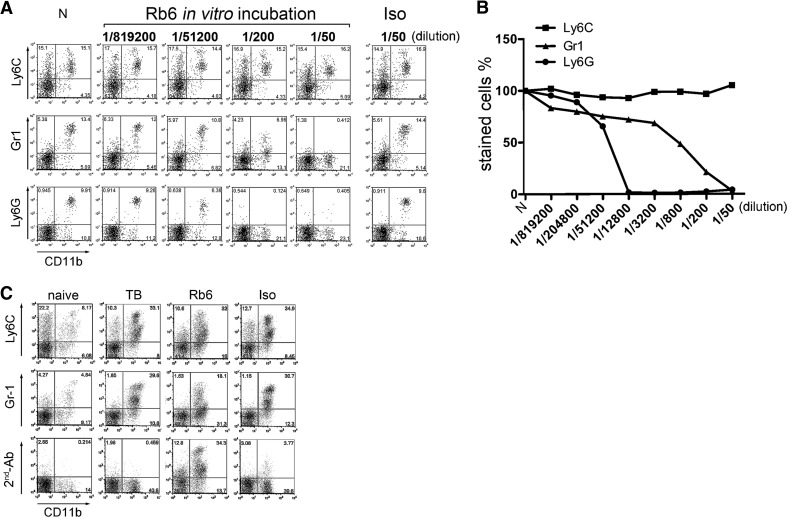
Murine MDSCs coexpress CD11b and Gr-1. Gr-1 antibody depletion (clone RB6-8C5) has been widely used in mice [2–5, 12]. The Gr-1 epitope on MDSC is represented by two molecules, Ly-6G and Ly-6C, which potentially allows costaining of cells with anti-Gr-1 and anti-Ly-6G or anti-Ly-6C. Therefore, we isolated splenocytes from tumor-bearing mice and incubated them with a different concentration of anti-Gr-1 in vitro. MDSCs were detected by flow cytometry using the following antibody combinations for staining: anti-CD11b plus anti-Ly-6C, anti-Ly-6G, or anti-Gr-1. As expected, MDSCs incubated with unlabeled anti-Gr-1 antibody could not be detected using the same antibody. Similarly, MDSCs were not detectable when MDSCs were stained with anti-Ly-6G. However, binding of RB6-8C5 had no effect on detection of MDSCs using anti-Ly6C (Fig. 1A and B).
Figure 1. Anti-Ly6C antibody stains RB6-8C5-bound MDSC.
Splenocytes from EL4 tumor bearing mice were isolated and preincubated with different concentration of RB6-8C5 antibody for 15 min and then stained with anti-CD11b-FITC plus anti-Ly6C-APC (HK1.4), anti-Ly6G-PE (1A8), or anti-Gr-1-APC (RB6-8C5), respectively. A rat IgG2b served as isotype control. N, Cells without antibody preincubation. Representative dot-plots from three independent experiments are shown in A, and competitive staining graph is shown in B. EL4 tumor-bearing mice were injected i.p. with 200 μg RB6-8C5 or isotype control. Two hours after treatment, mice were killed, and liver infiltrating cells were prepared and stained with Ly6C-APC, anti-Gr-1-APC (RB6-8C5), or a goat anti-rat IgG (2nd-Ab). Representative dot-plots from two independent experiments are shown in C. TB, Tumor-bearing mice without in vivo antibody treatment; Rb6, RB6-8C5-injected mice; Iso, rat IgG2b isotype antibody-injected mice.
Next, we analyzed the presence of hepatic MDSCs in tumor-bearing mice 2 h after i.p. injection of 200 μg RB6-8C5. As shown in Fig. 1C, the frequency of Ly6C+CD11b+ MDSCs was similar in tumor-bearing mice treated with RB6-8C5 or isotype control antibody (32% vs. 34.9%). Similar to the in vitro results, fewer cells were detected when MDSCs were stained using anti-Gr-1 (18.1% vs. 29.6% in isotype-treated mice). We further confirmed the presence of RB6-8C5-bound MDSCs in the liver by staining with anti-rat IgG-biotin, followed by Streptavidin/CD11b costaining. The frequency of double-positive cells (CD11b and anti-rat IgG) was similar (34.3%) to the frequency of Gr-1+CD11b+ or Ly6C+CD11b+ cells in untreated tumor-bearing mice (Fig. 1C), suggesting that all hepatic MDSCs were coated with RB6-8C5 after i.p. injection of 200 μg.
RB6-8C5 antibody depletes MDSC in spleen and peripheral blood but fails to deplete hepatic MDSC
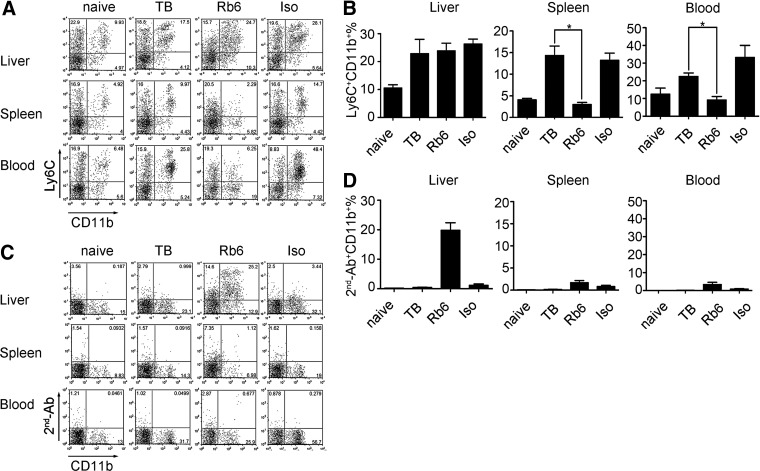
i.p. injection of RB6-8C5 has been used by many investigators to deplete MDSC [16, 20, 22, 28, 29], including hepatic MDSC [16, 20, 22, 28, 29]. We injected 200 μg RB6-8C5 i.p. into tumor-bearing mice in vivo. This dose was chosen based on our own results, as well as published data [16, 20, 22, 28, 29]. Twenty-four hours after treatment, MDSCs were analyzed at different sites using anti-Ly6C and anti-CD11b costaining. As expected, the frequency of MDSC was increased in tumor-bearing mice (9.97% vs. 4.92% in spleen, 25.8% vs. 6.48% in blood, and 17.5% vs. 9.93 in liver; Fig. 2A and B). Consistent with previous reports, RB6-8C5 injection completely eliminated the accumulated Ly6C+CD11b+ cells in spleen and peripheral blood, suggesting that the MDSC depletion was successful (Fig. 2A and B). Unexpectedly, the frequency of hepatic Ly6C+CD11b+ cells was not different in mice treated with RB6-8C5 or an isotype control (24.7% vs. 28.1%). This observation was confirmed by a second, independent analysis, when a costaining with anti-CD11b and anti-rat IgG was performed (Fig. 2C and D). As shown in Fig. 2C, whereas the frequency of cells detected by anti-CD11b and anti-rat IgG was only 1.12 (spleen) and 0.68 (blood), 25.2% of all hepatic-infiltrating immune cells stained positively, indicating the presence of RB6-8C5-bound hepatic MDSC. To rule out insufficient antibody dose as a cause of incomplete in vivo hepatic MDSC depletion, we repeated the experiments using 400 μg RB6-8C5 and obtained similar results (data not shown).
Figure 2. RB6-8C5 depletes MDSC in spleen and peripheral blood but not in liver of EL4 tumor-bearing mice.
EL4 tumor-bearing mice were injected i.p. with 200 μg RB6-8C5. Twenty-four hours later, mice were killed, and liver-infiltrating cells, splenocytes, and peripheral blood cells were prepared. MDSCs were detected by anti-Ly6C/anti-CD11b costaining. RB6-8C5-bound MDSCs were identified by staining with goat anti-rat IgG-biotin (2nd-Ab), followed by streptavidin/anti-CD11b costaining. A rat IgG2b antibody was injected as an isotype control. Representative dot-plots are shown in A and C. Cumulative results ± sem of four independent experiments are shown in B and D (naïve, n=4; TB, n=4; Rb6, n=7; Iso, n=7; *P<0.05).
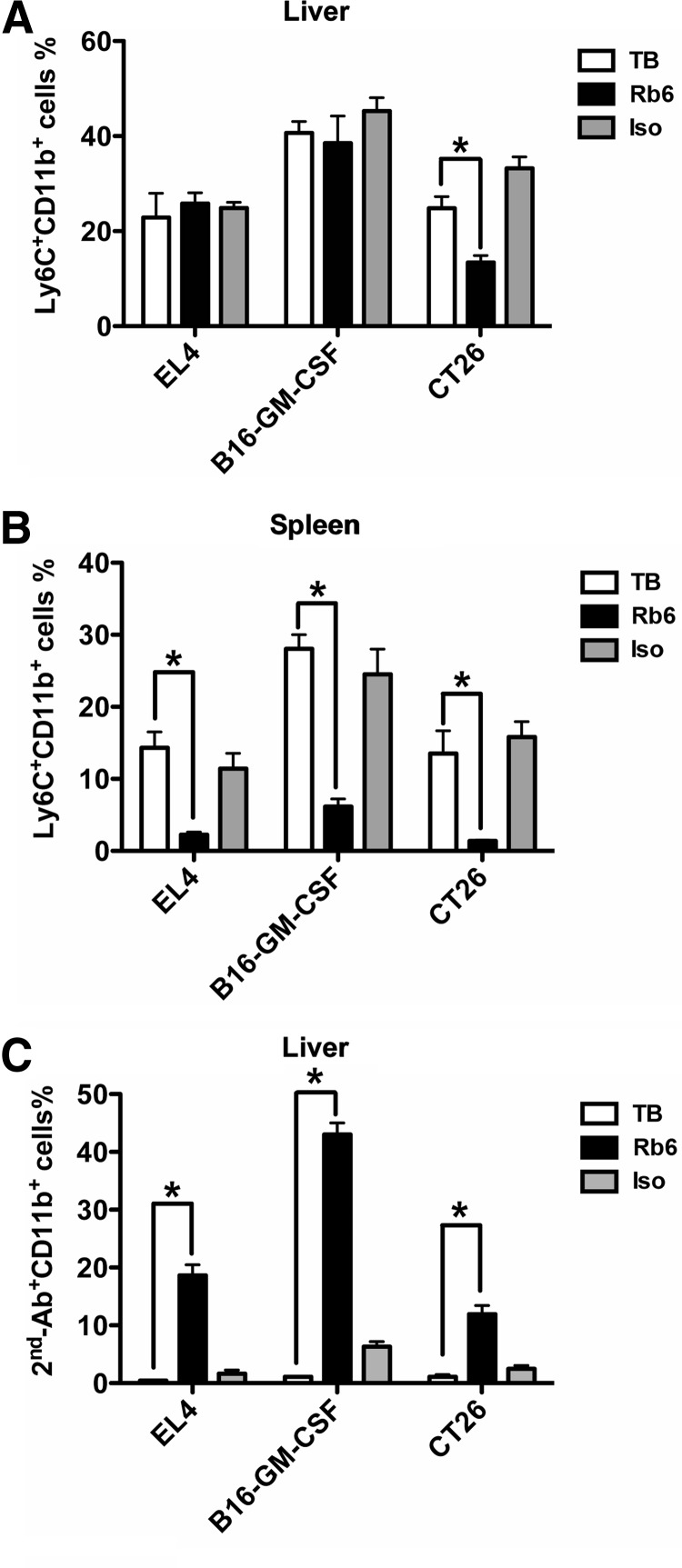
We examined further the effect of RB6-8C5 injection on hepatic MDSC in two other tumor models and in two different mouse strains—CT-26 colon carcinoma and B16 melanoma. As shown in Fig. 3 RB6-8C5 depletion had no effect on hepatic Ly6C+CD11b+ cell frequency (40.6% in tumor-bearing vs. 38.5% in RB6-8C5 mice) in mice with melanoma. In contrast, the number of Ly6C+CD11b+ cells was reduced in the spleen upon RB6-8C5 treatment (28.1% in tumor-bearing vs. 6.1% in RB6-8C5 mice). The existence of RB6-8C5-bound hepatic MDSC was confirmed by anti-rat IgG plus anti-CD11b costaining (43% in RB6-8C5 vs. 6.3% in isotype controls). In CT-26 tumor-bearing Balb/c mice, a significant number of hepatic Ly6C+CD11b+ cells were found after depletion (24.8% in tumor-bearing and 13.5% in RB6-8C5 mice). In contrast, splenic Ly6C+CD11b+ cells were eliminated completely (13.3% in tumor-bearing vs. 1.3% in RB6-8C5). RB6-8C5-bound MDSC also remained in the liver (11.9% in RB6-8C5 vs 2.5% in isotype controls). In summary, our data support that hepatic MDSCs remain in the liver after RB6-8C5 depletion.
Figure 3. Hepatic MDSCs resist RB6-8C5 depletion in B16-GM-CSF and CT-26 tumor model.
Mice-bearing EL4, B16-GM-CSF, or CT-26 tumor cells were injected i.p. with 200 μg RB6-8C5. Twenty-four hours later, mice were killed, and liver-infiltrating cells and splenocytes were prepared. MDSCs were detected by anti-Ly6C/anti-CD11b costaining (A and B). RB6-8C5-bound MDSCs were identified by staining with goat anti-rat IgG-biotin (2nd-Ab), followed by streptavidin/anti-CD11b costaining (C). A rat IgG2b antibody was injected as an isotype control. Cumulative results ± sem are shown (for EL4 tumor: TB, n=4; Rb6, n=7; Iso, n=7; for B16-GM-CSF tumor: TB, n=4; Rb6, n=4; Iso, n=4; for CT-26 tumor: TB, n=4; Rb6, n=6; Iso, n=6; *P<0.05).
RB6-8C5 antibody remains bound on the surface of hepatic MDSC for at least 4 days in vivo
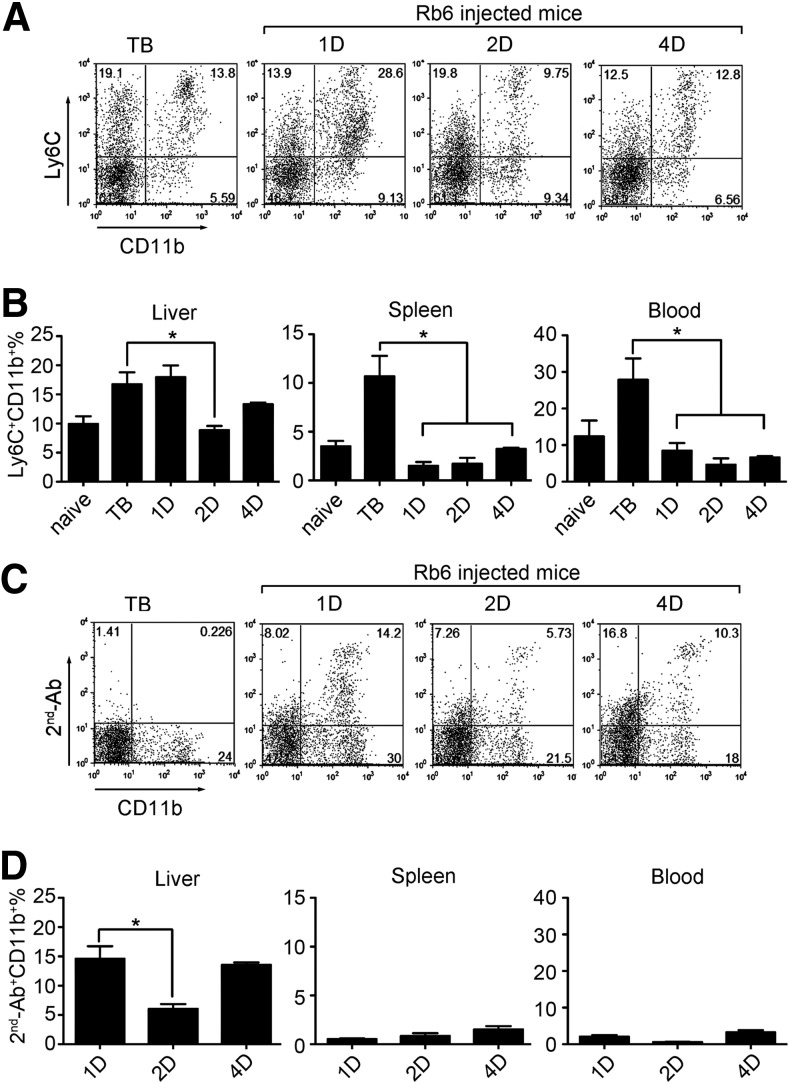
Our results clearly demonstrated the presence of hepatic MDSC 2 h after injection of RB6-8C5 in tumor-bearing mice. Next, we checked whether hepatic MDSCs could still be detected at later time-points and whether RB6-8C5 remained bound to their surface. For this, we analyzed hepatic MDSCs at 1, 2, and 4 days after RB6-8C5 treatment. As demonstrated in Fig. 4A and B, there was no difference in the frequency of hepatic MDSCs at 24 h. This number remained stable until Day 4, with a small drop after 2 days. In contrast, the frequency of Ly-6C+CD11b+ MDSCs in spleen dropped from 15% to 2%, 3%, and 4% on Days 1, 2, and 4, respectively, similar to the drop in peripheral blood from 30% to 8%, 3%, and 6%. Analysis of RB6-8C5-bound MDSCs using anti-rat IgG staining confirmed these findings (Fig. 4C and D). Whereas MDSC dropped to a very low level in peripheral blood and spleen, 1, 2, and 4 days after treatment, the frequency of hepatic MDSC remained stable with a small decrease 48 h after injection.
Figure 4. RB6-8C5 stays on hepatic MDSC surface for at least 4 days in vivo.
EL4 tumor-bearing mice were injected i.p. with 200 μg RB6-8C5. At different time-points after treatment (1D, 1 day; 2D, 2 days; 4D, 4 days), mice were killed, and liver-infiltrating cells, splenocytes, and peripheral blood cells were prepared. MDSCs in different compartments were stained as described above. Representative dot-plots of hepatic MDSCs are shown in A and C. Cumulative results ± sem are shown in B and D (naïve, n=3; TB, n=3; 1D, n=6; 2D, n=4; 4D, n=3; *P<0.05).
Cell death of hepatic MDSC is increased after depletion
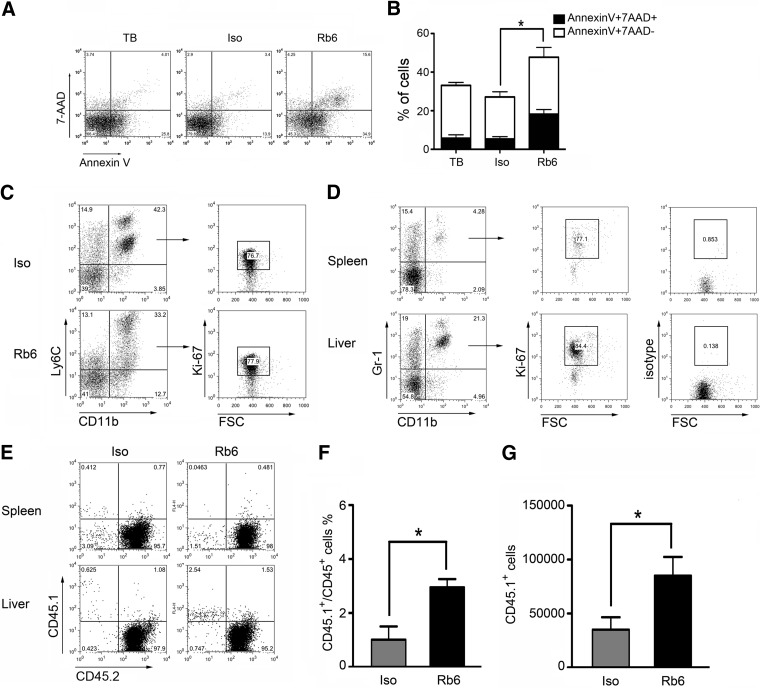
The small decrease in hepatic MDSC at 48 h prompted us to investigate the fate of hepatic MDSC upon RB6-8C5 injection in more detail. We stained RB6-8C5-bound hepatic MDSC with Annexin V and 7-AAD. As shown in Fig. 5A and B, the frequency of Annexin V-positive/7-AAD-negative and Annexin V-positive /7-AAD-positive cells was much higher after RB6-8C5 injection than in the isotype control-injected mice (36.4% and 18.2% in RB6-treated mice vs. 19.5% and 5.3% Annexin V/7-AAD+ cells in isotype controls). Whereas these data indicate that a portion of hepatic MDSCs dies following RB6-8C5 injection, the fact that viable MDSCs remain present in the liver also suggests that they are replaced rapidly. Indeed, we observed hepatic-proliferating MDSCs by Ki-67 staining (Fig. 5C), which was similar to results obtained from the livers and spleens of untreated mice (Fig. 5D).
Figure 5. RB6-8C5-bound hepatic MDSCs show higher cell death rate but are replaced rapidly by new MDSCs.
CT-26 tumor-bearing mice were injected i.p. with 200 μg RB6-8C5 or isotype control. Annexin V/7-AAD staining was used to detect cell death 24 h after injection. Hepatic MDSCs were identified by gating on Ly6C+CD11b+ cells. Representative dot-plots are shown in A. Cumulative results ± sem from two independent experiments are shown (B; TB, n=4; Iso, n=4; Rb6, n=4; *P<0.05). Cell proliferation of hepatic MDSC before and after RB6-8C5 depletion was detected by Ki-67 staining. Splenic MDSCs were used as a control. Representative dot-plots from two independent experiments are shown in C and D. FSC, Forward-scatter. (E–G) Adoptive transfer was performed to study MDSC migration to liver upon RB6-8C5 treatment. Donor CD45.1 bone marrow MDSCs were isolated as described in Materials and Methods. RB6-8C5 or isotype control antibody (200 μg) was injected into CD45.2 EL4 tumor-bearing mice, and 24 h later, 5 × 106 donor bone marrow-derived MDSCs were adoptively transferred. Three hours after cell transfer, mice were killed, and CD45.1 donor cells in liver were measured by flow cytometry. Representative dot-plots are shown in E. Cumulative results from two independent experiments (Iso, n=4; Rb6, n=4; *P<0.05) are indicated in F (relative numbers) and G (absolute numbers).
RB6-8C5 antibody treatment leads to enhanced migration of MDSC into the liver
To investigate whether hepatic MDSCs are reconstituted by local cell proliferation or by migration from the bone marrow, we adoptively transferred CD45.1 bone marrow MDSCs into EL4 tumor-bearing CD45.2 recipients after RB6-8C5 or isotype control treatment. The majority of transferred MDSCs migrated into the liver of mice treated with RB6-8C5 (Fig. 5E and F) as compared with isotype-treated controls. The calculation of the absolute number of donor cells confirmed the increase of MDSCs as well (Fig. 5G). Our results indicate that RB6-8C5 treatment enhances bone marrow MDSC accumulation into the liver and contributes to the quick reconstitution of the hepatic MDSC population.
RB6-8C5-bound MDSCs retain immunosuppressive function
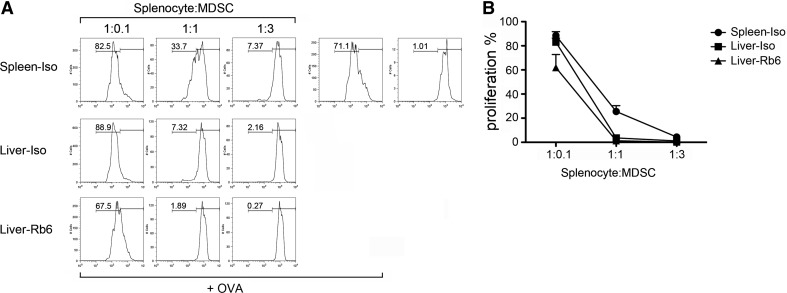
Finally, we analyzed the immunosuppressive function of hepatic MDSCs after RB6-8C5 injection. For this, MDSCs were isolated and added to antigen-stimulated OT-I splenocytes. As expected, the addition of splenic MDSCs from isotype-injected, tumor-bearing mice caused a dose-dependent reduction of CD8 T cell proliferation (Fig. 6A and B). Hepatic MDSCs from isotype antibody treatment also inhibited CD8 T cell proliferation. Unexpectedly, hepatic MDSCs suppressed T cell responses even stronger than splenic MDSCs. At a 1:1 ratio, hepatic MDSCs completely blocked CD8 T cell proliferation, whereas in the presence of splenic MDSCs, there was still 25% T cell proliferation. Finally, RB6-8C5-bound hepatic MDSCs exhibited a suppressive ability similar to nonbound hepatic MDSCs from the isotype treatment group at all three ratios tested (Fig. 6A and B).
Figure 6. RB6-8C5-bound hepatic MDSCs retain immunosuppressive function.
MDSCs from mice injected with RB6-8C5 or isotype antibody were isolated by autoMACS, as described in Materials and Methods. CFSE-labeled OT-I splenocytes were cocultued with splenic or hepatic MDSCs at indicated ratios. OVA peptide (0.1 μg/ml) was added to stimulate T cell proliferation. Two days later, proliferation of CD8 T cells was analyzed by flow cytometry. Representative flow cytometry histograms are shown in A. Cumulative results ± sem are from two independent experiments (B; TB, n=4; Iso, n=4; Rb6, n=4).
DISCUSSION
MDSCs are increased in tumor-bearing mice and have been shown to have a strong immune-suppressor function [1]. Increased numbers of MDSCs can be found at different sites in tumor-bearing mice, including spleen, liver, lung, blood, and bone marrow. The lack of specific markers for MDSCs makes it very challenging to perform experiments that would help us understand the function of these cells in vivo. Many investigators have used the Gr-1-specific antibody RB6-8C5 to deplete MDSCs in tumor-bearing mice [16, 19, 30]. Others have used chemotherapeutic agents [31] to target MDSCs or different compounds to reverse their effector function, such as iNOS or arginase [32, 33]. Alternatively, we have been able to demonstrate their function in adoptive-transfer experiments, in which effector cells were adoptively transferred together with MDSCs [4].
Recently, the liver has been found to be a preferred organ for MDSC accumulation in tumor-bearing mice [18, 19]. We have been able to confirm these findings, not only in tumor-bearing mice but also in mice with colitis (data not shown), indicating that the liver is a natural reservoir for MDSCs. Although the exact mechanism of hepatic MDSC accumulation remains unknown, the CCL2/CCR2 pathway seems to be responsible for this process [34, 35].
Antibody-mediated depletion of MDSCs using the RB6-8C5 antibody has been described by many investigators [16, 20, 22, 28, 29, 36], and in all of these cases, MDSC depletion was only verified in the peripheral blood but not in the liver.
We have used different staining protocols to demonstrate the presence of hepatic MDSC after RB6-8C5 injection. As expected, hepatic MDSCs could not be detected after RB6-8C5 injection when the same antibody was also used ex vivo. However, using anti-Ly6C, which recognizes a different epitope than RB6-8C5, as well as staining the RB6-8C5-bound cells with an anti-rat IgG secondary antibody, clearly indicated the presence of hepatic MDSCs after RB6-8C5 injection. In contrast, only a very low level of MDSC was detected in peripheral blood and spleen after RB6-8C5 injection in all three tumor models tested. An increased amount of RB6-8C5 (400 μg) used for depletion of hepatic MDSC did not improve depletion efficacy (data not shown). Together, these results demonstrate that RB6-8C5 fails to eliminate hepatic MDSC.
Our studies clearly demonstrate that hepatic MDSCs remain after RB6-8C5 depletion. Detailed analysis of MDSCs indicates only a slight decrease in frequency after 2 days with a full recovery of MDSCs after 4 days. Annexin V/7-AAD staining showed that hepatic MDSCs are susceptible to RB6-8C5-induced death. Interestingly, mice also show signs of liver damage at the same time (data not shown), and we are currently investigating this observation further.
The persistence of hepatic MDSCs, despite their increased death rate, indicates that there must be a mechanism to quickly replace dying MDSC in the liver, which does not exist in other organs. In support of this hypothesis, we have been able to find proliferating hepatic MDSC in the liver of RB6-8C5-treated mice by Ki-67 staining. Furthermore, preferential migration of MDSC into the liver was observed after depletion. In support of our findings, it has been reported that injection of RB6-8C5 antibody fails to deplete MDSC in bone marrow. Instead of eliminating MDSC, RB6-8C5 also remained on the surface of MDSC and induced cell expansion through STAT-1, STAT-3, and STAT-5 signaling [37].
Taken together, our results showed that anti-Gr-1 depletion by RB6-8C5 failed to effectively remove hepatic MDSC. RB6-8C5-bound MDSC retained immunosuppressive function. RB6-8C5 is not suitable to study hepatic MDSC in tumor-bearing mouse models, and other methods of MDSC depletion have to be used.
ACKNOWLEDGMENTS
This research was supported by the Intramural Research Program of the NCI, U.S. National Institutes of Health, and by the Initiative and Networking Fund of the Helmholtz Association within the Helmholtz Alliance on Immunotherapy of Cancer. We thank Austin Duffy for critical reading of this manuscript.
Footnotes
- 7-AAD
- 7-amino actinomycin D
- APC
- allophycocyanin
- HCC
- hepatocellular carcinoma
- MDSC
- myeloid-derived suppressor cell
- NCI
- National Cancer Institute
- RBC
- red blood cell
- Treg
- T regulatory cell
AUTHORSHIP
C.M. designed and performed the research, collected and analyzed data, and wrote the manuscript. T.K., J.G., M.P.M., and F.K. contributed toward analyzing the data and revising the manuscript. T.F.G. designed research, analyzed the data, and wrote the paper.
DISCLOSURES
The authors have no conflicting financial interests.
REFERENCES
- 1. Gabrilovich D. I., Nagaraj S. (2009) Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 9, 162–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chen S., Akbar S. M., Abe M., Hiasa Y., Onji M. (2011) Immunosuppressive functions of hepatic myeloid-derived suppressor cells of normal mice and in a murine model of chronic hepatitis B virus. Clin. Exp. Immunol. 166, 134–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hegde V. L., Nagarkatti P. S., Nagarkatti M. (2011) Role of myeloid-derived suppressor cells in amelioration of experimental autoimmune hepatitis following activation of TRPV1 receptors by cannabidiol. PLoS One 6, e18281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Haile L. A., von Wasielewski R., Gamrekelashvili J., Kruger C., Bachmann O., Westendorf A. M., Buer J., Liblau R., Manns M. P., Korangy F., Greten T. F. (2008) Myeloid-derived suppressor cells in inflammatory bowel disease: a new immunoregulatory pathway. Gastroenterology 135, 871–881, 881.e1–881.e5 [DOI] [PubMed] [Google Scholar]
- 5. Haile L. A., Gamrekelashvili J., Manns M. P., Korangy F., Greten T. F. (2010) CD49d is a new marker for distinct myeloid-derived suppressor cell subpopulations in mice. J. Immunol. 185, 203–10 [DOI] [PubMed] [Google Scholar]
- 6. Greten T. F., Manns M. P., Korangy F. (2011) Myeloid derived suppressor cells in human diseases. Int. Immunopharmacol. 11, 802–807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Almand B., Clark J. I., Nikitina E., van Beynen J., English N. R., Knight S. C., Carbone D. P., Gabrilovich D. I. (2001) Increased production of immature myeloid cells in cancer patients: a mechanism of immunosuppression in cancer. J. Immunol. 166, 678–689 [DOI] [PubMed] [Google Scholar]
- 8. Pak A. S., Wright M. A., Matthews J. P., Collins S. L., Petruzzelli G. J., Young M. R. (1995) Mechanisms of immune suppression in patients with head and neck cancer: presence of CD34(+) cells which suppress immune functions within cancers that secrete granulocyte-macrophage colony-stimulating factor. Clin. Cancer Res. 1, 95–103 [PubMed] [Google Scholar]
- 9. Young M. R., Newby M., Wepsic H. T. (1987) Hematopoiesis and suppressor bone marrow cells in mice bearing large metastatic Lewis lung carcinoma tumors. Cancer Res. 47, 100–105 [PubMed] [Google Scholar]
- 10. Bronte V., Mocellin S. (2009) Suppressive influences in the immune response to cancer. J. Immunother. 32, 1–11 [DOI] [PubMed] [Google Scholar]
- 11. Hoechst B., Voigtlaender T., Ormandy L., Gamrekelashvili J., Zhao F., Wedemeyer H., Lehner F., Manns M. P., Greten T. F., Korangy F. (2009) Myeloid derived suppressor cells inhibit natural killer cells in patients with hepatocellular carcinoma via the NKp30 receptor. Hepatology 50, 799–807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hoechst B., Ormandy L. A., Ballmaier M., Lehner F., Kruger C., Manns M. P., Greten T. F., Korangy F. (2008) A new population of myeloid-derived suppressor cells in hepatocellular carcinoma patients induces CD4(+)CD25(+)Foxp3(+) T cells. Gastroenterology 135, 234–243 [DOI] [PubMed] [Google Scholar]
- 13. Sinha P., Clements V. K., Bunt S. K., Albelda S. M., Ostrand-Rosenberg S. (2007) Cross-talk between myeloid-derived suppressor cells and macrophages subverts tumor immunity toward a type 2 response. J. Immunol. 179, 977–983 [DOI] [PubMed] [Google Scholar]
- 14. Ugel S., Delpozzo F., Desantis G., Papalini F., Simonato F., Sonda N., Zilio S., Bronte V. (2009) Therapeutic targeting of myeloid-derived suppressor cells. Curr. Opin. Pharmacol. 9, 470–481 [DOI] [PubMed] [Google Scholar]
- 15. Hu C. E., Gan J., Zhang R. D., Cheng Y. R., Huang G. J. (2011) Up-regulated myeloid-derived suppressor cell contributes to hepatocellular carcinoma development by impairing dendritic cell function. Scand. J. Gastroenterol. 46, 156–164 [DOI] [PubMed] [Google Scholar]
- 16. Li H., Han Y., Guo Q., Zhang M., Cao X. (2009) Cancer-expanded myeloid-derived suppressor cells induce anergy of NK cells through membrane-bound TGF-β 1. J. Immunol. 182, 240–249 [DOI] [PubMed] [Google Scholar]
- 17. Younos I., Donkor M., Hoke T., Dafferner A., Samson H., Westphal S., Talmadge J. (2011) Tumor- and organ-dependent infiltration by myeloid-derived suppressor cells. Int. Immunopharmacol. 11, 816–826 [DOI] [PubMed] [Google Scholar]
- 18. Ilkovitch D., Lopez D. M. (2009) The liver is a site for tumor-induced myeloid-derived suppressor cell accumulation and immunosuppression. Cancer Res. 69, 5514–5521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Connolly M. K., Mallen-St Clair J., Bedrosian A. S., Malhotra A., Vera V., Ibrahim J., Henning J., Pachter H. L., Bar-Sagi D., Frey A. B., Miller G. (2010) Distinct populations of metastases-enabling myeloid cells expand in the liver of mice harboring invasive and preinvasive intra-abdominal tumor. J. Leukoc. Biol. 87, 713–725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cheng L., Wang J., Li X., Xing Q., Du P., Su L., Wang S. (2011) Interleukin-6 induces Gr-1+CD11b+ myeloid cells to suppress CD8+ T cell-mediated liver injury in mice. PLoS One 6, e17631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lee J. M., Seo J. H., Kim Y. J., Kim Y. S., Ko H. J., Kang C. Y. (2010) Agonistic anti-CD137 monoclonal antibody treatment induces CD11bGr-1 myeloid-derived suppressor cells. Immune Netw. 10, 104–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bonder C. S., Ajuebor M. N., Zbytnuik L. D., Kubes P., Swain M. G. (2004) Essential role for neutrophil recruitment to the liver in concanavalin A-induced hepatitis. J. Immunol. 172, 45–53 [DOI] [PubMed] [Google Scholar]
- 23. Hatada S., Ohta T., Shiratsuchi Y., Hatano M., Kobayashi Y. (2005) A novel accessory role of neutrophils in concanavalin A-induced hepatitis. Cell. Immunol. 233, 23–29 [DOI] [PubMed] [Google Scholar]
- 24. Malchow S., Thaiss W., Janner N., Waetzig G. H., Gewiese-Rabsch J., Garbers C., Yamamoto K., Rose-John S., Scheller J. (2011) Essential role of neutrophil mobilization in concanavalin A-induced hepatitis is based on classic IL-6 signaling but not on IL-6 trans-signaling. Biochim. Biophys. Acta 1812, 290–301 [DOI] [PubMed] [Google Scholar]
- 25. Suzuki E., Kapoor V., Jassar A. S., Kaiser L. R., Albelda S. M. (2005) Gemcitabine selectively eliminates splenic Gr-1+/CD11b+ myeloid suppressor cells in tumor-bearing animals and enhances antitumor immune activity. Clin. Cancer Res. 11, 6713–6721 [DOI] [PubMed] [Google Scholar]
- 26. Vincent J., Mignot G., Chalmin F., Ladoire S., Bruchard M., Chevriaux A., Martin F., Apetoh L., Rebe C., Ghiringhelli F. (2010) 5-Fluorouracil selectively kills tumor-associated myeloid-derived suppressor cells resulting in enhanced T cell-dependent antitumor immunity. Cancer Res. 70, 3052–3061 [DOI] [PubMed] [Google Scholar]
- 27. Ko J. S., Zea A. H., Rini B. I., Ireland J. L., Elson P., Cohen P., Golshayan A., Rayman P. A., Wood L., Garcia J., Dreicer R., Bukowski R., Finke J. H. (2009) Sunitinib mediates reversal of myeloid-derived suppressor cell accumulation in renal cell carcinoma patients. Clin. Cancer Res. 15, 2148–2157 [DOI] [PubMed] [Google Scholar]
- 28. Liu Z. X., Han D., Gunawan B., Kaplowitz N. (2006) Neutrophil depletion protects against murine acetaminophen hepatotoxicity. Hepatology 43, 1220–1230 [DOI] [PubMed] [Google Scholar]
- 29. Xia S., Sha H., Yang L., Ji Y., Ostrand-Rosenberg S., Qi L. (2011) Gr-1+ CD11b+ myeloid-derived suppressor cells suppress inflammation and promote insulin sensitivity in obesity. J. Biol. Chem. 286, 23591–23599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nausch N., Galani I. E., Schlecker E., Cerwenka A. (2008) Mononuclear myeloid-derived “suppressor” cells express RAE-1 and activate natural killer cells. Blood 112, 4080–4089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Apetoh L., Vegran F., Ladoire S., Ghiringhelli F. (2011) Restoration of antitumor immunity through selective inhibition of myeloid derived suppressor cells by anticancer therapies. Curr. Mol. Med. 11, 365–372 [DOI] [PubMed] [Google Scholar]
- 32. Dugast A. S., Haudebourg T., Coulon F., Heslan M., Haspot F., Poirier N., Vuillefroy de Silly R., Usal C., Smit H., Martinet B., Thebault P., Renaudin K., Vanhove B. (2008) Myeloid-derived suppressor cells accumulate in kidney allograft tolerance and specifically suppress effector T cell expansion. J. Immunol. 180, 7898–7906 [DOI] [PubMed] [Google Scholar]
- 33. Ochoa A. C., Zea A. H., Hernandez C., Rodriguez P. C. (2007) Arginase, prostaglandins, and myeloid-derived suppressor cells in renal cell carcinoma. Clin. Cancer Res. 13, 721s–726s [DOI] [PubMed] [Google Scholar]
- 34. Huang B., Lei Z., Zhao J., Gong W., Liu J., Chen Z., Liu Y., Li D., Yuan Y., Zhang G. M., Feng Z. H. (2007) CCL2/CCR2 pathway mediates recruitment of myeloid suppressor cells to cancers. Cancer Lett. 252, 86–92 [DOI] [PubMed] [Google Scholar]
- 35. Obstfeld A. E., Sugaru E., Thearle M., Francisco A. M., Gayet C., Ginsberg H. N., Ables E. V., Ferrante A. W., Jr., (2010) C-C chemokine receptor 2 (CCR2) regulates the hepatic recruitment of myeloid cells that promote obesity-induced hepatic steatosis. Diabetes 59, 916–925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sander L. E., Sackett S. D., Dierssen U., Beraza N., Linke R. P., Muller M., Blander J. M., Tacke F., Trautwein C. (2010) Hepatic acute-phase proteins control innate immune responses during infection by promoting myeloid-derived suppressor cell function. J. Exp. Med. 207, 1453–1464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ribechini E., Leenen P. J., Lutz M. B. (2009) Gr-1 antibody induces STAT signaling, macrophage marker expression and abrogation of myeloid-derived suppressor cell activity in BM cells. Eur. J. Immunol. 39, 3538–3551 [DOI] [PubMed] [Google Scholar]