Abstract
Cydonia oblonga Miller (Quince) from Rosaceae family is a fruit tree cultivated in many countries mainly in Iran. This study was carried out to investigate the effect of quince juice (QJ) and quince hydroalcoholic extract (QHE) on ulcerative colitis (UC) induced by TNBS (trinitrobenzene sulfonic acid) in rats. Rats were grouped (n=6) and fasted for 36 h before colitis induction. TNBS was instilled into the colon with a hydroalcoholic carrier and then treatments were made for 5 days starting 6 h after colitis induction with different doses of QJ (200, 400, 800 mg/kg), QHE (200, 500 & 800 mg/kg) orally, QJ (400 mg/kg) and QHE (200 and 500 mg/kg) intraperitoneally. The colon tissue was removed and tissue damages were scored after macroscopic and histopathologic assessments. Albeit the examined doses of QJ and QHE were apparently effective to reduce the extent of UC lesions, only the greatest doses (500 and 800 mg/kg) resulted in significant alleviation. Weight/Length ratio as an illustrative of tissue inflammation and extravasation was also diminished with quince treatments while the results correlated with macroscopic and histopathologic evaluations. These data suggest that QJ and QHE were effective to diminish inflammation and ulcer indices in this murine model of acute colitis. Although QHE with different doses was effective in induced colitis, the dose and/or route of administration dependency was not confirmed. So quince fractions could be considered as a suitable anticolitic alternative, however further studies are needed to support this hypothesis for clinical setting.
Keywords: Cydonia oblonga, Inflammation, Plant extract, Quince, Rats, Ulcerative colitis
INTRODUCTION
Irritable bowel diseases (IBD) are a set of chronic inflammatory conditions with unclear etiology and pathogenesis. The most accepted hypothesis is that IBD origins from inappropriate and persistent activation of the mucosal immune system driven by the presence of intraluminal flora(1,2). IBD comprises from two distinct disorders: ulcerative colitis (UC) and Crohn's disease (CD). UC is an ulcero-inflammatory disease limited to the colon and affecting only the mucosa and sub-mucosa except in the most severe cases(3). UC is characterized by diffuse superficial inflammation of the colonic mucosa in the rectum and extends proximally to involve any contiguous length of the colon. Clear evidences exist for the activation of the immune response in IBD. The lamina propia is infiltrated with lymphocytes, macrophages and other cells of the immune system. The incidence and prevalence of ulcerative colitis vary greatly with geographic location(4).
Pharmacologic treatments of IBD, often involves drugs that belong to different therapeutic classes and have different but nonspecific mechanisms of anti-inflammatory action including: aminosalicylates, gluco-corticoids, anti-tumor necrosis factor and immunomodulators (azathiprine, mercap-topurine, methotrexate, cyclosporine and tacrolimus)(1,4). Alternative and comple-mentary therapies have also been used for disease treatment(5,6).
Cydonia oblonga Miller (Quince), from Rosaceae family is obtained from a tree cultivated in South Africa, Central Europe and Middle East. Iran supplies about 75% of the world production(7,8). It is recognized as an important dietary (nutrition) source and is traditionally used as a gastric tonic, anti-diarrheal, anti-inflammatory and ulcer healing agent especially within the gut, suitable for uterine and hemorrhoid bleeding, antiemetic and astringent(7,9). Various pharmacological studies have also shown antimicrobial activity(10), inhibitory effect on IgE immune reactions(11) antioxidant and antiulcerative effects for quince(12).
In this study we evaluated the anti-colitis effect of juice and extract of Cydonia oblonga with various doses and via oral and peritoneal injection routes. To the best of our knowledge, this is the first study on the effects of Cydonia oblonga in ulcerative colitis.
MATERIALS AND METHODS
Plant material and preparation of extract and juice
The quince fruit was purchased from the Najafabad local market (Isfahan, Iran) in November 2009. The fruits with their peels were sliced and air-dried at room temperature for extract preparation. Two hundred g of the slices were finely powdered and soaked in sufficient volume of ethanol/water (70/30) for 1 h. Extraction was then carried out using a percolator for 72 h to complete the extracting process(13). The extract was filtered and the solvents were evaporated using a rotary evaporator. To attain a semisolid concentrated extract, the fluid extract obtained in the previous step was further freeze-dried until a dry powder was produced. Juicer (Moulinex, France) was used to prepare quince juice.
Total phenol assay of the extract
The total phenols were determined by Folin-Cioculteau method described by Waterhouse and coworkers(14). Sodium carbonate solution (20%) was prepared. Gallic acid (as a reference for phenol compounds) stock solution was also prepared by dissolving 0.5 g of dry gallic acid in 10% hydroalcoholic solution in a 100 ml volumetric flask.
For construction of the calibration curve, gallic acid stock solution was diluted with water to obtain 0, 50, 100, 150, 250 and 500 mg/L concentrations. Test, reference and blank solutions were prepared and the absorbance of each sample was determined at 765 nm (Jenway, UK) against the blank. The absorbances were plotted against gallic acid concentrations and concentration of phenols in unknown samples was determined. Results are given as gallic acid equivalent (GAE) per g of the fruit.
Measurement of the pectin content of quince fruit
Twenty g of the dry fruit powder were extracted with boiling ethanol for 5 min. Ethanolic extract was then discarded. Afterwards, the powder was mixed thoroughly with 200 ml boiling water and extracted. The pH of the medium was adjusted to 6.5 with ammonia 1% and brought to volume by addition of distilled water. Finally, sufficient ethanol was added to achieve an 80% ethanolic solution. Pectin which was precipitated in this step was filtered,dried in room temperature and weighed and pectin content was determined(13).
Animals
Male Wistar rats (200 ± 25 g) bred in the animal house of Isfahan School of Pharmacy were used. The animals were housed singly in wire-bottomed cages under a uniform condition of temperature and humidity and fed with normal rat chow and tap water. All experiments were conducted according to the local ethics guidelines for research on animals and approved by the Research Committee of Isfahan University of Medical Sciences.
Chemicals
Dexamethasone was gifted from Iran Hormone Pharmaceutical Co. (Tehran, Iran). Trinitrobenzene sulfonic acid (TNBS) was purchased from Sigma-Aldrich (Buchs, Switzerland). Folin-Ciocalteau reagent and all organic solvents which were of analytical grade procured from Merck (Darmschtat, Germany).
Animal groups
Animals were randomly assigned to sham, control, test, and reference groups of 6 rats as follows:
Sham group; treated with vehicle (distilled water) orally (p.o.) (2 ml/kg) and intra-peritoneally (i.p.) (2.5 ml/kg) without colitis induction (normal group).
Control group; treated with vehicle p.o. (2 ml/kg) and i.p. (2.5 ml/kg), respectively after induction of colitis.
Extract group; treated with quince hydroalcoholic extract (QHE) at doses of 200, 500, and 800 mg/kg (p.o., 2ml/kg), and at doses of 200 and 500 mg/kg (i.p., 2.5 ml/kg).
Juice group; treated with quince juice (QJ) (p.o., 2ml/kg) at doses of 200, 400, and 800 mg/kg, and at doses of 200 and 400 mg/kg (i.p., 2.5 ml/kg).
Reference group; treated with dexa-methasone (p.o., 2 mg/kg, and i.p., 1 mg/kg)
All the treatments were started 6 h after induction of the colitis and continued daily for 5 consecutive days. The test plant samples including QHE and QJ were freshly prepared prior to administration.
Induction of colitis
Rats were fasted for 36 h with free access to water prior to induction of colitis. They were lightly anesthetized with ether and colitis was induced by intracolonic (rectal) instillation of 0.5 ml of TNBS (10 mg/rat in ethanol 50%) (v/v)(15). Then the rats were held in a head down position for 1 min to prevent anal leakage.
Evaluation of the colonic damage
Rats were euthanized using diethyl ether overdose at sixth day, 24 h after the last dose of treatment. The abdomen was opened and 8 cm of the colon, 3 cm up from the anus was excised, incised longitudinally and washed with normal saline solution. The wet colon was weighed and weight/length ratio was calculated for each sample. Ulcer area was measured using 3M® surgical transparent tape (St. Paul, USA), which was scaled by 1 mm2 cells. Macroscopic ulcer severity was scored according to Esmaily and coworkers as follows: 0: Normal appearance with no damage, 1: Localized hyperemia without ulceration, 2: Linear ulceration without significant inflammation, 3: Linear ulceration with inflammation at one site, 4: Two or more sites of ulceration and extending more than 1 cm along the length of colon, 5-8: Damage extending more than 2 cm along the length of the colon and the score was enhanced by 1 for each increased cm of involvement(15).
After macroscopic evaluation, colon samples were fixed in 10% formalin. Then the tissues were embedded in paraffin, processed and sectioned into 4 μm-thick slices and samples were stained with hematoxyllin and eosin (H&E). Total colitis index (TCI) was calculated by summing inflammation severity, inflammation extent and crypt damage. Microscopic studies were carried out using a Zeiss® microscope equipped with a Sony® color video camera for digital imaging by a pathologist (co-author) blind to the treatments(16).
Statistical analysis
Data analysis was made by SPSS (version 12.0) statistical software. Non-parametric data were analyzed by Mann-Whitney U test. Results are expressed as mean ± standard error of mean (SEM). Differences between groups were determined using one-way analysis of variance (ANOVA) with Scheffe multiple comparison test. The minimal level of significance was identified at P<0.05.
RESULTS
Analysis of extract and juice
A brownish extract with a pleasant odor was achieved and freeze-drying process yielded 12.02% (w/v) dried extract. The total phenol content determined by Folin-Ciocalteu method showed 8.55 mg GAE/g of the fruit (with peel) extract. The juice yielded 22.66% (w/v) dried product after freeze-drying. Pectin amount assay yielded 1.25% in dry fruit (with peel) powder.
Macroscopic assessment
As it is shown in Fig. 1 and Table 1, macroscopic observation in control group showed maximum ulcer area, ulcer severity and weight/length ratio which are indicative of highest level of damage produced by TNBS compared to sham (normal) group that showed no change. Data from the groups treated with dexamethasone (p.o., i.p.) as positive controls showed significant healing (P<0.001) in all macroscopic assessments (Table 1).
Fig. 1.
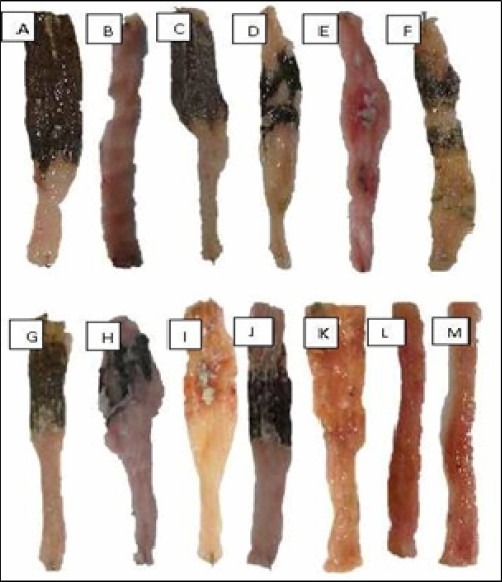
Macroscopic presentation of TNBS-induced colitis in rats. A: Vehicle treated colitis (2.5 ml/kg), B: Normal colon (Sham), C, D, E: Colitis treated with oral quince juice (200, 400, 800 mg/kg) respectively, F: Colitis treated with quince juice intraperitoneally (400 mg/kg), G, H, I: Colitis treated with oral quince hydroalcoholic extract (200, 500, 800 mg/kg) respectively, J, K: Colitis treated with quince hydroalcoholic extract intraperitoneally (200, 500 mg/kg) respectively, L: Colitis treated with oral dexamethason (2 mg/kg), M: Colitis treated with dexamethason intraperitoneally (1 mg/kg).
Table 1.
Macroscopic parameters of colitis induced by TNBS in rats and treated with Cydonia oblonga Miller (Quince) fractions.
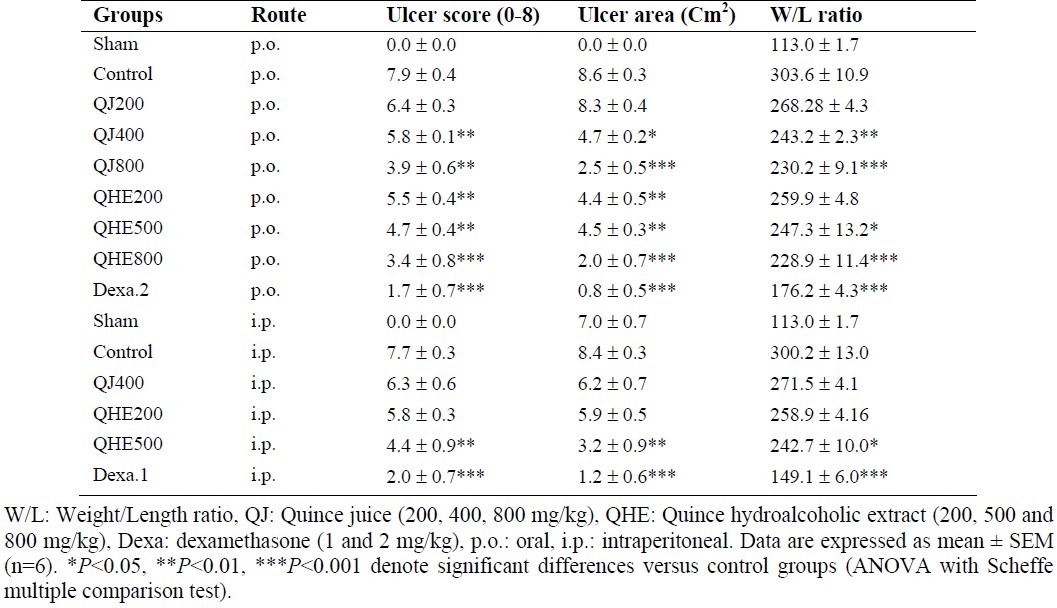
QJ and QHE except for the lowest doses administred (200 mg/kg), caused significant alleviation in macroscopic data (at least P<0.05) irrespective of the route of administration. QHE was effective to diminish macroscopic features even with the dose of 200 mg/kg after oral administration while QJ with the dose of 400 mg/kg was not statistically effective after intraperitoneal injection (Table 1).
Data analysis also showed that there was no significant differences (P>0.05) between the data of different administration routes with the same doses.
Histological assessment
As it is clear in Table 2 and Fig. 2 no signs of histological damage were observed in sham group. Control group data were demonstrative of severe inflammation and infiltration of leukocytes into the mucosal and sub-mucosal layers and so the highest tissue damage scores. Dexamethasone as the reference treatment could significantly decrease the inflammation extent and severity as well as crypt damage (at least P<0.01). In the case of plant fractions, QJ (800 mg/kg p.o.) and QHE (500 mg/kg and 800 mg/kg p.o.) caused significant decrease (at least P<0.05) in all microscopic parameters’ scores. Besides, QHE (500 mg/kg) by i.p. administration was effective to diminish total colitis index (Table 2). There was no significant difference (P>0.05) between the data of different administration routes with the same doses.
Table 2.
Microscopic parameters of colitis induced by TNBS in rats and treated with Cydonia oblonga Miller (Quince) fractions.
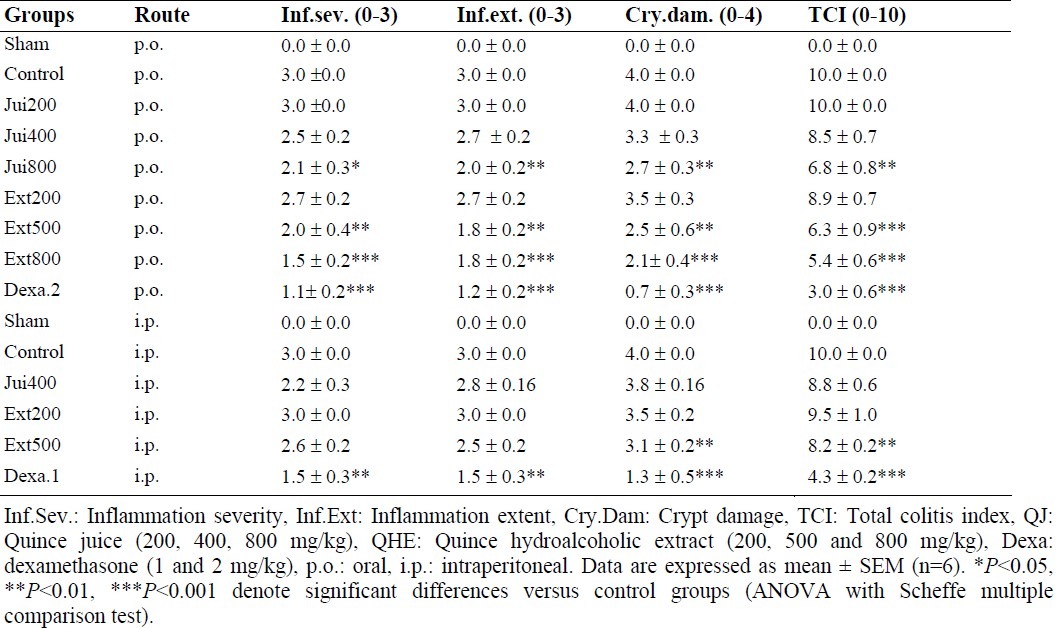
Fig. 2.
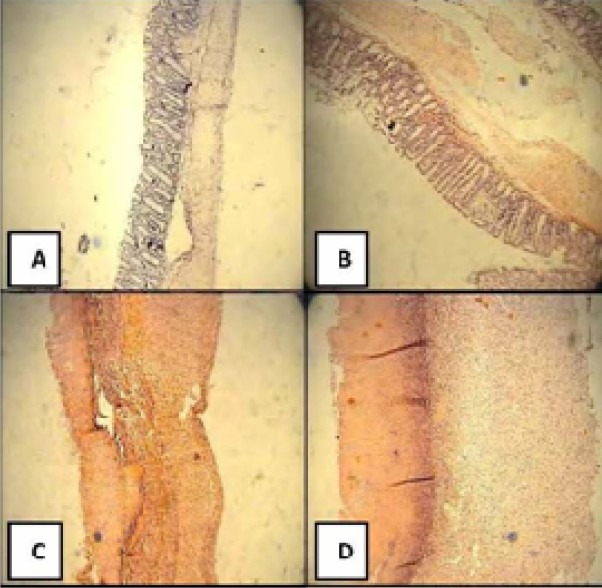
Histological sections of colons in selected groups of TNBS-induced colitis in rats. A: Normal colon treated with vehicle, 2.5 ml/kg (mucus layer and crypts are normal and leukocyte infiltration is abscent), B: Dexamethasone (1 mg/kg, i.p.) treated colitis, C: Quince juice treated colitis (800 mg/kg p.o.) D: Vehicle treated (control) colitis (2.5 ml/kg) (mucosal and submucosal inflammation as well as crypt damage and leukocyte infiltration are evident). H&E staining with low power (×10) magnification.
DISCUSSION
Recent investigations conducted on animal models of UC have had a significant role in the understanding of the disease underlying mechanisms and has led to identification of different therapeutic agents especially those with natural origin(17). TNBS induced colitis is a model with many similarities to human features UC especially in the point of immunological views(18). Ethanol carrier in this model causes disruption of epithelial layers and exposes the underlying layers to both TNBS and the bacterial component of the colon(18). The results of negative controls in comparison to normal (sham) groups indicated the consistency, effectiveness and suitability of the method(16).
The effects of different traditional medicines have been studied on TNBS-induced colitis including Rosmarinus officinalis(16), Folium syringae(19), Matricaria aurea(20) and Scutellariae radix(21). In the current study, was of interest to examine quince fruit juice for its effects on induced UC because it is abundantly used as a favorite fruity juice by people. With regard to the visual and microscopic examinations, it was found that QJ could alleviate colitis just with the highest dose (800 mg/kg) tested independent of the route of administration and. On the other hand, QHE was effective with the doses of 400 mg/kg and greater which was evident for all visual and pathologic evaluations. In the case of QHE, beneficial effects on colitis features seemed to be more efficient for samples given by oral route rather than intraperitoneal injection; however, this did not reach to a statistical significant.. Indeed better results observed by oral administration of quince fractions could be in favor of the patients because it is easier for the patients to take their medications orally. It is of noteworthy that our limitations in parenteral injection of QJ and QHE with larger doses persuade us to use low and middle doses of the test fractions in the current study. Therefore, more accurate conclusion about route-dependency effects needs more detailed and conclusive experiments. With regard to the applied doses of quince fractions, it was found that QHE was more effective than QJ because hydroalcoholic extract was capable of diminishing all colitis features at doses of 200 and 500 mg/kg after oral administration while QJ was generally non-effective at similar doses. Therefore, QHE could be considered as a better candidate for further detailed investigations in IBD therapy. Although macroscopic and microscopic examinations have distinct and consolidated places in experimental colitis evaluations, the results indicated that in most cases they were in accordance with each other(16,21–22). In this direction, the data of W/L ratio which is a good indecative of tissue edema and extravasation had reasonable correlation with ulcer area and severity findings(16,23).
Although the differences between healing effects of higher doses quince extract with those of negative control are significant, the differences between quince treatment and reference (dexamethason) groups did not reach to a significant level which may be due to the short duration of the treatment.
Quince fruit contains phenolic constituents including chlorogenic acid which is the main phenolic component of the fruit and has antioxidant(24) as well as strong anti-inflammatory(25) effects which can inhibit edema, inflammation, neutrophil migration and TNF-α expression(25,26). Rutin, quercetin and kaempferol are among(27,28) the well known quince flavonoids that have strong antioxidant and immunomodulatory effects(29). The role of oxidative stress has been confirmed in IBD. Decrement of antioxidant capacity like super oxide dismutase, hydrogen peroxidase and glutathione reducatse activity have also been reported in this disease(29). Flavonoids react with free radicals to form more stable radicals with lower toxicity. Besides, they can chelate Fe2+ which results in inhibiting free radicals effects(29). The inhibitory effects of flavonoids on mast cells, T cells, B cells, interferons, NK cells, basophils and neutrophils might have a significant role in the treatment of IBD(30). IL-5 and IL-13 are two important mediators in IBD pathology which originate from NK cells. These cells have cytotoxic effects and conduct the leukocytes migration to the vessels and with secretion of chemical mediators they can cause extensive tissue damage(31). Flavonoids especially quercetin, can also inhibit the secretion of inflammatory mediators like nitric oxide, INF-γ, IL-12 and TNF-α in which the latter has a principal role in IBD pathology(29,32).
Other active ingredients isolated from quince fruit in considerable amounts are tannins which could protect intestinal mucosal layers by precipitating their microproteins and protecting the layers against chemical injuries and proteolytic enzymes(33,34).
Pectin is another ingredient found in significant amount in quince(12,35) which is supposed to have active role in protection against the chemical induced colitis(35). Pectin can be fermented by bacteria within the colon to short chain fatty acids and these fatty acids can be consumed by epithelial cells to stimulate cell proliferation on colon biological layers(36,37). The beneficial effect of pectins against peptic ulcers was also suggested by Hamauzu and coworkers in the study of Cydonia oblonga anti-ulcerative effect(12).
CONCLUSION
The results indicated that both quince fractions including whole fruit juice (fruit with peel) and total extract (hydroalcoholic extract) were effective to alleviate colon inflammation and ulcers in TNBS experimental model which in turn support the conception of using Cydonia oblonga as a functional fruit in Iranian traditional medicine for gastrointestinal inflammatory ailments. Phenolic compounds and pectin as well as several other active components seems to be involved in the beneficial effects of quince, however accurate explanation needs more detailed and mechanistic examinations. Therefore, quince, especially its hydroalcoholic extract might be a good candidate for further studies to introduce this functional fruit as a complementary and alternative anticolitic agent.
ACKNOWLEDGMENT
This work was fully sponsored by the Research Council of Isfahan University of Medical Sciences, Isfahan, Iran.
REFERENCES
- 1.McQuaid KR. Drugs used in the treatment of gastrointestinal disease. In: Katzung BG, editor. Basic and clinical pharmacology. 10th ed. New York: McGraw Hill Companies; 2007. pp. 1029–1035. [Google Scholar]
- 2.Xavier RJ, Podolsky DK. Unraveling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427–434. doi: 10.1038/nature06005. [DOI] [PubMed] [Google Scholar]
- 3.Crawfors JM. The gastrointestinal tract. In: Kumar V, Fausto N, Abbas AK, editors. Robins and Cotran pathologic basis of diseases. 7th ed. New York: WB Saunders; 2005. pp. 797–876. [Google Scholar]
- 4.Stenson WF, Hanauer SB, Cohen RD. Gastroenterology. 15 ed. Wiley-Blackwell; 2008. Inflammatory bowel diseases; pp. 1386–1393. [Google Scholar]
- 5.D’Inca R, Garribba AT, Vettorato MG, Martin A, Martines D, Di Leo V, et al. Use of alternative and complementary therapies by inflammatory bowel disease patients in an Italian tertiary referral centre. Dig Liver Dis. 2007;39:524–529. doi: 10.1016/j.dld.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 6.Chen Q, Zhang H. Clinical study on 118 cases of ulcerative colitis treated by integration of traditional Chinese and Western medicine. J Tradit Chin Med. 1999;19:163–165. [PubMed] [Google Scholar]
- 7.Evans WC, Evans D, Trease GE. Trease and Evans pharmacognosy. 15th ed. New York: WB Saunders; 2002. p. 211. [Google Scholar]
- 8.Zargari A. Medicinal plants. 4th ed. Tehran: Tehran University Publications; 1986. pp. 243–246. [Google Scholar]
- 9.Al-Razi (Rhazes) M, Alhavi . In: Afsharypour S, translator. Tehran: Academy of Medical Science Publications; 2005. pp. 17–18. [Google Scholar]
- 10.Fattouch S, Caboni P, Tuberoso CIG, Angioni A, Dessi S, Marzouki N, Cabras P. Antimicrobial activity of Tunisian quince (Cydonia oblonga Miller) pulp and peel polyphenolic extracts. J Agric Food Chem. 2007;55:963–969. doi: 10.1021/jf062614e. [DOI] [PubMed] [Google Scholar]
- 11.Kawahara T, Lizuka T. Inhibitory effect of hot-water extract of quince (Cydonia oblonga) on immunoglobulin E-dependent late-phase immune reactions of mast cells. Cytotechnology. 2011;63:143–152. doi: 10.1007/s10616-010-9323-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hamauzu Y, Irie M, Kondo M, Fujita T. Antiulcerative properties of crude polyphenols and juice of apple and Chinese quince extracts. Food Chem. 2008;108:488–495. doi: 10.1016/j.foodchem.2007.10.084. [DOI] [PubMed] [Google Scholar]
- 13.Iranian herbal pharmacopeia. 1st ed. Tehran: Iranian Ministry of Health & Medical Education Publications; 2002. Iranian herbal pharmacopeia Committee; p. 9. (25-26,176-182,550-557). [Google Scholar]
- 14.Waterhouse AL. Current protocols in food analytical chemistry. New York: Wiley; 2001. Determination of total phenolics. [Google Scholar]
- 15.Esmaily H, Hosseini-Tabatabaei A, Rahimian R, Khorasani R, Baeeri M, Barazesh-Morgani A, et al. On the benefits of silymarine in murine colitis by improving balance of destructive cytokines and reduction of toxic stress in the bowel cells. Cent Eur J Biol. 2009;4:204–213. [Google Scholar]
- 16.Minaiyan M, Ghannadi AR, Afsharipour M, Mahzouni P. Effects of extract and essential oil of Rosmarinus officinalis L. on TNBS-induced colitis in rats. Res Pharm Sci. 2011;6:13–21. [PMC free article] [PubMed] [Google Scholar]
- 17.Jurjus AR, Khoury NN, Reimund JM. Animal models of inflammatory bowel disease. J Pharmacol Toxicol Methods. 2004;50:81–92. doi: 10.1016/j.vascn.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 18.Kawada M, Arihiro A, Mizoguchi E. Insights from advances in research of chemically induced experimental models of human inflammatory bowel disease. World J Gastroenterol. 2007;13:5581–5593. doi: 10.3748/wjg.v13.i42.5581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu X, Wang J. Anti-inflammatory effects of iridoid glycosides fraction of Folium syringae leaves on TNBS-induced colitis in rats. J Ethnopharmacol. 2011;133:780–787. doi: 10.1016/j.jep.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 20.Minaiyan M, Ghassemi-Dehkordi N, Mahzouni P, Ansari-Roknabady M. Effect of hydroalcoholic extract of Matricaria aurea (Loefl.) Shultz Bip on acetic acid-induced acute colitis in rats. Iran J Basic Med Sci. 2010;14:67–74. [Google Scholar]
- 21.Chung HL, Yue GGL, To KF, Su YL, Huang Y, Ko WH. Effect of Scutellariae radix extract on experimental dextran-sulfate sodium-induced colitis in rats. World Gastroenterol. 2007;13:5605–5611. doi: 10.3748/wjg.v13.i42.5605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim JM, Kang HW, Cha MY, Yoo D, Kim N, Kim IK. Novel guggulsterone derivative GG-52 inhibits NF-κB signaling in intestinal epithelial cells and attenuates acute murine colitis. Lab Invest. 2010;90:1004–1015. doi: 10.1038/labinvest.2010.54. [DOI] [PubMed] [Google Scholar]
- 23.Minaiyan M, Ghannadi AR, Mahzouni P, Nabi-Meibodi M. Anti-ulcerogenic effect of ginger (rhizome of Zingiber officinale Roscoe) hydroalcoholic extract on acetic acid-induced acute colitis in rats. Res Pharm Sci. 2008;3:15–22. [Google Scholar]
- 24.Sato Y, Itagaki S, Kurokawa T, Ogura J, Kobayashi M, Hirano T, et al. In vitro and in vivo antioxidant properties of chlorogenic acid and caffeic acid. Int J Pharm. 2011;403:136–138. doi: 10.1016/j.ijpharm.2010.09.035. [DOI] [PubMed] [Google Scholar]
- 25.Chagas-Paula DA, Oliveira RB, Da-Silva VC, Gobbo-Neto L, Gasparoto TH, Campanelli AP, et al. Chlorogenic acids from Tithonia diversifolia demonstrate better anti-inflammatory effect than indomethacin and its sesquiterpene lactones. J Ethnopharmacol. 2011;136:355–362. doi: 10.1016/j.jep.2011.04.067. [DOI] [PubMed] [Google Scholar]
- 26.Chauhan PS, Satti NK, Sharma VK, Dutt P, Suri KA, Bani S. Amelioration of inflammatory responses by chlorogenic acid via suppression of pro-inflammatory mediators. J Appl Pharm Sci. 2011;1:67–75. [Google Scholar]
- 27.Silva BM, Andrade PB, Ferreres F, Domingues AL, Seabra RM, Ferreira MA. Phenolic profile of quince fruit (Cydonia oblonga Miller) (pulp and peel) J Agric Food Chem. 2002;50:4615–4618. doi: 10.1021/jf0203139. [DOI] [PubMed] [Google Scholar]
- 28.Silva BM, Andrade PB, Martins RC, Valentão P, Ferreres F, Seabra RM, et al. Quince (Cydonia oblonga miller) fruit characterization using principal component analysis. J Agric Food Chem. 2005;53:111–122. doi: 10.1021/jf040321k. [DOI] [PubMed] [Google Scholar]
- 29.Nijveldt RJ, Van-Nood E, Van-Hoorn DEC, Boelens PG, Van-Norren K, Van-Leeuwen PAM. Flavonoids: a review of probable mechanisms of action and potential applications. Am J Clin Nutr. 2001;74:418–425. doi: 10.1093/ajcn/74.4.418. [DOI] [PubMed] [Google Scholar]
- 30.Middleton E, Kandaswami C. Effects of flavonoids on immune and inflammatory cell functions. Biochem Pharmacol. 1992;43:1167–1179. doi: 10.1016/0006-2952(92)90489-6. [DOI] [PubMed] [Google Scholar]
- 31.Baumgart DC, Carding SR. Inflammatory bowel disease: cause and immunobiology. Lancet. 2007;369:1627–1640. doi: 10.1016/S0140-6736(07)60750-8. [DOI] [PubMed] [Google Scholar]
- 32.Rao YK, Fang SH, Tzeng YM. Inhibitory effects of the flavonoids isolated from Waltheria indica on the production of NO, TNF-alpha and IL-12 in activated macrophages. Biol Pharm Bull. 2005;28:912–915. doi: 10.1248/bpb.28.912. [DOI] [PubMed] [Google Scholar]
- 33.Al-Rehailya AJ, Al-Howirinya TS, Al-Sohaibanib MO, Rafatullaha S. Gastroprotective effects of ‘Amla’ Emblica officinalis on in vivo test models in rats. Phytomedicine. 2002;9:515–522. doi: 10.1078/09447110260573146. [DOI] [PubMed] [Google Scholar]
- 34.Cobzac S, Moldovan M, Olah NK, Bobos L, Surducan E. Tannin extraction efficiency, from Rubus idaeus, Cydonia oblonga and Rumex acetosa, using different extraction techniques and spectrophotometric quantification. Seria F Chemia. 2005;8:55–59. [Google Scholar]
- 35.Otakar ROP, Balik J, Reznicek V, Jurikova T, Skardova P, Salas P, et al. Chemical characteristics of fruits of some selected quince (Cydonia oblonga Mill.) cultivars. Czech J Food Sci. 2011;29:65–73. [Google Scholar]
- 36.Rolandelli RH, Saul SH, Settle RG, Jacobs DO, Trerotola SO, Rombeau JL. Comparison of parenteral nutrition and enteral feeding with pectin in experimental colitis in the rat. Am J Clin Nutr. 1988;47:715–721. doi: 10.1093/ajcn/47.4.715. [DOI] [PubMed] [Google Scholar]
- 37.Roediger WE. The starved colon diminished mucosal nutrition, diminished absorption, and colitis. Dis Colon Rectum. 2010;33:858–862. doi: 10.1007/BF02051922. [DOI] [PubMed] [Google Scholar]


