Abstract
In recent years, the use of antifungal drugs in human medicine has increased, especially with the advent of AIDS epidemic. Efforts have focused on the development of new, less toxic and more efficacious antifungal drugs with novel mechanism of action. The purpose of this study was to synthesize of some new benzimidazole, benzotriazole and aminothiazole derivatives and to evaluate their activity against some species of Candida, Aspergillus and dermatophytes. The desired compounds were synthesized by the reaction of benzimidazole and benzotriazole with bromoalkanes and also by the reaction of an amide derivative of aminothiazole with 2-piperazino-1-ethanol in an efficient solvent in the presence of tetraethyl ammounim bromide or triethylamine) as catalyst. Chemical structures of all the new compounds were confirmed by spectrophotometric methods. Antifungal activities of the new compounds were evaluated by broth micro dilution method as recommended by CLSI. Among the tested compounds, 1-nonyl-1H-benzo[d]imidazole and 1-decyl-1H-benzo[d]imidazole exhibited the best antifungal activities. Of the examined synthetic compounds in different categories, benzimidazole derivatives established better antifungal activities than benzotriazole derivatives, and the piperazine analogue had no significant antifungal effect.
Keywords: Benzimidazole, Benzotriazole, Aminothiazole, Antifungal activity
INTRODUCTION
During the past two decades, the frequency of invasive and systemic fungal infections has increased dramatically due mainly to Candida species(1). The growing number of immunocompromised patients as a result of cancer chemotherapy, organ transplantation, and HIV infection are the major factors contributing to this incidence(2). Recently, the expansion of antifungal drug research has occurred because there is a critical need for new antifungal agents to treat these life-threatening invasive fungal infections(3). Since Candida albicans and Aspergillus fumigatus are the main causative fungi of the systemic mycosis, antifungal drugs for treating patients of deep mycosis should have a broad antifungal spectrum including at least these microorganisms. Unfortunately, none of the antifungal drugs which are currently available for the treatment of systemic mycoses are ideal in terms of efficacy, antifungal spectrum or safety(2).
Azole compounds are divided into the older imidazoles and the new triazoles(4). They inhibit fungal cytochrome P450 3A-dependent C14-α-demethylase which is responsible for the conversion of lanosterol to ergosterol, thereby depleting ergosterol in the fungal cell membrane(3). Interaction of triazoles with cytochrome P450 may cause disturbances of hepatic enzymes(4). Azole antifungal activity varies with each compound and clinical efficacy may not coincide with in vitro activity(3). Newer and less toxic antifungal agents are available for clinical use, but their clinical efficacy in some invasive fungal infections, is not optimal(5).
The incorporation of the benzimidazole nuclei is an important synthetic strategy in drug discovery. The high therapeutic properties of the related drugs have encouraged the medicinal chemists to synthesize the large number of novel chemotherapeutic agents(6).
In general, nitrogen and sulfur containing organic compounds and their metal complexes display a wide range of biological activity as antitumor, antibacterial, antifungal and antiviral agents(7).
In the previous reports, we described the preparation of a number of imidazole, benzimidazole and benzotriazole derivatives with biological interest(8–10). In the current study, we have synthesized some new derivatives of benzimazole, benzotriazole and aminothiazole as antifungal agents. In these new analogous an alkyl chain is substituted at the position 1 of benzimidazole and benzotriazole rings. A new compound of 2-aminothiazole also was prepared by the reaction of a piperazine moiety with an amide intermediate of 2-aminothiazole.
MATERIALS AND METHODS
All chemicals and solvents were purchased from Merck, Germany. 1HNMR spectra were obtained on a Bruker Avance DPX 500 MHZ instrument, USA. Thin-layer chromatography (TLC) was performed using 250 μm silica gel GF plates, Merck, Germany. All reactions were carried out under nitrogen gas. Microorganisms were obtained from Mycology and Parasitology department of the Shiraz University of Medical Sciences.
Chemistry
In the benzimidazole and benzotriazole categories, the azole rings reacted with appropriate bromoalkane in a suitable solvent at reflux temperature. 1,2,4-Triazoles can be alkylated on N1 or N4 and 1,2,3-benzotriazoles on N1 or N2 positins. The controlled regioselective alkylation of them is a difficult task. Our strategy was to modify the reported methods to get N1-substituted of the azoles in higher yield. Therefore, we employed acetonitrile as the solvent, and tetra ethyl ammonium iodide, anhydrous potassium carbonate, and sodium hydroxide as catalysts(10). Th e reaction mixture was worked up to get the crude product. Purification was carried out by plate chromatography. Chemical structures of the final compounds were confirmed by spectroscopy methods. For the aminothiazole analogue in the first step 3-chloropropionyl chloride was reacted with 2-aminothiazole to get an amide intermediate and in the second step, 2-piperazino-1-ethanol was reacted with the intermediate to afford the final compound.
General procedure
Benzimidazole and benzotriazole series
Benzimidazole or benzotriazole ring, bromoalkane, tetraethyl ammonium bromide (TEAB), sodium hydroxide and potassium carbonate in acetonitrile were refluxed for 48 h. The reaction mixture was then filtered and concentrated in vacuo. Water and chloroform was added, the organic layer was dried over Na2SO4, and concentrated in vacuo. The crude compound was purified by plate chromatography on silica gel using petroleum ether and ethyl acetate as solvent to obtain the final product (Scheme1).
Scheme 1.
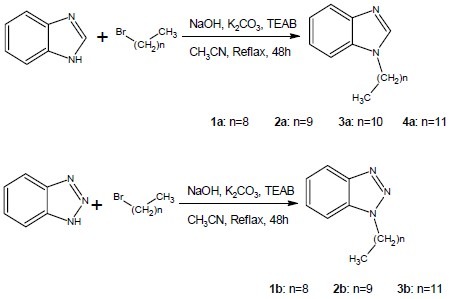
Synthesis of benzimidazole and benztoriazole derivatives.
Aminothiazole analogue
In the first step, 3-chloropropionyl chloride and triethylamine (TEA) were added dropwise to a solution of 2-aminothiazole in 1,4-dioxane and reacted at reflux temperature. The crude product filtrated and extracted by dichloro- methane and water. The organic layer quenched with saturated solution of NaCl in water and evaporated to get the amide intermediate. In the second step, piperazine 2-ethanol was reacted with the amide intermediate in the similar condition as benzimidazole and benzotriazole compounds reacted with bromoalkanes (Scheme 2).
Scheme 2.

Synthesis of aminothiazole analogues.
Synthesis of benzimidazole series (series a)
General procedure for the synthesis of benzimidazole series (series a)
A Solution of 590 mg benzimidazole (5 mmol), 0.95 ml of bromononane (5 mmol), 200 mg of NaOH (5 mmol), 690 mg of potassium carbonate (5 mmol), and 42 mg of TEAB (5 mmol) in 25 ml acetonitrile were heated under reflux for 48 h. Compounds 1a, 2a, 3a and 4a were purified by preparative thin layer chromatography using chloroform/ methanol: 95 ml/5ml.
1-Nonyl-1 H-benzo[d]imidazole (1a)
670 mg (55%) compound 1a was obtained as brown oily liquid. 1HNMR (DMSO-d6, 500 MHz): (δppm) 8.20 (s, 1H, imidazole), 7.56 (d, 2H, phenyl), 7.16-7.24 (m, 2H, phenyl), 4.21 (t, J=7.07 HZ, 2H, N- CH2), 1.76 (m,2H, N-CH2-CH2), 1.20 (m, 12H, N-CH2-CH2-(CH2)6), 0.81 (t, J=7.12 HZ, 3H,CH3). 13C-NMR: (125 MHz), δ (ppm) = 14.74, 22.92, 26.96, 29.37, 29.44, 29.73, 30.23, 32.08, 44.91, 111.15, 120.28, 122.14, 122.97, 134.66, 144.38, 144.82. MS (EI): M/Z (%): 243 (100M+), 227 (2.2), 213 (3), 199 (4.5), 130 (14), 117 (8).
1-Decyl-1 H-benzo[d]imidazole (2a)
900 mg (69%) compound 2a was obtained as yellow liquid. 1HNMR (DMSO-d6, 500MHz): (δppm) 8.20 (s, 1H, imidazole), 7.56 (d, 2H, phenyl) 7.16-7.24 (m, 2H, phenyl), 4.21 (t, J=7.07 HZ, 2H, N-CH2), 1.76 (m, 2H, N-CH2-CH2), 1.16 (m, 14H, N-CH2-CH2-(CH2)7), 0.82 (t, J=7.09 HZ, 3H, CH3). 13C-NMR: (125 MHz), δ(ppm) = 14.76, 22.94, 26.96, 29.36, 29.51, 29.74, 29.77, 30.23, 32.13, 44.91, 111.14, 120.283, 122.14, 122.96, 134.66, 144.38, 144.81. MS (EI): M/Z (%), 257 (100), 242 (1), 172 (10), 131 (11), 118 (9).
1-Undecyl-1 H-benzo[d]imidazole (3a)
890 mg (64%) compound 3a was obtained as light cream crystals, m.p. 38-40°C. 1HNMR (DMSO-d6, 500MHz): (δppm) 8.20 (s, 1H, imidazole), 7.55 (d, 2H, phenyl), 7.16-7.24 (m, 2H, phenyl), 4.20 (t, J=7.09 HZ, 2H, N-CH2), 1.76 (m,2H,N-CH2-CH2), 1.22 (m, 16H, N-CH2-CH2-(CH2)8), 0.82 (t, J=7.09 HZ, 3H, CH3). 13C-NMR: (125MHz), δ (ppm) = 14.75, 22.95, 26.97, 29.37, 29.56, 29.77, 29.80, 29.82, 30.23, 32.15, 44.91, 111.12, 120.28, 122.13, 122.95, 134.66, 144.39, 144.80. MS (EI): M/Z (%): 271 (100), 228 (6), 172 (8), 131 (13), 118 (6).
1-Dodecyl-1 H-benzo[d]imidazole (4a)
1100 mg (77%) compound 4a was obtained as cream liquid. 1HNMR (DMSO-d6, 500 MHz): (δppm) 8.19 (s, 1H, imidazole), 7.55 (d, 2H, phenyl), 7.15-7.23 (m, 2H, phenyl), 4.20 (t, J=7.08 HZ, 2H, N-CH2), 1.74 (m, 2H,N-CH2-CH2), 1.22 (m, 18H, N-CH2-CH2-(CH2)9), 0.82 (t, J=7.09 HZ, 3H, CH3). 13C-NMR: (125MHz), δ(ppm)=14.75, 22.96, 26.97, 29.38, 29.58, 29.78, 29.79, 29.79, 29.87, 30.23, 32.16, 44.91, 111.11, 120.28, 122.12, 122.94, 134.66, 144.38, 144.79. Ms (EI); M/Z (%): 285 (100), 256 (4), 228 (6), 214 (4), 200 (6), 186 (6), 172 (7), 131 (10), 117 (6).
Synthesis of benzotriazole series (series b)
General procedure for the synthesis of benzotriazole series (series b)
A Solution of 595 mg benzotriazole (5 mmol), 0.95 ml of bromononane (5 mmol), 200 mg of NaOH (5 mmol), 690 mg of potassium carbonate (5 mmol), and 42 mg of TEAB (0.20 mmol) in 25 ml acetonitrile were heated under reflux for 48 h. Compound 1b, 2b, and 3b were purified by preparative TLC using petroleum ether /ethyl acetate: 90 ml/10ml.
1-Nonyl-1H-1,2,3-benzotriazole (1b)
460 mg 37% compound 1b was obtained as light cream crystals, m.p. 32-35 °C. 1HNMR (DMSO-d6, 500 MHz): (δppm) 8.02 (d, 1H, phenyl), 7.87 (d, 1H, phenyl), 7.53 (t, 1H, phenyl), 7.38 (t, 1H, phenyl), 4.69 (t, J=6.98 HZ, 2H, N-CH2), 1.89 (m, 2H, N-CH2-CH2), 1.21 (m, 12H, N-CH2-CH2-(CH2)6), 0.81 (t, J=7.01 HZ, 3H, CH3). 13C-NMR: (125MHz), δ(ppm) = 14.75, 22.90, 26.85, 29.22, 29.37, 29.63, 30.03, 32.04, 48.26, 111.40, 119.96, 124.68, 127.92, 133.67, 196.07. MS (EI): M/Z (%) 244 (100), 187 (18), 174 (37), 145 (58), 132 (81), 118 (37), 91 (40), 77 (21).
1-Decyl-1H-1,2,3-benzotriazole (2b)
550 mg (42%) compound 2b was obtained as white crystals, m.p. 35-37°C. 1HNMR (DMSO-d6, 500 MHz): (δppm) 8.02 (d, 1H, phenyl), 7.08 (d, 1H, phenyl), 7.52 (t, 1H, phenyl), 7.37 (t, 1H, phenyl), 4.68 (t, J=6.97 HZ, 2H, N-CH2), 1.90 (m, 2H, N-CH2-CH2), 1.20 (m, 14H, N-CH2-CH2-(CH2)7), 0.80 (t, J=7.11 HZ, 3H, CH3). 13C-NMR: (125 MHz), δ(ppm) = 14.74, 22.91, 26.85, 29.22, 29.46, 29.68, 29.68, 30.03, 32.10, 48.25, 111.37, 119.95, 124.65, 127.89, 133.66, 146.02. MS, M/Z (%): 258 (100), 187 (11), 173 (12), 145 (30), 132 (46), 118 (7).
1-Dodecyl-1H-1,2,3-benzotriazole (3b)
640 mg (44%) compound 3b was obtained as light cream crystals, m.p. 40-42°C. 1HNMR (DMSO-d6, 500 MHz): (δppm) 8.02 (d, 1H, phenyl), 7.86 (d, 1H, phenyl), 7.52 (t, 1H, phenyl), 7.37 (t, 1H, phenyl), 4.68 (t, J=6.98 HZ, 2H, N-CH2), 1.88 (m, 2H, N-CH2-CH2), 1.21 (m, 18H,N-CH2-CH2-(CH2)9), 0.82 (t, J=7.09 HZ, 3H, CH3). 13C-NMR: (125 MHz), δ(ppm) = 14.76, 22.94, 26.86, 29.23, 29.54, 29.67, 29.73, 29.73, 29.82, 30.03, 32.14, 48.25, 111.37, 119.95, 124.65, 127.89, 133.66, 146.02. MS: M/Z (%): 286 (100), 257 (11), 216 (14), 187 (15), 173 (21), 145 (50), 132 (44), 118 (33), 91 (20), 77 (10).
Aminothiazole analogues (series c)
N1-(1,3-thiazol-2-yl)-3-Chloropropaneamide (1c)
1,3-Thiazol-2-amine (5 mmol, 500 mg) was dissolved in 1,4-dioxane (25 ml); then 3-chloropropanoylchloride (7.5 mmol, 0.198 ml) and TEAB (5.5 mmol, 0.8 ml) were added dropwise and the reaction mixture were refluxed at 102°C for 5 h. the reaction mixture were filtrated and extracted with dichloromethane and water. The organic layer was saturated by NaCl solution, dried using NaSO4 and evaporated to get 1c as brown liquid (650 mg, 70%). 1HNMR (DMSO-d6, 500 MHz): (δppm) 11.9 (1H, NH), 7.50 (d, J=5.00 HZ 1H, N-CH), 7.06 (d, J=5.00 HZ, 1H, S-CH), 3.94 (CH2Cl), 3.02 (CO-CH2). Ms (EI); M/Z (%): 190 (80), 154 (46), 126 (17), 100 (100).
N1- (1,3-thiazol-2-yl) -3- [4- (2-hydroxyethyl) piperazino]propaneamide (2c)
N1-(1,3-thiazol-2-yl)-3-Chloropropaneamide (1c) (3.5 mmol, 650 mg), 2-piperazino-1-ethanol (3.5 mmol, 0.43 ml), potassium carbonate (3.5 mmol, 483 mg) and TEAB (0.15 mmol, 31.5 mg) in acetonitrile (25 ml) were refluxed for 48 h. The reaction mixture were filtrated and extracted by dichloromethane and water. The organic layer was saturated by NaCl solution, dried using NaSO4 and evaporated to get 2c brown liquid (600 mg, 60%). 1HNMR (DMSO-d6, 500 MHz): (δppm) 7.98 (d, 1H, NH), 7.43 (d, J=5.00 HZ, 1H,N-CH), 7.15 (d, J=3.15 HZ 1H, S-CH), 3.46 (m, 5H, OH, CH2-OH,CO-CH2), 2.56 (m, 4H, CH2-CH2-OH,CO-CH2-CH2), 2.35 (m, 8H,N-[CH2-CH2]2-N). 13C-NMR: (125 MHz), δ(ppm) = 33.34, 53.23, 54.00, 54.00, 54.18, 59.36, 61.08, 114.06, 138.39, 158.74, 163.85, 171.05. MS (EI); M/Z (%), 283 (100), 251 (27), 183 (31), 153 (40), 142 (30), 111 (23), 99 (33).
Biological assay
Microorganisms
The antifungal activities of the synthesized compounds were determined against eleven standard strains of fungi including Candida albicans (ATCC 10261, CBS 1912), Candida krusei (ATCC 6258), Candida glabrata (ATCC 90030), Candida parapsilosis (ATCC 4344), Candida dubliniensis (CBS 8500), Candida tropicalis (ATCC 750), Cryptococcus neoformans (ATCC 9011), Aspergillus flavus (ATCC 64025), Aspergillus oryzae (ATCC) and Aspergillus fumigatus (ATCC 14110) as well as nine clinical isolates of yeasts identified by PCR-RFLP(11). Moreover, the inhibitory activities of the mentioned compounds against dermatophytes (Micro-sporum canis and Epidermophyton flocossum) which were identified by morphological and physiological tests were also examined. The susceptibility of the examined isolates of fungi against flocunazole (for Aspergillus species and yeasts) or griseofulvin (for dermatophytes) were examined by broth microdilution method(12). The interpretative breakpoints for susceptibility testing of fluconazole as recommended by clinical and laboratory standards institute (CLSI) were: Susceptible ≤8 μg/ml, Susceptible Dose Dependent 16-32 μg/ml and Resistant ≥64 μg/ml(12).
Determination of minimum inhibitory and fungicidal concentrations
Minimal inhibitory concentrations of the synthetized agents and selected drugs against standard and clinical species of the fungi were determined by broth microdilution method as recommended by CLSI, with some modifi-cations(12).
Briefly, the RPMI-1640 (with l-glutamine and phenol red, without bicarbonate) (Sigma, USA) was prepared and buffered at pH 7.0 with 0.165 mol 3-(N-morpholino) propane sulfonic acid (MOPS) (Sigma-Aldrich, Steinheim, Germany). Serial dilutions of the selected compound (0.5-256.0 μg/mL) were prepared in 96-well microtitre trays using RPMI-1640 media (Sigma, St. Louis, USA) buffered with MOPS (Sigma, St. Louis, USA). Double dilutions of fluconazole were also prepared for each of the tested Candida with the final concentration of 0.25-128 μg/mL.
Stock inoculums were prepared by suspending three colonies of the examined yeasts in 5 ml of sterile 0.85% NaCl, and adjusting the turbidity of the inoculums to 0.5 McFarland standard at 530 nm wavelength (this yields stock suspension of 1-5×106 cells/ml). For moulds (Aspergillus spp. and dermatophytes), conidia were recovered from the 7-day old cultures grown on potato dextrose agar by a wetting loop with Tween 20. The collected conidia were transferred in sterile saline and their turbidity was adjusted to OD=0.09-0.11 that yields 0.4-5×106 conidia/ml. Working suspension was prepared by making a 1/50 and 1/1000 dilution with RPMI of the stock suspension for moulds and yeasts, respectively. After addition of 0.1 ml of the inoculums to the wells, the trays were incubated at 30°C for 24-48 h in a humid atmosphere. 200 μl of the uninoculated medium was included as a sterility control (blank).
In addition, growth controls [medium with inoculums and 5% (v/v) DMSO (maximum DMSO concentration) but without the synthetic compounds] were also included. The growth in each well was compared with that of the growth control well. MICs were visually determined and defined as the lowest concentration of the compounds that produced ≥50 growth inhibitions. Each experiment was performed in triplicate. MFCs were of the lowest concentration that showed either no growth or fewer than 4 colonies, which corresponded to 98% killing activity of the initial inoculums. MFCs were also determined by culturing 10 μl from the wells showing no visible growth onto Sabouraud dextrose agar plates.
RESULTS
During the past two decades, a number of different classes of antifungal agents have been discovered. Since the discovery of amphotericin B, there has been much progress in this field(5). Recently the developments of resistance to currently available antifungal azoles have been reported. The azole compounds have also some clinical failures in the treatment of fungal infections(13). Benzimidazoles are regarded as a promising class of bioactive heterocyclic compounds that exhibit a range of biological activities(14). In the course of our search for therapeutically useful antifungal azoles, we synthesized some new derivatives of benzimidazole, benzo-triazole and aminothiazole (Table 1).
Table 1.
Synthesis of the new azole compounds.

The biological results are presented in Tables 2 and 3. As shown in Table 2, compounds 1a and 2a had the most antifungal effects against tested clinical species of Candida at 0.5-256 and 2-256 μg/ml. These two compounds also were effective against resistant species to fluconazole. Compounds 3a and 4a had moderate activity against tested clinical species of Candida at 0.5-256 μg/ml.
Table 2.
MIC (μg/ml) of the new azole compounds against different species of Candida.
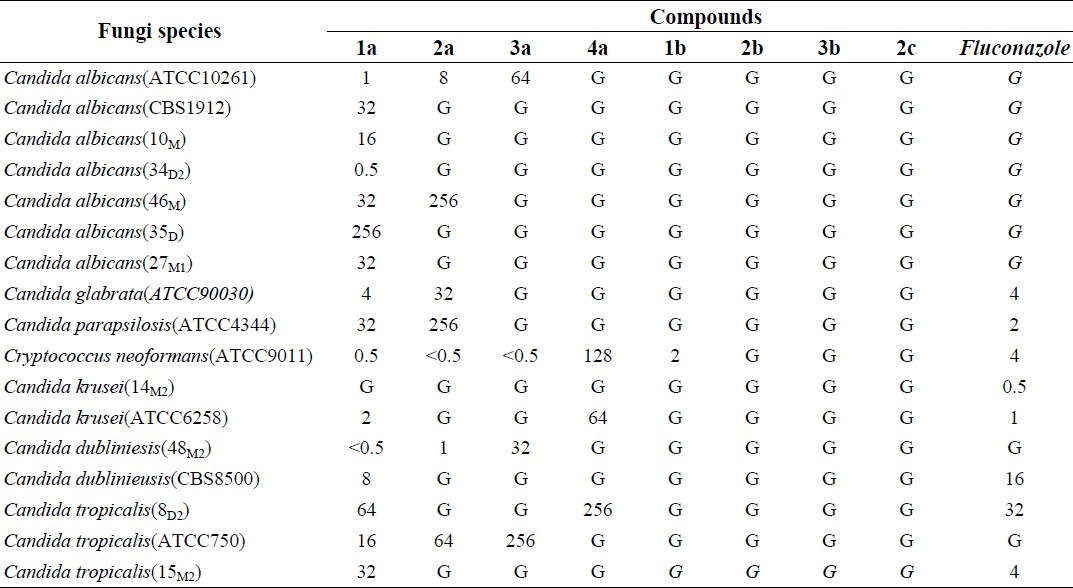
Table 3.
MIC (μg/ml) of the new azole compounds against different species of Aspergillus and dermatophytes.

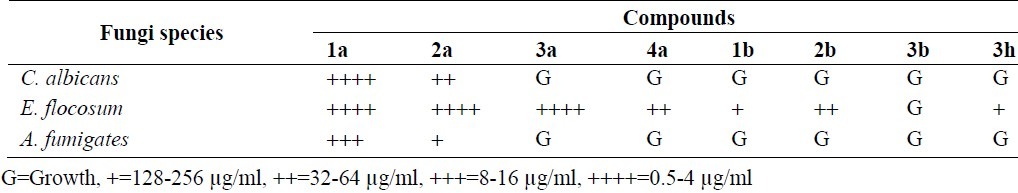
As shown in Table 3, compounds 1a and 2a had the most antifungal effects against Aspergillous species at 16-256 μg/ml. Compounds 1a, 2a and 3a had the most anti-fungal effects against dermatophytes species at 0.5-256 μg/ml. Other compounds showed desirable antifungal activity at 16-128 μg/ml.
DISCUSSION
Previously we reported that benzimidazole derivatives are more potent than benzotriazole compounds in the point of antifungal activity(15). The results also showed that alkylbenzimidazoles are more active than alkylbenzotriazole. In the benzimidazole category 1a and 2a affected all species of fungi. Compounds 3b had no antifungal effect. All tested compounds in this study except 3b and 2c showed desirable activity on C. neoformans. Some of Candida species such as C. albicans, C. krusei and C. tropicalis which are resistant to fluconazole and itraconazole were affected by the synthesized compounds (1a and 2a).
Che X and coworkers tested some phenoxy propyl piperazine derivatives and reported that these compounds showed good antifungal effect on some pathogenic and resistant fungi(16). In this study compound 2c (piperazine analogue) was affective only on the dermatophytes.
Although Ram Janam Singh showed that some benzimidazole and benzotriazole compounds were effective on C. albicans, Aspergillus nigeri and Trichophyton rubrum in solid agar technique(17) but in our study Aspergillouses species were affected only by 1a and 2a and not by the other compounds.
Hakan Goker and coworkers showed that addition of a 4-carbon atom chain with a phenyl moiety to benzimidazole ring increases the antifungal activity(18). Our results showed that antifungal activities of the benzimidazole derivatives were optimum with 9 carbon atoms in the alkyl chain and as the number of carbon atoms increases, the antifungal activities will decrease.
CONCLUSION
Some fungi which are resistant to fluconzole and itraconazole were affected by the compounds synthesized in this study (Table 4). We suggest further in vivo studies on these compounds to elucidate their effects and toxicity.
Table 4.
MIC (μg/ml) of the new azole compounds on the resistant species of fungi.
ACKNOWLEDGMENT
Financial assistance from the Shiraz University of Medical Sciences is gratefully acknowledged.
REFERENCES
- 1.Giraud F, Guillon R, Logé C, Pagniez F, Picot C, Borgne ML, et al. Synthesis and structure-activity relationships of 2-phenyl-1-[pyridinyl- and piperidinylmethyl) amino] -3-(1H-1,2,4-triazol-1-yl) propan-2-ols as antifungal agents. Bioorgan Med Chem Lett. 2009;19:301–304. doi: 10.1016/j.bmcl.2008.11.101. [DOI] [PubMed] [Google Scholar]
- 2.Masubuchi M, Ebiike H, Kawasaki KI, Sogabe S, Morikami K, Shiratori Y, et al. Synthesis and biological activities of benzofuran antifungal agents targeting fungal N-myristoyltransferase. Bioorgan Med Chem. 2003;11:4463–4478. doi: 10.1016/s0968-0896(03)00429-2. [DOI] [PubMed] [Google Scholar]
- 3.Andriole VT. Current and future antifungal therapy: New targets for antifungal therapy. Int J Antimicrob Ag. 2000;16:317–321. doi: 10.1016/s0924-8579(00)00258-2. [DOI] [PubMed] [Google Scholar]
- 4.De Pauw BE. New antifungal agents and preparations. Int J Antimicrob Ag. 2000;16:147–150. doi: 10.1016/s0924-8579(00)00221-1. [DOI] [PubMed] [Google Scholar]
- 5.Andriole VT. Current and future antifungal therapy: New targets for antifungal agents. J Antimicrob Chemoth. 1999;44:151–162. doi: 10.1093/jac/44.2.151. [DOI] [PubMed] [Google Scholar]
- 6.Khalafi-Nezhad A, Soltani Rad MN, Mohabatkar H, Asrari Z, Hemmateenejad B. Design, synthesis, antibacterial and QSAR studies of benzimidazole and imidazole chloroaryloxyalkyl derivatives. Bioorgan Med Chem. 2005;13:1931–1938. doi: 10.1016/j.bmc.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 7.Arjmand F, Mohani B, Ahmad S. Synthesis, antibacterial, antifungal activity and interaction of CT-DNA with a new benzimidazole derived Cu (II) complex. Eur J Med Chem. 2005;40:1103–1110. doi: 10.1016/j.ejmech.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 8.Khabnadideha S, Rezaeia Z, Khalafi-Nezhadb A, Pakshirc K, Heirana MJ, Shobeiria H. Design and synthesis of 2-methyl and 2-methyl-4-nitro imidazole derivatives as antifungal agents. Iran J Pharm Sci. 2009;5:31–36. [Google Scholar]
- 9.Khabnadideh S, Rezaei Z, Khalafi-Nezhad A, Pakshir K, Roosta A, Baratzadeh Z. Design and synthesis of imidazole and benzimidazole deerivatives as antifungal agents. Anti-Infective Agents in Medicinal Chemistry. 2008;7:215–218. [Google Scholar]
- 10.Rezaei Z, Khabnadideh S, Pakshir K, Hossaini Z, Amiri F, Assadpour E. Design, synthesis, and antifungal activity of triazole and benzotriazole derivatives. Eur J Med Chem. 2009;44:3064–3067. doi: 10.1016/j.ejmech.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 11.Mirhendi H, Makimura K, Zomorodian K, Maeda N, Ohshima T, Yamaguchi H. Differentiation of Candida albicans and Candida dubliniensis using a single-enzyme PCR-RFLP method. Jpn. J. Infect. Dis. 2005;58:235–237. [PubMed] [Google Scholar]
- 12.Wayne PA. Clinical and Laboratory Standards Institute. 2nd ed. 2007. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts; approved standard. CLSI M27-A7. [Google Scholar]
- 13.Upadhayaya RS, Jain S, Sinha N, Kishore N, Chandra R, Arora SK. Synthesis of novel substituted tetrazoles having antifungal activity. Eur J Med Chem. 2004;39:579–592. doi: 10.1016/j.ejmech.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 14.Ansari KF, Lal C. Synthesis, physicochemical properties and antimicrobial activity of some new benzimidazole derivatives. Eur J Med Chem. 2009;44:4028–4033. doi: 10.1016/j.ejmech.2009.04.037. [DOI] [PubMed] [Google Scholar]
- 15.Rezaei Z, Khabnadideh S, Zomorodian K, Pakshir K, Kashi G, Sanagoei N, et al. Design, synthesis and antifungal activity of some new imidazole and triazole derivatives. Archieve Der Pharmazei. 2011 doi: 10.1002/ardp.201000357. In Press. [DOI] [PubMed] [Google Scholar]
- 16.Che X, Sheng C, Wang W, Cao Y, Xu Y, Ji H, et al. New azoles with potent antifungal activity: Design, synthesis and molecular docking. Eur J Med Chem. 2009;44:4218–4226. doi: 10.1016/j.ejmech.2009.05.018. [DOI] [PubMed] [Google Scholar]
- 17.Singh RJ. Syntheses of some new 1, 2, 3-benzotriazoles as antimicrobial agents. Rasayan J Chem. 2009;2:598–601. [Google Scholar]
- 18.Göker H, Ku C, Boykin DW, Yildiz S, Altanlar N. Synthesis of some new 2-substituted-phenyl-1H-benzimidazole-5-carbonitriles and their potent activity against Candida species. Bioorgan Med Chem. 2002;10:2589–2596. doi: 10.1016/s0968-0896(02)00103-7. [DOI] [PubMed] [Google Scholar]


