Abstract
Entrapment of permeabilized whole cells within a matrix is a common method for immobilization. Chitosan possesses distinct chemical and biological properties, which make it a suitable matrix for entrapment and immobilization of penicillin G acylase (PGA). In the first step, Escherichia coli (ATCC 11105) cells were permeabilized using N-cetyl-N,N,N-trimethyl ammonium bromide (CTAB) (0.1% w/v, 45 min, 45 rpm) which then immobilized using glutaraldehyde (5% w/v) as cross-linker and chitosan (3% w/v) as the matrix. These conditions were established after preliminary trials with CTAB and glutaraldehyde concentrations in the range of 0.05-0.25% w/v and 1-9% v/v, respectively. The hydrolytic activity was assayed using Ehrlich reagent. Permeabilization of cells caused 9% increase in Penicillin G Acylase (PGA) conversion after 15 min compared to the intact cells. Although, immobilization on chitosan decreased the conversion compared to un-immobilized treated cells (13%), the new biocatalyst showed acceptable operational stability, maintaining more than 90% of the initial activity after 20 cycles. Optimum conditions for immobilization of E. coli cells were: CTAB 0.1% w/v and glutaraldehyde 5% v/v. A new combination method was successfully developed and optimized for immobilization of treated whole cells on chitosan matrix.
Keywords: Chitosan; Penicillin G acylase; E. coli (ATCC 11105); Immobilization; Glutaraldehyde; N-cetyl-N,N,N-trimethyl bromide (CTAB)
INTRODUCTION
Over the past several decades, enzyme catalysis has become increasingly important in industry. The main goal of using enzymes in industry is to develop environmentally friendly and cost benefit processes(1,2). However, high cost of isolation and purification, structural instability, sensitivity of enzymes to processes conditions (such as pH, temperature, shearing force and trace elements), short operational lifetime, difficult recovery processes and contamination of products with native enzymes are the major problems involved in industrial uses of intact enzymes(2,3). Therefore, enzymes are often used in immobilized forms(4). Based on the interactive force linking an enzyme to its solid support, enzyme immobilization can be performed physically or chemically. Adsorption of enzymes on a solid support and entrapment or encapsulation in a polymeric network is examples of physically immobilization techniques. Cross-linking; Cross-Linked Enzyme Aggregate (CLEA), Cross-Linked Enzyme Crystal (CLEC), Cross-Linked Spray-Dried Enzyme (CLSD) and carrier binding are common methods using chemical reactions for immobilization. Enzymes can be immobilized as either the isolated form or the whole cells(5) and the cells can be immobilized in a viable or a nonviable form. Immobilization of whole cells avoids expensive procedures for enzyme purification and preserves enzymes in its natural environment thus protecting them from inactivation. The major problem with whole cell biocatalysts is the limitation in diffusion of substrates and products through cell wall(6,7). Permeabilization of intact cells before immobilization is a method for processes that require use of single enzymes without cofactors which accumulate in periplasmic space (such as penicillin G acylase (PGA) in E. coli)(8).
The properties of immobilized enzymes are derived from the properties of both the enzyme and the support matrix. If a proper matrix is selected, the operational performance of the immobilized system can be greatly enhanced. Desirable characteristics of supports for an enzyme immobilization include: high affinity to proteins, availability of reactive functional group for direct reactions with enzymes and for chemical modifications, hydrophilicity, mechanical stability and rigidity and ease of preparation in different geometrical configurations (such as bead, fiber, film, membrane and powder)(9,10). Non-toxicity and biocompatibility of the materials are also required in pharmaceutical, food and medicinal industry. Furthermore, the material should be biodegradable and cost-benefit. Many of the carriers have been considered and studied for immobilizing enzymes. Chitin and chitosan are often ideal as they offer most of the aforementioned characteristics.
One of the successful examples of industrial enzymatic catalysis is the application of penicillin acylase (PA) in the production of 6-aminopenicillanic acid (6-APA) and 7-aminodeacetoxy cephalosporanic acid (7-ADCA), intermediates for a wide range of β-lactam semi-synthetic antibiotics. Bulk 6-APA can be achieved through either chemical routes or enzymatic hydrolysis. The industrial method for 6-APA bio-production was developed in 1960 and today all 6-APA is produced by the immobilized enzymatic route with PA(11).
The goal of current study is immobilization of PGA as permeabilized whole E. coli cells and involves three steps; treating the E. coli whole cells with CTAB (permeabilization), cross-linking with glutaraldehyde and beads preparation by neutralization the cross-linked mixture with NaOH-MeOH solution. The novelty of this study is related to combination of these three steps. The effects of glutaraldehyde and CTAB concentrations on preparation of beads and cell permeabilization were also studied in an attempt to optimize the immobilization conditions.
MATERIALS AND METHODS
Materials
E. coli (ATCC 11105) was obtained in lyophilized form from American Type Culture Collection (ATCC). Penicillin G potassium and 6-Aminopenicillanic Acid (6-APA) were gifts from Antibiotic-sazi Sari Company (Iran). Chitosan (75% deacylated, medium molecular weight), Glutaraldehyde, N-cetyl-N,N,N-trimethyl Bromide (CTAB), p-dimethyl amino benzaldehyde (ρ-DBA), phenylacetic acid (PAA), ammonium chloride, magnesium sulfate, ethanol, glacial acetic acid and sodium hydroxide were from Merck (Germany). All other chemicals and materials were of analytical grade.
Media and cultures
Lyophilized E. coli (ATCC 11105) was revitalized in Muller-Hinton broth (MHB) (24 h at 37°C). The cells were harvested by centrifugation at 4°C for 5 min at 10000 rpm and washed thoroughly with 0.1 M phosphate buffer pH 7 and resuspended in phosphate buffer. The resulting cell suspension was inoculated in a medium that had been previously optimized with respect to PGA production(12). This medium was consisted of: Phenylacetic acid (PAA) (0.2% w/v), MgSO4.7 H2O (0.02% w/v), yeast extract (0.5% w/v), NH4Cl (0.1% w/v), K2HPO4(0.1% w/v) and KH2PO4 (0.01% w/v) and the pH of medium was adjusted to 7.The cells were grown in shaking flask at 220 rpm, 24°C for 44 h. The cells were harvested by centrifugation (10000 rpm, 5 min and 4°C) and washed twice with 0.1 M phosphate buffer, pH 7.
Treatment with N-cetyl-N, N, N-trimethyl ammonium bromide
CTAB solutions with different concentrations, ranging from 0.05 to 0.25 % w/v with 0.05 intervals, were prepared in phosphate buffer (pH 7, 0.1 M, 4°C) containing 10 gwet cells from previous step. Each mixture was gently stirred at room temperature and treated cells were recovered after 45 min by centrifugation at 8000 rpm, 4°C for 10 min.
Preparation of chitosan beads
The pH-dependent solubility behavior of chitosan was used for preparation of the beads. The permeabilized cells (10 gwet cells) were added to 10 ml of chitosan solution (3% w/v) in acetic acid 3% v/v. Using a syringe, a portion of this mixture was added drop-wise to a solution containing NaOH (1M):MeOH (80/20) and glutaraldehyde (ranging from 1-9% v/v with 2 intervals) as a cross-linking agent and the remaining mixture was added to a medium devoid of glutaraldehyde under gentle stirring (45 rpm). After 1 h, the beads were washed thoroughly and stored in phosphate buffer at 4°C (pH 7, 0.1 M).
Hydrolytic activity assay
Hydrolytic activity assay was performed using Balasingham-Ehrlich method(13). According to this method, produced 6-APA from penicillin G hydrolysis reacts with p-dimethyl amino benzaldehyde (p-DBA) as the indicator, and produces a color compound, which can be measured by colorimetric assay. The amount of enzyme produces 1 μmol/min 6-APA upon addition of a solution of penicillin G (2%) at pH 7.8 is defined as 1 unit (U) of the hydrolytic activity.
Morphological characterization
The surface morphology of chitosan beads was observed by Scanning Electron Microscope (SEM). The beads were freeze-dried coated with gold and observed. The SEM was performed in Materials Department of Isfahan Technical University using scanning electron microscope (Phillips XL-20, Japan).
Operational stability
The operational stability of the immobilized biocatalysts and the cells treated with CTAB were analyzed by measuring hydrolytic activity of the biocatalysts in different batches. At the end of each batch, phosphate buffer (0.1 M, pH 7) was used to remove any substrate or product retained in the matrix and the immobilized biocatalyst was introduced into a fresh medium. The activity was measured at the end of each batch.
RESULTS
Effect of CTAB concentration on cell permeabilization
Fig. 1 shows the residual activity of treated cells at different concentrations of CTAB. By increasing the concentration ranging from 0.05 to 0.1% w/v the residual activity was increased.
Fig. 1.
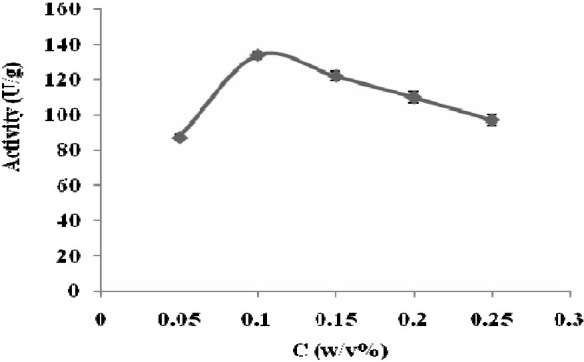
Effect of CTAB concentrations on activity of E. coli 11105 cells. The enzyme activity was assayed based on Balasingham method (pH 7.8, T 37°C). Data points are the averages of 3 repeats. Error bars represent standard errors.
Effect of glutaraldehyde concentration on cell immobilization
The residual activity of the immobilized biocatalyst was determined at different concentrations of glutaraldehyde. The conversions showed that the best concentration of glutaraldehyde used for immobilization of PGA on chitosan was 5% w/v (Fig. 2).
Fig. 2.
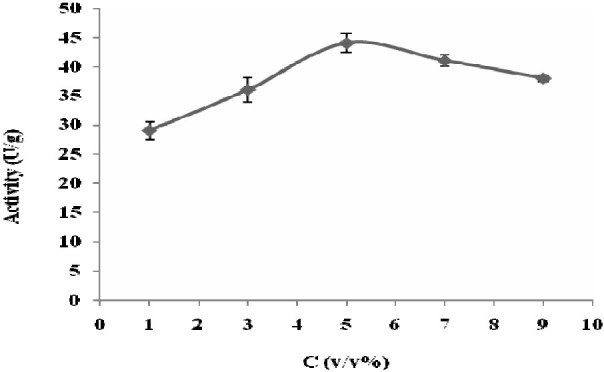
Effects of glutaraldehyde concentrations on activity of immobilized biocatalysts. The activity was determined based on Balasingham method (pH 7.8, T 37°C). Data points are the averages of 3 repeats. Error bars represent standard errors.
Beads size and shape
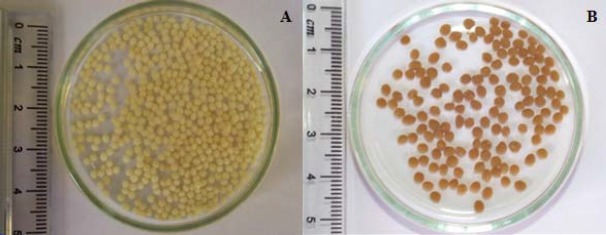
Treated cells were entrapped in chitosan matrix as described before. The beads prepared using glutaraldehyde, were brown and semi spherical (Fig. 3). The beads produced without glutaraldehyde were light beige and semi spherical. The average of diameters of 50 white beads and 50 brown beads were 1.70 and 2.32 mm, respectively. Nozzle diameter, the distance between nozzle and medium and stirring speed had important role on beads shape.
Fig. 3.
Digital photos of the prepared chitosan beads containing permeabilized E. coli 11105 cells. The beige beads (A) were prepared using NaOH:MeOH (80/20) and the brown beads (B) were prepared using NaOH-MeOH with glutaraldehyde (5% v/v).
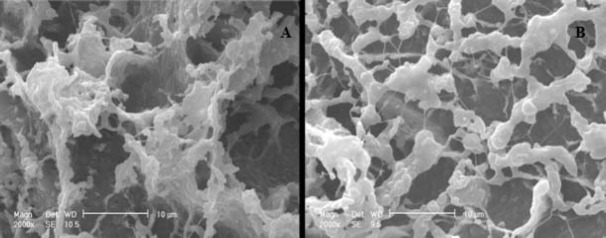
The SEM photos show that the pores in brown beads are spread uniformly over and are equal in size (Fig. 4). Therefore, transmission of the substrate (PG) and the final product (6-APA) between the medium and the biocatalysts entrapped in beads can easily occur. In beige beads prepared by NaOH:MeOH without glutaraldehyde, the size of pores is larger but they are not uniformly scattered. This could be due to the absence of glutaraldehyde in beads production procedures. Therefore, using glutaraldehyde produced uniform pores which could increase the substrate/product mass transfer. As a result, penicillin hydrolysis reaction can occur faster with better conversion.
Fig. 4.
SEM from beige (A) and brown (B) beads prepared using chitosan as matrix. The beads were freeze-dried, coated with gold and observed microscopically.
Hydrolytic activity of different forms of the biocatalysts
Intact cells, permeabilized cell and the two others immobilized biocatalysts (brown and beige beads) were added to substrate solution (penicillin G potassium 2%) and the conversion was calculated after 15 min for each biocatalyst (Fig. 5).
Fig. 5.
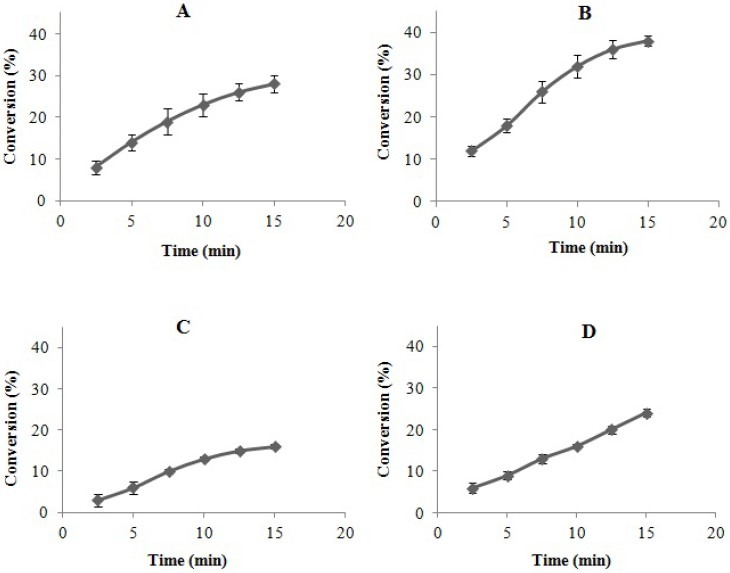
Conversion of different forms of the biocatalyst. The hydrolytic activity was performed at pH 7.8 and 37°C. The substrate solution was penicillin G potassium 2% (w/v). Data points are the mean of 3 repeats. Error bars represent standard deviations. A) Intact cells, B) CTAB-treated cells, C) beigebeads D) brown beads.
Operational stability
The immobilized enzyme and treated cells were used in hydrolytic batch reactions in order to determine the stability of biocatalyst in industrial applications. In treated cells, the residual activity after the fifth cycles of use was only 15% of the initial value (Fig. 6). However, the enzyme immobilized with glutaraldehyde showed good stability, and after 20 cycles of use, 90% of the initial activity was remained. Therefore, reuse of cells treated with CTAB resulted in a rapid loss of the cell-bound PA activity, but incubation of CTAB-treated cells immobilized with glutaraldehyde effectively prevented the leakage of enzyme from the cells. After 210 days storage in phosphate buffer at 4°C, only 16% of the activity was lost.
Fig. 6.
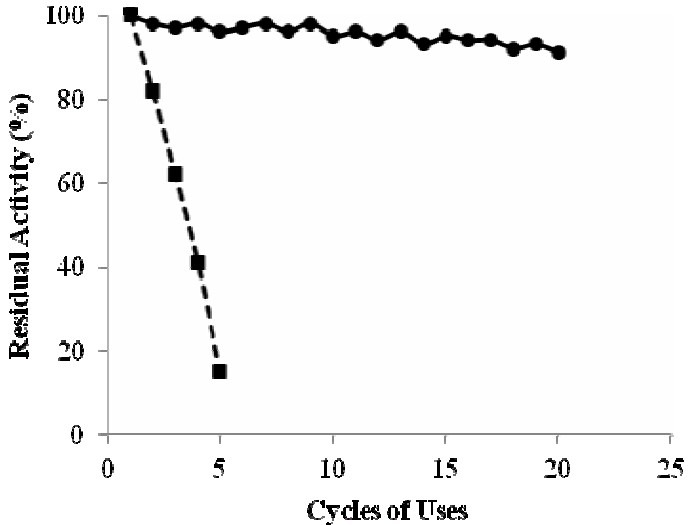
Operational stability of E. coli cells permeabilized with CTAB (0.1% w/v) and the cells immobilized using chitosan and glutaraldehyde (5% v/v). Squares (■) data point represent permeabilized cells but un-immobilized, and circles (●) are related to the immobilized cells. Each cycle was conducted at 37°C and pH 7.8 and one day intervals.
DISCUSSION
CTAB is a tertiary ammonium compound capable of dissolving proteins ant fatty acids in cell wall and cell membrane and make them porous. It appears the optimal concentration of CTAB for permeabilization of E. coli cells was 0.1% w/v. In this concentration, the maximum activity was achieved (134 U/gwet cells) which was 32 U/gwet cells higher than the maximum conversion of the intact cells. This could be attributed to the reduction of diffusional restriction imposed by cell wall. Prabhune and coworkers have also found out that the best concentration of CTAB for immobilization of PGA is 0.1% w/v (30 min, 4°C, gentle stirring)(14). Norouzian and coworkers have reported that treating cells with 0.1% w/v CTAB, resulted 40% increase in the relative rate of enzymatic hydrolysis of PGA compared to untreated cells(15).
Glutaraldehyde has been widely used as a cross-linking agent and seems to be one of the detrimental parameters for optimization of immobilization. Therefore, residual activity of the immobilized biocatalyst was determined at different concentrations of glutaraldehyde. The conversions showed that the optimal concentration of glutaraldehyde used for immobilization of PGA on chitosan was 5% w/v (Fig. 2). At this concentration, the activity was 56 U/g which decreased 90 U/g compared to intact cells. This reduction might be due to non-specific reaction of glutaraldehyde, which can change the conformation of the enzyme active and binding sites.
Glutaraldehyde is reacted with sulfunyl group of cysteine, α-amino group of lysine and phenolic and imidazole ring of histidine. Since the active site of PGA contains lysine, tryptophan, serine, arginine and glutamine, excess amounts of glutaraldehyde may cause conformational changes in the enzyme and its active site(16). At low concentrations, it is possible that both aldehyde groups of glutaraldehyde become cross-linked with only amino groups of chitosan and as a result, few free aldehyde groups would be available for interaction with the cell/enzyme.
Comparison the activities of immobilized cells using glutaraldehyde with CTAB-treated cells showed that glutaraldehyde effectively prevented the leakage of the enzyme from the cells. Immobilization of acid phosphatase in chitosan using glutaraldehyde showed that by increasing the glutaraldehyde concentration, the activity of biocatalyst initially decreased and then increased(17). This observation could be explained by the change in binding sites accessible for the enzyme immobilization. It has been reported that the optimal glutaraldehyde concentration is in the range of 0.1-1% w/v(17). When compared to our results, this lower amount of glutaraldehyde might be due to different nature of acid phosphatase and PGA (particularly in active site of enzymes). Another possible explanation for this difference is the protecting effect of E. coli cell from direct effect of glutaraldehyde on the native enzyme.
The molecular weight of substrate played an important role in enzyme activity. Low molecular weight compounds (300 Da) could be released easier from these beads. This limitation in mass transfer revealed the important role of uniform bead porosity caused by glutaraldehyde(18).
In general, immobilization results in partial loss of the activity due to the changes in the enzyme environment, physicochemical prope-rties of the matrix surrounding the enzyme or the cell, matrix-substrate interactions, and degradation reactions during immobilization processes (Fig. 5).
The maximum conversions of intact cells and permeabilized cells after 15 min were 29% and 38%, respectively. Therefore, using CTAB caused 9% increase in conversion compared to intact cells. This could be due to a reduction of the diffusional restriction imposed by cell wall (as discussed previously), the porosity of the beads and molecular weight of the substrate which could affect the activity of the biocatalyst. However, as a result of permeabi-lization of the cell wall, which causes release of the periplasmic content, the attachment of PGA to the cell, and reduction of diffusional restriction, permeabilized cells showed higher activity compared to the free cells.
Maximum conversion in the immobilized form was observed for biocatalysts immobilized with glutaraldehyde. Compared to the permeabilized cells, there was a 67% loss in the activity. The maximum activities of the biocatalysts immobilized with and without glutaraldehyde, were 44 U/g and 14 U/g, respectively. This may show the efficiency of glutaraldehyde in the immobilization processes.
Another point that should be considered is the trend of conversion lines which can be seen in Fig. 5. The activity of glutaraldehyde-immobilized cells (Fig. 5-D) was more linear than the others. This helps to predict the end of the reaction more precisely. The activity of the CTAB-treated cells immobilized within polyacrylamide beads was about 30% higher than the untreated cells(14). This might be attributed to the lower diffusional restrictions in the permeabilized cells. Our results showed that, permeabilization of the cells caused 23% increase in activity.
CONCLUSION
We have successfully permeabilized E. coli cells with CTAB, and immobilized in chitosan with glutaraldehyde as cross-linking agent. We have also optimized CTAB and glutaraldehyde concentrations. These results and the stability of the biocatalyst showed that the beads prepared using this method have potential for industrial applications. Use of continuous reactor can eliminate product inhibition and increase the activity of the biocatalysts and rate of the reaction. Thus, we recommend this type of reactor to be used in future works. Also we recommend using particles and freeze dried permeabilized cells instead of beads. This may allow superior loading of the cells, reduce the restriction of mass transfer and improve residual activity of the biocatalyst. The optimization of processing variables involved in bead preparation (i.e. chitosan molecular weight and degree of deacetylation and its concentration, environmental conditions; pH. Ionic strength, stirring speed) is also highly recommended.
REFERENCES
- 1.Martínez-Hernández JL, Mata-Gómez MA, CristóbalNoé, Aguilar-González CN, Ilyina A. A process to produce penicillin G acylase by surface-adhesion fermentation using mucorgriseocyanus to obtain 6-aminopenicillanic acid by penicillin g hydrolysis. Appl Biochem Biotechnol. 2010;160:2045–2053. doi: 10.1007/s12010-009-8768-8. [DOI] [PubMed] [Google Scholar]
- 2.Jin X, Wu Q, Chen Q, Chen C, Lin X. Immobilization of penicillin G acylase on a composite carrier with a biocompatible micro-environment of chitosan. J Chem Technol Biotechnol. 2008;83:1710–1716. [Google Scholar]
- 3.Adriano WS, Filho EHC, Silva JA, Giordano RLC, Gonçalves LRB. Stabilization of penicillin G acylase by immobilization on glutaraldehyde-activated chitosan. Braz J of Chem Eng. 2005;22:529–538. [Google Scholar]
- 4.Mateo C, Palomo JM, Fernandez-Lorente G, JGuisan JM, Fernandez-Lafuente R. Review: Improvement of enzyme activity, stability and selectivity via immobilization techniques. Enzyme Microb Technol. 2007;40:1451–1463. [Google Scholar]
- 5.Chandel AK, Rao LV, Narasua ML, Singh OV. Review: The realm of penicillin G acylase in β-lactam antibiotics. Enzyme Microb Technol. 2008;42:199–207. [Google Scholar]
- 6.Bernardino SMSA, Gallegos JFM, Santhagunam A, de Barros DPC, Fernandes P, Fonseca LP. Immobilization of penicillin G acylase by entrapment in a sol-gel matrix with magnetic properties. J Biotechnol. 2007;131:1–4. [Google Scholar]
- 7.Adikane HV, Thakar DM. Studies of penicillin G acylase immobilization using highly porous cellulose-based polymeric membrane. Appl Biochem Biotechnol. 2010;160:1130–1145. doi: 10.1007/s12010-009-8686-9. [DOI] [PubMed] [Google Scholar]
- 8.Krzeslaka J, Brauna P, Voulhouxb R, Coola RH, Quax WJ. Heterologous production of Escherichia coli penicillin G acylase in Pseudomonas aeruginosa. J Biotechnol. 2009;142:250–258. doi: 10.1016/j.jbiotec.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 9.Krajewsk B. Application of chitin and chitosan-based materials for enzyme immobilization: a review. Enzyme Microb Techol. 2004;35:126–139. [Google Scholar]
- 10.Felt O, Bori P, Gurny R. Chitosan: a unique polysaccharide for drug delivery. Drug Dev Ind Pharm. 1998;24:979–993. doi: 10.3109/03639049809089942. [DOI] [PubMed] [Google Scholar]
- 11.Van Langen LM. Penicillin acylase: Properties, application and prospects in the biocatalyst production of fine chemicals. Netherlands: Delft University of Technology; 2001. pp. 1–129. [Google Scholar]
- 12.Abedi D, Korbekandi H, Haghighi B, Kalantari S. A preliminary study on production of Penicillin G acylase from E. coli 11105. Thesis. Isfahan University of medical sciences. 2006:25–63. [Google Scholar]
- 13.Balasingham K, Warburton D, Dunnil P, Lilly MD. The isolation and kinetics of penicillin amydase from E. coli. Biochem Biophys Acta. 1972;276:250–256. doi: 10.1016/0005-2744(72)90027-7. [DOI] [PubMed] [Google Scholar]
- 14.Prabhune AA, Rao BS, Pandle AV, SivaRaman H. Immobilization of permeabilized Escherchia coli cell with penicillin acylase activity. Enzyme Microb Techol. 1992;14:161–163. doi: 10.1016/0141-0229(92)90176-o. [DOI] [PubMed] [Google Scholar]
- 15.Norouzian D, Javadpour S, Moazami N, Akbarzadeh A. Immobilization of whole cell penicillin G acylase in open pore gelatin matrix. Enzyme Microb Techol. 2002;30:26–29. [Google Scholar]
- 16.Chetan BP, Vilas GG. Adsorption and immobilization of penicillin G acylase on chitosan beads. Sep Scie Tech. 2004;39:2655–2675. [Google Scholar]
- 17.Juang RS, Wo FC, Tseng RL. Solute adsorption and enzyme immobilization on chitosan beads prepared from shrimp shell wastes. Bioresour Techol. 2001;80:187–193. doi: 10.1016/s0960-8524(01)00090-6. [DOI] [PubMed] [Google Scholar]
- 18.Gonzales SMI, Lang E, Carreno-Gomez B. Enzyme encapsulation on chitosan microbeads. Process Biochem. 1997;32:211–216. [Google Scholar]