Abstract
Quinazolinone ring system is renown because of its wide spectrum of pharmacological activities due to various substitutions on this ring system. In this study, the minimum inhibitory concentration of the synthesized compounds in our laboratory was determined by micro dilution Alamar Blue® Assay against six strains of bacteria (three Gram-positive and three Gram-negative) and three strains of fungi. Following a broth micro dilution minimum inhibitory concentration (MIC) test, Minimum Bactericidal Concentration (MBC) and Minimum Fungicidal Concentration (MFC) tests were performed. Cytotoxic effects of the compounds were measured using the MTT colorimetric assay on HeLa cell line. Results of antimicrobial screening showed that compounds had better bacteriostatic activity against Gram-negative bacteria. Results from MBC revealed that these compounds had more significant bacteriostatic than bactericidal activities. Nearly all screened compounds showed good activity against C. albicans and A. niger. Results from MFC indicated that these compounds had better fungistatic rather than fungicidal activities. The synthesized target molecules were found to exhibit different cytotoxicity in the range of 10 to 100 μM on HeLa cell line. Compounds 6 and 7 exhibited acceptable cytotoxicity approximately 50% at 10 μM concentration.
Keywords: Antibacterial, Antifungal, Cytotoxicity, Gram-negative, Gram-positive
INTRODUCTION
Quinazolinone is one of the most important and prosperous structures in medicinal chemistry. Quinazolinone derivatives have been used in medicine as antibacterial, antifungal, anti tuberculosis, anticancer and anti-inflammatory agents(1–6). The increased rate of resistance to ongoing antimicrobial agents and the advent of durable tumor cell to a wide range of cytotoxic drugs inspired us to the search for more effective agents. Quinazolinones have emerged as antimicrobial agents because of their broad spectrum of in vitro and in vivo chemotherapeutic activities(7,8). Substitution at position 3 of quinazolin-4(3H)-ones has been reported to be associated with anti microbial properties(5).
Introduction of bromine or chlorine atom at positions 6 and 8 can improve their antimicrobial activities(3). Deoxyvasicinone is an alkaloid with tricyclic 4(3H)-quinazolinone structure and has considerable antimicrobial activity(9).
Interest in quinazolines as anticancer agents has further increased since the discovery of raltitrexed (Tomudex®) as an antimetabolite drug used in cancer chemotherapy. It is an inhibitor of thymidylate synthase, which manufactured by AstraZeneca(10). Cytotoxic activity of the quinazolinone derivatives in various cell lines such as HeLa(2,5), L1210 (mouse lymphocytic leukemia)(11,12) and HT29 (human colon adeno-carcinoma)(13) have been reported.
In our previous work(14), we have reported the synthesiz of a series of novel quinazolinone derivatives (fused pyrazolo or pyridazino-quinazolinones and fused pyrrolo-quinazolinones) using chloroacyl chlorides and substituted anthranilic acids in order to elucidate the role of structural features and substituents on antimicrobial and/or cytotoxic activities of these compounds
In the current study, the minimum inhibitory concentration (MIC) of the synthesized compounds was determined by micro dilution Alamar Blue® Assay against six strains of bacteria (three Gram-positive and three Gram-negative) as well as the three strains of fungi. Following a broth microdilution MIC test, MBC (Minimum Bactericidal Concentration) and MFC (Minimum Fungicidal Concentration) tests were performed(15). Cytotoxic effects of the compounds were measured using the MTT (3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl tetrazolium bromide) colorimetric assay on HeLa cell line(16).
MATERIALS AND METHODS
Antimicrobial activity
Materials
Tested bacteria were three Gram-positive bacteria obtained from Persian Type Culture Collection (PTCC).: (Staphylococcus aureus PTCC 1337, Bacillus subtilis PTCC 1023 and Listeria monocitogenes PTCC 1165) and three Gram-negative bacteria: (Escherichia coli PTCC 1338, Pseudomonas aeruginosa PTCC 1074, and Salmonella entritidis PTCC 1091). Tested fungi were one yeast-like fungus (Candida albicans PTCC 5027) and two molds (Aspergillus niger PTCC 5021 and Aspergillus flavous PTCC 5003) obtained from PTCC. Mueller hinton agar, Mueller hinton broth and sabouraud dextrose agar were purchased from Merck. Roswell Park Memorial Institute (RPMI)-1640 culture medium was purchased from Gibco, USA.
Microplate alamar blue assay for antibacterial evaluation
MIC was determined by micro plate alamar blue assay (MABA) method. Mueller hinton agar was used to culture bacterial strains. The inocula of microorganisms (1.5 ×108 CFU/ml) were prepared from cultures. Microbial suspensions were adjusted to 0.5 Mc Farland standard turbidity(15,17). Compounds were dissolved in DMSO (0.5 ml) and diluted with water up to 1 ml to obtain concentration of 5120 μg/ml as stock solutions. The serial dilution method was used to obtain 2560 to 320 μg/ml concentrations(18). Mueller-Hinton broth was used as medium for bacterial growth. 20 μl of each concentration were distributed in 96-well plates with the exception of those wells acting as growth control (contain microorganisms and culture media) and positive control (contain microorganisms and standard antibiotic). After adding Alamar Blue® reagent (20 μl) to all of the 96 wells, total volume in each well became 200 μl. The final concentrations of compounds were (512-32 μg/ml) and the final concentration of inocula was 1.5 × 104 for bacteria(15,17). Plates were covered and sealed with parafilm and incubated for 24 h at 37°C. The MIC was defined as the lowest concentration, which prevented a color change from blue to pink. Ciprofloxacin was used as standard antibacterial drug(19,20).
Microplate alamar blue assay for antifungal evaluation
Sabouraud dextrose agar was used to culture fungal strains. The inocula of microorganisms were prepared from cultures. The turbidity was measured spectrometrically at 580 nm. The concentration of inocula with transmittance 0.5-0.6 is approximately (1.5 × 108 CFU/ml). RPMI 1640 was used as medium for fungal growth. 20 μl of each concentration were distributed in 96-well plates with the exception of those wells acting as growth control (contain microorganisms and culture media) and positive control (contain microorganisms and standard antifungal agent). After adding Alamar Blue® reagent (20 μl) to all of the 96 wells, total volume in each well became 200 μl. The final concentrations of compounds were (512-32 μg/ml) and the final concentrations of inocula were 1.5 × 105 for fungi. Plates were covered and sealed with parafilm and incubated for 48 h at 25°C. Ketoconazole was used as standard antifungal agent(15,17).
Following a broth micro dilution MIC test, from each well that shows no growth, contents were removed and spreaded onto mueller hinton agar plates for bacteria and saboraud dextrose agar for fungi to determine MBC and MFC results. The plates incubated for 24 h at 37°C for bacteria and 25°C for fungi(15,17).
Cytotoxic activity
Cell line and cell culture
HeLa cell line was obtained from the cultures maintained in the cytotoxicity laboratory of the school of pharmacy, Isfahan University. Roswell Park Memorial Institute (RPMI)-1640 culture medium, fetal calf serum (FCS) and trypsin-EDTA were purchased from Gibco, USA. Penicillin/streptomycin and 3-(4, 5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) were purchased from Sigma, USA. Doxorubicin vial was purchased from Merck, Germany. The absorbance was measured at 540 nm using an ELISA plate reader (Awarwness, USA).
Sample and culture media preparation
HeLa cells were grown in RPMI 1640 medium. Each 500 ml of the medium consisted of 5.2 g RPMI powder, 1 g of sodium bicarbonate, 5 ml of penicillin/streptomycin (10000 IU/ml/10 mg/ml) supplemented with 50 ml heat-inactivated fetal calf serum (FCS) in deionized water. The completed media was sterilized by filtering through 0.22 micron microbiological filters. The pH of the medium was then adjusted to 7.4 using concentrated HCl or NaOH and kept at 4°C before use(21). Cell lines were maintained in a humidified atmosphere of 5% CO2 and 95% air at 37°C.
The stock solutions of compounds (10 mM, 1 ml) were prepared in DMSO and appropriately diluted with the medium to obtain 10, 100, 1000 μM concentrations. Finally 20 μl of each dilution was added to the 96-well micro plate containing 180 μl of the cell suspensions in order to reach 1, 10, 100 μM concentrations. Doxorubicin was used as positive control at 1 μM final concentration in the wells.
In vitro cytotoxicity assay
The cytotoxic effects of compounds against HeLa cells were determined by a rapid colorimetric assay using MTT. The results were compared with untreated control. Briefly, after 2-3 subcultures, 180 μl of the cells (5 × 104 cells/ml of media) were seeded in 96-well micro plates and incubated for 24 h (37°C, air-humidified 5% CO2). 20 μl of different concentrations of the samples were then added and the micro-plates were further incubated for 48 h at the same condition. The first column of the micro-plate containing 180 μl of the cell suspension and 20 μl of the medium was regarded as negative control while the blank wells consisted of only 200 μl of the RPMI medium. To evaluate the cell survival, each well was then treated with 20 μl of MTT solution (5 mg/ml in phosphate buffer solution) for 3 h. Afterwards, the media in each well was gently replaced with 200 μl DMSO and pipetted up and down to dissolve the formazan crystals(16,21). The absorbance of each well was measured at 540 nm using an ELISA plate reader.
RESULTS
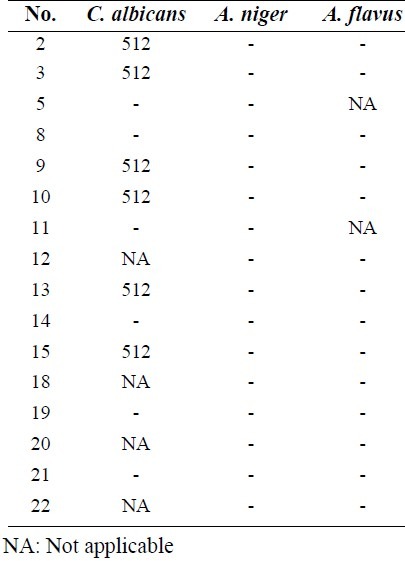
Antimicrobial results showed that compounds (Fig. 1) had better bacteriostatic activity against Gram-negative bacteria (Table 1). Results from MBC revealed that these compounds had more significant bacteriostatic than bactericidal activities (Table 2). Almost all screened compounds had good activity against C. albicans and A. niger (Table 3). According to MFC results these compounds were better fungistatic rather than fungicidal agents (Table 4).
Fig. 1.
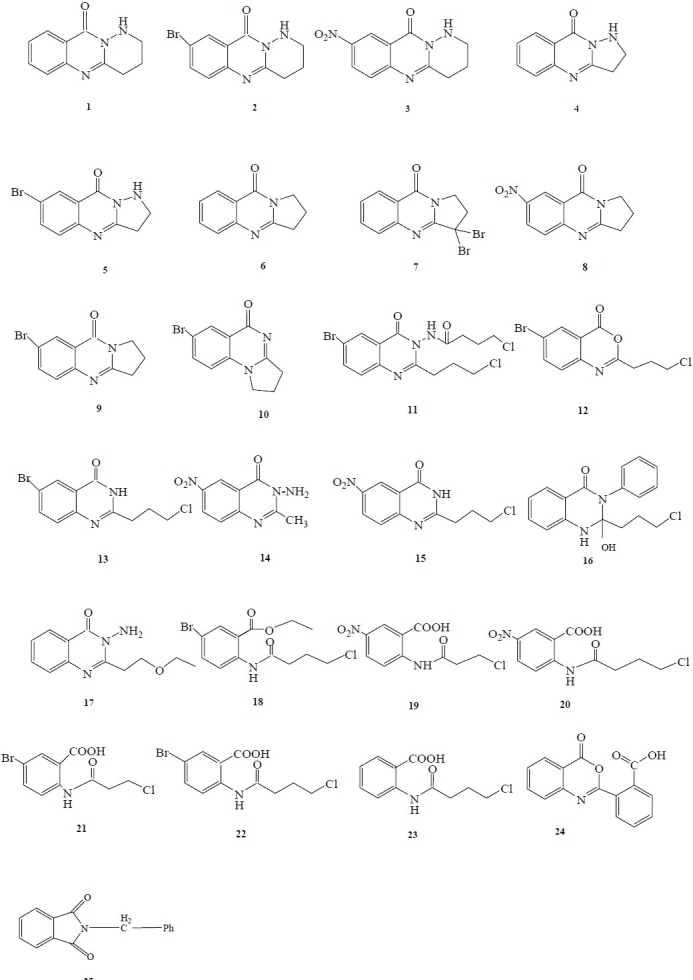
Screened compounds in antimicrobial and cytotoxicity tests
Table 1.
MIC results of synthesized compounds against tested bacteria
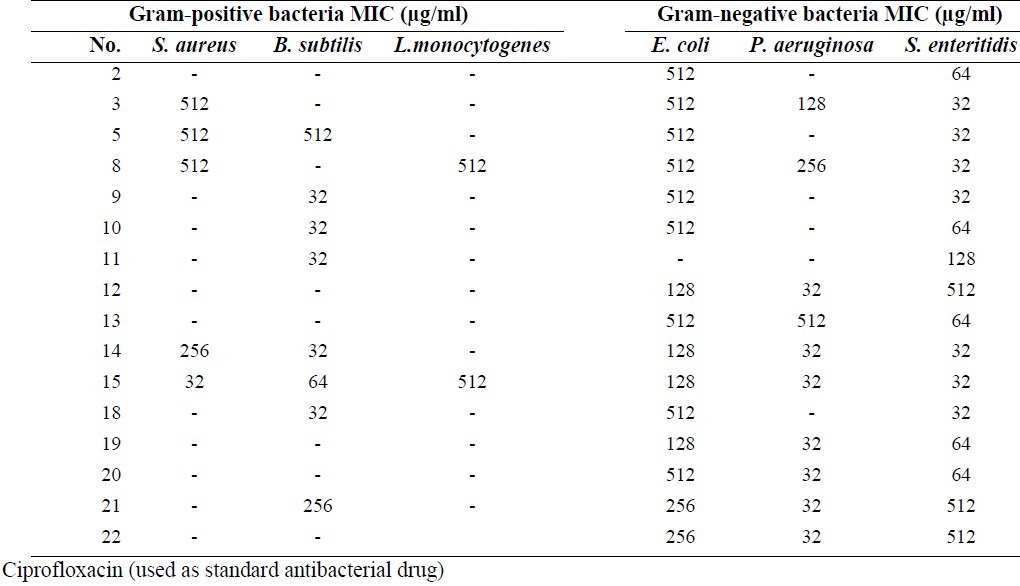
Table 2.
MBC results of synthesized compounds against tested bacteria
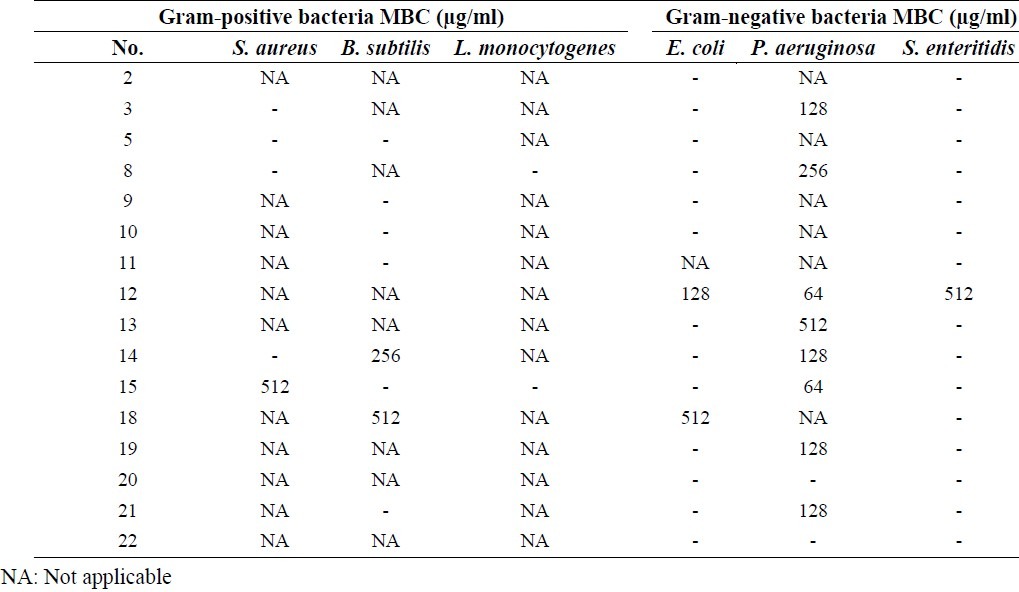
Table 3.
MIC results of synthesized compounds against tested fungi.
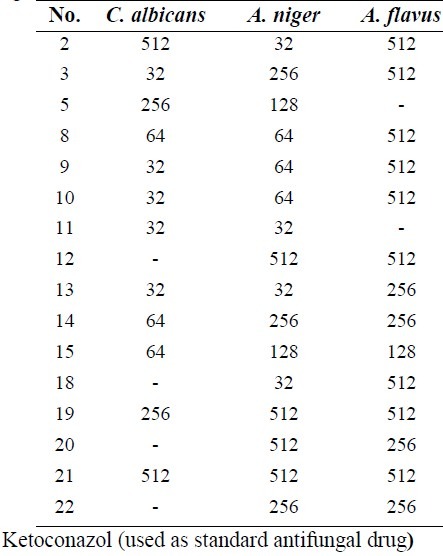
Table 4.
MFC results of synthesized compounds against tested fungi
The cytotoxicity of compounds were evaluated against HeLa cell line at different concentrations (final concentrations 1, 10, and 100 μM) using MTT assay. The MTT test is based on the ability of viable cells to produce formazan from the cleavage of the tetrazolium salt by functional mitochondria. This reduction takes place only when mitochondrial reductase enzymes are active and therefore conversion can be directly related to the number of viable cells(21). The percentage of cell viability (Table 5) was calculated using the following formula:
Table 5.
Summary of cytotoxic results
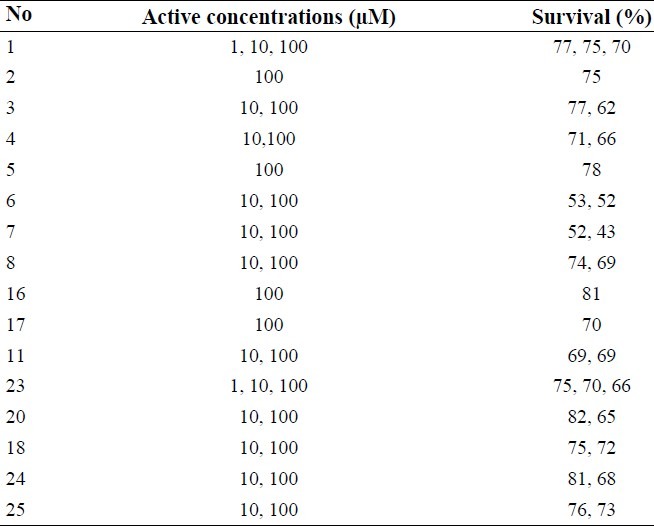

Absorbances were measured at 540 nm using an ELISA plate reader. Results were assessed by One-way ANOVA analysis. The synthesized target molecules were found to exhibit different cytotoxicity in the range of 10 to 100 μM in HeLa cell line.
DISCUSSION
The results of antibacterial tests against Gram positive bacteria showed that compounds 9, 10, 11, 14, 18 and 15 had the highest activities against B. subtitles at 32 or 64 μg/ml concentrations and compound 15 displayed high activity against S. aureus at 32 μg/ml concentration. Diverse examples of quinazolinone antimicrobial agents which exerted antibacterial effects against staphylococcus aureus at a very low concentration have been reported(3).
Results against Gram negative bacteria indicated that compounds 14, 15, 12 and anthranilic acid derivatives showed acceptable activities against P. aeruginosa. All anthranilic acid derivatives with a free carboxylic acid group and chlorine atom at the end of the side chain showed good activity against P. aeruginosa at 32 μg/ml concentration. Compounds 12 with benzoxazinone structure and chlorine atom at the end of the side chain exhibited activity against P. aeruginosa at 32 μg/ml concentration. This could be due to the unstability of benzoxazinone ring and possible cleavage to free carboxylic acid derivatives. All compounds containinge nitro or bromine substitutions on the phenyl ring of quinazolinone or anthranilic acid derivatives showed good activity against S. entritidis in a broad range of concentrations (from 32 to 512 μg/ml). It was reported that bromine or chlorine substitutions at positions 6 and 8 of the phenyl ring of quinazolinone can improve their antimicrobial activities(3). In anthranilic acid derivatives presence of a nitro group can increase activity against S. entritidis more than bromine atom as shown in compounds 19 and 20 compared to 21 and 22. Almost all of the screened compounds showed good activity against C. albicans and A. niger. Fused pyrolo-quinazolinone derivatives 8, 9 and 10 exhibited good activity against C. albicans and A. niger at 32 or 64 μg/ml concentrations. Fused pyridazine-quinazolinone derivatives 2 and 3 had acceptable antifungal activity against A.niger or C. albicans at 32 μg/ml concentration. Compounds 13 and 11 showed good antifungal activity against C. albicans and A.niger at 32 μg/ml concentration and compounds 14 and 15 had good activity against C. albicans at 64 μg/ml concentrations (Table 3).
The obtained results of cytotoxic tests (Table 5) indicated that fused pyridazine-quinazolinone derivatives 1, 2 and 3 exhibited significant differences in viability (P<0.05) compared to the negative control at 1, 10, 100 μM, 100μM and 10, 100 μM concentrations, respectively. It seems that bromine substitution on the phenyl ring could decrease the cytotoxic effect. In case of fused pyrazol-quinazolinone derivatives, compounds 4 and 5 showed significant differences in viability (P<0.05) compared to the negative control e observed at 10, 100 μM and 100 μM concentrations, respectively. Existence of bromine on the phenyl ring could not improve the cytotoxic effect in these derivatives. All tested compounds with fused pyrolo-quinazolinone structure 6, 7 and 8 exhibited significant differences (P<0.05) in viability in comparison with the negative control at 10, 100 μM concentrations. Among compounds with various substituents at position 3 of the quinazolinone ring, compound 11 showed significant differences (P<0.05) with the negative control at 10, 100 μM concentrations and compounds 16, 17 showed significant differences (P<0.05) in viability at 100 μM concentration. Existence of a chlorine atom at the end of the side chain and bromine on the phenyl ring could probably improve cytotoxic effect of this compound.
Compounds 24 and 25 showed significant differences (P<0.05) as compared with negative control at 10, 100 μM concentrations. In compounds 18, 20 and 23 with open ring structure, compound 23 showed significant differences (P<0.05) in viability relative to negative control at 1, 10, 100 μM concentrations while compounds 20 and 18 exhibited significant differences (P<0.05) at 10, 100 μM concentrations.
CONCLUSION
Amongst a series of new quinazolinone derivatives were previously synthesized in our laboratory, compounds 13 and 15 which displayed a structural similarity, showed different antimicrobial activities because of the differences in substitution on the phenyl ring. Both were good antifugal agents while 15 showed good antibacterial activities against Gram-positive and negative bacteria.
SAR studies indicated that the existence of a chlorine atom on the side chain of the newly synthesized compounds enhanced cytotoxicity. It was also found that the nitro substituted compounds were more cytotoxic than their bromo-containing counterparts.
ACKNOWLEDGMENT
This work was financially supported by the Research Council of Isfahan University of Medical Sciences.
REFERENCES
- 1.Patel JA, Mistry BD, Desai KR. Synthesis and antimicrobial activity of newer quinazolinone. Eur J Chem. 2006;3:97–102. [Google Scholar]
- 2.Jantova S, Stankovsky S, Spirkova k. In vitro antibacterial activity of ten series of substituted quinazolines. Biologia Bratislava. 2004;59:741–52. [Google Scholar]
- 3.Raghavendra NM, Thampi P, Gurubasavarajaswamy PM, Sriram D. Synthesis and antimicrobial activities of some novel substituted 2-imidazolyl–N -(4-oxo-quinazolin-3(4H)-yl) acetamides. Chem Pharm Bull. 2007;55:1615–1619. doi: 10.1248/cpb.55.1615. [DOI] [PubMed] [Google Scholar]
- 4.Laddha SS, Wadod Kar SG, Meghal SK. Studies on some biologically active substituted 4(3H)-quinazolinones. Part 1.Synthesis, characterization and anti-inflammatory, antimicrobial activity of 6,8-disubstituted 2-phenyl-3-[substituted- benzothiazol-2-yl]-4(3H)-quinazolinone. Arkivoc. 2006:1–20. [Google Scholar]
- 5.Dinakaran M, Selvam P, Declercq E, Sridhar SK. Synthesis, antiviral and cytotoxic activity of 6-bromo- 2,3-disubstituted 4(3H)-quinazolinones. Biol Pharm Bull. 2003;26:1278–1282. doi: 10.1248/bpb.26.1278. [DOI] [PubMed] [Google Scholar]
- 6.Cao SL, Feng YP, Jiang YY, Liu SY, Ding GY, Li RT. Synthesis and in vitro antitumor activity of 4(3H)-quinazolinone derivatives with dithiocarbamate side chains. Bioorg Med Chem Lett. 2005;15:1915–1917. doi: 10.1016/j.bmcl.2005.01.083. [DOI] [PubMed] [Google Scholar]
- 7.Kale AU, Kardile DP, Kalyane NV, Patel MR, Patel H. Synthesis and antimicrobial activity of some 2, 3-disubstituted quinazoline- 4(3H)- ones. Int J Pharm Applied Sci. 2010;1:85–90. [Google Scholar]
- 8.Mohamed MS, Kamel MM, Kassem EMM, Abotaleb N, El-moez SIA, Ahmed MF. Novel 6, 8-dibromo-4(3H) quinazolinone derivatives of anti-bacterial and anti-fungal activities. Eur J Med Chem. 2010;45:3311–3319. doi: 10.1016/j.ejmech.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 9.Potewar TM, Ingale SA, Srinivasan KV. Synthesis of tryptanthrin and deoxyvasicinone by a regioselective lithiation-intramolecular electrophilic reaction approach. Arkivoc. 2008:100–108. [Google Scholar]
- 10.Khalil A, Abdel hamide S, Al-Obaid A, El-subbagh HI. Substituted quinazolinones, Part2. Synthesis and invitro anticancer evaluation of new 2-substituted mercapto-3H-quinazolin analogs. Arch Pharm Pharm Med Chem. 2003;2:95–103. doi: 10.1002/ardp.200390011. [DOI] [PubMed] [Google Scholar]
- 11.Jiang JB, Hesson DP, Dusak BA, Dexter DL, Kang GJ, Hamel E. Synthesis and biological evaluation of 2-styrylquinazolin-4(3H)-ones, a new class of antimiotic anticancer agents which inhibit tubulin polymerization. J Med Chem. 1990;33:1721–1728. doi: 10.1021/jm00168a029. [DOI] [PubMed] [Google Scholar]
- 12.Raffa D, Edler M, Daidone G, Maggio B, Merickech M, Plescia S, et al. Synthesis, cytotoxicity, and inhibitory effects on tubulin polymerization of a new 3-heterocyclo substituted 2-styrylquinazolinones. Eur J Med Chem. 2004;39:299–304. doi: 10.1016/j.ejmech.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 13.Baek DJ, Kang TB, Kim HJ. Synthesis of nonclassical quinazolinone antifolates as thymidylate synthase inhibitors and their antitumor activity in vitro. Bull Korean Chem Soc. 2004;25:1898–1906. [Google Scholar]
- 14.Jafari E, Khodarahmi GA, Hakimelahi GH, Tsai FY, Hassanzadeh F. Synthesis of some new tricyclic 4(3H)-quinazolinone derivatives. Res Pharm Sci. 2011;6:93–100. [PMC free article] [PubMed] [Google Scholar]
- 15.Schwalbe R, Steele-Moore L, Goodwin AC. Antimicrobial susceptibility testing protocols. Newyork: Taylor & Francis Groups press; 2007. p. 64. (53-55, 58, 79). [Google Scholar]
- 16.Ghandrika PM, Yakaiah T, Ram rao AR, Narsaiah B, Reddy NC, Sridhar V, et al. Synthesis of novel 4,6-disubstituted quinazoline derivatives, their anti-inflammatory and anti-cancer activity (cytotoxic) against U937 lukemia cell lines. Eur J Med Chem. 2008;43:846–852. doi: 10.1016/j.ejmech.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 17.European committee for antimicrobial susceptibility testing (EUCAST) of the European socity of clinical microbiology and infectious diseases (ESCMID). Determination of minimum concentrations (MICS) of antibacterial agents by broth dilution. Clin Microbiol. Infec. 2003;9:1–7. [Google Scholar]
- 18.Khan A, Rahman M, Islam MS. Antibacterial, antifungal and cytotoxic activities of 3,5-diacetylambulin isolated from Amorphophallus campanulatus. Indian J Pharmacol. 2008;40:41–44. doi: 10.4103/0253-7613.40489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baker CN, Banerjee SN, Tenover FC. Evaluation of alamar colorimetric MIC method for antimicrobial susceptibility testing of Gram-negative bacteria. J Clin Microbiol. 1994;32:1261–1267. doi: 10.1128/jcm.32.5.1261-1267.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Franzblau SG, Witzig RS, Mclaughlin JC, Torres P, Madico G, Hernandez A, et al. Rapid, low technology MIC determination with clinical Mycobacterium tuberculosis isolates by using the microplate alamar blue assay. J Clin Microbiol. 1998:362–366. doi: 10.1128/jcm.36.2.362-366.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Freshney RI. Culture of animal cells: A manual of basic technique. 4th ed. United State: Wiley-Liss press; 1994. pp. 1–5. (9-15, 181-184, 309). [Google Scholar]


