Abstract
Sports-related concussion has gained widespread interest and media attention in recent years due to the potential dangers and long-term consequences. Despite several international consensus statements there remains a great deal of uncertainty surrounding these injuries. This paper is a review of recent literature on the topic of concussion, consisting of: biomechanics, pathophysiology, diagnosis and sideline management.
Keywords: concussion, sports, management, diagnosis
Abstract
Les commotions liées à l’activité sportive ont retenu l’intérêt du public et l’attention des médias au cours des dernières années en raison de leurs dangers potentiels et de leurs conséquences à long terme. Malgré plusieurs déclarations de consensus international, il existe toujours une grande part d’incertitude quant à ces blessures. Le présent article est une recension des écrits récents au sujet de la commotion et porte sur la biomécanique, la pathophysiologie, le diagnostic et le traitement depuis la ligne de côté.
Keywords: commotion, sports, traitement, diagnostic
Introduction
Sports-related concussion (SRC), as defined by the 3rd International Conference on Concussion in Sport, is a “complex pathophysiological process affecting the brain, induced by traumatic biomechanical forces”. It is caused by either a direct blow to the head or to another body region resulting in an abrupt acceleration and/or deceleration of the craniocervical complex.1,2 Impact may, or may not, result in a loss of consciousness and in fact, it has been demonstrated that 90% of concussions do not result in a loss of consciousness.3,4 It is estimated that each year between 1.6 and 3.8 million athletes in the United States suffer a concussion related to participation in sport however, this is still considered an underestimation due to many injuries going unrecognized and, therefore, unreported.5,6 Due to recent widespread media attention surrounding the topic of concussion as well as the potential short- and long-term associated complications, it is important for sports-based practitioners to be well versed in the management of these injuries. This paper presents a narrative review of the biomechanics, pathophysiology, and acute management of concussion injuries.
Biomechanics of Impact
Concussion is due to an abrupt linear and/or rotational acceleration or deceleration of the brain within the skull.1,2,7 Soccer is the most frequent cause of sport-related concussion in females in the United States accounting for roughly 8.2% of all high school sport-related concussions. Much of the biomechanical data on head impact, however, has come from studies of football players, who suffer approximately 50% of all US high-school concussions. 8 In a recent literature review examining high school and collegiate football players, it was found that the range of linear acceleration causing concussion ranged from 74.0g to 146.0g (g=force of gravity), and the angular accelerations ranged from 5,582.6 rad/s2 to 9,515.6 rad/s2.3 Using data from over 57,000 impacts, it was determined that an angular acceleration of >5,582 rad/s2 and a linear acceleration of >96.1g yielded the highest predictive value of concussion.9
Although concussion is primarily a head injury, the initial concern following an extreme acceleration to the head is a potentially catastrophic cervical spine injury.1,2 In a finite element model developed by Viano et al. (2007) it was found that after impact there is a rapid displacement of the head resulting in neck deformation and a build up of forces and moments that are transferred from the head to the torso.10 A study on injuries in the National Football League between 1996 and 2001 found that neck pain occurred concurrently with 12.6% of concussions.7
The cervical spine is not only a potential source of injury that we must be aware of, but it is also implicated as a factor in the concussion itself. In early animal studies using a standard concussive blow via pendulum apparatus, concussive injury was not observed if the head and neck were fixed in place. If the head was allowed to move, even slightly, the same impact led to a concussion injury.11 Similarly, during football impacts, head acceleration of the struck player is influenced by forces in the neck and it was determined that increased neck stiffness can decrease acceleration of the head.10
This has prompted the hypothesis that perhaps increased neck strength may decrease the risk of concussion; however, this was not shown to be the case in a recent study of adolescent hockey players.12 A likely reason for this being that a stronger neck, unless under direct muscular tension, doesn’t necessarily equate to increased stiffness. In order for increased neck strength to decrease the acceleration of the head caused by impact, the player must actively contract their cervical musculature prior to impact. EMG studies on muscle activation during whiplash injuries have shown activation of the sternocleidomastoid and cervical paraspinal musculature beginning between 79 milliseconds (ms) and 94 ms following a rear-end impact.13 Another study showed that activation of the cervical multifidus muscles occurred between 105 and 194ms following impact or auditory startle; however, there was a large amount of individual variability (range 34 to 571ms).14 Thus, being aware of an impending hit in advance with enough time to contract cervical stabilizer muscles may be a potential preventative strategy for concussion. This may be one reason why, in contact sports, the player delivering the hit is less likely to suffer from a concussion than the player on the receiving end. In extensive video analysis of concussive impacts in the NFL, it was found that during the typical impact, the striking player lined up his head, neck, and torso before striking, usually at an oblique angle to the head of the opponent. The struck player, unaware of the impeding hit, did not. This yielded a higher effective mass for the striking player, causing both a greater transfer of momentum as well as a statistically greater cranial acceleration in the struck player.7 Similarly, male and female soccer players in the NCAA were also found to have greater head-neck segment displacement when the force application was unknown; however, most head impacts in soccer are intentional, anticipated, and generally do not result in concussion.15
Studies examining intentional heading scenarios in soccer demonstrate cranial accelerations well below the concussion threshold ranges found in the football studies discussed above. For example, a study of girls youth soccer (under age 14) showed a mean linear acceleration of 20.4 g (range 4.5g to 62.9g) and a mean rotational acceleration of 1,940 rad/s2 during intentional headers.16 A separate study concluded that intentional heading scenarios in soccer have low impact accelerations, not exceeding 19.8g.17
There is a growing concern over the potential for multiple ‘subconcussive’ impacts, such as intentionally heading the ball in soccer, to be detrimental. This has not been extensively studied; however, a recent examination of 46 NCAA Division I football players, receiving a mean of 1177.3±772.9 head impacts and no diagnosed concussions over the course of a season, showed no difference between pre-and post-season neurocognitive examinations. 18 Another study of 63 high school soccer players following a series of headers in practices and games found them to be free of deficits as well as within the top 10% of normative values on neurocognitive testing upon study completion.19 Each of these studies demonstrates that, at this point, there are no significant neurological impairments as a result of subconcussive impacts; however, further research in this area is required.
Pathophysiology
Concussion injury results in rapid onset of short-lived neurological impairment that resolves spontaneously, usually within 7 to 10 days.2 Despite neurological impairment, conventional neuroimaging techniques rarely show any detectable injury suggesting neuronal dysfunction rather than cell death.2,5,20–22 Thus the complex and varied symptom presentation is considered to be due a reversible functional deficit, rather than actual structural damage. A large body of experimental evidence suggests that this neuronal dysfunction is a consequence of a complex bio- and neurochemical cascade, which is triggered by the traumatic insult.21,23,24
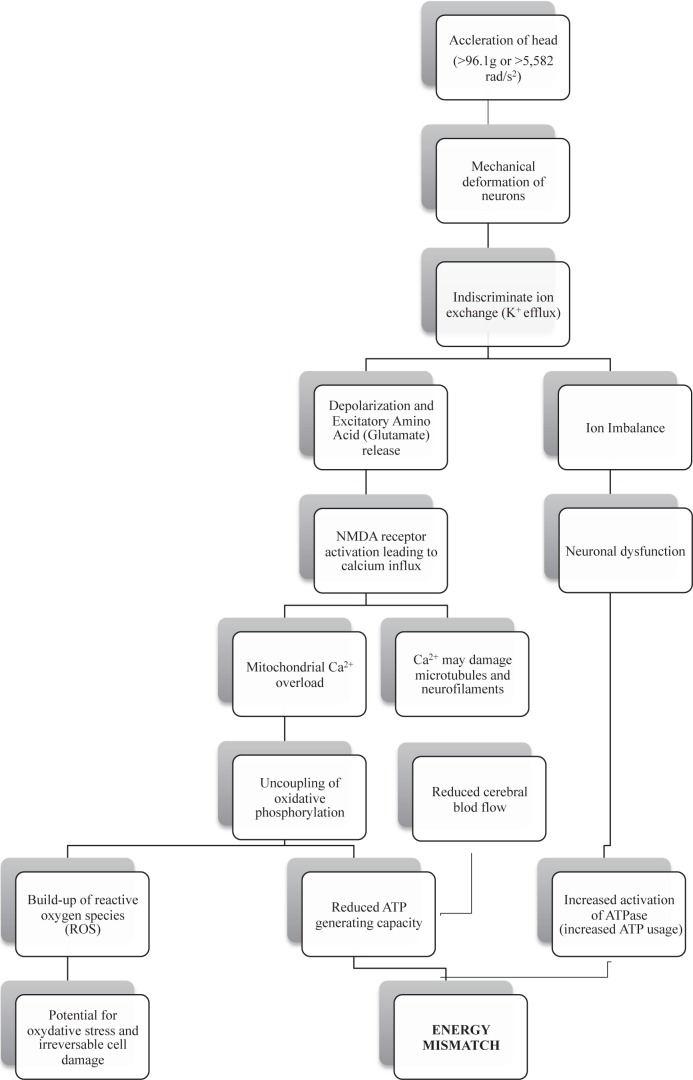
The neurometabolic cascade of concussion, first discussed by Giza and Hovda in 2001, has been shown to begin immediately after impact. During acceleration of the brain following a sufficient and significant impact, there is a sudden mechanical stretching and shearing of neurons.5,21,23 The brief loss of consciousness that is occasionally seen after impact has been attributed to these shear forces causing a transient neuronal disruption at the level of the brainstem and reticular activating system.5,25 Mechanical neuronal deformation on impact creates membrane defects and opens up formerly regulated ion channels, allowing an indiscriminant exchange of ions into and out of the cell; thus, depolarizing affected neurons. In consequence to the neuronal depolarization there is a widespread release of the excitatory amino acid glutamate. Expelled glutamate continues the cascade by promoting further potassium release and neuronal depolarization via activation of N-methyl-d-aspartate (NMDA) receptors. The result is a widespread excitation phase causing many of the transient signs of concussion such as confusion, vision and balance problems, and potentially a seizure or convulsive episode.5,21,23,25
Along with promoting the release of potassium, activated NMDA receptors also allow large amounts of calcium ions to flow into the cell, which can have a number of damaging effects. First, the large calcium influx overloads neuronal mitochondria resulting in dysfunction of the cells energy production capabilities. Mitochondrial calcium overload results in an uncoupling of oxidative phosphorylation within the electron transport chain. This essentially reduces the neurons capacity for generating adenosine triphosphate (ATP) and also leads to the creation of potentially damaging reactive oxygen species (ROS).21,23,26 If the buildup of ROS exceeds the cellular capacity to detoxify them, this may result in oxidative stress and irreversible cell damage and cell death.26 Secondly, increased intracellular calcium may also damage microtubules and neurofilaments within the axon, thereby disrupting neural connectivity.23
The brief excitation period following concussive impact is followed by a widespread neuronal suppression known as “spreading depression”, leading to cognitive deficits, amnesia, emotional lability, and fatigue.5,23,25 The end result of the entire cascade is a reversible ion imbalance along with potential axonal or neuronal damage. Ionic imbalance requires maximal function of the ATP dependent Na+/K+ pump to restore homeostasis. Typically, in the uninjured brain, oxidative metabolism runs at near maximum capacity; however, dysfunctional mitochondria and uncoupled oxidative phosphorylation forces the nerve cells to rely on less efficient anaerobic means of energy production. Anaerobic glycolysis produces approximately nine fold less ATP per one molecule of glucose; thus, creating a substantial increase in glucose demand to meet requirements.21,23,27 Animal studies examining post-impact glucose metabolism found an immediate hypermetabolism of glucose measuring as high as 46.6% higher in the cerebral cortex and 90.1% higher in the hippocampus compared with controls.28 Immediate demand for glucose is further complicated by a decrease in cerebral blood flow following head injury, creating a mismatch between demand and availability.23,29 Thus, during the period of greatest ATP demand there are tremendous barriers affecting production and supply. This results in an energy crisis whereby ATP is being used at a greater rate than it is being produced. (Figure 1)
Figure 1.
The net result of a concussive impact is neuronal ion imbalance causing cellular dysfunction and a cerebral energy deficit; a result of decreased ATP production coupled with increased ATP demand. This is by no means a complete description of the entire cascade that takes place during a concussion. For a more detailed description, the reader is encouraged to read the paper written by Giza and Hovda.23
Diagnosis
Determining if a concussion has occurred is the most important, and often the most difficult, part of the process. Concussion injury can present with a wide range of signs and symptoms (Table 1) and athletes are frequently untruthful about their presence.2,30 In a study of over 1000 high school sport-related concussions the most commonly reported symptoms were headache (94.3%), dizziness/unsteadiness (75.5%), difficulty concentrating (53.9%), confusion/disorientation (44.0%), and visual disturbance/sensitivity to light (34.4%). Loss of consciousness was reported in less than 5% of cases.31 Often, the signs and symptoms are transient, lasting under 15 minutes thus creating a difficult judgment call on the part of the practitioner. In grading systems of the past, transient symptoms lasting less than 15 minutes were considered to be grade I (one), or mild, concussions and athletes were allowed to return to play.32,33 The grading system of concussion has since been abandoned and athletes should not be allowed to return to play despite a potential resolution of symptoms.1,5,30
Table 1.
Signs and Symptoms of Concussion from the American College of Sports Medicine updated consensus statement – 20111. Reprinted with permission from Herring SA, Cantu RC, Guskiewicz KM, Putukian M, Kibler WB, Bergfeld JA, et al. Concussion (mild traumatic brain injury) and the team physician: a consensus statement – 2011 update. Medicine & Science in Sports & Exercise. 2011; 2412–22.
| Cognitive | Somatic | Affective | Sleep Disturbance |
|---|---|---|---|
|
|
|
|
The reaction of an athlete immediately after impact may provide valuable insight as to whether a concussion has occurred. Often times the athlete will show signs of confusion (going in the wrong direction), or difficulty with balance directly following impact. These signs of neurological impairment are indicative of concussion injury. Although quite rare, seizures and convulsions may occur immediately following impact. These are typically benign despite appearing quite dramatic, and usually require no more than standard concussion evaluation. Seizures occurring any time after the period directly following however, are cause for concern requiring prompt referral to the emergency department.34,35
According to the 3rd International Conference on Concussion in Sport (CIS), if an athlete exhibits any signs and symptoms, following an impact, which may be suggestive of a concussion, the athlete is to be removed from play immediately and evaluated.2 In the event of loss of consciousness (LOC), which interestingly is not considered a marker of increased injury severity2, the head and neck should be stabilized until the athlete regains consciousness. Orientation should be assessed immediately with continued stabilization of the cervical spine. Post-traumatic anterograde and retrograde amnesia may be indicative of injury severity and prolonged recovery.34,36 Once the initial concern of a cervical spine injury has been ruled out, a full examination should be done in a quiet area removed from the playing field and other distractions.
The initial examination should consist of the Sideline Concussion Assessment Tool 2nd Edition (SCAT2) as well as a full neurological exam consisting of cranial nerve, peripheral nerve, and cerebellar examinations. Neurological testing, aside from mental status and balance, are generally unremarkable with concussion4; however, these examinations are necessary to examine for more sinister pathology such as intracranial hemorrhage25. The face and skull should also be palpated for any tenderness, deformity, or other signs possibly indicative of fracture. The Canadian CT Head Rules35, should be used throughout the examination as indication for prompt referral to the emergency department. The Canadian CT Head Rules have been shown to have 100% sensitivity and significantly higher specificity than the New Orleans Criteria for determining the need for neurosurgical intervention (76.3% vs 12.1%) as well as clinically important brain injury (50.6% vs. 12.7%).35 Both the New Orleans and Canadian rules are included in table 2.
Table 2.
A comparison of the New Orleans Criteria and the Canadian CT Head Rule. Each of these guidelines are used in determination of which head injury patients should undergo further evaluation by means of neuroimaging.35 Reprinted with permission from Stiell et al.
| New Orleans Criteria | Canadian CT Head Rule |
|---|---|
CT (Computed Tomography) is required for patients with minor head injury with any 1 of the following findings. The criteria apply only to patients who have a Glasgow Coma Scale (GCS) score of 15
|
CT is only required for patients with minor head injury with any 1 of the following findings: Patients with minor head injury who present with a GCS score of 13 to 15 after witness loss of consciousness, amnesia, or confusion. High Risk for Neurosurgical Intervention
Medium Risk for Brain Injury Detection by CT
|
Sideline Concussion Assessment Tool, Second Ed
The SCAT2 is an easy to use outcome measure that consists of a 22-symptom questionnaire, questions of orientation, concentration, and memory, as well as balance and co-ordination tasks. Each component of the SCAT2 has a separate score that add up to a total score out of 100. It is beneficial to have preseason (baseline) concussion screens done on all athletes due to the large amount of variability seen even amongst uninjured groups. Two recent studies have found the average baseline SCAT2 score to be between 87 (±6.8) to 92.3 (±4.29) in high school athletes. Both of these studies showed that females tend to score higher on average than males, and individuals with a history of prior concussion tend to score lower than those without.37,38
Symptom Scores
Symptom scores are measured by subtracting the total number of symptoms present from 22, which is the total number of symptoms listed. A higher symptom score is therefore indicative of a lower number of symptoms. A study of high school athletes found that the average pre-injury baseline symptom score was 16.9 (+5.3) with no significant differences between age or gender.38 Another study of high school students found the average baseline symptom score to be 19.75 (+3.28) with the most frequent symptoms being fatigue or low energy (24%), trouble falling asleep (21%), difficulty concentrating (19%), difficulty remembering (17%) and being nervous or anxious (16%).37 In a study of nine to seventeen year old male and female hockey players there were a greater number of baseline symptoms reported in athletes who had a history of concussion in all groups except for the younger aged females. Similarly, the most common baseline symptom reported in this study was fatigue or low energy.39 These results demonstrate the variability seen in pre-injury concussion symptom reports in adolescent athletes and highlight the need for individual baseline evaluations.
Standard Assessment of Concussion (SAC)
The SAC consists of tests of orientation, immediate memory, and concentration.
On tests of immediate memory, females scored higher than males, remembering an average of 14.31/15 words versus 13.64/15 by males (p=0.00) at baseline. This difference was also significant when broken down by age cohort with females aged 13–15 as well as 16–19 having greater baseline word memory than male counterparts. Only forty-five percent of high school students scored 15/15 on immediate word memory at baseline.37
The concentration score consists of reciting a series of three, four, five, and six digit number strings in reverse order as well as saying the months of the year in reverse order. This same study found that on average 93.93% were able to recite three digits backwards, 76.64% were able to recite four, 41.12% were able to recite five, and only 17.29% were able to recite the six digit string. When asked to recite the months of the year in reverse order, only 67% of high school athletes were able to perform this task at baseline.37
Overall, the total score out of 30 on the SAC for this study demonstrated an average of 26.39 (+ 4.87) for females and 25.19 (+ 3.10) for males. Another study, which did not break the SAC down into individual components, reported the average score to be 26.9 (+ 2.3) for female and 26.4 (+ 2.7) for male high school athletes.38 Again, we see a large amount of variability in baseline scores for these adolescent athletes.
Balance Error Scoring System (BESS)
The BESS has been shown to be a valid and reliable side-line balance measure.38 The total test consists of holding three different positions (double leg stance, single leg stance, and tandem stance) each for 20 seconds with the eyes closed. During each test, any and all errors are counted and subtracted from 10. The total score is out of a possible 30. In one study of 214 high school athletes the average BESS score was 25.82 with females scoring higher than males (26.85+2.67 vs. 25.43+3.63, p= 0.01) (37). In another study of 1134 high school athletes, the average BESS score was 26.7(+2.9). Once again, females performed significantly better than males (27.3±2.7 vs. 26.6±2.9, p<0.001). It was also found in this study that ninth graders had significantly lower BESS scores than grades 10 through 12 indicating that age may play a factor. 38 It has also been shown that the Balance Error Scoring System (BESS) portion of the SCAT2 can be influenced by physical activity alone. Heavy physical exertion resulted in a significant decrease in single leg stance and tandem stance performance when performed immediately after exercise. A 15 minute rest or cool down period improved performance on these measures, therefore it may be advisable to delay BESS testing.40
Concussion injury can affect many different neural networks resulting in a wide range of possible of signs and symptoms.27 Clinicians should not expect every examination procedure to be significant in order to make a diagnosis. Any sign and/or symptom of neurological dysfunction following an impact meets the diagnostic criteria of a concussion and should be treated as such. The high amount of individual differences in pre-injury performance on these various measures demonstrates value of the baseline concussion testing for each individual athlete.
Management
The Third International Conference on Concussion in Sport (CIS) states that if a concussion is diagnosed, the athlete is not to return to play the same day except, in certain situations where adult athletes may be returned to play on the same day.2 Recent research would suggest, however, that no athlete suspected of having a concussion should be permitted to return to play the same day regardless of age or skill level, even with a clearance of symptoms. Considering the pathophysiology of the injury, there are a number of reasons why athletes suspected of having a concussion should not be permitted to return to play.
The first reason is delayed onset of symptoms. Numerous studies have demonstrated a delayed onset of symptoms and neurological deficits particularly in younger athletes. 41 Some athletes may have a brief period of symptoms immediately after impact and then a disappearance, only to have the symptoms return several hours or even a few days later.2,5,32 In animal studies examining postimpact cerebral metabolism, reductions in ATP were shown after only one minute, reaching statistically significant reductions by two hours compared to non-impacted controls. Maximum ATP depletion was not seen until six hours after injury.42 These findings suggest a delayed energy deficit, which anecdotally, fits well with reports of delayed symptom onset in many athletes. Lovell et al, 2004 studied 43 high school athletes who had disappearance of concussive signs and symptoms within 15 minutes after injury. All athletes were re-evaluated within 36 hours after injury and the results showed statistically significant increases in reported symptoms and neurological deficits as compared to pre-injury baseline data.32
Secondly, strenuous exercise following a concussion, has been shown to worsen outcome and prolong symptoms.2,27 Exercise modulates glucose uptake in the brain which may worsen the energy mismatch within the neurons. 27 On top of this, studies have shown that merely having an increased body temperature both before and after impact accentuates the amount of glutamate release and glutamate-induced damage.43,44 In moderate and severe brain injury, it is well established that inducing hypothermia can have a neuroprotective effect following injury.45,46 This has been less studied in concussion; however, a recent animal study found that having an increased body temperature prior to a mild traumatic brain injury and then maintaining that elevated body temperature for two hours post-impact had significantly larger cortical contusion areas and volumes, compared with normothermic and hypothermic conditions.44 Although attempting to induce hypothermic conditions in our athletes is beyond the scope of sideline management, the athlete should be removed from play, brought into the shade or air-conditioned environment and kept cool.
The final, and perhaps most feared, reason for removing the athlete from play is “Second Impact Syndrome” (SIS).47 Although extremely rare and controversial, this condition is believed to occur if an individual suffers a second concussion prior to fully recovering from the initial concussion. Theoretically, in younger athletes (under 20 years), the ionic imbalance and energy deficit of the initial concussion creates a neuronal environment that may not be capable of effectively managing a second neurometabolic cascade. Further calcium influx, mitochondrial dysfunction, and loss of cerebral blood-flow autoregulation ultimately results in massive cerebral swelling and death.21,23,47–49 The paucity of evidence surrounding second impact syndrome has led some to debate its existence. Despite limited evidence of true second impact syndrome causing of death, with only 17 case reports50, several studies have demonstrated periods of brain vulnerability following concussion. Decreased ATP levels within the brain signifying an incomplete recovery and ongoing metabolic deficiency mark this vulnerable period, during which, a second concussive impact leads to much greater ATP depletion as well as longer recovery times.23,24,51–53
In summary, premature return to play exposes athletes to additional risk of worsening of symptoms and/or risk of suffering a more serious brain injury due to a lowered threshold for re-injury as well as potential inability to effectively manage another neurometabolic cascade.21,32
In May of 2009, the state of Washington passed the Zachary Lystedt Law4,30 and 47 other states have since followed suit enacting their own concussion legislation. The original law is named after a middle school football player who suffered a significant brain injury in 2006 after returning to the game following a concussion. The law states that if a young athlete is suspected of having a concussion, they are to be removed from play and not permitted to return the same day; “when in doubt, sit them out”. The 2011 American College of Sports Medicine team physician consensus statement1 reiterates this point by stating that no athlete be allowed same day return-to-play. The law also states that no athlete shall be permitted to return until cleared by a licensed health care provider, which in many states has included chiropractors.
The injured athlete should be monitored via repeat neurological assessments over several (> 2) hours for improvement or deterioration of condition, paying particular attention to cognitive testing, as well as cranial nerve and balance testing.1,4,25 Conventional neuroimaging such as CT and MRI are not able to detect the presence of a concussion injury; however they are useful in examining for focal brain damage and intracranial hemorrhage.1,2,20 Severe or worsening headache, seizures, two or more episodes of vomiting, unsteady gait, slurred speech, weakness or numbness in the extremities, or a Glasgow Coma Scale (GCS) (Table 3) of less than 15 indicate a need for prompt referral to the emergency department for neuroimaging.4,25,34,35 Educate the athlete as well as a responsible care person on the condition, what to expect, signs of deterioration, importance of activity restriction, and step-wise management and return to play (Table 4). It is helpful to provide this information in writing.25
Table 3.
Glasgow Coma Scale
| Feature | Responses | Scores |
|---|---|---|
|
| ||
| Eye Opening (E) | Spontaneous | 4 |
| To speech | 3 | |
| To pain | 2 | |
| None | 1 | |
|
| ||
| Verbal Response (V) | Oriented | 5 |
| Confused conversation | 4 | |
| Words (inappropriate) | 3 | |
| Sounds (incomprehensible) | 2 | |
| None | 1 | |
|
| ||
| Best Motor Response (M) | Obey commands | 6 |
| Localise pain | 5 | |
| Flexion – Normal | 4 | |
| Flexion – Abnormal | 3 | |
| Extend | 2 | |
| None | 1 | |
|
| ||
| Total Score | Worst = 3/15, Best = 15/15 | |
Table 4.
Return to play stages as outlined by the 3rd International Conference on Concussion in Sport, held in Zurich in 2008.2 Each of these stages is to take at least 24 hours. If any symptoms are incurred at any one of the stages, the athlete is to take the rest of the day off and return to the previous stage the following day. The athlete is to remain at that stage for at least 24 hours. If symptom free, they may attempt the next stage again.
| Graduated Return to Play Protocol | ||
|---|---|---|
| Stage of Rehab | Functional Exercise to be attempted | Objectives to meet |
| 1. No Activity | Complete physical and cognitive rest | Recovery – remain at stage 1 until symptom free |
| 2. Light aerobic exercise* | Walking, swimming, stationary cycling (Intensity <70% of max heart (HR) rate) – no resistance training | Increase HR |
| 3. Sport-specific exercise | Skating, running, jumping (No head impact activities) | Add sport-specific movement |
| 4. Non-contact training drills | Progress to more complex training drills (passing, catching, dribbling, stick-handling etc.); May begin progressive resistance training | Exercise, coordination, and cognitive load –challenging multiple systems |
| 5. Full contact practice** | Following medical clearance, participate in normal training activities | Restore confidence and assess functional skills |
| 6. Return to play | Normal Game Play | |
Prior to returning to exercise, the authors recommend adding in a cognitive stage in which the athlete attempts a period of reading and light cognitive activities
Prior to returning the athlete to full contact, ensure that he or she has been sufficiently challenged cognitively and physically with high intensity exercise and complex training drills
Initial management includes a period of at least 24 hours of absolute physical and cognitive rest refraining from reading, watching television, going to school or work, and physical exercise until all symptoms have disappeared. The majority of symptoms are expected to resolve spontaneously over 7 to 10 days and until then all activities that require attention and concentration, or physical exertion, are to be avoided as physical and cognitive stress may exacerbate symptoms and delay recovery. 2,54 Acute use of medication should be done with caution as it may mask signs of worsening condition. Nonsteroidal anti-inflammatory medications should be avoided acutely in case of intracranial hemorrhage.4 Similarly, the use of sedatives, alcohol or recreational drugs should be avoided.54 There is still debate surrounding whether or not periodic waking of the injured athlete is necessary. Waking the athlete and assessing for cognitive decline throughout the night may catch a potentially catastrophic intracranial hemorrhage; however, some have argued that there may be more benefit to an uninterrupted sleep in terms of recovery.4,25,34
If the athlete is symptom free following the 24 hour rest period, they are then permitted to begin the stepwise return to play protocol as outlined at the 2008 CIS conference (Table 4).2 Each step is to be separated by at least 24 hours from the next. An exacerbation of symptoms at any one of the stages requires the athlete to go back to the previous stage for another 24-hour period. The entire return to play protocol as outlined by the CIS conference requires the athlete be away from game-play for a minimum of six days following a concussion provided they pass through all the stages with no return of symptoms. Recent studies, however, are beginning to suggest that this return to play protocol may be too lenient as symptomatology is a poor indicator of physiologic recovery.21,49,55,56
Studies using magnetic resonance spectroscopy have been able to measure the levels of a cerebral metabolite, N-Acetylaspartate (NAA), which has been shown to reflect ATP levels in the brain (R2 = 0.84).42 In human subjects following concussion, these studies have shown significantly reduced NAA on initial evaluation three days post-injury. Each of these studies have consistently shown significant decreases in NAA that do not return to healthy control levels until 30 days post-injury despite a normal symptom resolution between seven and ten days after injury.49,57 More interestingly, in a recently published case-series of 6 athletes involved in these studies who returned to sport prior to the end of the 30-day trial and suffered another concussion, showed much more symptoms and much longer symptom duration compared to their first concussions. Symptom resolution following the first concussion occurred between three and eight days post-injury for all subjects. Following the second impact, which occurred between nine and 21 days after the first concussion, symptom duration averaged 41 days (range 24 to 59 days). NAA levels in the doubly concussed athletes did not return to healthy control levels until 90 to 120 days later.58 It is unknown what type of return-to-play protocol these athletes followed, if any; however, this demonstrates that despite symptom resolution and more than six days between concussions, there was an incomplete metabolic recovery which lead to a much more severe second concussion. Unfortunately this technology is not available for clinical use leaving us to rely on clinical judgment as to whether an athlete is ready to safely return to sport. It is suggested by the authors that the clinician challenge the athlete with a variety of physically and cognitively demanding tasks in an attempt to provoke symptoms prior to allowing a return to contact.
In conclusion, the current literature on concussion injury primarily points to a metabolic ATP deficiency due to ionic imbalances created from the mechanical deformation of affected neurons. This injury may present with a wide range of signs and symptoms, any one of which, when preceded by a significant impulsive force delivered to the head, is indicative of concussion injury and the athlete should be removed from play. Initial examination should focus on, first, ruling out cervical spine injury and skull fracture, followed by SCAT2, cranial nerve, and cerebellar examinations. Severe or worsening headache, seizures, two or more episodes of vomiting, unsteady gait, slurred speech, weakness or numbness in the extremities, or a GCS of less than 15 indicate a need for referral to the emergency department.4,34,35 The athlete and caregiver should be educated and provided written information about signs of worsening condition as well as management of a concussion and return-to-play.
Footnotes
Sources of Support: none declared
References
- 1.Herring SA, Cantu RC, Guskiewicz KM, Putukian M, Kibler WB, Bergfeld JA, et al. Concussion (mild traumatic brain injury) and the team physician: a consensus statement – 2011 update. Medicine & Science in Sports & Exercise; 2011. pp. 2412–22. [DOI] [PubMed] [Google Scholar]
- 2.McCrory P, Meeuwisse W, Johnston K, Dvorak J, Aubry M, Molloy M, et al. Consensus statement on concussion in sport 3rd international conference on concussion in sport held in Zurich, November 2008. Clin J Sport Med. 2009:185–200. doi: 10.1097/JSM.0b013e3181a501db. [DOI] [PubMed] [Google Scholar]
- 3.Broglio SP, Surma T, Ashton-Miller JA. High school and collegiate football athlete concussions: A biomechanical review. Ann Biomed Eng. 2011;40(1):37–46. doi: 10.1007/s10439-011-0396-0. [DOI] [PubMed] [Google Scholar]
- 4.Scorza KA, Raleigh MF, O’Connor FG. Current concepts in concussion: evaluation and management. Am Fam Physician. 2012;85(2):123–32. [PubMed] [Google Scholar]
- 5.Khurana VG, Kaye AH. An overview of concussion in sport. J Clinical Neuroscience. 2012;19(1):1–11. doi: 10.1016/j.jocn.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 6.Langlois JA, Rutland-Brown W, Wald MM. The epidemiology and impact of traumatic brain injury: a brief overview. J Head Trauma Rehabil. 2006;21(5):375–8. doi: 10.1097/00001199-200609000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Pellman EJ, Viano DC, Tucker AM, Casson IR, Waeckerle JF. Concussion in professional football: reconstruction of game impacts and injuries. Neurosurgery. 2003;53(4):799–794. doi: 10.1093/neurosurgery/53.3.799. [DOI] [PubMed] [Google Scholar]
- 8.Marar M, McIlvain NM, Fields SK, Comstock RD. Epidemiology of concussions among United States high school athletes in 20 sports. Am J Sports Medicine. 2012;40(4):747–55. doi: 10.1177/0363546511435626. [DOI] [PubMed] [Google Scholar]
- 9.Broglio SP, Schnebel B, Sosnoff JJ, Shin S, Fen X, He X, et al. Biomechanical properties of concussions in high school football. Medicine & Science in Sports & Exercise. 2010;42(11):2064–71. doi: 10.1249/MSS.0b013e3181dd9156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Viano DC, Casson IR, Pellman EJ. Concussion in professional football: Biomechanics of the struck player – Part 14. Neurosurgery. 2007;61(2):313–28. doi: 10.1227/01.NEU.0000279969.02685.D0. [DOI] [PubMed] [Google Scholar]
- 11.Denny-Brown DE. Experimental Concussion: (Section of Neurology) Proc R Soc Med. 1941;34(11):691–692. doi: 10.1177/003591574103401102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mihalik JP, Guskiewicz KM, Marshall SW, Greenwald RM, Blackburn JT, Cantu RC. Does cervical muscle strength in youth ice hockey players affect head impact biomechanics? Clin J Sport Med. 2011;21(5):416–21. doi: 10.1097/JSM.0B013E31822C8A5C. [DOI] [PubMed] [Google Scholar]
- 13.Brault JR, Siegmund GP, Wheeler JB. Cervical muscle response during whiplash: evidence of a lengthening muscle contraction. Clin Biomech (Bristol, Avon) 2000;15(6):426–35. doi: 10.1016/s0268-0033(99)00097-2. [DOI] [PubMed] [Google Scholar]
- 14.Siegmund GP, Blouin J-S, Carpenter MG, Brault JR, Inglis JT. Are cervical multifidus muscles active during whiplash and startle? An initial experimental study. BMC Musculoskelet Disord. 2008;9(1):80. doi: 10.1186/1471-2474-9-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mansell J, Tierney RT, Sitler MR, Swanik KA, Stearne D. Resistance training and head-neck segment dynamic stabilization in male and female collegiate soccer players. J Athl Train. 2005;40(4):310–9. [PMC free article] [PubMed] [Google Scholar]
- 16.Hanlon E, Bir C. Real-time head acceleration measurement in girls youth soccer. Medicine & Science in Sports & Exercise; 2011. Published ahead of print. [DOI] [PubMed] [Google Scholar]
- 17.Shewchenko N. Heading in football. Part 1: Development of biomechanical methods to investigate head response. Br J Sports Medicine. 2005;39(Supplement 1):i10–i25. doi: 10.1136/bjsm.2005.019034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gysland SM, Mihalik JP, Register-Mihalik JK, Trulock SC, Shields EW, Guskiewicz KM. The relationship between subconcussive impacts and concussion history on clinical measures of neurologic function in collegiate football players. Ann Biomed Eng. 2011;40(1):14–22. doi: 10.1007/s10439-011-0421-3. [DOI] [PubMed] [Google Scholar]
- 19.Kontos AP, Dolese A, Elbin RJ, III, Covassin T, Warren BL. Relationship of soccer heading to computerized neurocognitive performance and symptoms among female and male youth soccer players. Brain Inj. 2011;25(12):1234–41. doi: 10.3109/02699052.2011.608209. [DOI] [PubMed] [Google Scholar]
- 20.Gonzalez PG, Walker MT. Imaging modalities in mild traumatic brain injury and sports concussion. PM&R. 2011;3(10):S413–24. doi: 10.1016/j.pmrj.2011.08.536. [DOI] [PubMed] [Google Scholar]
- 21.Signoretti S, Lazzarino G, Tavazzi B, Vagnozzi R. The pathophysiology of concussion. PM&R. 2011;3(10):S359–68. doi: 10.1016/j.pmrj.2011.07.018. [DOI] [PubMed] [Google Scholar]
- 22.Difiori JP, Giza CC. New techniques in concussion imaging. Curr Sports Med Rep. 2010;9(1):35–9. doi: 10.1249/JSR.0b013e3181caba67. [DOI] [PubMed] [Google Scholar]
- 23.Giza CC, Hovda DA. The neurometabolic cascade of concussion. J Athl Train. 2001;36(3):228–35. [PMC free article] [PubMed] [Google Scholar]
- 24.Signoretti S, Vagnozzi R, Tavazzi B, Lazzarino G. Biochemical and neurochemical sequelae following mild traumatic brain injury-summary of experimental data and clinical implications – 2010. Neurosurg Focus. 2010;29(5):1–12. doi: 10.3171/2010.9.FOCUS10183. [DOI] [PubMed] [Google Scholar]
- 25.Ropper AH, Gorson KC. Concussion. N Engl J Med. 2007;356(2):166–72. doi: 10.1056/NEJMcp064645. [DOI] [PubMed] [Google Scholar]
- 26.Vagnozzi R, Marmarou A, Tavazzi B, Signoretti S, Di Pierro D, Del Bolgia F, et al. Changes of cerebral energy metabolism and lipid peroxidation in rats leading to mitochondrial dysfunction after diffuse brain injury. J Neurotrauma. 1999;16(10):903–13. doi: 10.1089/neu.1999.16.903. [DOI] [PubMed] [Google Scholar]
- 27.Majerske CW, Mihalik JP, Ren D, Collins MW, Reddy CC, Lovell MR, et al. Concussion in sports-postconcussive activity levels, symptoms, and neurocognitive performance – 2008. J Athl Train. 2008;43(3):1–10. doi: 10.4085/1062-6050-43.3.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yoshino AA, Hovda DAD, Kawamata TT, Katayama YY, Becker DPD. Dynamic changes in local cerebral glucose utilization following cerebral conclusion in rats: evidence of a hyper- and subsequent hypometabolic state. Brain Res. 1991;561(1):106–19. doi: 10.1016/0006-8993(91)90755-k. [DOI] [PubMed] [Google Scholar]
- 29.Len TK, Neary JP. Cerebrovascular pathophysiology following mild traumatic brain injury. Clinical Physiology and Functional Imaging. 2010;31:85–93. doi: 10.1111/j.1475-097X.2010.00990.x. [DOI] [PubMed] [Google Scholar]
- 30.Doolan AW, Day DD, Maerlender AC, Goforth M, Gunnar Brolinson P. A review of return to play issues and sportsrelated concussion. Ann Biomed Eng. 2011;40(1):106–13. doi: 10.1007/s10439-011-0413-3. [DOI] [PubMed] [Google Scholar]
- 31.Meehan WP, d’Hemecourt P, Collins CL, Comstock RD. Assessment and management of sport-related concussions in United States high schools. Am J Sports Medicine. 2011;39(11):2304–10. doi: 10.1177/0363546511423503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lovell MR, Collins MW, Iverson GL, Johnston KM, Bradley JP. Grade 1 or “ding” concussions in high school athletes. Am J Sports Medicine. 2004;32(1):47–54. doi: 10.1177/0363546503260723. [DOI] [PubMed] [Google Scholar]
- 33.Practice parameter: the management of concussion in sports (summary statement). Report of the Quality Standards Subcommittee. Neurology. 1997:581–5. doi: 10.1212/wnl.48.3.581. [DOI] [PubMed] [Google Scholar]
- 34.Halstead ME, Walter KD. The council on sports medicine and fitness. Sport-related concussion in children and adolescents. Pediatrics. 2010;126(3):597–615. doi: 10.1542/peds.2010-2005. [DOI] [PubMed] [Google Scholar]
- 35.Stiell IG, Clement CM, Rowe BH, Schull MJ, Brison R, Cass D, et al. Comparison of the Canadian CT Head Rule and the New Orleans Criteria in patients with minor head injury. JAMA. 2005;294(12):1511–8. doi: 10.1001/jama.294.12.1511. [DOI] [PubMed] [Google Scholar]
- 36.Asplund CA, McKeag DB, Olsen CH. Sport-related concussion: factors associated with prolonged return to play. Clin J Sport Med. 2004;14(6):339–43. doi: 10.1097/00042752-200411000-00003. [DOI] [PubMed] [Google Scholar]
- 37.Jinguji TM, Bompadre V, Harmon KG, Satchell EK, Gilbert K, Wild J, et al. Sport Concussion Assessment Tool – 2: Baseline values for high school athletes. Br J Sports Medicine. 2012;46(5):365–70. doi: 10.1136/bjsports-2011-090526. [DOI] [PubMed] [Google Scholar]
- 38.Valovich McLeod TC, Bay RC, Lam KC, Chhabra A. Representative baseline values on the Sport Concussion Assessment Tool 2 (SCAT2) in adolescent athletes vary by gender, grade, and concussion history. Am J Sports Medicine. 2012;40(4):927–33. doi: 10.1177/0363546511431573. [DOI] [PubMed] [Google Scholar]
- 39.Schneider KJ, Emery CA, Kang J, Schneider GM, Meeuwisse WH. Examining Sport Concussion Assessment Tool ratings for male and female youth hockey players with and without a history of concussion. Br J Sports Medicine. 2010;44(15):1112–7. doi: 10.1136/bjsm.2009.071266. [DOI] [PubMed] [Google Scholar]
- 40.Schneiders AG, Sullivan SJ, Handcock P, Gray A, McCrory PR. Sports concussion assessment: the effect of exercise on dynamic and static balance. Scandinavian J Medicine & Science in Sports. 2010;22(1):85–90. doi: 10.1111/j.1600-0838.2010.01141.x. [DOI] [PubMed] [Google Scholar]
- 41.Sahler CS, Greenwald BD. Traumatic brain injury in sports: A review. Rehabilitation Research and Practice. 2012:1–10. doi: 10.1155/2012/659652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Signoretti S, Marmarou A, Tavazzi B, Lazzarino G, Beaumont A, Vagnozzi R. N-Acetylaspartate reduction as a measure of injury severity and mitochondrial dysfunction following diffuse traumatic brain injury. J Neurotrauma. 2001;18(10):977–91. doi: 10.1089/08977150152693683. [DOI] [PubMed] [Google Scholar]
- 43.Suehiro E, Fujisawa H, Ito H, Ishikawa T, Maekawa T. Brain temperature modifies glutamate neurotoxicity in vivo. J Neurotrauma. 1999;16(4):285–97. doi: 10.1089/neu.1999.16.285. [DOI] [PubMed] [Google Scholar]
- 44.Sakurai A, Atkins CM, Alonso OF, Bramlett HM, Dietrich WD. Mild hyperthermia worsens the neuropathological damage associated with mild traumatic brain Injury in rats. J Neurotrauma. 2012;29(2):313–21. doi: 10.1089/neu.2011.2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Choi HA, Badjatia N, Mayer SA. Hypothermia for acute brain injury—mechanisms and practical aspects. Nat Rev Neurol. 2012:1–9. doi: 10.1038/nrneurol.2012.21. [DOI] [PubMed] [Google Scholar]
- 46.Dietrich WD, Bramlett HM. The evidence for hypothermia as a neuroprotectant in traumatic brain injury. Neurotherapeutics. 2010;7(1):43–50. doi: 10.1016/j.nurt.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cantu RC. Second-impact syndrome. Clin Sports Med. 1998;17(1):37–44. doi: 10.1016/s0278-5919(05)70059-4. [DOI] [PubMed] [Google Scholar]
- 48.Cantu RC, Register-Mihalik JK. Considerations for return-to-play and retirement decisions after concussion. PM&R. 2011;3(10):S440–4. doi: 10.1016/j.pmrj.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 49.Vagnozzi R, Signoretti S, Cristofori L, Alessandrini F, Floris R, Isgro E, et al. Assessment of metabolic brain damage and recovery following mild traumatic brain injury: a multicentre, proton magnetic resonance spectroscopic study in concussed patients. Brain. 2010;133(11):3232–42. doi: 10.1093/brain/awq200. [DOI] [PubMed] [Google Scholar]
- 50.McCrory PP, Davis GG, Makdissi MM. Second impact syndrome or cerebral swelling after sporting head injury. Curr Sports Med Rep. 2012;11(1):21–3. doi: 10.1249/JSR.0b013e3182423bfd. [DOI] [PubMed] [Google Scholar]
- 51.Longhi L, Saatman KE, Fujimoto S, Raghupathi R, Meaney DF, Davis J, et al. Temporal window of vulnerability to repetitive experimental concussive brain injury. Neurosurgery. 2005;56(2):364–74. doi: 10.1227/01.neu.0000149008.73513.44. [DOI] [PubMed] [Google Scholar]
- 52.Vagnozzi R, Signoretti S, Tavazzi B, Cimatti M, Amorini AM, Donzelli S, et al. Hypothesis of the postconcussive vulnerable brain: experimental evidence of its metabolic occurrence. Neurosurgery. 2005 Jul;57(1):164–71. doi: 10.1227/01.neu.0000163413.90259.85. [DOI] [PubMed] [Google Scholar]
- 53.Vagnozzi R, Tavazzi B, Signoretti S, Amorini AM, Belli A, Cimatti M, et al. Temporal window of metabolic brain vulnerability to concussions. Neurosurgery. 2007;61(2):379–89. doi: 10.1227/01.NEU.0000280002.41696.D8. [DOI] [PubMed] [Google Scholar]
- 54.Makdissi M. Sports related concussion-management in general practice. Aust Fam Physician. 2010;39(1–2):12–7. [PubMed] [Google Scholar]
- 55.Gall B. Exercise following a sport induced concussion. Br J Sports Medicine. 2004;38(6):773–7. doi: 10.1136/bjsm.2003.009530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gall B, Parkhouse W, Goodman D. Heart rate variability of recently concussed athletes at rest and exercise. Medicine & Science in Sports & Exercise. 2004;36(8):1269–74. doi: 10.1249/01.mss.0000135787.73757.4d. [DOI] [PubMed] [Google Scholar]
- 57.Vagnozzi RR, Signoretti SS, Tavazzi BB, Floris RR, Ludovici AA, Marziali SS, et al. Temporal window of metabolic brain vulnerability to concussion: a pilot 1H-magnetic resonance spectroscopic study in concussed athletes – part III. CORD Conference Proceedings. 2008;62(6):1286–6. doi: 10.1227/01.neu.0000333300.34189.74. [DOI] [PubMed] [Google Scholar]
- 58.Lazzarino G, Vagnozzi R, Signoretti S, Manara M, Floris R, Amorini AM, Ludovici A, Marziali S, McIntosh TK, Tavazzi B. The importance of restriction from physical activity in the metabolic recovery of concussed brain. In: Argrawal A, editor. Brain injury: Pathogenesis, monitoring, recovery, and management. Rejika,Croatia: InTech; 2012. pp. 501–522. [Google Scholar]