Abstract
Carum copticum from Apiaceae family has several biological effects including analgesic, anti-inflammatory, anxiolytic and antispasmodic activities. This study was designed to evaluate its effect on suppression of naloxone-induced morphine withdrawal signs. Hydroalcoholic and polyphenolic extracts were prepared according to the standard methods. Male mice (25-30 g) were made dependent by subcutaneous injection of increasing doses (30-90 mg/kg) of morphine. Withdrawal syndrome was elicited by naloxone (5 mg/kg, i.p.) and number of jumpings and also presence of ptosis, hyperventilation, piloerection, tremor and diarrhea were evaluated during a 30 min period started immediately after naloxone injection. The hydroalcoholic extract at doses of 1 and 2 g/kg and the polyphenolic extract at doses of 0.5, 1 and 2 g/kg significantly (P<0.05) inhibited jumpings. Both extracts inhibited tremor significantly (P<0.01). Also the maximum applied dose of the extracts significantly (P<0.05) reduced ptosis. These results clearly show that Carum copticum is effective in suppression of morphine withdrawal and further studies are needed to find out the responsible constituents and also the mechanism of their actions.
Keywords: Carum copticum, Morphine withdrawal, Apiaceae
INTRODUCTION
Opioid withdrawal signs are bothersome and therefore drugs such as methadone, buprenorphine and clonidine have been administered to alleviate these signs. These drugs can also facilitate entry of opioid addicts into recovery and/or rehabilitation programs(1–3).
In addition to chemical drugs, several medicinal herbs including Crocus sativus, Cuminum cyminum, Ferula gummosa, Otostegia persica, Portulaca oleraceae and Rosmarinus officinalis have been investigated for their suppressive effects on opioid withdrawal signs in animal models(4–9). Carum copticum (L.) C. B. Clarke or ajowan caraway from Apiaceae family is a worldwide used plant that grows in different parts of the Middle East, Indian subcontinent and Iran. It is used as a remedy in traditional iranian medicine for treating several gastrointestinal and nervous disorders like flatulence and colic pains. The C. copticum fruits are commonly known as “Zenyan” in Iran(10–12). Chemical constituents and pharmacological and biological activities of the plant have been the subject of several researches over the years. Phytochemical studies have revealed that tannins, flavonoids, saponins and essential oils are the prominent components of the fruits(11–16). C. copticum extracts and essential oil are reported to have anti-cholinergic, tracheal relaxant, antispasmodic, bronchodilator, antitussive, anxiolytic, anticonvulsant, analgesic, anti-inflammatory, antihypertensive, hepatoprotective, antimicrobial, antiviral, antioxidant and antimutagenic effects(11,14–16,17–24).
The purpose of the present study was to evaluate the possible effects of the total and polyphenolic extracts of C. copticum fruits on morphine withdrawal signs in mice.
MATERIALS AND METHODS
Plant material
Dried fruits of C. copticum were purchased from the local market of city of Isfahan and cultivated in Kashan, Iran. The plant material was identified in the Science and Research Branch of the Herbarium Department of Tehran Islamic Azad University by Dr. Iraj Mehregan and a voucher specimen was deposited in the herbarium department of Barij Essence Pharmaceutical Co., Kashan for future evidence (Herbarium number: 198-1)
Preparation of extracts
The plant extracts were prepared as described earlier(25–27) with little modification. For preparation of total hydroalcoholic extract, dried and powdered fruits (100 g) was soaked by adequate volume of ethanol:water (7:3 v/v) and the extraction was carried out for 72 h to obtain full extract using percolation method. The extract was then shuddered, filtered and evaporated in a rotary evaporator under reduced pressure until a semisolid extract yielded at 48.3 % w/w.
For preparation of p olyphenol-rich extract, same plant materials were weighed out. Extraction of polyphenolic compounds was carried out in two steps, first with ethanol:water (9:1 v/v) and second with ethanol:water (1:1 v/v). At each step solvent was added to make slurry with the fruits powder and then was left for 48 h. The two extracts were then combined and evaporated. The resultant extract was then cleared of low polarity contaminants like xanthophylls, chlorophylls and fats by extraction in a separating funnel with chloroform three times. This solvent-extracted aqueous layer, containing the bulk of the flavonoids and other phenolic compounds, was then evaporated to dryness under vacuum in an evaporator. Evaporation and solvent removal of the extract gave a semisolid mass yielded 10.4 % w/w. These extracts were stored in a refrigerator.
Animals
Male albino mice (25-35 g) were provided by the animal house of School of Pharmacy and Pharmaceutical Sciences, Isfahan University of Medical Sciences (Isfahan, Iran). They were maintained in 12/12 h light/dark cycle at 21 ± 2°C with free access to food and water. All experiments were carried out in accordance with local guidelines for the care of laboratory animals of Isfahan University of Medical Sciences (Isfahan, Iran).
Morphine dependence
Dependence was induced by subcutaneous injection of morphine to mice at doses of 30 and 45 mg/kg on day 1 and 60 and 90 mg/kg on day 2 (8:00 am and 6:00 pm). On day 3, a single dose of morphine (90 mg/kg) was administered at 8:00 am(7).
Naloxone-precipitated withdrawal syndrome
Withdrawal signs were elicited by i.p. injection of naloxone hydrochloride (5 mg/kg) 2 h after the last injection of morphine. Counted and checked signs were evaluated during a 30 min period starting just after naloxone injection. Jumpings were counted and checked signs including diarrhea, hyperventilation, ptosis, tremor and piloerection were evaluated over 3-10 min periods with one point given for the presence of each sign during each period (range of scores: 0-3)(7).
Statistical analysis
The data were expressed as mean ± S.E.M. One-way ANOVA followed by Duncan test was used for comparison of data and P values less than 0.05 were considered significant. The Mann-Whitney U test was used for comparison of checked signs data. All statistical calculations were done with SPSS for windows (SPSS 13) software.
RESULTS
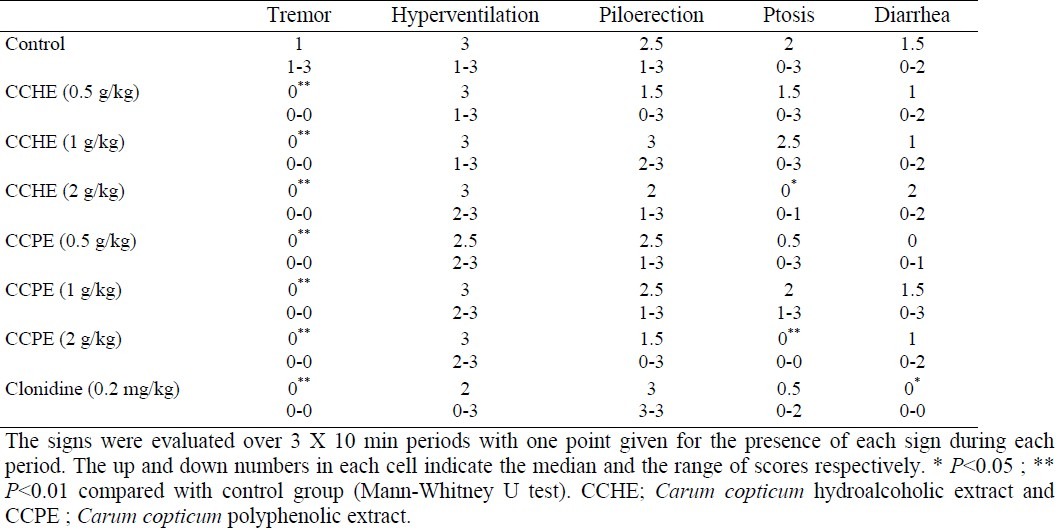
Hydroalcoholic extract at doses of 1 and 2 g/kg significantly (P < 0.05) reduced number of naloxone-induced jumpings. Compared with control group, these doses produced 66% and 73% reduction of jumpings respectively. Clonidine as the reference drug reduced jumpings by 84% (Fig. 1). The effect of polyphenolic extract of C. copticum on naloxone-elicited jumpings is shown in Fig. 2. As it is seen, the polyphenolic extract at doses of 0.5, 1 and 2 g/kg significantly (P < 0.05) inhibited jumpings. Effects of hydroalcoholic and polyphenolic extracts on non-countable signs including tremor, hyperventilation, piloerection, ptosis and diarrhea are summarized in Table 1. Both extracts significantly (P < 0.01) inhibited tremor. Also the maximum applied dose of the extracts significantly (P < 0.05) reduced ptosis.
Fig. 1.
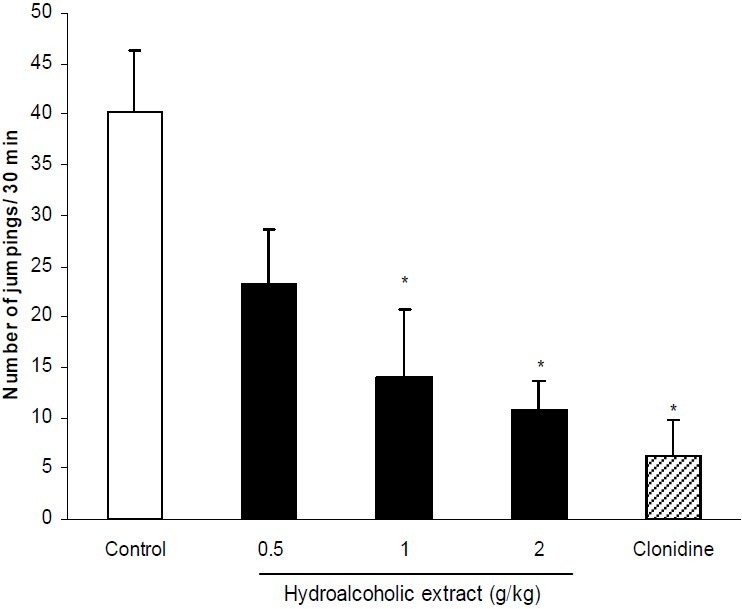
Effect of different doses of hydroalcoholic extract of C. copticum on naloxone-induced jumping. Morphine dependent mice (n=6) received different doses of hydroalcoholic extract of C. copticum or vehicle via oral route 90 min prior to naloxone (5 mg/kg, i.p.) and number of jumpings were recorded during a 30 min period. Clonidine (0.2 mg/kg, p.o.) was used as standard drug. Data are mean ± S.E.M. of 6 mice in each group. * P<0.05 compared with control group (ANOVA and Duncan).
Fig. 2.
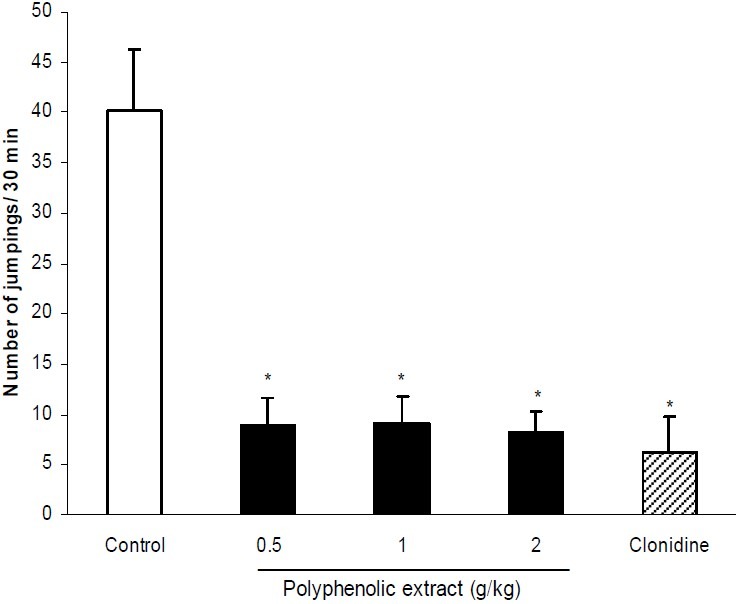
Effect of different doses of polyphenolic extract of C. copticum on naloxone-induced jumping. Morphine dependent mice (n=6) received different doses of polyphenolic extract of C. copticum or vehicle via oral route 90 min prior to naloxone (5 mg/kg, i.p.) and number of jumpings were recorded during a 30 min period. Clonidine (0.2 mg/kg, p.o.) was used as standard drug. Data are mean ± S.E.M. of 6 mice in each group. * P<0.05 compared with control group (ANOVA and Duncan).
Table 1.
Effect of C. copticum hydroalcoholic and polyphenolic extracts on checked signs of naloxone-induced morphine withdrawal in mice (n=6)
DISCUSSION
C. copticum contains essential oil and various polyphenols, including flavonoids. Thymol and its precursors, para-cymene and gamma-terpinene are the prominent compounds of its essential oil(11–13,15,23). These natural compounds are present in several members of Apiaceae and Lamiaceae families and ameliorate morphine tolerance and dependence in mice(5,7,9). As it was mentioned in result section, polyphenolic extract of C. copticum inhibited jumpings. Our results are in agreement with previous reports indicating the suppressive effects of polyphenolic compounds on morphine withdrawal(28,29).
Some flavonoids including quercetin, flavone, catechin and chrysin have been reported to block naloxone-induced contracture of guinea pig ileum after exposure to morphine(28). Naidu and coworkers reported that quercetin can reverse naloxone-induced jumps of morphine dependent mice and they concluded that this effect may be via suppression of nitric oxide synthase activity(29). Also some flavonoids potentiate brain GABAA receptors and increase GABA-induced currents in rat cortical neurons. Flavonoids may also be capable of modulating glutamate excitotoxicity via attenuation of calcium influx or by direct scavenging of reactive oxygen species(30).
In general various systems including purinergic(31,32), adrenergic(33), dopaminergic(34,35), excitatory amino acids(36–39) and nitric oxide(40) are involved in suppression of opioid withdrawal syndrome. On the basis of the above-mentioned evidences, it is possible that the alleviative effects of C. copticum extracts on withdrawal syndrome in mice are exerted via potentiation of GABA neurotransmission, suppression of glutamate receptors and/or suppression of nitric oxide pathway(15,28,30). However, the exact mechanism of action of this plant is not known and further investigations are needed to clarify it.
CONCLUSION
It can be concluded that C. copticum and especially its flavonoids have a modest potential in alleviating morphine withdrawal syndrome and further studies are required to identify the effective components.
ACKNOWLEDGMENT
This work was financially supported by Research Council of Isfahan University of Medical Sciences, Isfahan, Iran.
REFERENCES
- 1.Ebner R, Schreiber W, Zierer C. Buprenorphine or methadone for detoxification of young opioid addicts. Psychiatr Prax. 2004;31:108–110. doi: 10.1055/s-2004-828448. [DOI] [PubMed] [Google Scholar]
- 2.Janiri L, Mannelli P, Persico AM, Serretti A, Tempesta E. Opiate detoxification of methadone maintenance patients using lefetamine, clonidine and buprenorphine. Drug Alcohol Depend. 1994;36:139–145. doi: 10.1016/0376-8716(94)90096-5. [DOI] [PubMed] [Google Scholar]
- 3.Umbricht A, Hoover DR, Tucker MJ, Leslie JM, Chaisson RE, Preston KL. Opioid detoxification with buprenorphine, clonidine, or methadone in hospitalized heroin-dependent patients with HIV infection. Drug Alcohol Depend. 2003;69:263–272. doi: 10.1016/s0376-8716(02)00325-3. [DOI] [PubMed] [Google Scholar]
- 4.Hosseinzadeh H, Jahanian Z. Effect of Crocus sativus L.(Saffron) stigma and its constituents, crocin and safranal, on morphine withdrawal syndrome in mice. Phytother Res. 2010;24:726–730. doi: 10.1002/ptr.3011. [DOI] [PubMed] [Google Scholar]
- 5.Haghparast A, Shams J, Khatibi A, Alizadeh AM, Kamalinejad M. Effects of the fruit essential oil of Cuminum cyminum Linn.(Apiaceae) on acquisition and expression of morphine tolerance and dependence in mice. Neurosci Lett. 2008;440:134–139. doi: 10.1016/j.neulet.2008.05.060. [DOI] [PubMed] [Google Scholar]
- 6.Ramezani M, Hosseinzadeh H, Mojtahedi K. Effects of Ferula gummosa Boiss. fractions on morphine dependence in mice. J Ethnopharmacol. 2001;77:71–75. doi: 10.1016/s0378-8741(01)00263-x. [DOI] [PubMed] [Google Scholar]
- 7.Hajhashemi V, Rabbani M, Asghari GR, Karami-Saravi Z. Effects of Otostegia persica (Burm.) Boiss on morphine withdrawal syndrome in mice. Iranian J Pharm Res. 2004;3:171–175. [Google Scholar]
- 8.Karimi Gh, Ziaee T, Nazari A. Effect of Portulaca oleraceae L.extracts on the morphine dependence in mice. Iranian J Basic Med Sci. 2008;10:229–232. [Google Scholar]
- 9.Hosseinzadeh H, Ramezani M, Shahsavand Sh. Effects of Rosmarinus officinalis L.aerial parts extract and fractions on morphine withdrawal syndrome in mice. J Med Plants. 2006;5:27–35. [Google Scholar]
- 10.Amin Gh. Tehran: Publications of Tehran University of Medical Sciences; 2005. Popular medicinal plants of Iran; pp. 168–169. [Google Scholar]
- 11.Hejazian SH. Analgesic effect of essential oil (EO) from Carum copticum in mice. World J Med Sci. 2006;1:95–99. [Google Scholar]
- 12.Samsam Shariat SH. Iranian Herbal Pharmacopoeia. Tehran: Iranian Ministry of Health Publications; 2003. Trachyspermi coptici Fructus; pp. 397–403. [Google Scholar]
- 13.Gersbach PV, Reddy N. Non-invasive localization of thymol accumulation in Carum copticum (Apiaceae) fruits by chemical shift selective magnetic resonance imaging. Annals Bot. 2002;90:253–257. doi: 10.1093/aob/mcf179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gilani AH, Jabeen Q, Ghayur MN, Janbaz KH, Akhtar MS. Studies on the antihypertensive, antispasmodic, bronchodilator and hepatoprotective activities of the Carum compticum seed extract. J Ethnopharmacol. 2005;98:127–135. doi: 10.1016/j.jep.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 15.Rezvani ME, Roohbakhsh A, Mosaddegh MH, Esmailidehaj M, Khaloobagheri F, Esmaeili H. Anticonvulsant and depressant effects of aqueous extracts of Carum comticum seeds in male rats. Epilepsy Behav. 2011;22:220–225. doi: 10.1016/j.yebeh.2011.07.017. [DOI] [PubMed] [Google Scholar]
- 16.Zahin M, Ahmad I, Aqil F. Antioxidant and antimutagenic activity of Carum copticum fruit extracts. Toxicol in Vitro. 2010;24:1243–1249. doi: 10.1016/j.tiv.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 17.Boskabady MH, Shaikhi J. Inhibitory effect of Carum copticum on histamine (H1) receptors of isolated guinea-pig tracheal chains. J Ethnopharmacol. 2000;69:217–227. doi: 10.1016/s0378-8741(99)00116-6. [DOI] [PubMed] [Google Scholar]
- 18.Boskabady MH, Jandaghi P, Kiani S, Hasanzadeh L. Antitussive effect of Carum opticum in guinea pigs. J Ethnopharmacol. 2005;97:79–82. doi: 10.1016/j.jep.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 19.Dashti-Rahmatabadi MH, Hejazian SH, Morshedi A, Rafati A. The analgesic effect of Carum copticum extract and morphine on phasic pain in mice. J Ethnopharmacol. 2007;109:226–228. doi: 10.1016/j.jep.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 20.Hejazian SH, Morowatisharifabad M, Mahdavi SM. Relaxant effect of Carum copticum on intestinal motility in ileum of rat. World J Zoology. 2007;2:15–18. [Google Scholar]
- 21.Hejazian SH, Mosaddegh MH, Dashti Rahmatabadi MH. Antinociceptive effects of Carum copticum extract in mice using formalin test. World Appl Sci J. 2008;3:215–219. [Google Scholar]
- 22.Kharade SM, Khetmar SS, Desai PS, Lokhande RS, Patil SS. Evaluation of anxiolytic activity of Carum copticum by using elevated plus maze and open field method. Int Res J Pharm. 2011;2:165–168. [Google Scholar]
- 23.Kazemi Oskuee R, Behravan J, Ramezani M. Chemical composition, antimicrobial activity and antiviral activity of essential oil of Carum copticum from Iran. AJP (Avicenna Journal of Phytomedicine) 2011;1:83–90. [Google Scholar]
- 24.Thangam C, Dhananjayan R. Antiinflammatory potential of the seeds of Carum copticum L. Indian J Pharmacol. 2003;35:388–391. [Google Scholar]
- 25.Ghannadi A, Hajhashemi V, Jafarabadi H. An investigation of the analgesic and anti-inflammatory effects of Nigella sativa seed polyphenols. J Med Food. 2005;8:488–493. doi: 10.1089/jmf.2005.8.488. [DOI] [PubMed] [Google Scholar]
- 26.Hajhashemi V, Ghannadi A, Sedighifar S. Analgesic and anti-inflammatory properties of the hydroalcoholic, polyphenolic and boiled extracts of Stachys lavandulifolia. Res Pharm Sci. 2007;2:92–98. [Google Scholar]
- 27.Hajhashemi V, Ghannadi A, Mousavi S. Antinociceptive study of extracts of Platanus orientalis leaves in mice. Res Pharm Sci. 2011;6:123–128. [PMC free article] [PubMed] [Google Scholar]
- 28.Capasso A, Piacente S, Pizza C, Sorrentino L. Flavonoids reduce morphine withdrawal in vitro. J Pharm Pharmacol. 1998;50:561–564. doi: 10.1111/j.2042-7158.1998.tb06199.x. [DOI] [PubMed] [Google Scholar]
- 29.Naidu PS, Singh A, Joshi D, Kulkarni SK. Possible mechanisms of action in quercetin reversal of morphine tolerance and dependence. Addict Biol. 2003;8:327–336. doi: 10.1080/13556210310001602248. [DOI] [PubMed] [Google Scholar]
- 30.Hanrahan JR, Chebib M, Johnston GA. Flavonoid modulation of GABA (A) receptors. Br J Pharmacol. 2011;163:234–245. doi: 10.1111/j.1476-5381.2011.01228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Michalska E, Malec D. Agonists and antagonists of adenosine receptors and morphine withdrawal syndrome in rats. Pol J Pharmacol. 1993;45:1–9. [PubMed] [Google Scholar]
- 32.Capasso A, Loizzo A. Purinoreceptors are involved in the control of morphine withdrawal. Life Sci. 2001;69:179–188. doi: 10.1016/s0024-3205(01)01313-3. [DOI] [PubMed] [Google Scholar]
- 33.Ambrosio E, Igalesias V, Garcia-Lecumberri C, Orensanz L, Algauacil LF. Effect of yohimbine on the development of morphine dependence in the rat: lack of involvement of cortical beta-adrenoceptor modifications. Pharmacol Biochem Behav. 1997;56:487–491. doi: 10.1016/s0091-3057(96)00243-2. [DOI] [PubMed] [Google Scholar]
- 34.Puri S, Lal H. Effect of dopaminergic stimulation or blockade on morphine withdrawal aggression. Psychopharmacologia. 1973;32:113–120. doi: 10.1007/BF00428682. [DOI] [PubMed] [Google Scholar]
- 35.Lal H, Numan R. Blockade of morphine withdrawal body shakes by haloperidol. Life Sci. 1976;18:163–167. doi: 10.1016/0024-3205(76)90020-5. [DOI] [PubMed] [Google Scholar]
- 36.Wange L, Milne B, Jhamandas K. Involvement of excitatory amino acid pathways in the expression of precipitated opioid withdrawal in the rostral ventrolateral medulla: an in vivo voltametric study. Brain Res. 1995;697:130–142. doi: 10.1016/0006-8993(95)00803-x. [DOI] [PubMed] [Google Scholar]
- 37.Tokuyama S, Wakabayashi H, Ho IK. Direct evidence for a role of glutamate in the expression of the opioid withdrawal syndrome. Eur J Pharmacol. 1996;295:123–129. doi: 10.1016/0014-2999(95)00645-1. [DOI] [PubMed] [Google Scholar]
- 38.Gonzalez P, Cabello P, Germany A, Norris B, Contreas E. Decrease of tolerance to, and physical dependence on morphine by glutamate receptor antagonists. Eur J Pharmacol. 1997;332:257–262. doi: 10.1016/s0014-2999(97)01099-6. [DOI] [PubMed] [Google Scholar]
- 39.Tokuyama S, Zhu H, Oh S, Ho IK, Yamamoto T. Further evidence for a role of NMDA receptors in the locus coeruleus in the expression of withdrawal syndrome from opioids. Neurochem Int. 2001;39:103–109. doi: 10.1016/s0197-0186(01)00019-5. [DOI] [PubMed] [Google Scholar]
- 40.Herman BH, Vocci F, Bridge P. The effects of NMDA receptor antagonists and nitric oxide synthase inhibitors on opioid tolerance and withdrawal: Medication development issues for opiate addiction. Neuropsychopharmacol. 1995;13:269–293. doi: 10.1016/0893-133X(95)00140-9. [DOI] [PubMed] [Google Scholar]


