Abstract
Several species of Prangos are traditionally used as emollient, carminative, tonic, anti-flatulent, anthelmintic and anti-thrombotic agents. Osthole, a coumarin isolated from Prangos, are believed to be responsible for some of its effects. In this research, relaxant effects of Prangos ferulacea extract and osthole on rat uterus contraction induced by KCl, acetylcholine (ACh), oxytocin and electrical field stimulation (EFS) was investigated and compared with atropine and salbutamol. P. ferulacea acetonic extract concentration-dependently relaxed uterine contraction induced by KCl (IC50=13 ± 0.81 μg/ml), ACh (IC50=12 ± 1.38 μg/ml), oxytocin (IC50=16 ± 3.14 μg/ml) and EFS (IC50=11 ± 1.5 μg/ml). However, the extract at lower concentration (2.5 μg/ml) potentiated the EFS response. Osthole only had inhibitory effect on rat uterus and its relaxant effect was observed at lower concentration in comparison with P. ferulacea extract. Osthole in a similar way inhibited the response to KCl (IC50=4 ± 0.13 μg/ml), ACh (IC50=4 ± 0.8 μg/ml), oxytocin (IC50=4 ± 0.8 μg/ml) and EFS (IC50=1.5 ± 0.5 μg/ml). Our results demonstrated that osthole acted directly on uterus smooth muscle to induce relaxation, whereas P. ferulacea caused both contraction and relaxation of rat uterine smooth muscle. The relaxation of osthole might be mediated through Ca2+ channel blocking activity as it inhibited the response to KCl. Mechanisms other than Ca2+ channel blocking appeared to be responsible for ACh relaxation effect of osthole.
Keywords: Prangos ferulacea, Osthole, EFS, KCl, Ach, Oxytocin
INTRODUCTION
The genus Prangos (Jashir in Persian) belongs to the Umbelliferae family and consists of fifty species(1). They have been used in traditional medicine as emollient, carminative(2), tonic, anti-flatulent, anthelmintic, antifungal and antibacterial agents(3,4). Phytochemical investigations on different species of Prangos have led to the isolation of coumarins(5–7) and volatile oils(8–12) from different parts of the plants.
Prangos ferulacea (L.) Lindl. is a plant found in the Mediterranean and Middle-east regions including Iran(13). It is traditionally used as flavoring additive in yogurt(14). P. ferulacea consumption is suspicious of abortive activity(15). Fifty-two components were identified in essential oil from the roots of P. ferulacea among which δ-3-carene (22.5%), β-phellandrene (11.8%), α-pinene (8.6%), terpinolene (7.2%), p-cymene (6.3%) and myrcene (4.5%) were found to be the major ones(16). A number of coumarin components are also identified in the root extract of P. ferulacea including osthole, isoimperatiorin, oxypeucedanin, psoralen, and gosferol(17).
Osthole is also identified as a component of other plants which are used in Chinese folk medicine, and believed to have anti-inflammatory, anti-osteoporosis and anti-tumor activities(18–20). It has been reported that osthole suppressed the contraction response of tracheal, ileum and taeniae coli smooth muscles(21,22). In addition, osthole inhibits vascular smooth muscle contraction and lowers the blood pressure in animals as well as inhibiting platelet aggregation(23–25). There is also a short abstract about relaxant effect of osthole on rat uterus but the result can not be validated(26). If this is the case, then P. ferulacea extract which is a source of osthole(17) should have inhibitory effect on uterus motility. However, so for the pharmacological effect of P. ferulacea on uterus smooth muscle motility has not been reported. Therefore, the objective of this research was to investigate the effect of P. ferulacea extract on isolated uterus smooth muscle contraction for comparison with osthole and two standard drugs.
MATERIALS AND METHODS
Plant material
Roots of P. ferulacea were collected from Yasouj in Kohgiluyeh-Boirahmad province in June 2010, at an altitude of 1800 meter above sea level. The plant was identified at the Botany Department of Yasouj University and a voucher specimen (No. 2408) was deposited at the Herbarium of the School of Pharmacy and Pharmaceutical Sciences, Isfahan University of Medical Sciences, Isfahan, Iran. The air dried plant material was roughly cut and ground to the coarse powder.
Extraction, isolation and identification of osthole
500 g of aerial parts was extracted with acetone for two days (5L × 4). The extract was concentrated to bear a viscous mass which was then put in -20°C for two days and filtered chilled in which the filtrate resulted in a solid mass after drying. The latter was fractioned by vacuum liquid chromatography (Silica gel, MESH 230-400) using a gradient of heptane: ethyl acetate (H:EtOAc) from 100:0 to 0:100 to afford several fractions. One of the fractions eluted with H:EtOAc (7:3) bore a mixture of two coumarins, osthole and isoimperatorin in which further purification using silica as adsorbent and H:EtOAc as eluents resulted in pure osthole. The identity of compound was determined using HNMR and CNMR spectroscopy and comparison of data with what has been previously reported(17,27).
Uterine contractile assessment
Experiments were conducted on adult non-pregnant female Wistar rats (180-230 g) bred in School of Pharmacy animal house. All animals were handled in compliance with the principles of the guide for care and use of laboratory animal care(28). A day before experiment, rats were given 17-β-oestradiol (100 μg/kg, S.C.) and housed in a cage with free access to food and water at room temperature. On the day of experiment, a rat was killed by a blow on the head, followed by exsanguination. Both uterine horns were removed and placed in oxygenated Tyrode's solution at room temperature. The uterine horns were separated from each other. A uterine horn was mounted for isotonic contraction under 1 g tension in 50 ml organ bath (Harvard, England) containing Tyrode's solution and continuously gassed with O2 at 37°C. Uterine contraction was measured using a Harvard isotonic transducer and recorded on a Harvard Universal Oscillograph (England) pen recorder device. The other uterine horn was used as vehicle treated time matched control tissue.
After calibrating the oscilograph, 3 successive washes was given to the tissue and allowed to relax to a stable base line. Following a resting period of about 30 min, inhibitory effect of P. ferulacea extract and osthole, were examined on isolated rat uterine contraction induced by KCl, oxytocin, acetylcholine (ACh) and electrical field stimulation (EFS) as described below and compared with the standard drugs salbutamol, atropine and dibucaine (for methodology see references 29,30).
Effect of drugs on spasm induced by potassium chloride
Uterine horns were exposed to KCl (80 mM) to induce tonic contraction. After 15 min equilibration, relaxants were added in a cumulative manner to the bath until maximum inhibition was obtained. The time matched control tissues were treated with equivalent volume of relaxant vehicle.
Effect of drugs on spasm induced by oxytocin
Phasic spasm of uterine was induced by adding oxytocin (0.002 IU/ml) into the bath. As before, after 15 min equilibration, relaxants were added to the bath in a cumulative manner until maximum inhibition was achieved. The time matched control tissues were treated with equivalent volume of relaxant vehicle.
Effect of drugs on spasm induced by acethylcholine (ACh)
Uterine contraction was induced by single concentration of ACh (2 μM). Acetylcholine was in contact with the tissue for 30 sec before it was washed off with fresh Tyrode's solution. Initial contraction to ACh was determined and repeated twice for consistency in 15 min intervals, then ACh responses in the presence of increasing concentration of relaxant were assessed in non-cumulative manner, until maximum inhibitory responses was obtained. The same protocol was repeated for vehicle treated time matched control tissues.
Effect of drugs on spasm induced by electrical field stimulation (EFS)
EFS was performed with trains of rectangular pulses from a stimulator (made in school of Pharmacy workshop) 6 V and 50 Hz for 1 sec. A train of 5 successive EFS pulses were delivered at 1 min intervals to the uterine horn suspended in the organ bath via a couple of platinum electrode wires fixed alongside the tissue holder. The same protocol as for ACh was used for EFS studies.
Drugs and Solutions
The following drugs were used in this research: P. ferulacea extract, osthole, atropine sulfate, salbutamol (Sigma), acethylcholine hydrochloride (Sigma), oxytocin ampule (Aburaihan Pharm., Iran), dibucaine hydrochloride (Sigma), and estradiol valerate (Aburaihan Pharm., Iran). 17-β-oestradiol was prepared in arachis oil as 100 μg/ml stock solution. The extract and the osthole were made up as 10 mg/ml stock solution in dimethyl sulphoxide (DMSO), dilution being made in DMSO. Oxytocin (1 IU/ml), KCl (2 M), atropine (10 mM), salbutamol (1 mM) and dibucaine (10 mM) stock solutions were made up in distilled water and further diluted with distilled water. Acetylcholine was made up as 100 mM stock solution and acidified by 1% acetic acid, and further serial dilution was made in distilled water.
Tyrode's solution composed of (mM): NaCl, 136.9; KCl, 2.68; CaCl2, 1.8; MgCl2, 1.05; NaHCO3, 11.9; NaH2PO4, 0.42 and glucose 5.55, was made up in distilled water. Unless stated, all chemicals and drugs were from Merck.
Measurements and statistical analysis
The contractile response to KCl, oxytocin, ACh and EFS were measured as maximum amplitude from pretreatment baseline and expressed as the percentage of the initial response in the absence of drugs or vehicle for each tissue. First EFS response sometime was mixed with spontaneous activity, thus, the mean amplitude of other four responses to EFS was used for the calculation of contraction response. All the values are quoted as mean ± standard error of the mean (SEM). The significance of differences between the mean was calculated by ANOVA for repeated measures or two tailed Student's t-test. Differences was considered statistically significant for P<0.05.
The IC50 value (drug concentration causing 50% of maximum response) of relaxant in isolated uterus were calculated for each tissue and mean and SEM was determined for each group of results. Sigma plot computer program was used for statistical analysis and drawing the graphs for calculation of IC50 values.
RESULTS
Rat isolated uterus suspended under 1 g tension in vitro, after a few minuets exhibited spontaneous and irregular rhythmic activity with variable amplitude and duration. The spontaneous activity was subsided by washing or after induced contraction.
Effect of drugs on spasm induced by potassium chloride
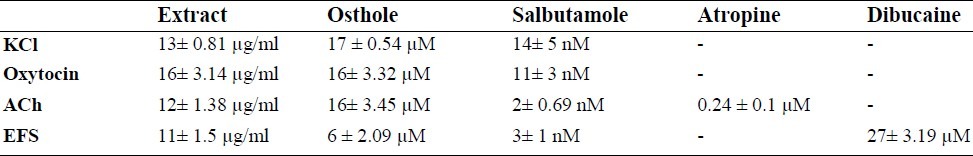
KCl (80 mM) caused a sustained tonic contraction which maintained for the duration of study. The extract (5-40 μg/ml) and the osthole (0.6-20 μg/ml) applied cumulatively into the organ bath caused a concentration dependent relaxation of rat uterus tonic contraction. Analysis of the concentration response curve revealed a mean IC50 value of 13 ± 0.81 μg/ml (n=6) and 4 ± 0.13 μg/ml (17 ± 0.54 μM, n=6) respectively (Fig. 1 A). The maximal inhibition of osthole was achieved at concentration of 20 μg/ml in the bath while the maximum effect of the extract was seen at bath concentration of 40 μg/ml. Salbutamol (0.1 nM - 10 μM) in a similar way inhibited tonic contraction induced by KCl. Atropine (50 nM - 55 μM) has no significant effect on tonic contraction induced by KCl. Statistically analysis revealed no significant difference in contraction of vehicle treated time matched control tissues (the highest used concentration of DMSO was 1%)
Fig. 1.
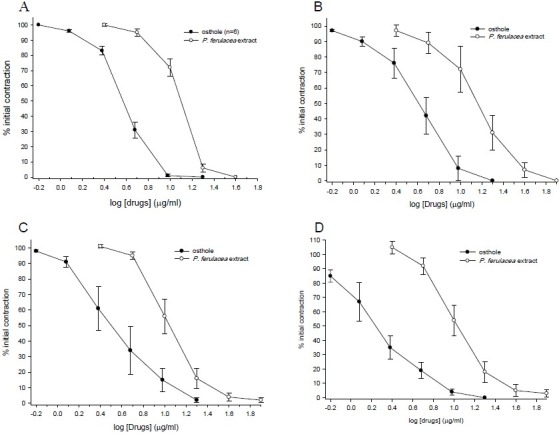
Inhibitory effect of P. ferulacea extract and osthole on tension developments in the isolated uterus of nonpregnant rat treated with A: KCl (80 mM), B: oxytocin (0.002 IU/ml) C: acetylcholine (ACh, 2 μM), and D: electrical field stimulation (EFS: 6 V, 50 Hz, 1 S duration). Contractile response was measured relative to the baseline. Ordinant scales: spasm remaining as a % of the contraction prior to compounds addition. Abscissa scales: log10 concentration of compounds. Each point is mean of six experiments and the vertical lines show the SEM. The inhibition of contraction is statistically significant in comparison to initial control contraction (P<0.001). The maximum concentration of DMSO used was 1% in all cases.
Effect of drugs on spasm induced by oxytocin
Oxytocin (0.002 IU/ml), caused a relatively regular rhythmic contraction on rat uterus. Both the extract (5-80 μg/ml) and the osthole (0.6-20 μg/ml) concentration-dependently. inhibited the rhythmic contraction induced by oxytocin (Fig. 1 B). Over the course of the study, sometimes slight gradual attenuation was observed in contraction induced by oxytocin in vehicle treated time matched control tissue. However, there was no statistically significant changes when the mean results were compared (ANOVA). Atropine (50 nM - 55 μM) had no significant effect on contraction induced by oxytocin in comparison with time matched control tissues, while salbutamol concentration-dependently reduced the oxytocin response, however, complete inhibition was not achieved and maximum inhibition was seen at 1 μM concentration (77%), increased salbutamol concentration to 10 μM had no further inhibitory effect of oxytocin induced contraction.
Effect of drugs on spasm induced by acythylcholine (ACh)
Acetylcholine caused a rapid contraction of rat uterus reaching its peak within 30 S of the contact time. Atropine (50 nM - 12.8 μM) concentration-dependently inhibited the response to ACh. At concentration of 12.8 μM, atropine abolished the response to ACh. Both the extract of P. ferulacea and osthole concentration-dependently inhibited contraction induced by ACh with IC50=12 ± 1.38 μg/ml (n=6) and IC50=4 ± 0.8 μg/ml (16 ± 3.4 μM, n=6), respectively (Fig. 1 C). Relaxation of the uterus began with 5 mg/ml extract in the bath, reduced to 4 ± 2.6% with 40 μg/ml bath concentration. Salbutamol also concentration-ependently (0.1 nM – 400 nM) inhibited the response to ACh (The IC50 values are compared in Table 1). No significant changes were observed in the ACh time matched control tissues treated with the vehicle.
Table 1.
Comparison of the IC50 values (±SEM) of Prangos ferulacea extract, osthole, salbutamole, atropine and dibucaine on contraction induced by KCl, oxytocin, acethylcholine (ACh) and electrical field stimulation (EFS) in rat isolated uterus (n=6).
Effect of drugs spasm induced by electrical field stimulation (EFS)
Rat uterine contracted rapidly to EFS, reaching a peak within 10 sec followed by full rapid relaxation to the baseline. The EFS responses were similar to EFS response described by Houdeau and coworkers(29). Atropine at concentrations which blocked ACh responses in rat uterus (50 nM to 12.8 μM) only partially attenuated the EFS responses. Increasing concentration of atropine had no further inhibitory effect of EFS induced contraction in rat uterus. Dibucaine (10, 20, 40, and 80 μM) concentration-dependently reduced the response to EFS, while at 10, 20 and 40 μM bath concentrations dibucaine had no inhibitory effect on uterine response to ACh. Nevertheless, with 80 μM dibucaine in the bath ACh response was reduced by 21 ± 7.9% while the EFS response was reduced by 89 ± 2.6%.
Relaxant effect of P. ferulacea extract and osthole at concentration ranges which inhibited the KCl responses were also examined on EFS response. The extract (5 μg/ml to 160 μg/ml) concentration-dependently inhibited contraction responses to EFS (IC50=11 ± 1.5 μg/ml). At its highest used concentration the extract totally removed the EFS response (see Fig 1 D). From time to time it was observed that the extract may also potentiate the EFS response, therefore, we have examined the effect of lower concentration of the extract (0.25, 0.5, 1 and 2.5 μg/ml) on EFS and ACh responses. These experiments revealed that the inhibitory effect of the extract may have started at 0.25 μg/ml bath concentration. However, as there were some stimulatory components in the extract, it reversed the inhibition of EFS response (Fig. 2). At 2.5 μg/ml bath concentration, the stimulatory effect of the extract overcame the inhibition and EFS response was significantly potentiated (see Fig. 2). Thereafter, the inhibitory response was dominant.
Fig. 2.

Stimulatory effect of P. ferulacea extract on tension development in the isolated uterus of non-pregnant rat treated with electrical field stimulation (EFS: 6 V, 50 Hz, 1 S duration) and acetylcholine (ACh). Contractile response was measured relative to the baseline. Ordinant scales: % spasm of the contraction prior to compounds addition. Abscissa scales: log10 concentration of P. ferulacea. Each point is mean of six experiments and the vertical lines show the SEM. *P<0.05 in comparison with initial concentration (Student's t-test).
Osthole at concentration ranges of 0.6-20 μg/ml, had a similar pattern of inhibition to EFS and at its highest used concentration removed the EFS response. The IC50 values are compared in Table 1.
DISCUSSION
Spontaneous abortion and preterm labor can be a problem in pregnancy(31). Consumption of some plant material might be a cause, while other plant materials are being used in folk medicine for treatment of this condition(30). We have demonstrated that P. ferulacea extract is a relaxant of isolated rat uterus of non-pregnant rat, inhibiting contraction induced by high concentration K+ ions, oxytocin, ACh and EFS stimulation. Osthole is one of the components isolated from P. ferulacea extract. It had similar pattern of inhibitory effect on rat uterus contraction evoked by above stimuli. However, it was much more potent than the extract and the ratio of potency at IC50 levels were 3.3, 3, 4, and 7.3 for inhibiting KCl, ACh, oxytocin and EFS responses respectively. Comparison of inhibitory concentrations of osthole and extract of P. ferulacea (see Fig. 1) suggest that osthole is mainly responsible for the inhibitory effect of the extract.
Inhibition of EFS response by selective concentrations of local anesthetic, dibucaine, without inhibiting the effect of ACh, support the suggestion that the parameters used for EFS is mainly stimulating the neurons within the isolated uterus because the myometrium was still capable of contraction to exogenous ACh. At higher concentration dibucaine, however, has local activity on smooth muscle(32).
The contractile responses of the uterus vary at different stages of the menstrual cycle and during pregnancy(33). Therefore, in this study, all the animals used, were non pregnant and in order to synchronized their cycle, all the rats were pretreated with estrogen. Isolated uterus pretreated with estrogen contracted rhythmically in vitro. The contraction originates in the myometrial cells which act as pacemakers and give rise to conducted action potential(29). The electrophysiological activity of these pacemaker cells is regulated by the sex hormones and the nerve supplying the uterus. Both noradrenergic and cholinergic innervations are found in uterus(34). The nerve supplying to the uterus includes both excitatory and inhibitory sympathetic components(35). Adrenalin acting on β2-adrenoceptors inhibits uterine contraction, whereas noradrenaline acting on α-adrenoceptors, stimulated contraction(36). Rat uterus is also supplied by parasympathetic nerves, which contribute to the control of muscular activity(35). Acetylcholine released from these nerve fibers evokes the contraction of the uterus acting through muscarinic receptors which is a G-protein coupled receptor and by increasing activity of phospholipase C cause release of Ca2+ from intracellular stores(37). The cholinergic nerve control of uterine activity is layer specific and predominant in the caudal uterine horn(38).
Oxytocin is a peptide hormone of the posterior pituitary gland and stimulates the contractions of uterus. Uterine smooth muscles are much more sensitive to oxytocin when it is primed with estrogen(31). Estrogen induces oxytocin receptor synthesis and consequently uterus treated with estrogen is highly sensitive to oxytocin(31). Oxytocin acts on the oxytocin receptor on uterine smooth muscle cells and cause release of intracelullar Ca2+ and protein kinase C activation(31). Oxytocin caused regular contraction and uterus muscle relaxes completely between contractions with low doses of oxytocin. High doses of oxytocin may cause a sustained contraction(34).
High concentration of KCl causes smooth muscle depolarization and consequently activation of voltage gated calcium channels and influx of Ca2+ into the cells(39). Atropine, a muscarinic antagonist(34), at concentrations which abolished the response to ACh had no effect on the KCl response but contraction evoked by EFS was partially reduced by atropine, indicating that ACh has a role in spasm induced by EFS. The remaining response is probably due to release of noradrenaline from sympathetic neurons acting through α-adrenoceptors. Salbutamol on the other hand, is a selective β2-adrenoceptor agonist and as expected it was a relaxant of rat uterus(34). The relaxant effect of osthole was more similar to salbutamol than atropine. Both inhibited contractions induced by KCl, ACh, EFS and oxytocin. Comparing the relaxant effect of P. ferulacea extract, osthole and the standard drugs used in this study, may give some idea about possible site of action. Inhibition of tonic contraction induced by KCl, rhythmic contraction induced by oxytocin, and phasic contraction induced by ACh, as well as EFS indicate that more general intracellular inhibitory mechanism may have involved. It is therefore, not surprising that it has inhibitory effect on other smooth muscles. For example osthole suppressed the contraction response curves of tracheal smooth muscle of guinea-pig. The researchers concluded that osthole exerts a non-specific relaxant effect on the trachealis by inhibiting the cAMP and cGMP dependent phosphodiesterases(21). It has also been reported that osthole inhibits vascular smooth muscle contraction and lowers blood pressure in animal studies. Again the researchers suggested that the mechanism might by due to Ca2+ channel blocking properties and by elevating cGMP levels in vascular smooth muscle(24). A similar mechanism of action could also explain the relaxant effect of osthole in rat uterus. Components other than osthole must be responsible for potentiating the EFS response. As P. ferulacea had no potentiating effect on ACh response, it is very likely that potentiation is mediated through intramural neurons.
CONCLUSION
Osthole is a major component of P. ferulacea extract and is responsible for the relaxant effect of the extract on the rat uterus. Osthole is a coumarin and as mentioned above it also has anticoagulant effect. As coumarins are contraindicated in pregnancy(40) we can not suggest its use for prevention of preterm labor. On the other hand, the P. ferulacea extract had a potential to increase neuronal contractile response of rat uterus, therefore, the general believe that it may causes abortion is substantiated in this study. Identification of the stimulatory components is recommended for future studies.
ACKNOWLEDGMENT
We would like to thank the research department of Isfahan University of Medical Sciences, Isfahan, I.R.Iran, for financially supporting this project.
REFERENCES
- 1.Evans WC. 13th ed. Bailliere Tindall, London: 1989. Trease and Evans’ Pharmacognosy; p. 205. [Google Scholar]
- 2.Zargari A. Vol. 2. Tehran: Tehran University Publications; 1988. Medicinal Plants; p. 553. [Google Scholar]
- 3.Ulubelen A, Topcu G, Tan N, Olcal S, Johansson C, Ucer M, et al. Biological activities of a Turkish medicinal plant, Prangos platychlaena. J Ethnopharmacol. 1995;45:193–197. doi: 10.1016/0378-8741(94)01215-l. [DOI] [PubMed] [Google Scholar]
- 4.Bouaoun D, Hilan C, Garabeth F, Sfeir R. Antimicrobial activity of the essential oil of the wild plant, Prangos asperula Boiss. Phytotherapia. 2007;5:129–134. [Google Scholar]
- 5.Razavi SM, Nazemiyeh H, Hajiboland R, Kumarasamy Y, Delazar A, Nahar L, et al. Coumarins from the aerial parts of Prangos uloptera (Apiaceae) Brazil J Pharmacognosy. 2008;18:1–5. [Google Scholar]
- 6.Eshbakova KA, Saidkhodzhaev AI, Baser KHC, Duman H, Vdovin AD, Abdullaev ND. Furocoumarins from Prangos ferulacea. Chem Nat Comp. 2006;42:102–103. [Google Scholar]
- 7.Tada Y, Shikishima Y, Takaishi Y, Shibata H, Higuti T, Honda G, et al. Coumarins and γ-pyrone derivatives from Prangos pabularia: antibacterial activity and inhibition of cytokine release. Phytochemistry. 2002;59:649–654. doi: 10.1016/s0031-9422(02)00023-7. [DOI] [PubMed] [Google Scholar]
- 8.Sefidkon F, Khajavi MS, Malackpour B. Analysis of the oil of Prangos ferulacea (L.) Lindl. J Essent Oil Res. 1998;10:81–82. [Google Scholar]
- 9.Baser KHC, Ozek T, Demirci B, Duman H. Composition of the essential oil of Prangos heyniae H.Duman et M. F. Watson, a new endemic from Turkey. Flavour Fragr J. 2000;15:47–49. [Google Scholar]
- 10.Sajjadi SE, Mehregan I. Chemical composition of of the essential oil of Prangos asperula Boiss. subsp. Haussknechtii (Boiss.) Herrnst. et Heyn fruits. Daru. 2003;11:79–81. [Google Scholar]
- 11.Sefidkon F, Najafpour Navaii M. Chemical composition of the oil of Prangos uloptera DC. J Essent Oil Res. 2001;13:84–85. [Google Scholar]
- 12.Masoudi S, Aghajani Z, Yari M, Rustaiyan A. Volatile constituents of Prangos latiloba Korov. J Essent Oil Res. 1999;11:767–768. [Google Scholar]
- 13.Ghahreman A. Tehran: Research Institute of Forests and Rangelands Publication; 1986. Flora of Iran; p. 845. [Google Scholar]
- 14.Coruh N, Sagdicoglu Celep AG, Ozgokce F. Antioxidant properties of Prangos ferulaceae (L.) Lindl., Chaerophyllum macropodum Boiss. and Heracleum persicum Desf. from Apiaceae family used as food in Eastern Anatolia and their inhibitory effects on glutathione-S-transferase. Food Chem. 2007;100:1237–1242. [Google Scholar]
- 15.Kazerooni T, Mousavizadeh K, Abdollahee A, Sarkarian M, Sattar A. Abortifacient effect of prangos ferulacia on pregnant rats. Contraception. 2006;73:554–556. doi: 10.1016/j.contraception.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 16.Sajjadi SE, Shokoohinia Y, Gholamzadeh S. Chemical Composition of the Essential Oil of the Root of Prangos ferulacea (L.) Lindl. Chemija. 2011;22:178–180. [Google Scholar]
- 17.Gholamzadeh S. Pharm D Thesis. Isfahan, Iran: School of Pharmacy and Pharmaceutical Sciences, Isfahan University of Medical Sciences; 2011. Phytochemical investigation of the root of Prangos ferulacea (L.) Lindl. [Google Scholar]
- 18.Liu J, Zhang W, Zhou L, wang X, Lian Q. Antiinflammatory effect and mechanism of osthole in rats. Zhong Yao Cai. 2005;28:1002–1006. [PubMed] [Google Scholar]
- 19.Kuo PL, Hsu YL, Chang CH, Chang JK. Osthole-mediated cell differentiation through bone morphogenetic protein-2/p38 and extracellular signal-regulated kinase 1/2 pathway in human osteoblast cells. J Pharmacol Exp Ther. 2005;14:1290–1299. doi: 10.1124/jpet.105.085092. [DOI] [PubMed] [Google Scholar]
- 20.Okamoto T, Kobayashi T, Yoshida S. Chemical aspects of coumarin compounds for the prevention of hepatocellular carcinomas. Curr Med Chem Anticancer Agents. 2005;5:47–51. doi: 10.2174/1568011053352622. [DOI] [PubMed] [Google Scholar]
- 21.Teng CM, Lin CH, Ko FN, Wu TS, Huang TF. The relaxant action of osthole isolated from Angelica pubescens in guinea-pig trachea. Naunyn Schmiedebergs Arch Pharmacol. 1994;349:202–208. doi: 10.1007/BF00169838. [DOI] [PubMed] [Google Scholar]
- 22.Li L, Zhuang FE, Zhao GS, Zhoa DK. Effects of osthole on the isolated guinea-pig ileum and taeniae coli. Yao Xue Xue Bao. 1993;28:899–904. [PubMed] [Google Scholar]
- 23.Ko FN, Wu TS, Liou MJ, Huang TF, Teng CM. Vasorelaxation of rat thoracic aorta caused by osthole isolated from Angelica pubescens. Eur J Pharmacol. 1992;219:29–34. doi: 10.1016/0014-2999(92)90576-p. [DOI] [PubMed] [Google Scholar]
- 24.Hoult JR, Paya M. Pharmacological and biochemical actions of simple coumarins: natural products with therapeutic potrntial. 1996;27:713–722. doi: 10.1016/0306-3623(95)02112-4. [DOI] [PubMed] [Google Scholar]
- 25.Teng CM, Ko FN, Wang JP, Lin CN, Wu TS, Chen CC, Huang TF. Antihaemostatic and antithrombotic effect of some antiplatelet agents isolated from Chinese herbs. J Pharm Pharmacol. 1991;3:667–669. doi: 10.1111/j.2042-7158.1991.tb03561.x. [DOI] [PubMed] [Google Scholar]
- 26.Li L, Zhuang F, Zhao G, Zhoa D. Effects of osthole on the contractive function of uterine smooth muscle. J Xi’an Med Univ (Chinese) 1994;15:164–167. [Google Scholar]
- 27.Sajjadi SE, Zeinvand H, Shokoohinia Y. Isolation and identification of osthol from the fruits and essential oil composition of the leaves of Prangos asperula Boiss. Res Pharm Sci. 2009;4:19–23. [Google Scholar]
- 28.Guide for the Care and use of Laboratory animals. Washington DC: The National Academies Press; 2010. Committee for the update of the guide for the care and use of laboratory animals, National Research Council. [Google Scholar]
- 29.Houdeau E, Rossano B, Prud’homme MJ. Regional and muscle layer variations in cholinergic nerve control of the rat myometrium during the oestrous cycle. Auton Neurosci. 2003;104:1–9. doi: 10.1016/s1566-0702(02)00233-3. [DOI] [PubMed] [Google Scholar]
- 30.Sadraei H, Ghannadi A, Takei-Bavani M. Effects of Zataria multiflora and Carum carvi essential oils and hydroalcoholic extract of Passiflora incarnatem, Berberis integerrima and Crocus sativus on rat isolated uterus contractions. Int J Aromather. 2003;13:121–127. [Google Scholar]
- 31.Parker KL, Schimmer BP. Pituitary hormones and their hypothalamic releasing hormones. In: Brunton LL, Lazo JS, Parker KL, editors. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 11th ed. New York, McGraw-Hill: 2006. pp. 1489–1510. [Google Scholar]
- 32.Luciani S, Furlanut M. Interaction between dibucaine and K+ on smooth muscle. Pharmacol Res Commun. 1974;6:147–153. doi: 10.1016/s0031-6989(74)80022-6. [DOI] [PubMed] [Google Scholar]
- 33.Zoubina EV, Fan Q, Smith PG. Variations in uterine innervation during the estrous cycle in rat. J Comp Neurol. 1998;397:561–571. [PubMed] [Google Scholar]
- 34.Rang HP, Dale MM, Ritter JM, Moore P. 6th ed. Edinburgh London: Churchill Livingstone; 2007. Pharmacology; pp. 144–361. (367, 456). [Google Scholar]
- 35.Sato Y, Hotta H, Nakayama H, Suzuki H. Sympathetic and parasympathetic regulation of uterine blood flow and contraction in the rat. J Auton Nerv Syst. 1996;59:151–158. doi: 10.1016/0165-1838(96)00019-7. [DOI] [PubMed] [Google Scholar]
- 36.Stjernquist M, Owman C.H. Cholinergic and adrenergic neural control of smooth muscle function in the non-pregnant rat uterine cervix. Acta Physiol Scand. 1985;124:429–436. doi: 10.1111/j.1748-1716.1985.tb07679.x. [DOI] [PubMed] [Google Scholar]
- 37.Batra S. Influence of chronic estrogen treatment on the density of muscarinic cholinergic receptors and calcium channels in the rabbit uterus. J Endocrinol. 1990;125:185–189. doi: 10.1677/joe.0.1250185. [DOI] [PubMed] [Google Scholar]
- 38.Hollings worth M. Mechanical responses of rat isolated uterine horns to transmural stimulation. Br J Pharmacol. 1975;55:41–46. doi: 10.1111/j.1476-5381.1975.tb07607.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Diamond J, Holmes TG. Effects of potassium chloride and smooth muscle relaxants on tension and cyclic nucleotide levels in rat myometrium. Can J Physiol Pharmcol. 1975;53:1099–1107. doi: 10.1139/y75-153. [DOI] [PubMed] [Google Scholar]
- 40.Majerus PW, Tollefsen DM. Blood coagulation and anticoagulant, thrombolytic and antiplatelet drugs. In: Brunton LL, Lazo JS, Parker KL, editors. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 11th ed. New York, McGraw-Hill: 2006. pp. 1467–1488. [Google Scholar]


