Abstract
In this study antibacterial, antifungal and cytotoxic effects of some new 2,3-disubstituted 4(3H)-quinazolinone derivatives have been evaluated. The in vitro antibacterial and antifungal tests of new synthesized compounds were performed using MABA method against six strains of bacteria (three Grampositive and three Gram-negative) and three strains of fungi. Also Minimum Bactericidal Concentration (MBC) and Minimum Fungicidal Concentration (MFC) tests were performed. All synthesized compounds indicated mild antibacterial effects especially against Gram-negative bacteria. The most sensitive bacteriumwas E. coli. All strains of tested fungi were sensitive to the synthesized compounds mostly at 32 μg/ml and there was no significant differences in the sensitivity of the tested compounds. MBC and MFC data indicated that tested compounds act as bactriostatic and fungistatic agents. Cytotoxic activity of the compounds was screened at 1, 10 and 100 μM concentrations against HeLa cells using the MTT colorimetric assay. While the synthesized compounds did not show significant cytotoxic activities, compounds 7a3 and 7a4 reduced cell viability to about 50% at 100 μM concentration. The present study revealed that most of the new synthesized compounds possess good antifungal effects and they could be considered as valuable candidates for further structural modification to design more potent antifungal agents.
Keywords: 4(3H)-Quinazolinone, Antibacterial, Antifungal, Cytotoxicity
INTRODUCTION
Currently one of the most important health problems is drug resistance due to the increased use and misuse of antibiotics and also lack of immunity in cases such as Acquired Immune Deficiency Syndrome (AIDS) and organ transplantation. Therefore, developing of new antimicrobial agents is unavoidable and many researchers have focused their efforts to find new and effective antibiotics from natural and synthetic sources. In addition, cancer is the second major life threatening disease in both developing and undeveloped countries. Although many advances have been made in the treatment of some types of cancers, the continued efforts of the researchers to discover a new anticancer agent is also critically important(1).
Quinazolines are a group of fused heterocyclic compounds which have attracted interest of researchers due to their valuable biological properties including antifungal(2–4), antibacterial(4–6), and cytotoxic(7) activities.
There are many reports indicating that 2,3-disubstituted 4(3H)-quinazolinones have valuable antimicrobial properties. Structure activity relationship (SAR) studies have shown that positions 2 and 6 of quinazoline structure are important and position 3 should be presented for better antimicrobial activities. The 3-substitution includes phenyl rings, bridged phenyl rings, heterocyclic rings and different aliphatic systems(8,9). Quinolones perform their antibacterial activities by inhibition of bacterial DNA gyrase which is an enzyme essential for bacterial DNA replication(10,11). Due to the structure similarity between quinazolinones and quinolones, antibacterial effects of quinazolinones could be a result of inhibitory activity against DNA replication. Also antibacterial activity was observed in a group of quinazoline derivatives as a result of DNA polymerase III inhibitory effect. The inhibition of chromosomal DNA replication results in bacterial cell death(12).
It is also reported that quinazoline derivatives bearing aromatic rings and some nitrogen containing groups in their structures are potential antitumor agents through different mechanisms including inhibition of several factors such as epidermal growth factor receptor (EGFR)(1,13), tubulin polymerization(14–16), poly (ADP-ribose) polymerase-1(PARP-1)(17,18) and dihydrofolate reductase (DHFR) and thymidylate synthase (TS)(19–21) which are essential for cell growth.
In the present study 15 newly synthesized quinazolinones which were developed in our laboratory(22) were evaluated for their antibacterial, antifungal and cytotoxic activities. All of quinazolinones tested here contained aromatic substituents at position 3 and hydrazid moieties at position 2 of their structures (Fig. 1).
Fig. 1.
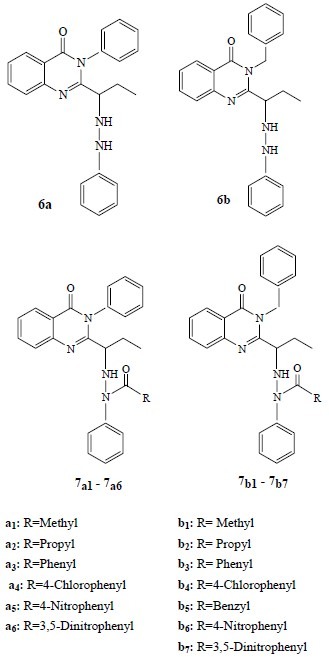
Structure of new synthesized compounds studied in this work
MATERIALS AND METHODS
The following microorganisms were used as diluted samples with broth culture: Staphylococcus. aureus, Bacillus subtilis, Listeria. monocytogenes, Escherichia. coli, Pseudomonas. aeruginosa, Salmonella. Enteritidis, Candida. albicans, Aspergillus. niger and Aspergillus. flavus. All micro-organisms were obtained from Persian Type Culture Collection (PTCC).
Mueller-Hinton broth (Merk, Germany) and RPMI-1640 (Gibco, Scotland) were used for growth of bacteria and fungi respectively. 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT), and dimethyl-sulfoxide (DMSO) were purchased from Merck, Germany. Absorbances were determined with an ELISA plate reader ( Awareness, USA). HeLa cells were purchased from Pasteure Institute, Tehran, Iran.
Antibacterial and antifungal evaluation
The in vitro antibacterial and antifungal activities of the synthesized compounds (Fig. 1) were studied by Microplate Alamar Blue Assay (MABA) using 96-wells microplates(23–25).
Briefly 4-5 isolated colonies were selected from an overnight bacterial culture and were diluted in broth to turbidity comparable to that of a 0.5 McFarland turbidity standard (approximately 1.0×108 CFU/ml). Finally inoculum density equal to 1.0×105 CFU/ml was prepared. 20 μl of each bacterial dilution was distributed in all 96 wells of microplate including positive control (containing standard antibiotic) and growth control (containing culture broth without testing materials). Then 20 μl of each concentration of the synthesized compounds were added to two neighbor wells except for positive and growth control wells. After adding alamar blue (20 μl) to all of 96 wells the total volume in each well reaches to 200 μl. The final concentrations of the tested compounds were (512, 256, 128, 64 and 32 μg/ml). After incubation, results were recorded as MIC (minimum concentration of each synthesized compound which completely inhibited growth of microorganism).
Antifungal activity was determined as antibacterial assay except with some modifications. The final size of inoculum was 1.0×106 CFU/ml for fungi and the turbidity was measured spectrometrically at 580 nm.
Following the MIC test, from each well which did not show growth, 50 μl of suspension was spreaded onto a plate containing appropriate medium. The plates were incubated overnight and the minimum drug concentrations able to kill 99% of bacteria and fungi were reported as Minimum Bactericidal Concentration (MBC) and Minimum Fungicidal Concentration (MFC) respectively(23). The antimicrobial tests were repeated three times.
In vitro cytotoxicity assay
HeLa cells were cultured in RPMI 1640 medium that supplemented with 50 ml heat-inactivated fetal calf serum (FCS) and sterilized by filtering through 0.22 μm filters. The HeLa cells were maintained in a humidified atmosphere of 5% CO2, 95% air at 37°C(26,27).
The stock solutions of the synthesized compounds were prepared by dissolving in the minimum volume of dimethyl sulfoxide (DMSO) and phosphate buffered saline (PBS) was then added to reach the appropriate volume.
The cytotoxic effects of compounds against HeLa cells were evaluated using a rapid colorimetric MTT assay. Mitochondrial succinic dehydrogenase enzyme of live cells would metabolically reduce the yellow soluble 3- (4, 5-dimethylthiazol -2 -yl) -2, 5- diphenyl tetrazolium bromide (MTT) salt into a blue insoluble formazan product. The blue solid was dissolved in DMSO and measured spectrophotometrically in this assay. The results were compared with untreated control(26,27).
Briefly, after 2-3 subcultures, 180 μl of the cells (5×104 cells/ml of media) was seeded in 96 well microplates and incubated for 24 h. Each three wells of microplate was devoted to a certain concentration and 20 μl of each compound concentration was added to 180 μl of the cell suspensions in microplate wells. The final concentrations of the compounds were 1, 10 and 100 μM.
Doxorubicin was used as positive control and the wells containing DMSO (1%) and cell suspension was regarded as the negative control. The blank wells were consisted of 200 μl of the RPMI medium. The microplates were further incubated for 48 h. To evaluate cell survival, each well was then incubated with 20 μl of MTT solution (5 mg/ml in PBS) for 3 h and the media in each well was replaced with 200 μl DMSO and pipetted up and down to dissolve the formazan crystals. The absorbance of each well was measured at 540 nm using an ELISA reader. Each experiment was repeated three times. The percentage of cell viability was calculated using the following formula:

The results are the mean of three triplicate experiments. Analysis of variance (ANOVA) followed by Tukey test was used to determine the differences between various groups. The significance level was set at P<0.05.
RESULTS
Tables 1–4 present the antimicrobial effects of new 2, 3-disubstituted 4(3H)-quinazolinone derivatives. All synthesized compounds have mild to high antibacterial effects especially against Gram-negative bacteria. The most sensitive bacterium was E. coli. Compounds 6a and 6b showed weaker antibacterial effects than other compounds. All compounds showed antibacterial effects at concentrations higher than 128 μg/ml for B. subtilis . The tested compounds indicated no inhibitory effects against L. monocytogenes and S. aureus. Gram-positive bacteria used in the current study were insensitive to the tested compounds.
Table 1.
The minimum inhibitory concentration (MIC) of the tested compounds against selected Gram-positive and Gram-negative bacteria
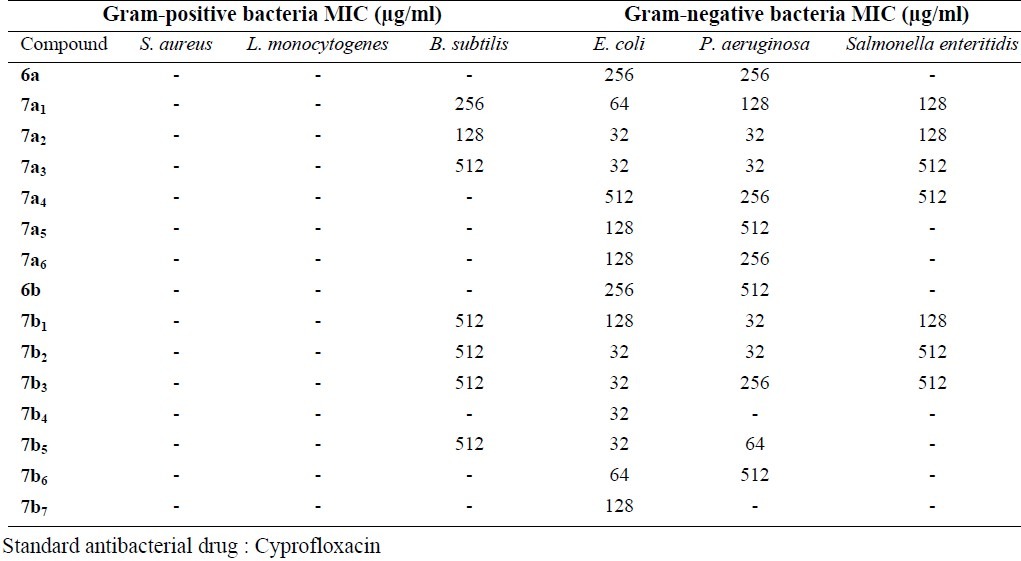
Table 4.
Minimum Fungicidal Concentration (MFC) results of the tested compounds

Table 2.
Minimum Bactericidal Concentration (MBC) results of the tested compounds
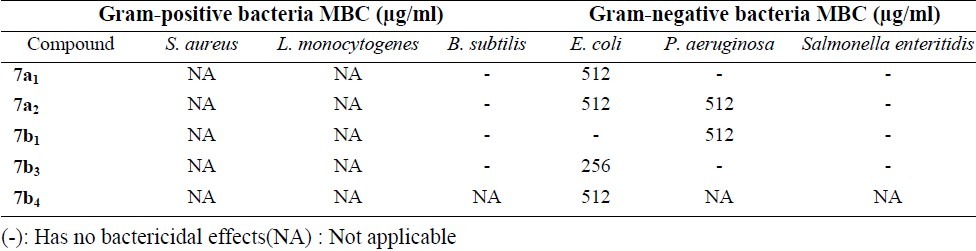
Table 3.
The minimum inhibitory concentration (MIC) of the tested compounds against selected fungi
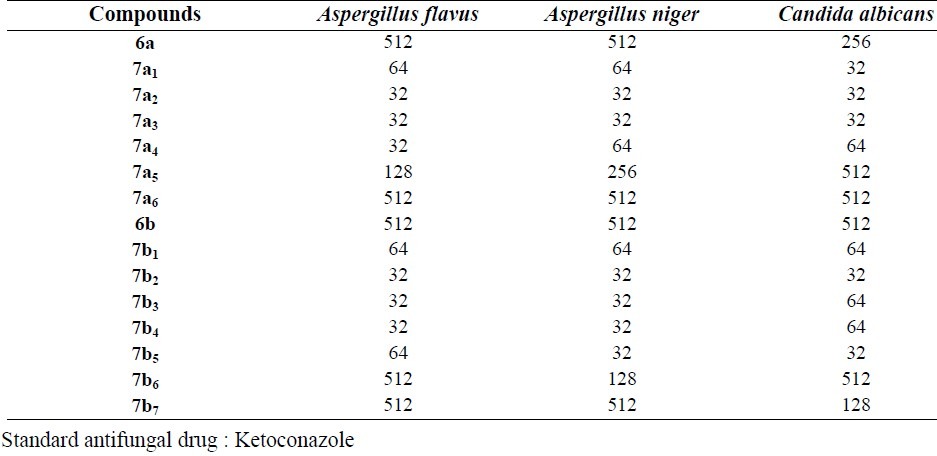
Except compounds 6a , 7a5, 7a6, 6b , 7b6, 7b7, all other compounds showed good antifungal effects. All three strains of fungi were sensitive to the tested compounds mostly at 32 μg/ml and there were no significant differences in the sensitivity level of the tested compounds. Synthesized compounds did not show antibacterial and antifungal effects at concentrations lower than 32 μg/ml.
Despite reasonable fungistatic and bactriostatic effects tested compounds did not show significant bactericidal and fungicidal effects according to the MBC and MFC results.
Although the synthesized compounds did not show significant cytotoxic effects, compounds 7a3 and 7a4 reduced cell viability to about 50% at 100 μM concentration. The cytotoxic results are shown in Figs. 2–4 and comparative results are presented in Table 5.
Fig. 2.
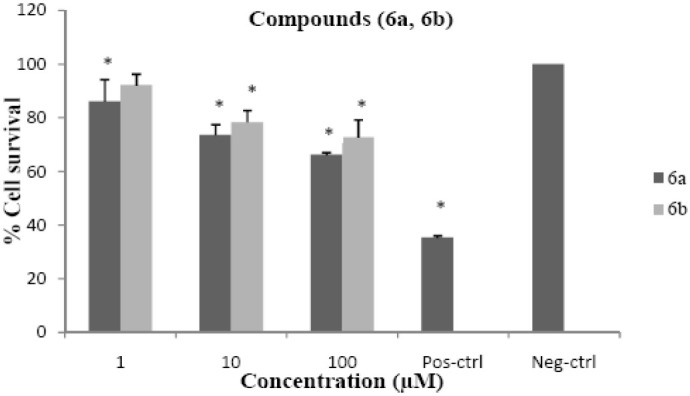
Cytotoxic effects of compounds 6a and 6b on HeLa cell line following exposure to different concentrations of compounds 6a and 6b, cell viability was assessed using the MTT method. Data are presented as mean ± SD, *P<0.05, n=3, Pos-ctrl=Positive control, Neg-ctrl=Negative control
Fig. 4.
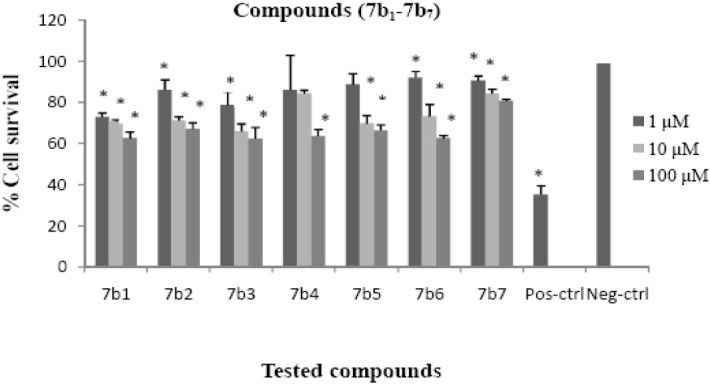
Cytotoxic effects of compounds 7b1-7b7 on HeLa cell line following exposure to different concentrations of compounds 7b1-7b7, cell viability was assessed using the MTT method. Data are presented as mean ± SD, *P<0.05, n=3, Pos-ctrl=Positive control, Neg-ctrl=Negative control
Table 5.
The percentage of cell viability following exposure to different concentrations of compounds.
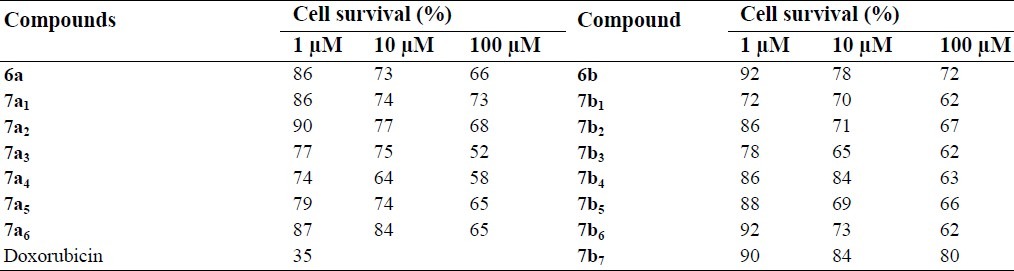
Fig. 3.
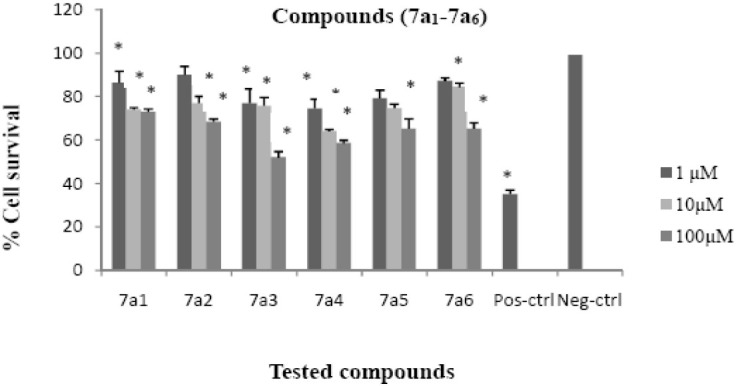
Cytotoxic effects of compounds 7a1-7a6 on HeLa cell line following exposure to different concentrations of compounds 7a1-7a6, cell viability was assessed using the MTT method. Data are presented as mean ± SD, *P<0.05, n=3, Pos-ctrl=Positive control, Neg-ctrl=Negative control
DISCUSSION
In comparison with some other 2,3-disubstituted quinazolinones which have some similarity in structure to the tested compounds, for example, a number of nitrogen containing groups and aromatic substituents in position 2 and 3, the studied compounds were equal or more effective in antimicrobial evaluations(28–30). It seems that the presence of nitrogen containing substituents in position 2 is beneficial for antimicrobial effects because some synthesized quinazolinones which have nitrogen containing substituents at position 2(30) are more potent than their 3-substituted counterparts. In antimicrobial assay, compounds 6a and 6b (Fig. 1) are less effective than NH substituted compounds (7a1-7a6 and 7b1-7b7). Apparently substitution on phenyl bonded NH is necessary to have better antimicrobial effects. On the other hand it seems that the size of the substituent is important because compounds with bigger substitution showed lower antimicrobial effects. According to the results of in vitro antibacterial tests which were accomplished in this study it seems that Gram-negative bacteria are more sensitive to the synthesized compounds.
Most of the studied compounds indicated high antifungal effects. The results of antifungal tests against Candida albicans, Aspergillus niger and Aspergillus flavus were the same and interestingly the tested compounds are more promising as potential antifungal agents.
All of the new compounds were evaluated for cytotoxic activities against the HeLa cells using MTT assay. In this assay cytotoxic activities were expressed as percentage of cell survival and were measured at 3 different concentrations. Only two compounds 7a3, 7a4 reduced cell viability to about 50% at 100 μM and other compounds did not show significant cytotoxicity. Compounds 7a3 and 7a4 showed significant difference (P<0.05) in viability in comparison to the negative control at concentrations of 1, 10, 100 μM. According to the cytotoxic results only two compounds 7a3, 7a4 which have phenyl ring at position 3 and benzoyl and 4-chloro benzoyl, respectively, as N-bonded groups showed moderate cytotoxicity whereas compounds with the same groups but different substituents at position 3 did not reduce the viability of cells similar to 7a3, 7a4. Consequently it is possible to say that cell growth inhibitory effects of the compounds may be attributed to substituent property at position 3 of quinazolinone ring and the acyl group attached to the NH of phenyl hydrzine moiety.
CONCLUSION
According to the biological evaluations, tested compounds showed no significant cytotoxic effects while they possess good antifungal activities. The lack of high cytotoxicity could be beneficial as potential antifungal agents should have no toxicity on human cells. The synthesized compounds could be considered as valuable templates for further modification or derivatization to design more potent antifungal agents by the addition of different heterocyclic groups such as imidazole and triazole to the quinazolinone structure.
ACKNOWLEDGMENT
This work has been performed in the Department of Medicinal Chemistry and financially supported by the Research Council of Isfahan University of Medical Sciences.
REFERENCES
- 1.El-Azab AS, Al-Omar MA, Abdel-Aziz AAM, Abdel-Aziz NI, El-Sayed MAA, Aleisa AM, et al. Design, synthesis and biological evaluation of novel quinazoline derivatives as potential antitumor agents: Molecular docking study. Eur J Med Chem. 2010;45:4188–4198. doi: 10.1016/j.ejmech.2010.06.013. [DOI] [PubMed] [Google Scholar]
- 2.Ouyang G, Zhang P, Xu G, Song B, Yang S, Jin L, et al. Synthesis and antifungal bioactivities of 3-alkylquinazolin-4-one derivatives. Molecules. 2006;11:383–392. doi: 10.3390/11060383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brossi A. New York: Academic Press; 1986. The alkaloids: Chemistry and Pharmacology; p. 131. [Google Scholar]
- 4.Pandeya SN, Sriram D, Nath G, De Clercq E. Synthesis, antibacterial, antifungal and anti-HIV evaluation of Schiff and Mannich bases of isatin derivatives with 3-amino-2-methylmercapto quinazolin-4 (3H)-one. Pharm Acta Helvet. 1999;74:11–17. doi: 10.1016/s0031-6865(99)00010-2. [DOI] [PubMed] [Google Scholar]
- 5.Panneerselvam P, Rather BA, Ravi Sankar Reddy D, Ramesh Kumar N. Synthesis and anti-microbial screening of some Schiff bases of 3-amino-6, 8-dibromo-2-phenylquinazolin-4 (3H)-ones. Eur J Med Chem. 2009;44:2328–2333. doi: 10.1016/j.ejmech.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 6.Armarego WL. New York: wiley-Interscience; 1967. Fused Pyrimidines; p. 512. [Google Scholar]
- 7.Aly MM, Mohamed YA, El-Bayouki KAM, Basyouni WM, Abbas SY. Synthesis of some new 4 (3H)-quinazolinone-2-carboxaldehyde thiosemicarbazones and their metal complexes and a study on their anticonvulsant, analgesic, cytotoxic and antimicrobial activities-Part-1. Eur J Med Chem. 2010;45:3365–3373. doi: 10.1016/j.ejmech.2010.04.020. [DOI] [PubMed] [Google Scholar]
- 8.Dinakaran M, Selvam P, DeClercq E, Sridhar SK. Synthesis, antiviral and cytotoxic activity of 6-bromo-2, 3-disubstituted-4 (3H)-quinazolinones. Biol Pharm Bull. 2003;26:1278–1282. doi: 10.1248/bpb.26.1278. [DOI] [PubMed] [Google Scholar]
- 9.Raghavendra NM, Thampi P, Gurubasavarajaswamy PM, Sriram D. Synthesis and Antimicrobial Activities of Some Novel Substituted 2-Imidazolyl-N-(4-oxo-quinazolin-3 (4H)-yl)-acetamides. Chem Pharm Bull. 2007;55:1615–1619. doi: 10.1248/cpb.55.1615. [DOI] [PubMed] [Google Scholar]
- 10.Hooper DC, Wolfson JS. Mode of action of the quinolone antimicrobial agents: review of recent information. Reviews of Infectious Diseases. 1989;11:902–911. doi: 10.1093/clinids/11.supplement_5.s902. [DOI] [PubMed] [Google Scholar]
- 11.Shen L, Kohlbrenner W, Weigl D, Baranowski J. Mechanism of quinolone inhibition of DNA gyrase.Appearance of unique norfloxacin binding sites in enzyme-DNA complexes. J Biol Chem. 1989;264:2973. [PubMed] [Google Scholar]
- 12.Guiles J, Sun X, Critchley IA, Ochsner U, Tregay M, Stone K, et al. Quinazolin-2-ylamino-quinazolin-4-ols as novel non-nucleoside inhibitors of bacterial DNA polymerase III. Bioorg Med Chem Lett. 2009;19:800–802. doi: 10.1016/j.bmcl.2008.12.038. [DOI] [PubMed] [Google Scholar]
- 13.Lüth A, Löwe W. Syntheses of 4-(indole-3-yl) quinazolines-A new class of epidermal growth factor receptor tyrosine kinase inhibitors. EurJ Med Chem. 2008;43:1478–1488. doi: 10.1016/j.ejmech.2007.09.018. [DOI] [PubMed] [Google Scholar]
- 14.Raffa D, Edler MC, Daidone G, Maggio B, Merickech M, Plescia S, et al. Synthesis, cytotoxicity, and inhibitory effects on tubulin polymerization of a new 3-heterocyclo substituted 2-styrylquinazolinones. Eur J Med Chem. 2004;39:299–304. doi: 10.1016/j.ejmech.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 15.Kamal A, Bharathi EV, Reddy JS, Ramaiah MJ, Dastagiri D, Reddy MK, et al. Synthesis and biological evaluation of 3, 5-diaryl isoxazoline/isoxazole linked 2, 3-dihydroquinazolinone hybrids as anticancer agents. Eur J Med Chem. 2011;46:691–703. doi: 10.1016/j.ejmech.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 16.Xia Y, Yang ZY, Hour MJ, Kuo SC, Xia P, Bastow KF, et al. Antitumor agents. Part 204: Synthesis and biological evaluation of substituted 2-aryl quinazolinones. Bioorg Med Chem Lett. 2001;11:1193–1196. doi: 10.1016/s0960-894x(01)00190-1. [DOI] [PubMed] [Google Scholar]
- 17.Iwashita A, Hattori K, Yamamoto H, Ishida J, Kido Y, Kamijo K, et al. Discovery of quinazolinone and quinoxaline derivatives as potent and selective poly (ADP-ribose) polymerase-1/2 inhibitors. FEBS Lett. 2005;579:1389–1393. doi: 10.1016/j.febslet.2005.01.036. [DOI] [PubMed] [Google Scholar]
- 18.Orvieto F, Branca D, Giomini C, Jones P, Koch U, Ontoria JM, et al. Identification of substituted pyrazolo [1, 5-a] quinazolin-5 (4H)-one as potent poly (ADP-ribose) polymerase-1 (PARP-1) inhibitors. Bioorg Med Chem Lett. 2009;19:4196–4200. doi: 10.1016/j.bmcl.2009.05.113. [DOI] [PubMed] [Google Scholar]
- 19.Jantová S. Induction of cytotoxicity and ssDNA breaks by 9-bromo-5-morpholino-tetrazolo [1, 5-c] quinazoline in tumor cells cultured in vitro. Toxicol in vitro. 2003;17:457–463. doi: 10.1016/s0887-2333(03)00066-3. [DOI] [PubMed] [Google Scholar]
- 20.Al-Omary FAM, Abou-zeid LA, Nagi MN, Habib ESE, Abdel-Aziz AAM, El-Azab AS, et al. Non-classical antifolates. Part 2: Synthesis, biological evaluation, and molecular modeling study of some new 2, 6-substituted-quinazolin-4-ones. Bioorg Med Chem. 2010;18:2849–2863. doi: 10.1016/j.bmc.2010.03.019. [DOI] [PubMed] [Google Scholar]
- 21.Cao SL, Feng YP, Jiang YY, Liu SY, Ding GY, Li RT, et al. Synthesis and in vitro antitumor activity of 4(3H)-quinazolinone derivatives with dithiocarbamate side chains. Bioorg Med Chem Lett. 2005;15:1915–1917. doi: 10.1016/j.bmcl.2005.01.083. [DOI] [PubMed] [Google Scholar]
- 22.Hassanzadeh F, Rahmani Khajouei M, Hakimelahi G, Jafari E, Khodarahmi G. Synthesis and cytotoxicity evaluation of some new 2, 3- disubstituted 4(3H)-quinazolinone derivatives. J Res Pharm Sci. 2012;1:23–30. [PMC free article] [PubMed] [Google Scholar]
- 23.Schwalbe R. New York: CRC; 2007. Antimicrobial susceptibility testing protocols; pp. 75–79. [Google Scholar]
- 24.Baker CN, Tenover FC. Evaluation of Alamar colorimetric broth microdilution susceptibility testing method for staphylococci and enterococci. J Clin Microbiol. 1996;34:2654–2659. doi: 10.1128/jcm.34.11.2654-2659.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.To WK, Fothergill AW, Rinaldi MG. Comparative evaluation of macrodilution and Alamar colorimetric microdilution broth methods for antifungal susceptibility testing of yeast isolates. J Clin Microbiol. 1995;33:2660–2664. doi: 10.1128/jcm.33.10.2660-2664.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 27.Freshney RI. John Wiley and Sons, Inc; 1994. Culture of animal cells: a manual of basic technique; pp. 205–216. [Google Scholar]
- 28.Nanda AK, Ganguli S, Chakraborty R. Antibacterial Activity of Some 3-(Arylideneamino)-2-phenyl quinazoline-4(3H)-ones: Synthesis and Preliminary QSAR Studies. Molecules. 2007;12:2413–2426. doi: 10.3390/12102413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kadi AA. Synthesis and antimicrobial activity of some new quinazolin-4 (3H)-one derivatives. J Saudi Chem Soc. 2011;15:95–100. [Google Scholar]
- 30.Pandey SK, Singh A. Antimicrobial studies of some novel quinazolinones fused with [1, 2, 4]-triazole,[1, 2, 4]-triazine and [1, 2, 4, 5]-tetrazine rings. Eur J Med Chem. 2009;44:1188–1197. doi: 10.1016/j.ejmech.2008.05.033. [DOI] [PubMed] [Google Scholar]


