Abstract
Trinitrobenzene sulfonic acid (TNBS)-induced colitis is one of the most common methods for studying inflammatory bowel disease in animal models. Several factors may, however, affect its reproducibility, rate of animal mortality, and macroscopic and histopathological outcomes. Our aim was to validate the main contributing factors to this method and compare the effects of different reference drugs upon remission of resultant colon injuries. TNBS was dissolved in 0.25 ml of ethanol (50% v/v) and instilled (25, 50, 100 and 150 mg/kg) intracolonically to the male Wistar rats. After determination of optimum dose of TNBS in male rats and assessment of this dose in female rats, they were treated with reference drugs including dexamethasone [1 mg/kg, intraperitoneally (i.p.) and 2 mg/kg, orally (p.o.)], Asacol (mesalazine, 100 mg/kg, p.o.; 150 mg/kg, enema) and hydrocortisone acetate (20 mg/kg, i.p.; 20 mg/kg, enema) which started 2 h after colitis induction and continued daily for 6 consecutive days. Thereafter, macroscopic and microscopic parameters and clinical features were assessed and compared in different groups. We found that the optimum dose of TNBS for the reproducibility of colonic damage with the least mortality rate was 50 mg/kg. Amongst studied reference drugs, hydrocortisone acetate (i.p.), dexamethasone (i.p. and p.o.) and Asacol (p.o.) significantly diminished the severity of macroscopic and microscopic injuries and could be considered effective for experimental colitis studies in rats . Our findings suggest that optimization of TNBS dose is essential for induction of colitis under the laboratory conditions; and gender exerts no impact upon macroscopic and histological characteristics of TNBS-induced colitis in rats. Furthermore, the enema forms of hydrocortisone and Asacol are not appropriate reference drugs.
Keywords: Animal model, Experimental colitis, Inflammatory bowel disease, Optimization, TNBS
INTRODUCTION
Inflammatory bowel disease (IBD) including Crohn's disease (CD) and ulcerative colitis (UC), is a chronic inflammatory disorder of the intestinal tract whose etiology has not yet been fully elucidated(1,2). Recent studies have demonstrated that IBD pathogenesis is multifactorial. In fact, genetic, immune and environmental factors contribute to its pathogenesis(3–4). IBD is characterized by massive cellular infiltrates which is associated with abnormalities of the immune system including increased number of CD4+ T-lymphocytes, mast cells, neutrophils, and eosinophils(5). These conditions give rise to inflammation, ulceration, edema, diarrhea along with blood and/or mucus, fever, and gastric dysmotility(6). No medical cure has been developed for IBD and treatment focuses on producing and maintaining remission(3–5). Conventional pharmacotherapy for both types of IBD is treatment with aminosalicylates and corticosteroids(7,8). Moreover, Immuno-suppressive agents and biological response modifiers are considered as alternative therapies(9). Nonetheless, available medicines are not universally effective and result in marked deleterious effects(10). These challenges have thus heightened the need for research in order to adopt new therapeutic approaches for the treatment of IBD.
More recently, literature has emerged those findings about the key cellular and molecular mechanisms predisposing to IBD in animal models. These models represent certain pathophysiological features of human UC and CD involving weight loss and diarrhea accompanied by blood and/or mucus, fever, shortened colon, crypt abnormalities, gastric dysmotility and infiltration of inflammatory cells(11).
However, the experimental animal models couldn’t fully reflect the complexity of the disease present in human(12) and each method has its own disadvantages. Morris and coworkers constructed a simple and reproducible rat model of acute and chronic colonic inflammation and ulceration by means of intracolonical injection of 2,4,6-trinitrobenzenesulfonic acid (TNBS) in ethanol(13). The major advantages of this model include proposing a simple process and reproducible colonic damage, short duration of the experiment, long-lasting damage accompanied by inflammatory cell infiltration and ulcers(14). In addition, this model mimics both acute and chronic phases of ulcerative colitis which is one of the hallmarks of human ulcerative colitis. There is compelling evidence that dysregulation of the mucosal immune system is a major contributing factor to the pathogenesis of IBD. In this regard, a few murine models of IBD have shown that alterations in immune system functions result from a failure of regulation by T-helper cells and lead to acute and chronic inflammation in the intestine(15). In comparison with other animal models of IBD, TNBS is an efficient method. It can mimic the pattern of inflammation with human IBD and is widely applicable to mice, rats and guinea pigs(11,16). On the other hand, TNBS model of colitis suffers from some disadvantages (e.g. the absence of spontaneous relapse which is the hallmark of human ulcerative colitis) as do many other methods. In addition, the reproducibility of the model is dependent upon the dose of TNBS(17).
Recent studies have provided evidence with regard to the mechanism of ulceration produced by this method of colonic inflammation and subsequently assessed the efficacy of new therapeutic regimens. It is worth noting that the dose of TNBS selected for induction of colitis varies in different studies(18–20). In fact, doses of 50, 100 and 150 mg/kg of TNBS have been reported(20–22) which indicate that the severity of colitis is probably dependent on the selected TNBS doses. Corticostroids and sulfasalazine are selected as reference drugs in most studies conducted on colitis. However, their efficacy in remission of experimental ulcerative colitis is sometimes controversial and has not been compared based on various routes of administration (i.e. i.p., p.o. or enema)(23–24). Therefore, the present study was set out to determine the optimum dose of TNBS under our laboratory conditions, sex-related difference in the severity of colitis, and therapeutic effects of some reference drugs in remission of this immune-based animal model of IBD.
MATERIALS AND METHODS
Animals
Male and female Wistar rats (200 ± 25 g, 12-week-old) bred in the animal house of the School of Pharmacy and Pharmaceutical Sciences of Isfahan University of Medical Sciences were used. Animals were housed individually in metabolic cages, under uniform and controlled temperature, humidity and lighting conditions and fed standard pelleted chow and water ad libitum. All experimental protocols were approved by the Animal Care Committee of the Isfahan University of Medical Sciences, Iran.
Chemicals
Dexamethasone and hydrocortisone acetate were provided by Iran Hormone Pharmaceutical Co. (Tehran, Iran). Trinitrobenzene sulfonic acid (TNBS) was purchased from Sigma (St. Louis, MO, USA). Hydrocortisone acetate enema was obtained from Valeant Pharmaceutical Co. (Saint-Laurent, Canada). Asacol® (mesalazine) was purchased from Iran Daru Co. (Tehran, Iran) and Tillotts (Tillotts pharma AG Ziefen, Switzerland) for p.o. (mesalazine microgranules) and enema administrations, respectively. Suspension of reference drugs were freshly prepared using 0.2% Tween 80 in normal saline as a vehicle for p.o. and i.p. administrations.
Induction of colitis with various TNBS doses in male and female rats
In the present work , thirty male Wistar rats were recruited and divided into 5 groups of 6 animals in each. Groups were categorized based on the dose of TNBS/saline instilled intracolonically. In order to induce the colitis, TNBS with doses of 25, 50, 100 and 150 mg/kg was administered intracolonically to male rats in groups 1a, 2a, 3a and 4a, respectively. Group 5a constituted male rats that served as control group and received normal saline intracolonically. After deter-mination of optimum dose of TNBS in male rats, it was evaluated in female rats (n=6).
All animals were fasted for 36 h prior to induction of colitis. Colitis was induced based on method of Morris and coworkers(13). Under light ether anesthesia, rats were positioned on their right side. Thereafter, TNBS (25, 50, 100 and 150 mg/kg; dissolved in ethanol 50% v/v), was intracolonically administered in a volume of 0.25 ml, via a polyethylene catheter inserted 8 cm proximal to the anus. Rats were kept in the head-down position for 2-3 min to preclude immediate anal leakage of the instillate and then returned to their cages with free access to food and water. Control rats were treated with enema of 0.25 ml of 0.9 % saline.
Treatment groups
In order to suggest the best experimental colitis treatment, some of the main reference drugs were evaluated subsequent to assessment of optimum dose of TNBS and effect of gender upon severity of colitis. Seventy two rats were assigned into 12 groups of 6 animals each. Two h after induction of colitis, following treatments were carried out: Dexamethasone (1 mg/kg, i.p; 2 mg/kg, p.o.), Asacol (100 mg/kg, p.o.; 150 mg/kg, enema) and hydrocortisone acetate enema (20 mg/kg, i.p.; 20 mg/kg). Control groups were treated with oral (5 ml/kg), intraperitoneal (2 ml/kg) and enema vehicle (5 ml/kg) without induction of colitis. In 50 mg/kg-TNBS-treated groups, animals received vehicle orally (5 ml/kg), intraperitoneally (2 ml/kg), and as enema (5 ml/kg), after induction of colitis. Treatments were performed on daily basis for 7 consecutive days.
Measurements of body weight changes, food/water intake and diarrhea
Animals were kept separately in metabolic cages. Food and water consumptions were recorded in g and ml, respectively. Body weight, food and water intake were assessed during the experiment (prior to the administration of TNBS or normal saline and subsequent to the procedure daily for 7 days). The fecal output was scored by arbitrary criteria defined as: 1) formed stools, 2) loose stools and 3) diarrhea . Percent of body weight loss was then measured.
Macroscopic examination
All animals were sacrificed by ether inhalation on day 7. It was followed by opening their abdomen and examining the appearance of colon. Thereafter, distal colon was removed, opened longitudinally and gently cleaned of fecal content using normal saline. Wet weight (mg) of the distal colon (8 cm from the anus) and weight/ length ratio (mg/cm) were recorded for each specimen. The severity of macroscopically visible colonic damage was assessed by means of the scoring system showed in Table 1, according to Ballester and coworkers, with some modifications(20).
Table 1.
Scoring criteria applied to the macroscopic injuries in the rat colon.
Histopathological examination
Colon tissues were individually fixed in 10% formalin, dehydrated, paraffin embedded, processed, sectioned in slices with 4 μm thickness and stained with H&E (haematoxylin and eosin). Total colitis index was derived by summing 3 sub-scores (inflammation severity, inflammation extent, crypt damage) on H&E-stained and coded sections using a modification of a validated scoring system presented by Cooper, Dieleman and coworkers(25,26).
The microscopic and histological scoring was carried out by pathologist coworker blind to the treated groups. In fact, it was implemented using a Zeiss® microscope equipped with a Sony® color video camera for digital imaging.
Statistical analysis
Data management and analysis was performed using the SPSS statistical package (Version 17.0). Non-parametric data were analyzed by Mann-Whitney U test.
Results were reported as mean ± standard error of mean (SEM). Comparison between groups was made using One-way analysis of variance (ANOVA) with Tukey as post hoc test. A P value <0.05 was considered significant.
RESULTS
Effect of various TNBS doses on macroscopic and microscopic parameters in male rats
Changes in food consumption, water intake and diarrheal status
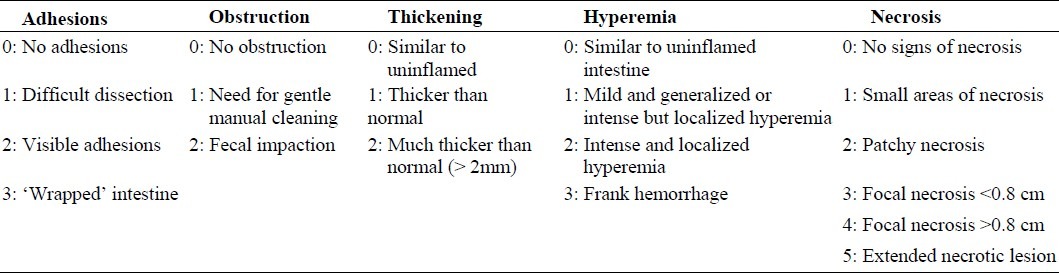
During the initial 4 days subsequent to induction of colitis, TNBS-treated rats at doses of 50 and 100 mg/kg showed a significant increase in the diarrhea index, in comparison with control group. In these groups, there was a reduction from 4th to 7th day in diarrhea index which was high as compared to control group (P<0.01) (Fig. 1A). During all days of experiment, no significant difference was seen in diarrheal status between these two groups.
Fig. 1.
Daily diarrheal status (A), food intake (B) and water intake in male rats (C), before (day 0) and during 7 days after induction of colitis with various doses of TNBS. Values are means ± SEM (n=6).***P<0.001, **P<0.01 and *P<0.05 compared with controls; diarrheal status (1. formed stools, 2. loose stools and 3. diarrhea).
No significant difference was seen between diarrheal statuses of 25 mg/kg-TNBS treated rats and male control groups during the experimental period.
Moreover, diarrheal status of 150 mg/kg-TNBS-treated rats in all 7 days was significantly high, in contrast to control groups (P<0.001) (Fig. 1A).
We found that the food consumption of TNBS-treated rats with doses of 50 and 100 mg/kg was significantly less than that of control rats over the initial 4 days after induction of colitis (P<0.001). However, the average food intake of TNBS-treated-rats in these groups returned to control levels by day 5. During the experimental period, no significant difference was found in the food intake between 50 and 100 mg/kg-TNBS-treated rats. The difference in food intake between male 25 mg/kg-TNBS-treated rats and control group during the experimental period was not statistically significant. Addit-ionally, food intake in male 150 mg/kg-TNBS-treated rats was significantly low, in comparison with control group (P<0.001) (Fig. 1B).
Water intake in TNBS-treated rats with doses of 50 and 100 mg/kg was significantly (P<0.001) higher on days 1, 2, 3, and 4 after induction of colitis, compared to the period before induction of colitis. The average water intake in these TNBS-treated rats also returned to control levels on day 5 (Fig. 1C). During all days of experiment, no significant difference in water intake was observed between these two groups (50 and 100 mg/kg of TNBS). Additionally , water intake in 150 mg/kg-TNBS-treated rats in all days of experiment was significantly high, in comparison with control group (P<0.001). The difference in water intake between 25 mg/kg-TNBS-treated rats and control group during the experimental period was not statistically significant (Fig. 1C).
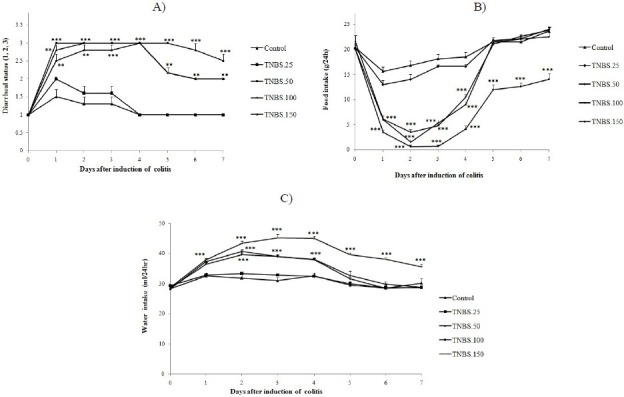
The dose of 50 mg/kg of TNBS was determined to be the optimum dose for induction of colitis in male rats. Therefore, the effect of this dose was also assessed in female rats. As a result, no significant difference was found between male and female 50 mg/kg-TNBS-treated rats during 7 days of study in food/ water intake and diarrheal status (Fig. 2A–2C).
Fig. 2.
Daily diarrheal status (A), food intake (B) and water intake (C) in male/female rats before (day 0) and during 7 days after induction of colitis with TNBS (50 mg/kg).Values are means ± SEM (n=6).***P<0.001, **P<0.01 and *P<0.05 compared with controls.
Macroscopic and microscopic assessment of colitis induced by different doses of TNBS
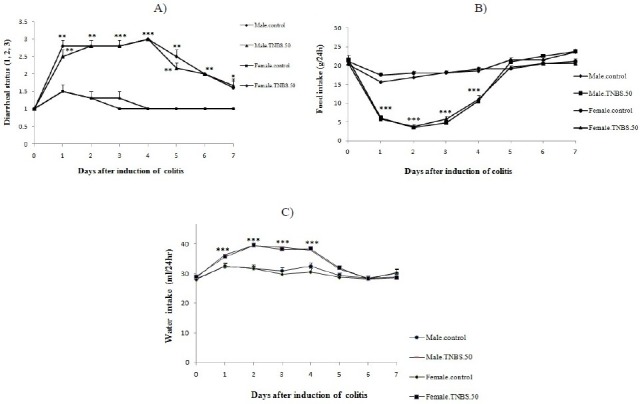
As it is evident in Fig 3 and Table 2, TNBS treatment with doses of 50, 100, 150 mg/kg produced severe inflammation, ulceration, wall thickening and necrosis in colitis rats; whereas, colons of control rats were normal. There was also considerable loss of body weight in these groups compared to control group (P<0.001). We found that in 25 mg/kg-TNBS-treated group, colonic weight/length ratio, ulcer severity and loss of body weight exhibited no significant difference, in comparison to control group. It is worth mentioning that during the study, two rats died in 150 mg/kg-TNBS-treated groups, whereas there was no mortality in other groups.
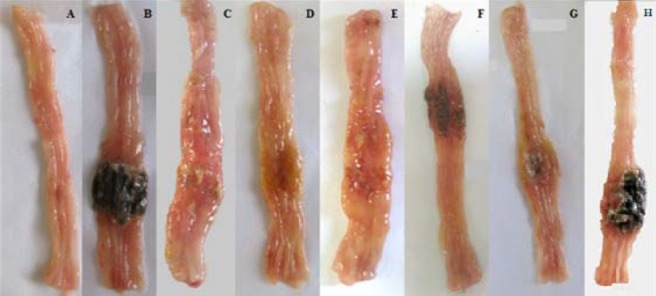
Fig. 3.
Macroscopic presentation of TNBS-induced colitis in rats. Normal colon treated with normal saline, 2 ml/kg (A);TNBS-treated-colons with doses of 25 mg/kg (B); 50 mg/kg (C); 100 mg/kg (D); 150 mg/kg (E) TNBS. Colon tissue in control rat was normal (A). TNBS (25 mg/kg) treated rat showed no damage in colon tissue (B) while there were considerable inflammation, ulceration, wall thickening and necrosis with greater doses of TNBS: 50 (C), 100 (D), 150 (E) mg/kg, and the severity of these damage were more prominent in 150 mg/kg-TNBS-treated rats.
Table 2.
Macroscopic and microscopic parameters of TNBS (25-150 mg/kg)-induced colitis in male/female rats.
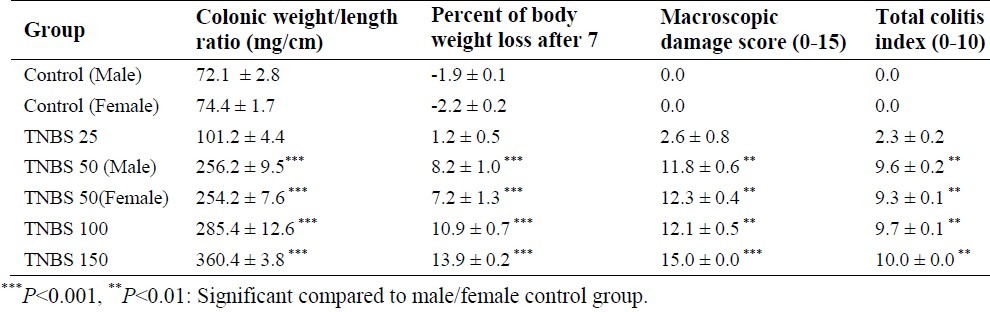
Evaluation of clinical and macroscopic parameters showed that there was no significant difference between 50 and 100 mg/kg-TNBS-treated groups. However, colitis severity of 150 mg/kg TNBS-treated rats was significantly higher than that of 50 and 100 mg/kg TNBS-treated rats (Table 2). In addition, no significant difference was seen between male and female 50 mg/kg-TNBS- treated rats in terms of macroscopic parameters during 7 days of the study (Table 2).
In the case of microscopic evaluation, severe and intense transmural inflammation and/or diffuse necrosis, inflammatory granulomas and submucosal neutrophils infiltration were observed in male rats receiving TNBS with doses of 50 and 100 mg/kg (Table 2, Fig. 4).
Fig. 4.
Macroscopic presentation of TNBS-induced colitis in rats. A. Normal colon treated with vehicle, 2 m/kg; B. TNBS (50 mg/kg) treated rats; C. Dexamethasone (1 mg/kg, i.p.) treated colitis; D. Dexamethasone (2 mg/kg, p.o.) treated colitis; E. Hydrocortisone acetate (20 mg/kg, i.p.) treated colitis; F. Hydrocortisone acetate (20 mg/kg, enema) treated colitis; G. Asacol (100 mg/kg, p.o.) treated colitis; H. Asacol (150 mg/kg, enema) treated colitis.
We observed noticeable ulcers, extensive necrosis of the colonic mucosa and inflam-matory granulomas in colons of male groups treated with TNBS with a dose of 150 mg/kg. Further more, neutrophil infiltration was obviously present in mucus and sub-mucosal layers. No significant difference was found in total colitis index between 50, 100 and 150 mg/kg-TNBS-treated rats.
In addition, there was no significant difference between male and female 50 mg/kg-TNBS-treated rats in terms of microscopic parameters during 7 days of study (Table 2).
Effect of reference drugs on remission of ulcerative colitis induced by TNBS (50 mg/kg)
Macroscopic assessment
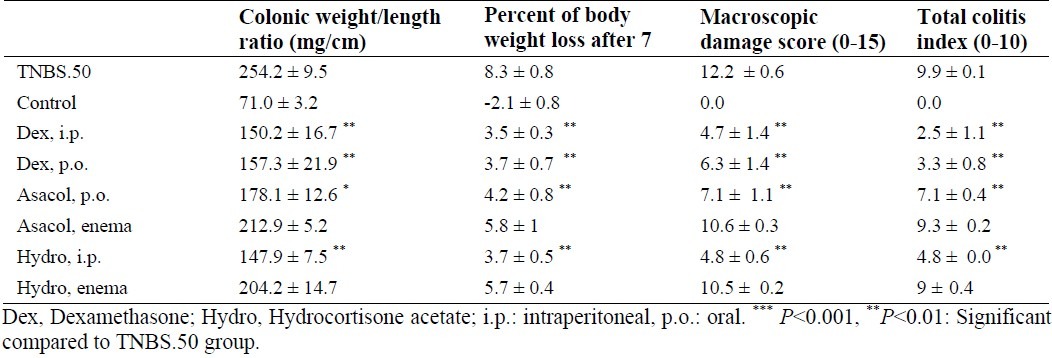
Amongst reference drugs, dexamethasone (1 mg/kg, i.p.; 2 mg/kg, p.o.) and hydrocortisone acetate (20 mg/kg, i.p.) significantly diminished weight/length ratio and body weight loss, compared to untreated TNBS-induced-colitis rats (P<0.001). More to the point, in these groups, total colitis index and ulcer severity were significantly lower than those of TNBS-treated group (P<0.01).
Hydrocortisone acetate in enema form (20 mg/kg) exerted no effect upon clinical and microscopic features of colitis lesions (Table 3, Fig. 4). Asacol declined weight/length ratio and percentage of body weight loss in colitis rats after oral administration (100 mg/kg) (Table 3). It also exerted no influence upon aforementioned parameters in enema form (150 mg/kg).
Table 3.
Effects of dexamethasone (i.p.,1 mg/kg; p.o., 2 mg/kg), hydrocortisone acetate (i.p.,20 mg/kg; enema, 20 mg/kg) and Asacol (p.o.,100 mg/kg; enema, 150 mg/kg) on the microscopic and macroscopic parameters of TNBSinduced colitis in rats (50 mg/kg).
Histological assessment
Fig. 5 and Table 3 indicate that there was no histological damage in control groups. In 50 mg/kg TNBS-treated groups, microscopic assessments exhibited severe, intense transmural inflammation and/or diffuse necrosis, inflammatory granulomas and submucosal neutrophils infiltration.
Fig. 5.
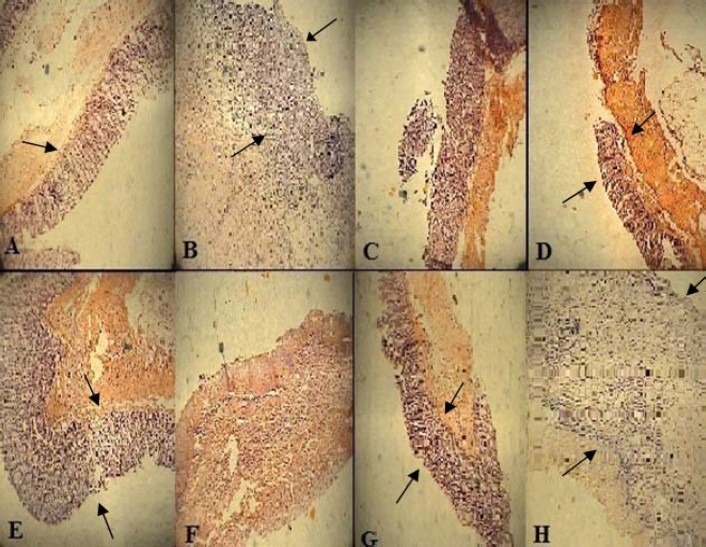
Microscopic presentation of TNBS-induced colitis in rats. A. Normal colon treated with vehicle, 2 ml/kg, mucus layer and crypts are normal and leucocyte infiltration is absent; B. Control colitis treated with vehicle, 2 ml/kg, mucosal and submucusal inflammation as well as crypt damage and leucocyte infiltration are evident; C. Dexamethasone, 1 mg/kg (i.p.); D. Dexamethasone, 2 mg/kg (p.o.); E. Hydrocortisone acetate, 20 mg/kg (i.p.); F. hydrocortisone acetate, 20 mg/kg (enema); G. Asacol, 100 mg/kg (p.o.); H. Asacol, 150 mg/kg (enema);. H&E staining with low power (×10) magnification.
Oral administration of dexamethasone and Asacol as well as i.p. injection of hydrocortisone acetate and dexamethasone diminished total colitis index (inflammation severity, inflammation extent, crypt damage) in injured colons (P<0.01).
Hydrocortisone acetate and Asacol in enema forms did not significantly affect the remission of microscopic injuries.
DISCUSSION
Recently, researchers have shown an increased interest in investigating the effect of different drugs used for the treatment of IBD. In order to understand how drugs affect the colitis, animal models of colitis are normally applied. Intracolonic administration of TNBS mixed with ethanol acting as “barrier breaker” is known as one of the most common methods to investigate IBD conditions in animal models(13,27). TNBS can, in fact, mimic many of macroscopic and histological characteristics of human IBD(12,28). In spite of the fact that ethanol is used to break the mucosal barrier, TNBS alone is responsible for haptenization of colonic autologous or microbiota proteins and causes them to become immunogenic to the host immune system(12). This experimental model is also simple, reproducible, and useful for assessment of T helper cell-dependent mucosal immune responses(12) and extensively applied for examining novel therapeutic regimens predominantly in 3 to 14-day protocols(24).
Some studies reported that TNBS-induced colitis model is associated with overproduction of interferon-γ (IFNγ) and interleukin-2 (IL-2). Colitis seen in this model is induced by a T-helper type-1 (Th1-type) response, as a major CD model(29). It must be noticed that hapten-induced, typical Th1-type colitis model with TNBS is characterized as both Th1- and Th2-type cytokine-mediated colitis and therefore can mimic both acute and chronic inflammations in the colon(15).
Present study was designed to specify the best dose of TNBS for induction of colitis, and to determine ideal reference drugs for the treatment of TNBS-induced colitis. We found a significant difference in the severity of inflammation between TNBS treatments with four test doses. The TNBS treatment with the dose of 25 mg/kg did not impose macroscopically visible and histological colonic injures. During the experiment, macroscopic and microscopic colon tissue damage caused by TNBS treatment with the dose of 100 mg/kg was more severe than that of produced with the dose of 50 mg/kg; even so, there was no significant difference in clinical features and macroscopic and histological injuries produced by these two doses. Moreover, treatment with TNBS with the dose of 150 mg/kg produced massive clinical features and macroscopic and histological damages. This dose was the only one that resulted in 33% mortality in the rats.
Present study demonstrated that intrarectal TNBS administration induced inflammation resembling ulcerative colitis and its effect was largely dose-dependent. In this regard, our results corroborated findings of Morris et al who reported that TNBS-induced colitis follows a dose-dependent manner in a range of 25-150 mg/kg in rat.(13).
Ideally, an optimum animal model of IBD should resemble the clinical course and histopathology of the disease; it shouldn’t cause high rate of mortality and not to be too expensive by providing widely available animals and chemical substances. Treatment with TNBS at its highest dose (150 mg/kg) caused mortality in 33% of animals. In this regard, our findings were not in consistent with those of Morris and co-workers who demonstrated that TNBS treatment with the dose of 150 mg/kg caused mortality in only 3% of the rats. They also demonstrated that treatment with TNBS at a dose of 150 mg/kg was ideal for induction of colitis. Moreover, in their study, amongst four doses of TNBS (25, 50, 100 and 150 mg per rat), a significant weight loss was only demonstrated at 150 mg/kg. We, however, observed that treatment with TNBS at doses of 50, 100 and 150 mg/kg caused significant weight loss. Treatment with TNBS with the dose of 50 mg/kg appeared to be optimal for induction of colitis, although no significant difference in the severity of microscopic and macroscopic injuries was found between TNBS treatments at doses of 50 and 100 mg/kg.
It should be mentioned that little is known about the effect of gender on severity of colon injuries in TNBS-induced colitis in rats. In different studies both genders have been used for induction of colitis(30–32). In the present study, we observed that the gender did not affect clinical, macroscopic and histological characteristics of TNBS-induced colitis in rats.
Our results did not buttress the hypothesis of Morris and coworkers showing the probable effect of sex disparities upon severity of colitis. Therefore, we suggest that both genders be utilized in this model of induction of colitis. However it seems that male rats are preferred due to lack of hormonal fluctuations seen in female rats.
Furthermore, in animal models of diseases, ideal reference drugs are required to assess the efficacy of novel therapeutic agents. Cortico-steroids (i.e., prednisolone, dexamethasone, hydrocortisone acetate) and aminosalicylates (i.e., sulfasalazine and 5-aminosalicylic acid analogues) are the most commonly prescribed medicines for abating wide range of mild to severe human ulcerative colitis(33,34). New formulations of these drugs have recently been introduced to increase their release at sites of inflammation while decreasing their systemic absorption. The efficacy of these new forms was also evaluated in many clinical trials(35). Asacol and hydrocortisone acetate are marketed in the form of enemas for rectal administration throughout the world(36). In short-term animal studies, however, the effectiveness of administration of these two drugs as enema is not well-documented. We found that hydrocortisone acetate (i.p.), dexamethasone (i.p., p.o.) and Asacol (p.o.) significantly diminished the severity of macroscopic and microscopic injuries.
Corticosteroids affect cellular and humoral immune function; inhibit the production and action of cytokines and inflammatory mediators(33,37). Aminosalicylates inhibit the production of reactive oxygen species and scavenge reactive oxygen metabolites. Hence, it is expected that oxygen reactive metabolites play a significant role in TNBS-induced colitis pathogenesis(33,38).
Therefore, these drugs are suitable reference drugs for experimental colitis studies in rats. On the other hand, our findings showed that enema forms of Asacol and hydrocortisone acetate as topical route of administrations did not exert significant effect upon remission of colon injuries.
It is suggested that ineffectiveness of these drugs in enema form may be associated with their poor availability of active drugs in injured mucosal tissue(33). Secretary diarrhea which increases enteric peristaltic movements results in shortened time of absorption and reduced colonic mucosa access to drugs. Therefore, this causes less efficiency of enema forms of applied drugs.
In addition, it should be noted that the volume and viscosity of Asacol may be important determinants of its efficacy. On the grounds that the efficacy of Asacol is affected by direct contact to the inflamed mucosa, the drug in a low volume is exclusively delivered to recto-sigmoid junction. This might limit the access of proximal sigmoid colon to drugs. Conversely, a larger enema volume may be associated with higher proximal coating of the colon. Furthermore, the higher viscosity of enema may be correlated with more proximal distribution of the drug(39). Nonetheless, this hypothesis should be interpreted with caution due to the short duration of our study. In addition, preventive therapy with these dosage forms may yield acceptable results.
CONCLUSION
To recapitulate briefly, the most obvious finding to emerge from this study is that treatment with TNBS with the dose of 50 mg/kg is an optimum dose for induction of colitis. It should thus be noted that the optimization of TNBS dose is essential for induction of colitis under laboratory conditions. In addition, our findings showed that gender did not affect macroscopic and histological characteristic of TNBS-induced colitis in rats. It was also shown that the enema forms of hydrocortisone and Asacol may not be useful as reference drugs; however systemic administrations are suitable for experimental colitis studies in rats.
ACKNOWLEDGMENT
This work was financially supported by School of Pharmacy and Pharmaceutical Sciences, Isfahan University of Medical Sciences.
REFERENCES
- 1.Seibel J, Molzberger AF, Hertrampf T, Leschowski UL, Diel P. Oral treatment with genistein reduces the expression of molecular and biochemical markers of inflammation in a rat model of chronic TNBS-induced colitis. Eur J Nutr. 2009;48:213–220. doi: 10.1007/s00394-009-0004-3. [DOI] [PubMed] [Google Scholar]
- 2.Hosseini-Tabatabaei A, Abdollahi M. Potassium channel openers and improvement of toxic stress: Do they have role in the management of inflammatory bowel disease? Inflamm Allergy Drug Targets. 2008;7:129–135. doi: 10.2174/187152808785748164. [DOI] [PubMed] [Google Scholar]
- 3.Podolsky DK. Inflammatory bowel disease. N Engl J Med. 2002;347:417–429. doi: 10.1056/NEJMra020831. [DOI] [PubMed] [Google Scholar]
- 4.Baumaqart DC, Sandborn WJ. Inflammatory bowel disease: clinical aspects and established and evolving therapies. Lancet. 2007;369:1641–1657. doi: 10.1016/S0140-6736(07)60751-X. [DOI] [PubMed] [Google Scholar]
- 5.Forbes E, Murase T, Yang M, Matthaei KI, Lee J, Lee AN, et al. Immunopathogenesis of experimental ulcerative xolitis is mediated by eosinophil peroxidase. J Immunol. 2004;172:5664–5675. doi: 10.4049/jimmunol.172.9.5664. [DOI] [PubMed] [Google Scholar]
- 6.Grisham MB. Molecular and cellular aspects of intestinal inflammation: clinical implications in inflammatory bowel disease. Inflam Bow Dis. 2000;1:1–14. [Google Scholar]
- 7.Langmead L, Rampton DS. Complementary and alternative therapies for inflammatory bowel disease. Aliment Pharm Therap. 2006;23:341–349. doi: 10.1111/j.1365-2036.2006.02761.x. [DOI] [PubMed] [Google Scholar]
- 8.Sands BE. Therapy of inflammatory bowel disease. Gastroenterology. 2000;118:S68–S82. doi: 10.1016/s0016-5085(00)70007-2. [DOI] [PubMed] [Google Scholar]
- 9.Braus NA, Elliott DE. Advances in the pathogenesis and treatment of IBD. Clin Immunol. 2009;4:1–9. doi: 10.1016/j.clim.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dubinsky MC. Targeting therapy in pediatric inflammatory bowel disease. Curr Treat Options Gastroenterol. 2004;7:391–405. doi: 10.1007/s11938-004-0052-y. [DOI] [PubMed] [Google Scholar]
- 11.Hibi T, Ogata H, Sakuraba A. Animal models of inflammatory bowel disease. J Gastroenterol. 2002;37:409–417. doi: 10.1007/s005350200060. [DOI] [PubMed] [Google Scholar]
- 12.Wirtz S, Neurath MF. Mouse models of inflammatory bowel disease. Adv Drug Deliver Rev. 2007;59:1073–1083. doi: 10.1016/j.addr.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 13.Morris GP, Beck PL, Herridge MS, Depew WT, Szewczuk MR, Wallace JL. Hapten-induced model of chronic inflammation and ulceration in the rat colon. Gastroenterology. 1989;96:795–803. [PubMed] [Google Scholar]
- 14.Zheng L, Gao ZQ, Wang SX. A chronic ulcerative colitis model in rats. World J Gastroenterol. 2000;6:150–152. doi: 10.3748/wjg.v6.i1.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dohi T, Fujihashi K. Type 1 and 2 T Helper Cell-mediated Colitis. Curr Opin Gastroenterol. 2006;22:651–657. doi: 10.1097/01.mog.0000245545.80160.0f. [DOI] [PubMed] [Google Scholar]
- 16.Linden DR, Chen JX, Gershon MD, Sharkey KA, Mawe GM. Serotonin availability is increased in mucosa of guinea pigs with TNBS-induced colitis. Am J Physiol Gastrointest Liver Physiol. 2003;285:G207–G216. doi: 10.1152/ajpgi.00488.2002. [DOI] [PubMed] [Google Scholar]
- 17.Stevenson CS, Marshall LA, Morgan DW. 2nd ed. Berlin: Birkhauser Verlag; 2006. In vivo Models of Inflammation; pp. 150–151. [Google Scholar]
- 18.Carvalho AT, Souza H, Carneiro AJ, Branco MC, Madi K, Schanaider A, et al. Therapeutic and prophylactic thalidomide in TNBS-induced colitis: Synergistic effects on TNF-α, IL-12 and VEGF production. World J Gastroenterol. 2007;13:2166–2173. doi: 10.3748/wjg.v13.i15.2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dworzanski T, Celinski K, Korolczuk A, Slomka M, Radej S, Czechowska G, et al. Influence of the peroxisome proliferator-activated receptor Gamma (PPAR-γ) agonist, rosiglitazone and antagonist, biphenol-a-diglicydyl ether (badge) on the course of inflammation in the experimental model of colitis in rat. J Physicol Pharmacol. 2010;61:683–693. [PubMed] [Google Scholar]
- 20.Ballester I, Daddaoua A, Posadas RL, Nieto A, Sua´rez MD, Zarzuelo A, et al. The bisphosphonate alendronate improves the damage associated with trinitrobenzenesulfonic acid-induced colitis in rats. Brit J Pharmacol. 2007;151:206–215. doi: 10.1038/sj.bjp.0707227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Minaiyan M, Ghannadi AR, Afsharipour M, Mahzouni P. Effects of extract and essential oil of Rosmarinus officinalis L. on TNBS-induced colitis in rats. Res Pharm Sci. 2011;6:13–21. [PMC free article] [PubMed] [Google Scholar]
- 22.Esmaily H, Hosseini-Tabatabaei A, Rahimian R, Khorasani R, Baeeri M, Barazesh-Morgani AR, et al. On the benefits of silymarin in murine colitis by improving balance of destructive cytokines and reduction of toxic stress in the bowel cells. Cent Eur J Biol. 2009;4:204–213. [Google Scholar]
- 23.Li JH, Yu JP, Yu HG, Xu XM, Yu LL, Liu J, et al. Melatonin reduces inflammatory injury through inhibiting NF-κB activation in rats with colitis. Mediat Inflamm. 2005;4:185–193. doi: 10.1155/MI.2005.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Menozzi A, Pozzoli C, Poli E, Lazzaretti M, Grandi D, Coruzzi G. Long-term study of TNBS-induced colitis in rats: Focus on mast cells. Inflamm Res. 2006;55:416–22. doi: 10.1007/s00011-006-6017-y. [DOI] [PubMed] [Google Scholar]
- 25.Cooper H, Murthy S, Shah R, Sedergran D. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest. 1993;69:238–249. [PubMed] [Google Scholar]
- 26.Dieleman L, Palmen M, Akol H, Bloemena E, Pena A, Meuwissen S. Chronic experimental colitis induced by dextran sulfate sodium (DSS) is characterized by Th1 and Th2 cytokines. Clin Exp Immuno. 1998;114:385–391. doi: 10.1046/j.1365-2249.1998.00728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu F, Qian J, Pan G. The establishment of TNBS-induced experimental colitis. Acta Acad Med Sci. 1998;20:271–278. [PubMed] [Google Scholar]
- 28.Blumberg RS, Saubermann LJ, Strober W. Animal models of mucosal inflammation and their relation to human inflammatory bowel disease. Curr Opin Immunol. 1999;11:648–656. doi: 10.1016/s0952-7915(99)00032-1. [DOI] [PubMed] [Google Scholar]
- 29.Hibi T, Ogata H, Sakuraba A. Animal models of inflammatory bowel disease. J Gastroenterol. 2002;37:409–417. doi: 10.1007/s005350200060. [DOI] [PubMed] [Google Scholar]
- 30.Ameho CK, Adjei AA, Harrison EK, Takeshita K, Morioka T, Arakaki Y, et al. Prophylactic effect of dietary glutamine supplementation on interleukin 8 and tumour necrosis factor á production in trinitrobenzene sulphonic acid induced colitis. Gut. 1997;41:487–493. doi: 10.1136/gut.41.4.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peterson TC, Cleary CE, Shaw AM, Malatjalian DA, Veldhuyzen SJ. Therapeutic role for bismuth compounds in TNBS-induced colitis in the rat. Dig Dis Sci. 2000;45:466–473. doi: 10.1023/a:1005476619923. [DOI] [PubMed] [Google Scholar]
- 32.Azooz OG, Farthing MGJ, Savage MO, Ballinger AB. Delayed puberty and response to testosterone in a rat model of colitis. Am J Physiol Regulatory Integrative Comp Physiol. 2001;281:R1483–R1491. doi: 10.1152/ajpregu.2001.281.5.R1483. [DOI] [PubMed] [Google Scholar]
- 33.Wood JL. Inflammatory bowel disease. N Engl J Med. 1996;334:841–848. doi: 10.1056/NEJM199603283341307. [DOI] [PubMed] [Google Scholar]
- 34.Kuhbacher T, Fölsch UR. Practical guidelines for the treatment of inflammatory bowel disease. World J Gastroenterol. 2007;13:1149–1155. doi: 10.3748/wjg.v13.i8.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Allgayer H. Sulfasalazine and 5-ASA compounds. Gastroenterol Clin North Am. 1992;21:643–658. [PubMed] [Google Scholar]
- 36.Hanauer SB, Stathopoulos G. Risk-benefit assessment of drugs used in the treatment of inflammatory bowel disease. Drug Saf. 1991;6:192–219. doi: 10.2165/00002018-199106030-00005. [DOI] [PubMed] [Google Scholar]
- 37.Minaiyan M, Ghannadi A, Etemad M, Mahzouni P. A study of the effects of Cydonia oblonga Miller (Quince) on TNBS-induced colitis in rats. Res Pharm Sci. 2012;7:103–110. [PMC free article] [PubMed] [Google Scholar]
- 38.Varshosaz J, Emami J, Fassihi A, Tavakoli N, Minaiyan M, Ahmadi F. Effectiveness of budesonide-succinate-dextran conjugate as a novel prodrug of budesonide against acetic acid-induced colitis in rats. Int J Colorectal Dis. 2010;25:1159–1165. doi: 10.1007/s00384-010-1026-2. [DOI] [PubMed] [Google Scholar]
- 39.Hanauer SB, Marteau P. Paris: John Libbey Eurotext Limited; 2000. Ulcerative colitis: focus on topical treatment. Efficacy and tolerance of rectal 5-aminosalicyclic acid. [Google Scholar]


