Abstract
A series of ortho-hydroxypyridine-4-ones were prepared in high yields and evaluated for antioxidant and iron chelating activities. N1-H hydroxypyridinones Va, Vb, and Ve were the best radical scavengers in DPPH free radical scavenging assay. Compound Vb was proved to be the most potent compound in hydrogen peroxide scavenging assay. All of the synthesized compounds had very close chelating ability, compounds containing N1-CH3 hydroxypyridinone ring were stronger chelating agents.
Keywords: Ortho-Hydroxypyridine-4-one, Antioxidant activity, Iron chelating activity, Radical scavenging activity
INTRODUCTION
The importance of researches on the roles of free radical species in biological systems has been significantly increased in the last two decades. The production of these species in naturally occurring biological reactions is an inevitable phenomenon in aerobic metabolic processes. The presence of a singlet electron in free radicals renders them extreme reactivity which may cause severe oxidative damages to the biomolecules such as lipids, proteins and DNA(1). Various disorders such as cancer, atherosclerosis, di abetes, alzehimer, parkinson and disease related to aging process are long term manifestations of these damages(2). Since this kind of damages can exert irreversible adverse effects on cells, there are some antioxidant mechanisms and compounds with endogenous and exogenous origin to deactivate or neutralize these reactive species. Gluthation reductase (GSH), superoxide dismutase (SOD) and catalase are well-known enzymes built in these mechanisms. Radical scavenging compounds such as α-tocopherol, ascorbic acid, carotenoids, and natural polyphenols are some examples of exogenous antioxidant compounds. These enzymes and antioxidant compounds help the body to equilibrate the generation and neutralization of free radicals. When these defensive mechanisms become insufficient or defective, design of potent and safe antioxidant molecules seems to be necessary to hold this balance again(3,4).
Oxidative reactions have multiple dimensions which make it possible to have different plans for designing antioxidant substances. Molecules with free radical scavenging abilities are the most-studied antioxidant agents. It is revealed that many different substances are involved in the process of free radical generation, among them metal ions with specific roles in biological reactions are especially important. Iron is the fundamental catalyst of Fenton and Haber-Weiss reactions which produce the hydroxyl radical which is the most destructive free radical(5). Substances which can chelate iron cations potentially suppress oxidative reactions to alleviate the destroying effects of free radicals. Ortho-Hydroxypyridinones comprise one of the well-known chelating moieties in medicinal chemistry. 3(5)-hydroxypyridin-4-ones have the most chelating ability among the three classes of ortho-hydroxypyridinones: 1-hydroxypyridin-2-ones, 3-hydroxypyridin-2-ones, and 3(5)-hydroxypyridin-4-ones. These compounds like other high affinity Fe3+ chelating agents bind both Fe3+ and Fe2+ under most physiological conditions(6). 3(5)-hydroxypyridin-4-one derivatives form five-membered chelate rings with iron in which the metal is coordinated by two vicinal oxygen atoms, thus these bidentate chelators form neutral and stable 3: 1 complexes with iron cations and inhibit their redox activity(3).
Co-administration of iron chelating compounds, to prevent Fenton chemistry induced by free iron, and radical scavenging agents, to neutralize the free radicals, has been one of the strategies to control oxidative stress conditions. Prevention of post-ischemic cardiac injury using a combination of 1 ,2- dimethyl -3- hydroxypyridin -4- one (deferiprone) and antioxidant flavonoid (+) cyanidanol-3(7) and more recently, combined treatment with deferiprone and the antioxidant idebenone in individuals with Friedreich's ataxia(8), are some reported cases of this combination therapy. Design of compounds possessing the dual ability of iron chelation and radical scavenging has been another strategy to control oxidative conditions. Some chemical entities possess both iron chelating and antioxidant abilities. For example, the antioxidant activity of flavonoids is attributed to a combination of iron chelation and free radical scavenging activities(9). Iron-chelating-radical-scavenging 8-hydroxyquinoline derivatives with neuro-protective properties are under investigation for neurodegenerative disorders such as Alzehimer and Parkinson disease(10). Famous 3-hydroxypyridin-4-one derivatives (e.g. deferiprone, CP20, or CP08, Fig. 1) in contrast to many iron chelators lack a remarkable radical scavenging ability(11).
Fig. 1.
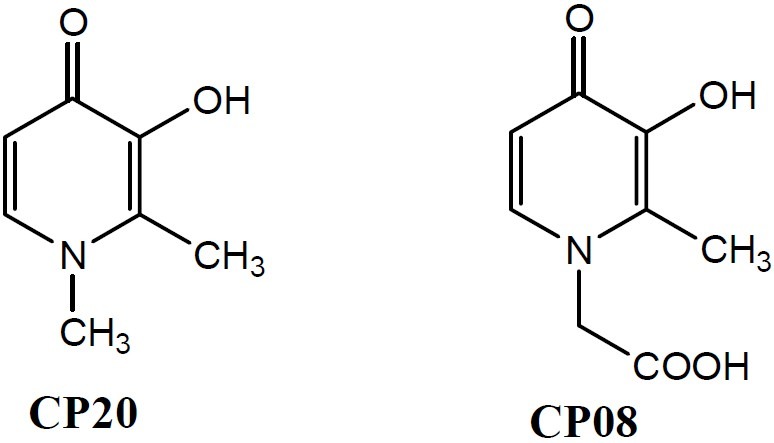
CP20 and CP08: Two well-known 3-hydroxy pyridin-4-one derivatives
There are few reports on the design of small molecules with 3(5)-hydroxypyridin-4-one scaffold to render them this dual activity. A world patent in 1999 claimed that ortho-hydroxypyridinone derivatives possess iron chelating and antioxidant activities. The inventors provide compounds which may be used in the treatment of oxidative stress, particularly oxidative damage to the central nervous system as neuroprotective agents(12). In a more recent article, functionalized ortho-hydroxypyridinones with the desired dual activity are provided to combat neuro-degeneration associated with Alzheimer's disease(13).
Here, we report on the synthesis of some novel 5-hydroxypyridin-4-one derivatives. These compounds are chelating agents due to the presence of the ortho-hydroxypyridinone ring in their structure. All of these compounds have at least one labile hydrogen atom in the auxiliary moiety attached to the hydroxy pyridin-4-one ring. This renders them a radical scavenging ability which is greater than what is expected for the main scaffold. These compounds are evaluated as free radical scavenging and Fe2+ chelating agents. The H2O2 scavenging power of these compounds is determined as well.
MATERIALS AND METHODS
Chemistry
All chemicals used for the synthesis of the compounds were supplied by Merck or Sigma. Melting points were determined on a Mettler capillary melting point apparatus and were uncorrected. The IR spectra were recorded with a WQF-510 Ratio Recording FT-IR spectrometer as a KBr disc (γ, cm-1). The 1HNMR spectra (DMSO-d6) were recorded on a Bruker 400 MHz spectrometer. Chemical shifts (δ) are reported in ppm downfield from the internal standard tetramethylsilane (TMS). Electrospray mass spectra (ESI-MS) were obtained in negative and positive ion mode on a SHIMADZU LCMS-2010 EV spectrometer using methanol and dimethylsulfoxide as solvent, a capillary voltage of 4500 V and a cone voltage of 10 V. The purity of the compounds was checked by thin layer chromatography (TLC) on silica gel plate using chloroform and methanol. The procedure for the synthesis of the desired compounds is depicted in Scheme 1.
Scheme 1.
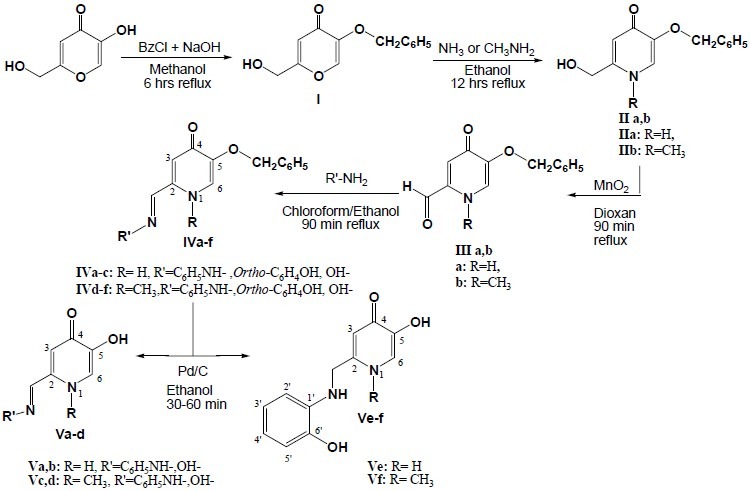
General procedure for the synthesis of the studied compounds
Synthesis of 5-(benzyloxy)-2-(hydroxymethyl)-4H-pyran-4-one (I)
This compound was prepared according to the instruction already published(14).
Synthesis of 5-Benzyloxy-2-(hydroxymethyl)-pyridin-4(1H)-one (IIa) and 5-(benzyloxy)-2-(hydroxymethyl)-1-methylpyridin-4(1H)-one (IIb)
These compounds were prepared according to the method reported by Ma and coworkers(15).
Synthesis of 5-(benzyloxy)-4-oxo-1,4-dihydro pyridine -2- carbaldehyde (IIIa) and 5-(Benzyloxy) -1- methyl -4- oxo -1, 4-dihydro pyridine-2-carbaldehyde (IIIb)
Aldehyde IIIa was prepared by the oxidation of compound IIa using manganese dioxide as described by Becker (16). Aldehyde IIIb was prepared by the same method as IIIa.
General procedure for the synthesis of IVa-f derivatives
2.063 g (8.7 mmol) of IIIa or IIIb was dissolved in 30 ml chloroform and 10 ml ethanol. 8.7 mmol of the proper -NH2 containing compound was added to this solution. The mixture was then refluxed and the progression of the reaction was monitored by TLC. The reaction was completed within 90 min. The product was filtrated off, washed with chloroform and recrystallized in methanol. The obtained crystals were dried under vacuum.
General procedure for the synthesis of Va-f derivatives
0.500 g of each of IVa-f was dissolved in 100 ml of absolute ethanol. Then 5.00 g of 10% palladium/charcoal was added. Hydrogen was bubbled into the reaction mixture using a balloon connected to the reaction vessel. The mixture was shaken vigorously at room temperature for 30-60 min. When the reaction was completed, palladium/charcoal was filtrated off and washed 3 times by ethanol. The product was crystallized from the filtrate and dried under reduced pressure (Scheme 1).
Antioxidant evaluation
DPPH free radical scavenging assay
This assay was performed according to the method described by Blois and coworkers(17). Briefly, 4 ml of different concentrations of methanolic solution of standard or test compounds (0.4 to 0.00625 mg/ml) were added to 2 ml of DPPH methanolic solution (60 mM). The mixture was shaken vigorously and allowed to stand for 30 min, and then the absorbance of the resulting solution was measured at 517 nm using a UV/Vis-spectrophotometer. Scavenging of DPPH free radicals was calculated as:
DPPH scavenging activity (%) = [(Ac – At)/ Ac] × 100
where, Ac is the absorbance of the control tube (containing all reagents except the test compound), and At is the absorbance of the test tube. Ascorbic acid, gallic acid, BHT and BHA were used as standards.
Hydrogen peroxide scavenging assay
H2O2 scavenging power was determined according to the method of Ruch and coworkers(18). This method is based on the ability of a compound to convert hydrogen peroxide to water. A 40 mM solution of hydrogen peroxide was prepared in saline phosphate buffer (pH 7.4) and concentrations were determined spectrophotometrically at 230 nm. 100 μl of 0.312 mg/ml methanolic solutions of the test compounds or standards was added to 2 ml of hydrogen peroxide solution (40 mM) and the absorbance was determined at 230 nm after 20 min against a blank solution (100 μl of methanolic solutions of test compounds or standards and 2 ml of saline phosphate buffer). The hydrogen peroxide scavenging for compounds and standards was calculated using the following equation:
H2O2 scavenging activity (%) = [(Ac-At)/Ac] × 100
Where Ac is the absorbance of the control and At is the absorbance of tested compounds or standards. Ascorbic acid and gallic acid were used as standards.
Iron chelating ability
The chelating effects were determined according to the method of Dinis and coworkers(19). 500 μl of the standard or test compounds (12.5 μg/ml) was added to 50 μl of FeCl2 solution (2 mM). The reaction was initiated by adding 20 ml of ferrozine methanolic solution (5 mM). The mixture was shaken vigorously and left at room temperature for 10 min. The absorbance of the solution was measured spectrophotometrically at 562 nm. The chelation ability of the prepared compounds was calculated based on the inhibition of ferrozine-Fe2+ complex formation using the following formula:
Metal chelating activity (%) = [(Ac – As)/ Ac] × 100
where, Ac is the absorbance of the control (control contained 50 μl of FeCl2, 20 μl of ferrozine and 500 μl of methanol), and at is the absorbance of the test compound. EDTA and gallic acid were used as controls.
RESULTS
Chemistry
5-Hydroxypyridine-4-one derivatives des-cribed here were synthesized following the synthetic routes outlined in scheme 1. Reaction of kojic acid (5-Hydroxy-2-(hydroxymethyl)-4H-pyran-4-one) with benzyl chloride resulted in the protected form of this molecule (I) which was then reacted with ammonia or methyl amine to produce the corresponding pyridin-4(1H)-one derivatives (IIa,b). Oxidation of these alcohols with activated manganese (IV) oxide in dioxane afforded the desired aldehydes (IIIa,b). These aldehydes were prepared to be used as intermediates for the preparation of some 5-hydroxypyridine-4-one derivatives containing appropriate moieties for stabilizing the radical species which would be formed from the reaction of these compounds with free radicals. Thus, IIIa and IIIb were reacted with phenyl hydrazine, 2-aminophenol and hydroxylamine to afford the desired final compounds after debenzylation of the 5-benzyloxy group of the obtained molecules. The structures of all compounds were confirmed by FTIR, 1HNMR and mass spectroscopy. Carbon atoms are numbered sequentially to facilitate the assignment of protons in 1HNMR (Scheme 1). Compounds IVa-c were revealed to be as two isomers as shown in Fig. 2.
Fig. 2.
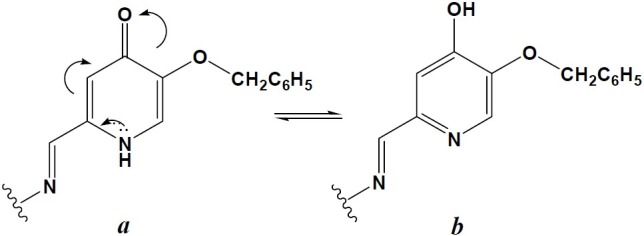
The observed tautomerism in compounds IVa-c
5- (Benzyloxy)-1-methyl-4- oxo -1,4- dihydro pyridine-2-carbaldehyde (IIIb)
White crystals; yield 80%; m.p. 227-229°C; IR (KBr) cm-1: 3047 (C-H, aromatic, str.), 2893 (C-H, aliphatic, str.), 1716 (C=O, aldehyde, str.), 1618 (C=O ketone, str.), 1583 (shouldered, C=C aromatic, C=C alkene), 1437 (C=C, aromatic, bend); 1HNMR (DMSO-d6): δ 9.68 (s, 1H, CHO), 7.44 (s, 1H, C6-H), 7.16-7.33 (m, 5H, CH2-C6H5), 6.75 (s, 1H, C3-H), 5.07 (s, 2H, O-CH2-C6H5), 3.85 (s, 3H, NCH3).
5-Benzyloxy-2-[(phenylaminoimino)methyl] pyridin-4(1H)-one (IVa)
Yellow crystals; yield 74%; m.p. 236-237°C; a,b tautomers: a/b=100/40. IR (KBr) cm-1: 3381 (N-H, str.), 3222 (O-H, str.), 3051 and 3033 (C-H, aromatic, str.), 2968 and 2931(C-H, aliphatic, str.), 1630 (C=O, str.), 1593 (C=C, aromatic, str.), 1529 (shouldered, C=C alkene, C=N), 1493 (C=C, aromatic, bend); 1HNMR (DMSO-d6): δ 11.15 [s, 1H, =N-NH- (a)], 10.87 [s, 1H, N1-H- (a)], 10.76-10.78 [m, 2H, =N-NH- (b), C4-OH (b)], 8.08 [s, 1H, -CH=N- (b)], 7.00-7.70 [m, 12H, -CH2-C6H5 (a,b), -CH=N- (a) and C6-H (b)], 6.80 [bs, 2H, C6-H (a), C3-H (b)], 6.31 [1H, C3-H (a)], 5.171 [s, 2H, -CH2-C6H5 (b)], 5.032 [s, 2H, -CH2-C6H5 (a)]; ESI-MS (+) m/z (%): 320.1(M + H+) (95), 342.2 (M + Na+) (100).
5-Benzyloxy-2-[(2-hydroxyphenylimino)methyl] pyridin-4(1H)-one (IVb)
Pale yellow crystals; yield 79%; m.p. 235-236°C; a,b tautomers: a/b=100/30. IR (KBr) cm-1: 3234 (O-H, str.), 3066 (N-H, str.), 3032 (C-H, aromatic, str.), 2866 (C-H, aliphatic, str.), 1633 (C=O, str.), 1603 (C=C, aromatic, str.), 1541 (shouldered, C=C alkene, C=N), 1487 (C=C, aromatic, bend); 1HNMR (DMSO-d6): δ 11.67 [s, 1H, N1-H (a)], 10.68 [s, 1H, C4-OH (b)], 9.10-9.30 [m, 2H, disubstituted phenyl-OH (a,b)], 8.65 [s, 1H, -CH=N- (a)], 8.45 [s, 1H, -CH=N- (b)], 6.87-8.30 [m, 20H, CH2-C6H5 (a,b), C6-H (b), C3-H (a), disubstituted phenyl ring hydrogens (a,b)], 6.64 [s, 1H, C6-H (a)], 5.23 [s, 2H, - CH2-C6H5 (b)], 5.08 [s, 2H, -CH2-C6H5 (a)]; ESI-MS (-) m/z (%): 319.1(M - H+) (100).
5- Benzyloxy -4- oxo -1,4- dihydropyridine-2-carbaldehyde oxime (IVc)
White crystals; yield 82%; m.p. 231-232°C; a,b tautomers: a/b=100/50. IR (KBr) cm-1: 3278, 3168 (N-H, str.), 3064, 3035 (C-H, aromatic, str.), 2868 (C-H, aliphatic, str.), 2629 (O-H, str.), 1637 (C=O, str.), 1601 (C=C, aromatic, str.), 1537 (shouldered, C=C alkene, C=N), 1483 (C=C, aromatic, bend); 1HNMR (DMSO-d6): δ 12.57 [s, 1H, =N-OH (a)], 11.95 [s, 1H, N1-H (a)], 11.35 [s, 1H, =N-OH (b)], 10.65 [s, 1H, C4-OH (b)], 7.10-8.30 [m, 15H, CH=N- (a,b), CH2-C6H5 (a,b), C3-H (a), C6-H (a,b)], 6.35 [s, 1 H, C3-H (b)], 5.0-5.2 [m, 4H, CH2-C6H5 (a,b)]; ESI-MS (-) m/z (%): 243.1(M - H+) (100).
5-Benzyloxy-2-[(phenylaminoimino)methyl]-1-methylpyridin-4(1H)-one (IVd)
Yellow crystals; yield 72%; m.p. 241-242°C; IR (KBr) cm-1: 3433 (O-H, str.), 3169 (N-H, str.), 3044, (C-H, aromatic, str.), 2936 (C-H, aliphatic, str.), 1619 (C=O, str.), 1570 (C=C, aromatic, str.), 1523 (C=C alkene), 1497 (C=N), (1448 (C=C, aromatic, bend); 1HNMR (DMSO-d6): δ 10.88 (s, 1H, =N-NH-), 7.61 (m, 1H, CH=N-), 7.34-7.45 (m, 5 H, CH2C6H5), 7.26 (t, 2H, C3’-H, C5’-H), 7.08 (d, 2H, C2’-H, C6’-H), 6.83 (t, 1H, C4’-H), 6.59 (s, 1H, C6-H), 5.03 (s, 2H, CH2C6H5), 3.77 (s, 3H, NCH3).
5-Benzyloxy-2-[(2-hydroxyphenylimino)methyl]-1-methylpyridin-4(1H)-one (IVe)
Yellow crystals; yield 69%; m.p. 228-229°C; IR (KBr) cm-1: 3434 (O-H, str.), 3066 (C-H, aromatic, str.), 2948 (C-H, aliphatic, str.), 1605 (shouldered, C=O, C=C, aromatic, str.), 1553(C=N), 1453 (C=C, aromatic, bend); 1HNMR (DMSO-d6): δ 9.25 (bs, 1H, C2’-OH), 8.68 (s, 1H, -CH=N-), 7.71 (s, 1H, C6-H), 7.31-7.50 (m, 5H, CH2-C6H5), 7.26 (d, J=7.50 Hz, 1H, C6’-H), 7.14 (t, J=7.50 Hz, 1H, C4’-H), 7.05 (s, 1H, C3-H), 6.92 (d, J= 8.00 Hz, 1H, C3’-H), 6.86 (t, J= 7.50 Hz, 1H, C5’-H), 5.09 (s, 2H, CH2-C6H5), 3.95 (s, 3H, NCH3).
5- Benzyloxy -1- methyl -4- oxo -1,4- dihydro pyridine-2-carbaldehyde oxime (IVf)
White crystals; yield 83%; m.p. 239-240°C; IR (KBr) cm-1: 3157 (N-H, str.), 3062 (C-H, aromatic, str.), 2998,2928,2867 (C-H, aliphatic, str.), 2692 (O-H, str.), 1620 (C=O, str.), 1564 (shouldered, C=C alkene, C=N), 1490 (C=C, aromatic, bend); 1HNMR (DMSO-D6): μ 12.36 (s, 1H, OH), 8.24 (s, 1H, -CH=N-), 7.68 (s, 1H, C6-H), 7.33-7.43 (m, 5H, CH2-C6H5), 6.60 (s, 1H, C3-H), 5.01 (s, 2H, CH2-C6H5), 3.72 (s, 3H, NCH3).
5- Hydroxy -2- [(phenylaminoimino)methyl] pyridin-4(1H)-one (Va)
Yellow crystals; yield 86%; m.p. 235-236°C; IR (KBr) cm-1: 3311 (N-H, str.), 3290 (N-H, str.), 1639 (C=O, str.), 1593 (C=N, str.), 1520 (C=C, aromatic, str.), 1479 (C=C, aromatic, bend) 1HNMR (DMSO-d6): δ 11.2 (s, 1H, -NH-N=), 10.81 (s, 1H, N1-H), 7.57 (s, 1H, CH=N-), 7.23 (bs, 5H,-C6H5), 6.80 (s, 1H, C6-H), 6.33 (s, 1H, C3-H), 4.0-6.0 (bs,1H, OH). ESI-MS (-) m/z (%): 228.1 (M - H+) (100).
5- Hydroxy -4- oxo -1,4- dihydropyridine-2-carbaldehyde oxime (Vb)
White crystals; yield 86%; m.p. 262-263°C; IR (KBr) cm-1: 3402 (O-H, str.), 3261 (O-H, str.), 3132 (N-H, str.), 1624 ( C=O, str.), 1537 (C=N, str.), 1473 (C=C, aromatic); 1HNMR (DMSO-d6): δ 8.22 (s, 1H, -CH=N-), 7.55 (s, 1H, C6-H), 6.74 (s, 1H,C3-H). ESI-MS (-) m/z (%): 153.1 (M - H+) (100).
5-Hydroxy-2-[(phenylaminoimino)methyl]-1-methylpyridin-4(1H)-one (Vc)
Yellow crystals; yield 72%; m.p. 241-242°C; IR (KBr) cm-1: 3440 (O-H, str.), 3244 (N-H, str.), 3194 (C-H, aromatic, str.), 2966 and 2925 (C-H, aliphatic, str.), 1616 (C=O, str.), 1599 (C=C, aromatic, str.), 1525 (shouldered, C=C alkene, C=N), 1481(C=C, aromatic, bend); 1HNMR (DMSO-d6): δ 10.86 (s, 1H, NH), 7.80 (s, 1H, -CH=N-), 7.44 (s, 1H, C3-H), 6.80-7.30 (m, 5H, CH2-C6H5), 6.61 (s, 1H, C6-H), 6.05(bs, 1H, OH), 3.75 (s, 3H, NCH3).
5-Hydroxy-1-methyl-4-oxo-1,4-dihydropyridine -2-carbaldehyde oxime (Vd)
White crystals; yield 85%; m.p. 245-246°C; IR (KBr) cm-1: 3446 (O-H, str.), 2956 (C-H, aliphatic, str.), 1541 (shouldered, C=C alkene, C=N); 1HNMR (DMSO-d6): δ 8.24 (s, 1H, -CH=N-), 7.49 (s, 1H, C6-H), 6.59 (s, 1H, C3-H), 4.20-5.50 (bs, 1H, OH), 3.71 (s, 3H, NCH3).
5-Hydroxy-2-[(2-hydroxyphenylamino)methyl] pyridin-4(1H)-one (Ve)
Pale yellow crystals; yield 82%; m.p. 255-256°C; IR (KBr) cm-1: 3396 (N-H, str.), 3286 (N-H, str.), 3070 (C-H, aromatic, str.), 2850, 2727 (C-H, aliphatic, str.), 1608 (C=O, str.), 1556, 1523 (C=C, aromaic); 1HNMR (DMSO-d6): δ 11.10 (s, 1H, N1-H), 7.27 (s, 1H, C6-H), 6.67 (d, J= 7.60 Hz, 1H, C2’-H), 6.563(t, J= 7.60 Hz, 1H, C4’-H), 6.423(t, J= 7.60 Hz, 1H, C3’-H), 6.345(d, J= 7.60 Hz, 1H, C5’-H), 6.16 (s, 1H, C3-H), 5.41 (bs, 1H, NH-CH2), 4.15(d, J= 5.20 Hz, 2H, NH-CH2). ESI-MS (+) m/z (%): 231.1 (M + H+) (100).
5-Hydroxy -2-[(2-hydroxyphenylamino)methyl] -1-methylpyridin-4(1H)-one (Vf)
Pale yellow crystals; yield 85%; m.p. 244-245°C; IR (KBr) cm-1: 3387 (O-H, str.), 3157 (N-H, str.), 3044 (C-H, aromatic, str.), 2950, 2930 (C-H, aliphatic, str.), 1597 (C=O, str.), 1556 (C=N), 1443(C=C, aromatic, bend); 1HNMR (DMSO-d6): δ 7.42 (s, 1H, C6-H), 6.44-6.72 (m, 4H, NH-C6H4-OH), 6.14 (s, 1H, C3-H), 5.25 (bs, 1H, NH-CH2), 4.27 (d, J= 2.80 Hz, 2H, NH-CH2), 3.67 (s, 3H, NCH3).
Antioxidant evaluation
The antioxidant potential of the synthesized compounds was assessed using two different methodologies, namely DPPH free radical and hydrogen peroxide scavenging assays.
DPPH free radical scavenging assay
DPPH scavenging evaluation is a standard assay in antioxidant activity studies and offers a rapid technique for screening the radical scavenging activity of specific compounds. This is a common antioxidant assay method which determines the ability of the compound to react with a free radical to give hydrogen to it.
A freshly prepared methanolic solution of DPPH has a deep purple color with an absorption maximum at 517 nm. This color generally disappears when an antioxidant is present in the medium. Thus, antioxidant molecules can react with DPPH free radicals (by providing hydrogen atoms or by electron donation via a free radical attack on this molecule) and convert them to colorless/ odt bleached product(20,21).
The DPPH free radical scavenging activity of methanolic solutions of 5-hydroxy-pyridine-4-one derivatives Va-f were examined and compared in Table 1 and Fig. 3.
Table 1.
IC50 values for DPPH scavenging ability of the compounds Va-f

Fig. 3.
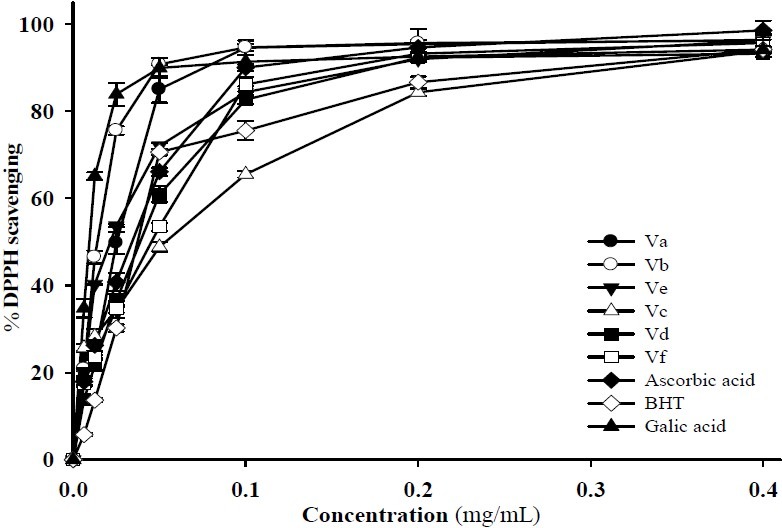
% DPPH scavenging activity of the compounds Va-f (0.00625-0.4 mg/ml)
From analysis of Table 1 and Fig. 3, we can conclude that compounds with no substituent at N1 atom of the pyridinone ring (Va, Vb and Ve) exhibited more scavenging effects on DPPH radicals compared with compounds having methyl substituent on this atom. Among them compound Va (IC50=0.013 mg/ml) was the most potent one.
Hydrogen peroxide scavenging assay
The method provided by Ruch and coworkers which is based on the ability of a compound to convert hydrogen peroxide to water was used for determination of H2O2 scavenging power. Hydrogen peroxide is a weak oxidizing agent capable of oxidizing the essential thiol (-SH) groups of proteins, thus inactivating a few enzymes. It rapidly passes through the cell membranes and inside the cell reacts with Fe2+ to form hydroxyl radical which exerts several toxic effects(22). Thus, it is advantageous for cells to control the amount of hydrogen peroxide to prevent oxidative stress conditions.
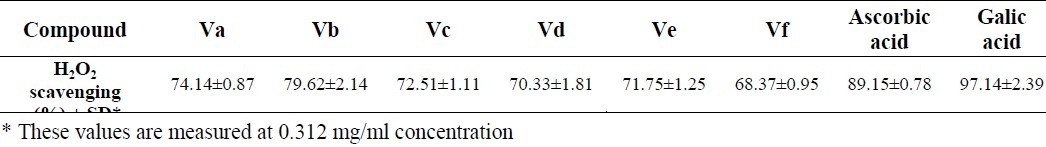
The values for hydrogen peroxide scavenging power of compounds Va-f are provided in Table 2. It is understood from this table that Vb was the most potent compound in this assay.
Table 2.
H2O2 scavenging activity values for the compounds Va-f
Iron chelation ability
Ferrous ion can initiate biological damages by the Fenton reaction. Inasmuch as H2O2, by itself, is a poorly reactive oxidant, it participates in the direct formation of the hydroxyl radicals in the presence of free iron ions; as it can be explained by Fenton reaction as follows:
![]()
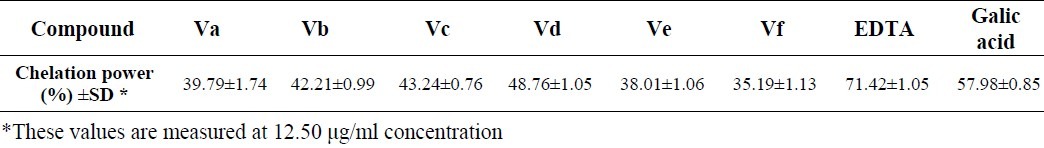
Ferrozine forms red colored complexes with ferrous ions. When chelating agents is present in the medium this complex formation is interrupted and this leads to the decrease of the red color. Measuring the amount of color reduction allows determining the chelating effect of the coexisting compounds. The formation of the ferrozine-Fe2+ complex was interrupted in the presence of Va-f, indicating their chelating activity. The results of this assay are provided in Table 3. Ferrous iron chelating agents form coordinate bonds with Fe2+; which reduces the redox potential of this cation, and thereby stabilizes the oxidized form of Fe2+. This property renders iron chelators effective secondary antioxidant ability(23).
Table 3.
Fe2+ chelating power for the compounds Va-f
DISCUSSION
The antioxidant potency of the compounds can be explained according to the differences in their chemical structures after categorizing them in two chemical classes. Three of the synthesized compounds contain N1-H hydroxypyridinone ring and the other ones have a methyl group substituted on the nitrogen atom of the ring. In both antioxidant assays performed in this research, there was a dominant difference between the results for these two groups. Compounds with N1-H show more antioxidant potency than those with N1-CH3 moiety. The proofs to explain this phenomenon can be provided considering the mechanism of DPPH free radical scavenging assay provided in Fig. 4.
Fig. 4.
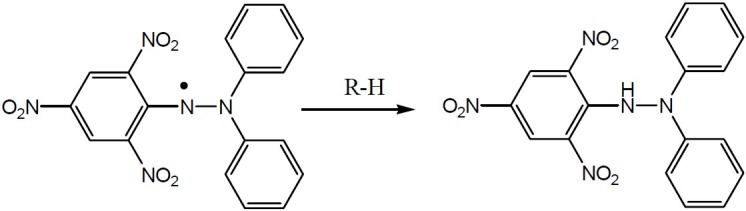
DPPH reaction with an antioxidant
The hydrogen abstracted from R-H in Fig. 4 is a labile hydrogen and the most potent compounds in this assay (Va,b,e) possess several hydrogens of this type. A common hydrogen in these compounds is the N1-H of hydroxypyridinone ring which is absent in the less active compounds (Vc, Vd, and Vf). Compound Vb was proved to be the most potent compound in hydrogen peroxide scavenging assay. All the synthesized compounds proved to have very close chelating ability, and compounds containing N1-CH3 hydroxypyridinone ring were stronger chelating agents. The reason was the inductive effect of the methyl group which pushes more electrons to the oxygen attached to the C-4 position of the ring making it a good ligand for iron chelation. The other reason is the fact that N1-H derivatives loose a proton which results in a neutral molecule with a hydroxyl group, instead of a negative oxygen, as it is formed in N1-CH3 derivatives, in the C-4 position of the hydroxypyridinone ring. The latter case performs definitely better in iron chelation.
CONCLUSION
In sum, the ortho-hydroxypyridinones prepared in this research seem to be promising antioxidant iron chelator agents for in vitro studies of oxidative stress conditions.
ACKNOWLEDGMENT
This research was performed by the financial support of Pharmaceutical Research Center, Isfahan University of Medical Sciences, Isfahan, Iran.
REFERENCES
- 1.Guelman LR, Pagotto RM, Di Toro CG, Zieher LM. Deferoxamine antioxidant activity on cerebellar granule cells γ-irradiated in vitro. Neurotoxicol Teratol. 2004;26:477–483. doi: 10.1016/j.ntt.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 2.Sjödin B, Hellsten-Westing Y, Apple FS. Biochemical mechanisms for oxygen free radical formation during exercise. Sports Med. 1990;10:236–254. doi: 10.2165/00007256-199010040-00003. [DOI] [PubMed] [Google Scholar]
- 3.Reeder BJ, Hider RC, Wilson MT. Iron chelators can protect against oxidative stress through ferryl heme reduction. Free Radic Biol Med. 2004;44:264–273. doi: 10.1016/j.freeradbiomed.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 4.Loganathan JK, Gunasundari D, Hemalatha M, Shenbhagaram R, Kaviyarasan V. Antioxidant and phytochemical potential of wild edible mushroom Termitomyces reticulatus: individual cap and stipe collected from eastern part of India. IJPSR. 2010;1:62–72. [Google Scholar]
- 5.Palmer C. New York: Plenum Press; 1997. Metals and Oxidative Damage in Neurological Disorders; pp. 205–236. [Google Scholar]
- 6.Hider RC. Potential protection from toxicity by oral iron chelators. Toxicol Lett. 1995;82/83:961–967. doi: 10.1016/0378-4274(95)03606-7. [DOI] [PubMed] [Google Scholar]
- 7.van der Kraaij AMM, van Eijk HG, Koster JF. Prevention of postischemic cardiac injury by the orally active iron chelator 1,2 -Dimethyl-3 -Hydroxy- 4-Pyridone (L1) and the antioxidant (+)-cyanidanol-3. Circulation. 1989;80:158–164. doi: 10.1161/01.cir.80.1.158. [DOI] [PubMed] [Google Scholar]
- 8.Velasco-Sánchez D, Aracil A, Montero R, Mas A, Jiménez L, O’Callaghan M, et al. Combined therapy with idebenone and deferiprone in patients with Friedreich's ataxia. Cerebellum. 2011;10:1–8. doi: 10.1007/s12311-010-0212-7. [DOI] [PubMed] [Google Scholar]
- 9.van Acker SA, van Balen GP, van den Berg DJ, Bast A, van der Vijgh WJ. Influence of iron chelation on the antioxidant activity of flavonoids. Biochem Pharmacol. 1998;56:935–943. doi: 10.1016/s0006-2952(98)00102-6. [DOI] [PubMed] [Google Scholar]
- 10.Zheng H, Weiner LM, Bar-Am O. Design, synthesis, and evaluation of novel bifunctional iron-chelators as potential agents for neuroprotec-tion in Alzheimer's, Parkinson's, and other neurodegenerative diseases. Bioorg Med Chem. 2005;13:773–783. doi: 10.1016/j.bmc.2004.10.037. [DOI] [PubMed] [Google Scholar]
- 11.Kayyali R, Pannala AS, Khodr H, Hider RC. Comparative radical scavenging ability of bidentate iron (III) chelators. Biochem Pharmacol. 1998;55:1327–1332. doi: 10.1016/s0006-2952(97)00602-3. [DOI] [PubMed] [Google Scholar]
- 12.David B, Nat M, Suneel G, Alan P, Richard P, Craig M. Ortho-hydroxypyridinone derivatives as iron chelating and antioxidant agents. PAT-WO9923075. 1999 [Google Scholar]
- 13.Schugar H, Green DE, Bowen ML, Scott LE, Storr T, Böhmerle K, et al. Combating Alzheimer's disease with multifunctional molecules designed for metal passivation. Angew Chem. 2007;119:1746–1748. doi: 10.1002/anie.200603866. [DOI] [PubMed] [Google Scholar]
- 14.Streater M, Taylor PD, Hider RC, Porter J. Novel 3-hydroxy-2(1H)-pyridinones. Synthesis, iron (III)-chelating properties and biological activity. J Med Chem. 1990;33:1749–1755. doi: 10.1021/jm00168a033. [DOI] [PubMed] [Google Scholar]
- 15.Ma YM, Luo W, Quinn PJ, Zudong L, Hider RC. Design, synthesis, physicochemical properties, and evaluation of novel iron chelators with fluorescent sensors. J Med Chem. 2004;47:6349–6362. doi: 10.1021/jm049751s. [DOI] [PubMed] [Google Scholar]
- 16.Becker HD. The conversion of kojic acid into comenaldehyde and comenic acid. Acta Chem Scand. 1962;16:78–82. [Google Scholar]
- 17.Blois MS. Antioxidant determinations by the use of a stable free radical. Nature. 1958;181:1199–1200. [Google Scholar]
- 18.Ruch RJ, Cheng SJ, Klaunig JE. Prevention of cytotoxicity and inhibition of intracellular communication by antioxidant catechins isolated from Chinese green tea. Carcinogenesis. 1989;10:1003–1008. doi: 10.1093/carcin/10.6.1003. [DOI] [PubMed] [Google Scholar]
- 19.Dinis TCP, Maderia VMC, Almeida LM. Action of phenolic derivatives (acetaminophen, salicylate and 5-Amino salycilate) as inhibitor of membrane lipid peroxidation and as peroxyl radical scavengers. Arch Bichem Biophys. 1994;315:161–169. doi: 10.1006/abbi.1994.1485. [DOI] [PubMed] [Google Scholar]
- 20.Amarowicz R, Pegg RB, Rahimi-Moghaddam P, Barl B, Weil JA. Free-radical scavenging capacity and antioxidant activity of selected plant species from the Canadian prairies. Food Chemistry. 2004;84:551–562. [Google Scholar]
- 21.Siddhuraju P, Becker K. The antioxidant and free radical scavenging activities of processed cowpea (Vigna Unguiculata (L.) Walp.) seed extracts. Food Chemistry. 2007;101:10–19. [Google Scholar]
- 22.Halliwell B, Gutteridge JMC. 4th ed. Oxford, Clarendon Press; 2006. Free Radicals in Biology and Medicine; pp. 419–420. [Google Scholar]
- 23.Gordon MH. The mechanism of antioxidant action in vitro. In: Hudson B.J.F, editor. Food Antioxidants. London: Elsevier Applied Science; 1990. pp. 1–18. [Google Scholar]


