Abstract
Standardization of induction of oxidative stress with Fenton mixture (FM) in isolated perfused rat kidney and the antioxidant effect of Terminalia arjuna bark in the isolated oxidatively stressed rat kidney has been evaluated. Six groups each containing eight isolated perfused rat kidneys were used for the present study and the oxidative stress was induced by perfusing the isolated kidneys with FM. The antioxidant effect of Terminalia arjuna at the dose of 250 and 500 mg/kg was evaluated in oxidative stress induced isolated kidneys. A significant (P<0.05) increase in lipid peroxidation, gluatamate pyruvate transaminase, glutamate oxaloacetate transaminase were observed in oxidative stress induced isolated kidney. On perfusion with extract, the oxidative stress was decreased with increasing in antioxidants while the marker enzymes were found to maintain the normal level. It was concluded from the present study that hydroalcholic extract of Terminalia arjuna bark at the dose of 250 and 500 mg/kg showed significant antioxidant potential in isolated perfused rat kidneys.
Keywords: Terminalia arjuna, Fenton mixture, Rat, Antioxidant, Isolated perfused kidney
INTRODUCTION
Antioxidants are micronutrients that have gained importance in recent years due to their ability to neutralize free radicals or their actions. Free radicals have been implicated in the etiology of several major human ailments, including cancer, cardiovascular diseases, neural disorders, diabetes and arthritis(1). Due to the recent trends towards the development of healthy foods in the form of functional foods’, one of the desirable properties in a dietary component is considered to be of its antioxidant effect(2).
Oxygen radicals in the body are reactive, short lived and play a role in most major health problems. A number of radicals are formed in biological systems via a range of different processes. The oxygen containing radicals involved in oxidative stress together with some non radical species are collectively called reactive oxygen species (ROS) which are often toxic intermediates in different metabolic processes. Examples of ROS known to induce damages in vivo are H2O2, organic hydroperoxides, hypochlorous acid, nitric oxide radical, superoxide radical, alkoxyl radicals and the hydroxyl radical. ROS can be derived from numerous sources in vivo including normal respiration, photochemical reactions and enzymatic reactions. A large number of enzymes have been shown to be capable of generating ROS which include the cytochromes P450, various oxidases, peroxidases, lipogenases and dehydrogenases(3).
The free radicals react with cell membrane lipids causing oxidative destruction, initiate lipid peroxidation in renal tissues and ultimately cause nephrotoxicity. The antioxidant enzymes protect the cells from risky effects of free radicals. The increased activities of antioxidant enzymes are known to produce protective responses by scavenging free radicals in renal tissues(4,5). One of the most hazardous radicals is the extremely reactive hydroxyl radical, with an almost diffusion limited half life of about one nanosecond and has been shown to play a major role in a number of diseases. Oxidative stress induced by hydroxyl radicals is believed to be the main cause of damage to the neurons in Parkinson's disease. Oxidation of proteins and enzymes may contribute to the buildup of glutamate in stroke. The reaction of hydroxyl radicals with nucleic acids causes strand breaks and alters bases and thereby, contributes to carcinogenesis. Accumulation of oxidized proteins and enzymes has also been observed during the process of aging(6).
In the present study, the main source of in vivo hydroxyl radicals is probably generated from the so called Haber-Weiss reaction, which initiates the Fenton reaction between Fe2+ and hydrogen peroxide(3). Hydroxyl radicals are generated as Fe2+ donates an electron to hydrogen peroxide. The iron is oxidized to Fe3+ and becomes inactive for further reaction. In a chemical Fenton system, a reducing agent such as ascorbic acid, is usually added to regenerate Fe2+(7).
Terminalia arjuna (Combretaceae family) is a deciduous and evergreen tree, standing 20-30 m above ground level(8). It is found in abundance throughout Indo-sub-Himalayan tracts of Uttar Pradesh, South Bihar, Madhya Pradesh, Delhi and Deccan region near ponds and rivers(9). Among different species of Terminalia, the bark of Terminalia arjuna has its own characteristic features (10). The therapeutic use of Terminalia arjuna for cardiac ailments was based on empirical observations recorded in various treatises of ancient medicine. Several late publications regarding its potential antimutagenic properties have also been reported(11). Terminalia arjuna bark powder also has hypolipidaemic effect(12).
The efficacy of Terminalia arjuna as an anti-ischemic agent and as a potent antioxidant preventing LDL cholesterol oxidation and reperfusion ischemic injury to the heart, and its potential to reduce atherogenic lipid levels have been amply demonstrated in various experimental and clinical studies(13–18).
In the present study, the evaluation of the antioxidant effect of Terminalia arjuna bark in isolated perfused rat kidney is attempted which is the first attempt to investigate the antioxidant activity of Terminalia arjuna in isolated perfused rat kidney.
MATERIALS AND METHODS
Collection and identification of Terminalia arjuna bark
Bark of Terminalia arjuna was collected from Thovalai, Kanyakumari District, Tamilnadu State, India. The plant material was identified at Centre for Advanced Research in Indian System of Medicine (CARISM), SASTRA University, Thanjavur, Tamilnadu, India. The specimen of the plant material is also maintained in the same Department for future reference. The Voucher number of the specimen is 0094.
Extraction of plant material
The collected bark was dried under shade for one week and coarsely powdered. 100 g of the coarsely powdered of raw material was soaked in 1000 ml of ethanol: water (70:30) for 7 days. The extract was filtered and the solvent was removed by distillation. The final trace of solvent was removed by rotary vacuum evaporator. The yield of extract was calculated as 27.7% w/w. The concentrated residue was used for the following experiments. The extract was dissolved in physiological salt solution (PSS).
Screening of qualitative and quantitative phytochemical of components
The qualitative analysis of hydroalcholic extract of Terminalia arjuna bark were performed(19). The quantitative estimation of total phenolics(20), total tannins(20), and proteins were carried out by Folin-Ciocalteau, Folin-Danis, and Folin-Ciocalteau methods, respectively(21).
Vitamin C, vitamin E, and total carbohydrate were respectively analysed by DNPH(22), Dipyridyl(23), and Anthrone methods(24).
Perfusion apparatus
In this system the isolated kidney was suspended in a 2×20 cm (internal dimensions) water-jacketed chamber with a coarse sintered glass filter disk sealed into the lower portion. A mixture of moistened O2:CO2 (95:5) was delivered by small diameter tubing to the lower portion of the chamber by the aerator.
An 18-gauge hypodermic needle, approximately 2.5 cm long, was used as the arterial cannula. The perforate entered the renal circulation by way of the cannulated renal artery and on leaving the kidney, flowed down the surface of the chamber and through the filter. Satisfactory pressure and flow rate were obtained in the subsequent perfusion by making a preliminary adjustment of the reservoir to produce appropriate pressure. This device permits perfusion by gravity flow. This chamber was at a height of approximately 76 cm above the kidney(25,26).
Perfusate composition
A modified Krebs-Henseleit bicarbonate buffer(27) (pH 7.4; temperature, 38°C) was prepared to obtain the following final concentrations: 118 mM sodium chloride, 4.7 mM potassium chloride, 2.4 mM calcium chloride, 1.2 mM potassium phosphate, 0.5 mM calcium-EDTA, 11 mM glucose, 25 mM sodium hydrogen carbonate, and 1.2 mM magnesium sulfate. The perfusion medium was filtered successively through filters, prior to use and was equilibrated with mixture of O2: CO2 (95:5) at 37°C for 1 h before and throughout the perfusion.
Preparation of Fenton mixture (FM)
The method of preparation of FM is similar to that reported earlier(28). About 13.9 mg FeSO4.7 H2O, 75 mg of sodium EDTA, and 30 μl of 50% H2O2 were added to 10 ml of 0.1 M dipotassium hydrogen orthophosphate solution and the reaction mixture was kept in a water bath at 40°C for 20 min. By continuous stirring, the solution was used as a source of hydroxyl free radical.
Operative procedure
All the animal experiments were performed after getting clearance from Institutional Animal Ethical Commitee (IAEC Approval No. 6/SASTRA/IAEC/RPP). Albino Wistar rats weighing 350-450 g were anaesthetized by an intraperitoneal injection of sodium pentobarbitone (40 mg/kg). A midline laparotomy incision was made from pelvis to sternum on the animal. The right kidney was used for perfusion, because the mesenteric artery arises from the aorta at the same level as the right renal artery and a cannula can be passed from one to the other without blood loss and without stopping the blood flow to the kidney.
To expose the major abdominal vessels and the right kidney, fat and peri-vascular tissues were cleared away by blunt dissection. The adrenal branch of the right renal artery was tied and loose ligatures were placed around the following blood vessels: inferior vena cava, just below the liver; aorta, above the mesenteric artery; mesenteric artery, near the aorta; mesenteric artery, further from the aorta; right renal artery, at its origin from the aorta; inferior vena cava, between the left and right renal vein; inferior vena cava, below the left renal vein; inferior vena cava, more distally still and left renal vein. Then, heparin (2 ml, 200 units) was injected into the lower inferior vena cava; afterwards the opening in the wall of the vein was then closed by means of a ligature passed over the point of the injecting needle.
Cannulation
The right renal artery was cannulated via the mesenteric artery. An 18-gauge hypodermic needle, approximately 2.5 cm long was used as the arterial cannula. The end was slightly beveled, with the edges filed to produce a smooth tip. As the cannula was pushed into the renal artery, a hemostat holding back washout perfusate was released and perfusion was begun; there was immediate clearing of blood from the kidney. The inferior vena cava was rapidly cut-off and the kidney was completely freed from the animal. The kidney was trimmed of adhering tissue and after turning the stopcock to permit circulation of the perfusate; the kidney was suspended within a thermostatically controlled cabinet at 37°C.
Grouping of kidneys
The kidneys were randomly assigned to the following treatment groups:
Group I – Normal - 15 ml of PSS + 50 ml of PSS + 50 ml of PSS
Group II – Control - 15 ml of PSS + 50 ml of FM + 50 ml of PSS
Group III – 15 ml of PSS + 50 ml of FM + 50 ml of drug solution (250 mg/kg)
Group IV – 15 ml of PSS + 50 ml of FM + 50 ml of drug solution (500 mg/kg)
Group V - 15 ml of PSS + 50 ml of PSS + 50 ml of drug solution (250 mg/kg)
Group VI - 15 ml of PSS + 50 ml of PSS + 50 ml of drug solution (500 mg/kg)
Biochemical parameters
After completion of perfusion, the kidneys were homogenized in ice cold 0.1 M 10% Tris HCl buffer (pH 7.4). The homogenate was centrifuged at 4°C at 1500 rpm for 10 min. The supernatant was used for the estimation of lipid peroxidation(29), catalase (30), glutathione peroxidase(31), reduced glutathione(32), protein(21), glutamate pyruvate transaminase(33), glutamate oxaloacetate transaminase(34).
Statistical analysis
Statistical analysis was carried out using SPSS software version 12.0. Bars in the graphs are mean ± SD. Significant difference was evaluated using One Way ANOVA with Duncan multiple range test (DMRT). P<0.05 was considered as significant difference.
RESULTS
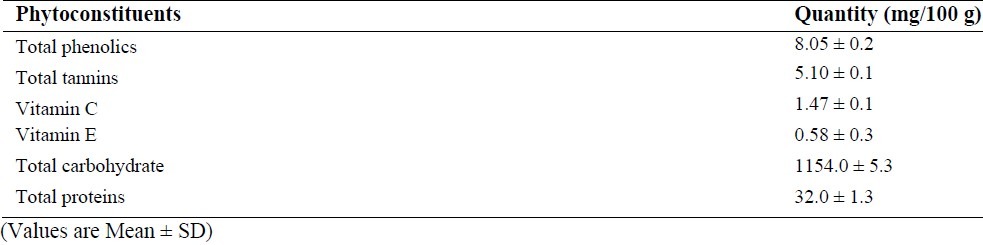
The qualitative phytochemical analysis showed the presence of alkaloids, carbohydrates, glycosides, flavonoids, tannins and saponins. The quantitative estimation of total phenolics, tannins, vitamin C and vitamin E was found to be 8.05, 5.10, 1.47 and 0.58 mg /100 g respectively. In our study, we have observed increased levels of thiobarbituric acid reactive substance (TBARS) in fenton perfused kidneys (Group II). The decrease in the activities of antioxidant enzymes is in close relationship with the induction of lipid peroxidation(35). But there is a consistent dip in the level of TBARS (P<0.05, Fig. 1) in drug perfused kidneys showing the antioxidant effect of Terminalia arjuna bark extract. The decreased TBARS level of Terminalia arjuna is reported earlier(36). Catalase level was found to be decreased significantly (P<0.05, Fig. 2) in 250 and 500 mg/kg drug perfused kidneys against oxidative stressed kidneys. The levels of antioxidant enzymes such as glutathione and glutathione peroxidase are markedly decreased (P<0.05) in FM treated group when compared to normal. But these levels are significantly increased (P<0.05) in kidneys perfused with Terminalia arjuna extract at the dose 500 mg/kg against oxidative stress induced kidneys (P<0.05, Figs. 3 and 4). This might be due to the antioxidant activity of Terminalia arjuna bark. In Group VI kidney GSH level is found to be increased against normal kidney. This result further furnishes the antioxidant activity of Terminalia arjuna. The increased level of GSH by Terminalia arjuna is reported by Gauthaman and coworkers(37). Significant difference has not been observed in Group III against Group II kidneys. In our study, both GPT and GOT level are found to be increased significantly in fenton perfused kidneys, when compared with normal kidneys and it may be due to the damage caused by the oxidative stress. This change is completely recovered by the drug of both doses by decreasing these levels (Figs. 5 and 6). The phytochemical constituents detected in the plant materials (Table 1) could be responsible for their antioxidant activity though their exact mode of action for its renoprotective is poorly understood be responsible for their antioxidant activity though their exact mode of action for its renoprotective is poorly understood.
Fig. 1.
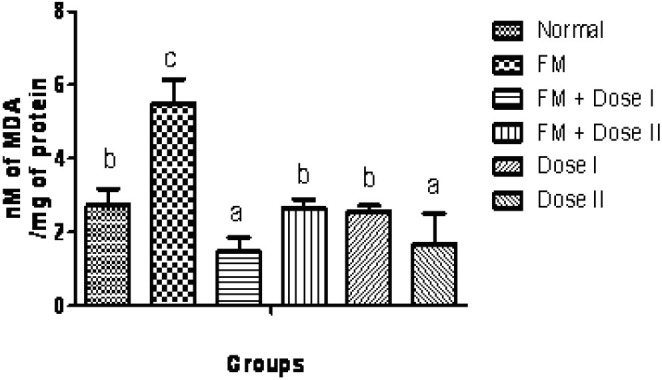
Effect of Terminalia arjuna bark extract on TBARS level in isolated perfused kidney. n=8, Bars not sharing common superscript differ significantly at P<0.05
Fig. 2.
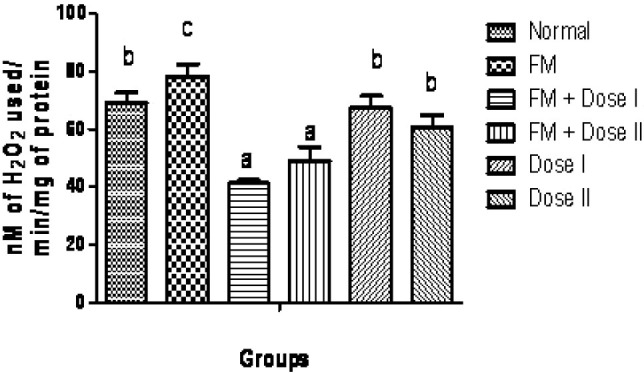
Effect of Terminalia arjuna bark extract on catalase level in isolated perfused kidney. n=8, Bars not sharing common superscript differ significantly at P<0.05
Fig. 3.
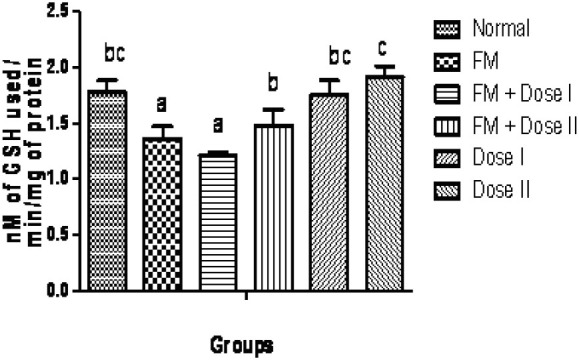
Effect of Terminalia arjuna bark extract on glutathione peroxidase level in isolated perfused kidney. n=8, Bars not sharing common superscript differ significantly at P<0.05
Fig. 4.
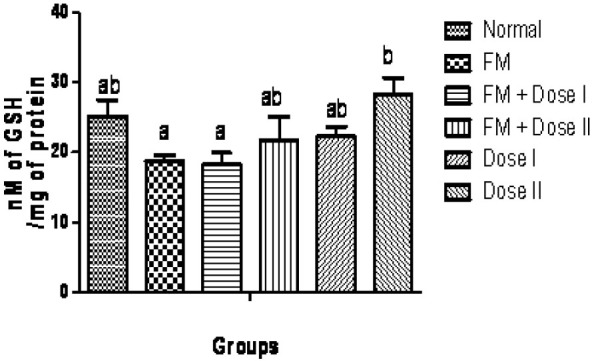
Effect of Terminalia arjuna bark extract on glutathione level in isolated perfused kidney. n=8, Bars not sharing common superscript differ significantly at P<0.05
Fig. 5.
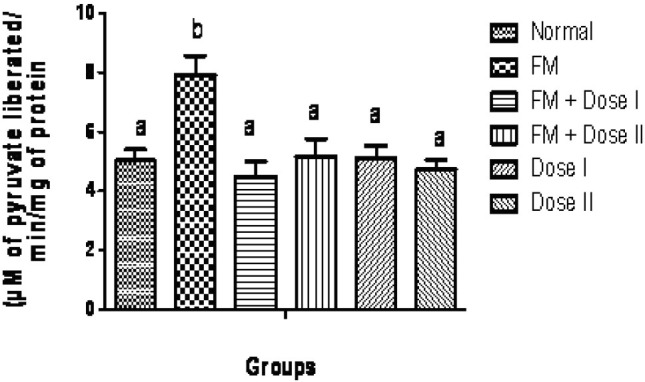
Effect of Terminalia arjuna bark extract on glutamate pyruvate transaminase level in isolated perfused kidney. n=8, Bars not sharing common superscript differ significantly at P<0.05
Fig. 6.
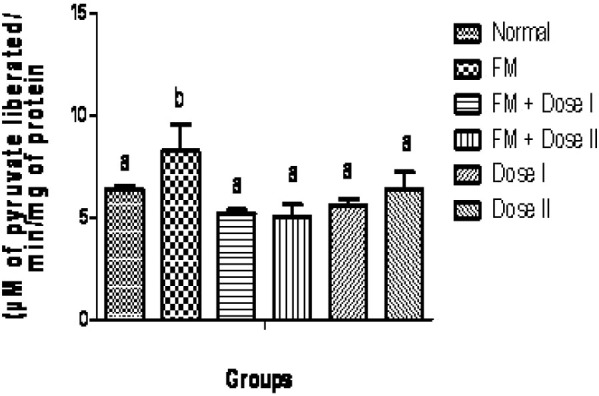
Effect of Terminalia arjuna barks on glutamate oxaloacetate transaminase level in isolated perfused kidney. n=8, Bars not sharing common superscript differ significantly at P<0.05
Table 1.
Quantitative estimation of phyto-constituents in hydroalcholic bark extract of Terminalia arjuna
DISCUSSION
ROS may attack any type of molecules, but their main target appears to be polyunsaturated fatty acids, which is the precursor of lipid peroxide formation(34). The formation of ROS can oxidize biological macromolecules such as DNA, proteins, lipids and carbohydrates. To circumvent this injure, living organisms developed antioxidant defense systems consisting of ROS catabolizing enzymes and antioxidants. Oxidative stress can be defined as the pathogenic result of an oxidant creation that devastates the endogenous antioxidant defense system and this condition has been linked to the cause and development of chronic diseases(38).
Iron an important catalyst with its labile electron system, is well suited to catalyze redox reactions(39). The combination of H2O2 and a ferrous salt made use of Fenton chemistry is considered to be the major mechanism by which the highly reactive hydroxyl radical is generated in biological systems(40). Hydroxy radical, a strong electrophilic compound, can easily react with the double bond of unsaturated lipids and propagate the chain reaction of lipid auto-oxidation(41). Significant difference in GSH level has not been observed in low-dose treated kidneys when compared with FM infused kidney and this might be due to the utilization of GSH by kidneys from oxidative stress produced by FM. Pereira and De Oliveira have explained that without the protection from oxidative injury afforded by glutathione, cells might be damaged or killed(42). Likewise, glutathione peroxidase level is also considerably increased in the drug (500 mg/kg ) treated organs against diseased kidney. Glutathione peroxidase level of Group VI animals is found to be increased against normal animals.
This result has further confirmed the antioxidant activity of Terminalia arjuna. The increased activity of Glutathione peroxidase by Terminalia arjuna treatment has been reported earlier by Devi and coworkers(43). This might be due to the utilization of catalase to protect the organ from damage caused by hydrogen peroxide produced from FM. GPT is found exclusive in cytosol of the liver cells and kidney cells. The presence of GPT in plasma is directly related to membrane damage(44). The decreased level of GOT and GPT by Terminalia arjuna is also reported earlier(45).
Polyphenols, the major phytoconstituents present in most of plant species, possess antioxidant property. This activity is supposed to be mainly due to their redox properties(46), which play an important role in scavenging free radicals, quenching singlet and triplet oxygen, or decomposing peroxides. The results obtained in the current study also confirm the presence of these polyphenolic compounds in hydroalcholic extract of Terminalia arjuna bark and its renoprotective property might be attributed to the presence of these valuable phytoconstituents. Although some studies have revealed a correlation between phenolic content and antioxidant capacity(47), but the antioxidant activity could also be due to the presence of other phytoconstituents namely alpha tocopherol, vitamin C and tannin contents and this is evident from our phytochemical analysis. Polyphenol tannic acid belongs to hydrolysable form able to inhibit hydroxyl radical formation or prevent 2-deoxyribose degradation, when compared with iron chelators and classical hydroxyl radical scavenger(48).
Gil and coworkers has also reported earlier that the antioxidant capacity of pomegranate juice is mainly due to the presence of hydrolysable tannins(49). Above all, the antioxidant property of Terminalia arjuna in isolated perfused kidney might also be due to the presence of polyphenolic compounds and is evident from our phytochemical analysis.
CONCLUSION
The present study shows that the bark extract of Terminalia arjuna has a definite modulatory effect on the antioxidant milieu of isolated rat kidney. In conclusion, the pharmacological activities of the Terminalia arjuna barks showed significant antioxidant effects and further pharmacological and biological studies are needed to elucidate the mechanisms of its action.
ACKNOWLEDGMENT
The authors are grateful to Prof. R. Sethuraman, Vice-Chancellor, SASTRA University and his able administrative colleagues for allowing us to complete this work.
REFERENCES
- 1.Devasagayam TPA, Tilak JC, Boloor KK, Sane KS, Ghaskadbi S, Lele RD. Free radicals and antioxidants in human health: current status and future prospects. J Assoc Phys India. 2004;52:794–804. [PubMed] [Google Scholar]
- 2.Peter KV. 1st ed. USA: CRC Press, Boca Raton, FL; 2001. Handbook of Herbs and Spices. [Google Scholar]
- 3.Kehrer JP. The Haber-Weiss reaction and mechanism of toxicity. Toxicology. 2000;149:3–50. doi: 10.1016/s0300-483x(00)00231-6. [DOI] [PubMed] [Google Scholar]
- 4.Rao M, Kumar MM, Rao MA. In vitro and in vivo effects of phenolic antioxidants against cisplatin-induced nephrotoxicity. J Biochem (Tokyo) 1999;125:383–390. doi: 10.1093/oxfordjournals.jbchem.a022298. [DOI] [PubMed] [Google Scholar]
- 5.Sakac V, Sakac M. Free oxygen radicals and kidney diseases-part I. Med Pregl. 2000;53:463–474. [PubMed] [Google Scholar]
- 6.Foley P, Riederer P. Influence of neurotoxins and oxidative stress on the onset and progression of Parkinson's disease. J Neurol. 2000;247:82–94. doi: 10.1007/pl00007766. [DOI] [PubMed] [Google Scholar]
- 7.Ghiselli A. Aromatic Hydroxylation: Salicylic Acid as a Probe for Measuring Hydroxyl Radical Production. In: Armstrong D, editor. Free Radical and Antioxidant Protocols. 1st ed. Vol. 108. Humana Press; 1998. pp. 89–100. [DOI] [PubMed] [Google Scholar]
- 8.Nadkarni AK, Nadkarni KM. 1st ed. Bombay, India: Popular Book Depot; 1908. Indian Materia Medica. [Google Scholar]
- 9.Chopra RN, Chopra IC, Handa KL, Kapur LD. Terminalia arjuna W&A (Combretaceae) In: Chopra RN, Chopra IC, Handa KL, Kapur LD, editors. Chopra's Indigenous Drugs of India. 1st ed. Calcutta, India: UN Dhur & Sons; 1958. pp. 421–423. [Google Scholar]
- 10.Parkinson CE. Indian Terminalias of the Section Pentaptera. Indian Forest Records (New Series) Botany. 1936 [Google Scholar]
- 11.Woodman OL, Chan ECH. Vascular and anti-oxidant actions of flavonols and flavones. Clin Exp Pharmacol Physiol. 2004;31:786–790. doi: 10.1111/j.1440-1681.2004.04072.x. [DOI] [PubMed] [Google Scholar]
- 12.Khanna AK, Chander C, Kapoor NK. Terminalia arjuna: an Ayurvedic cardiotonic regulates lipid metabolism in hyperlipidemic rats. Phytother Res. 1996;10:663–665. [Google Scholar]
- 13.Alan L, Miller ND. Botanical influences on cardiovascular disease. Altern Med Rev. 1998;3:422–431. [PubMed] [Google Scholar]
- 14.Dwivedi S, Agarwal MP. Antianginal and cardioprotective effects of Terminalia arjuna, an indigenous drug, in coronary artery disease. J Assoc Physicians India. 1994;42:287–289. [PubMed] [Google Scholar]
- 15.Bharani A, Ganguly A, Bhargave KD. Salutary effect of Terminalia arjuna in patients with severe refractory heart failure. Int J Cardiol. 1995;49:191–199. doi: 10.1016/0167-5273(95)02320-v. [DOI] [PubMed] [Google Scholar]
- 16.Dwivedi S, Jauhari R. Beneficial effects of Terminalia arjuna in coronary artery disease. Indian Heart J. 1997;49:507–510. [PubMed] [Google Scholar]
- 17.Ram A, Lauria P, Gupta R, Kumar P, Sharma VN. Hypocholesterolaemic effects of Terminalia arjuna tree bark. J Ethnopharmacol. 1997;55:165–169. doi: 10.1016/s0378-8741(96)01493-6. [DOI] [PubMed] [Google Scholar]
- 18.Khanna AK, Ramesh C, Kapoor NK. Terminalia arjuna: an Ayurvedic cardiotonic regulates lipid metabolism in hyperlipidaemic rats. Phytotherapy Res. 1996;10:663–665. [Google Scholar]
- 19.Harborne JB. 3rd ed. London: Chapman and Hall; 1998. Phytochemcial methods: A Guide to Modern Techniques of Plant Analysis. [Google Scholar]
- 20.Bray HG, Thorpe WV. Analysis of phenolic compounds of interest in metabolism. Meth Biochem Anal. 1954;1:27–52. doi: 10.1002/9780470110171.ch2. [DOI] [PubMed] [Google Scholar]
- 21.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin-phenol reagent. J Biol Chem. 1951;193:265–75. [PubMed] [Google Scholar]
- 22.Khan MM, Rahman MM, Murad ATM, Begum SA. Determination of vitamin C content in various fruits and vegetables by UV-spectrophotometric method at Sylhet area, Bangladesh. J Environ Sci. 2005;11:190–193. [Google Scholar]
- 23.Baker H, Frank O. 1st ed. Wiley, New York: 1968. Clinical Vitaminology: Methods and Interpretation. [Google Scholar]
- 24.Yemm EW, Willis AJ. The estimation of carbohydrates in plant extracts by anthrone. Biochem J. 1954;57:769–773. doi: 10.1042/bj0570508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morgan HE, Henderson MJ, Regen DM, Park CR. Regulation of glucose uptake in muscle. J Biol Chem. 1961;236:253–261. [PubMed] [Google Scholar]
- 26.Gayathri K, David Raj C, Jipnomon J, Dhevi R, Mohamed Shabi M, Subashini U, et al. Antioxidant activity of Nelumbo nucifera (Gaertn) flowers in isolated perfused rat kidney. Rev Bras Farmacogn. 2009;19:224–229. [Google Scholar]
- 27.Nishiitsutsuji-uwo JM, Ross BD, Krebs HA. Metabolic activities of the isolated perfused rat kidney. Biochem J. 1967;103:852–862. doi: 10.1042/bj1030852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Venkatesham A, Sharath Babu P, Vidya Sagar J, Krishna R. Effect of reactive oxygen species on cholinergic receptor function. Indian J Pharmacol. 2005;37:366–370. [Google Scholar]
- 29.Okhawa H, Oohishi N, Yagi N. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Annu Rev Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 30.Sinha AK. Colorimetric assay of catalase. Anal Biochem. 1972;47:389–394. doi: 10.1016/0003-2697(72)90132-7. [DOI] [PubMed] [Google Scholar]
- 31.Wendel A. Glutathione peroxidase. Meths Enzymol. 1981;77:325–333. doi: 10.1016/s0076-6879(81)77046-0. [DOI] [PubMed] [Google Scholar]
- 32.Ellman GL. Tissue sulphydryl groups. Arch Biochem Biophys. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 33.Reitman S, Frankel S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol. 1957;28:56–63. doi: 10.1093/ajcp/28.1.56. [DOI] [PubMed] [Google Scholar]
- 34.Gutteridge JMC. Free radicals damage to lipids, amino acids, carbohydrates and nucleic acids, determined by TBA reactivity. Int J Biochem. 1982;14:649–654. doi: 10.1016/0020-711x(82)90050-7. [DOI] [PubMed] [Google Scholar]
- 35.Jagetia GC, Rajanikant GK, Rao SK, Baliga MS. Alteration in glutathione, glutathione peroxidase, superoxide dismutase, and lipid peroxidation by ascorbic acid in the skin of mice exposed to fractionated gamma radiation. Clin Chim Acta. 2003;332:111–121. doi: 10.1016/s0009-8981(03)00132-3. [DOI] [PubMed] [Google Scholar]
- 36.Karthikeyan K, Sarala Bai BR, Gauthaman K, Sathish KS, Niranjali Devaraj S. Cardioprotective effect of the alcoholic extract of Terminalia arjuna bark in an in vivo model of myocardial ischemic reperfusion injury. Life Sci. 2003;73:2727–2739. doi: 10.1016/s0024-3205(03)00671-4. [DOI] [PubMed] [Google Scholar]
- 37.Gauthaman K, Maulik M, Kumari R, Manchanda SC, Dinda AK, Maulik SK. Effect of chronic treatment with bark of Terminalia arjuna: a study on the isolated ischemic-reperfused rat heart. J Ethnopharmacol. 2001;75:197–201. doi: 10.1016/s0378-8741(01)00183-0. [DOI] [PubMed] [Google Scholar]
- 38.Droge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 39.Kanner J, Hazan B, Doll L. Catalytic “Free” Iron Ions in Muscle Foods. J Agri Food Chem. 1988;6:412–415. doi: 10.1007/978-1-4684-5568-7_45. [DOI] [PubMed] [Google Scholar]
- 40.Halliwell B, Gutteridge JMC. The chemistry of free radicals and related ‘reactive species’. In: Halliwell B, Gutteridge J.M.C, editors. Free Radicals in Biology and Medicine. 3rd ed. Oxford: Oxford University Press; 1999. pp. 36–100. [Google Scholar]
- 41.Choe E, Min DB. Chemistry and reactions of reactive oxygen species in foods. Crit Rev Food Sci Nutr. 2006;46:1–22. doi: 10.1080/10408390500455474. [DOI] [PubMed] [Google Scholar]
- 42.Pereira CF, De Oliveira CR. Oxidative glutamate toxicity involves mitochondrial dysfunction and perturbation of intracellular Ca2+ homeostasis. J Neurosci Res. 2000;37:227–236. doi: 10.1016/s0168-0102(00)00124-3. [DOI] [PubMed] [Google Scholar]
- 43.Devi RS, Narayan S, Vani G, Shyamala Devi CS. Gastroprotective effect of Terminalia arjuna bark on diclofenac sodium induced gastric ulcer. Chem Biol Interact. 2007;167:71–83. doi: 10.1016/j.cbi.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 44.Pari L, Karthikesan K. Protective role of caffeic acid against alcohol-induced biochemical changes in rats. Fundam Clin Pharm. 2007;21:355–361. doi: 10.1111/j.1472-8206.2007.00505.x. [DOI] [PubMed] [Google Scholar]
- 45.Subasini U, Rajamanickam GV, Dubey GP, Prabu PC, Savariraj Sahayam C, Mohamed Shabi M, et al. Hydroalcoholic Extract of Terminalia arjuna: A Potential Hepatoprotective Herb. J Biol Sci. 2007;7:255–262. [Google Scholar]
- 46.Zheng W, Wang SY. Antioxidant activity and phenolic compounds in selected herbs. J Agric Food Chem. 2001;49:5165–5170. doi: 10.1021/jf010697n. [DOI] [PubMed] [Google Scholar]
- 47.Yang JH, Lin HC, Mau JL. Antioxidant properties of several commercial mushrooms. Food Chem. 2002;77:229–235. [Google Scholar]
- 48.Lopes GK, Schulman HM, Hermes-Lima M. Polyphenol tannic acid inhibits hydroxyl radical formation from Fenton reaction by complexing ferrous ions. Biochim Biophys Acta. 1999;1472:142–152. doi: 10.1016/s0304-4165(99)00117-8. [DOI] [PubMed] [Google Scholar]
- 49.Gil MI, Tomás-Barberán FA, Hess-Pierce B, Holcroft DM, Kader AA. Antioxidant activity of Pomegranate juice and its relationship with phenolic composition and processing. J. Agric. Food Chem. 2008;48:4581–4589. doi: 10.1021/jf000404a. [DOI] [PubMed] [Google Scholar]


