Abstract
The killer cell immunoglobulin-like receptor (KIR) gene cluster exhibits extensive allelic and haplotypic diversity. Variation at the locus is associated with an increasing number of human diseases, reminiscent of the HLA loci. Characterization of diversity at the KIR locus has progressed over the past several years, particularly since the sequence of entire KIR haplotypes have become available. To determine the extent of KIR haplotypic variability among individuals of northern European descent, we genotyped 59 CEPH families for presence/absence of all KIR genes and performed limited allelic subtyping at several KIR loci. A total of 20 unique haplotypes differing in gene content were identified, the most common of which was the previously defined A haplotype (f=0.52). Several unusual haplotypes that probably arose as a consequence of unequal crossing over events were also identified. Linkage disequilibrium (LD) analysis indicated strong negative and positive LD between several pairs of genes, values that may be useful in determining haplotypic structure when family data are not available. These data provide a resource to aid in the interpretation of disease association data involving individuals of European descent.
Keywords: KIR haplotypes, CEPH, Linkage disequilibrium
Natural killer (NK) cells are central to the innate immune system and participate in early responses against infected or transformed cells through production of cytokines and direct cytotoxicity (Bancroft 1993; Biron et al. 1999; Trinchieri 1989). They also interact with components of the adaptive immune system including T cells and dendritic cells (Raulet 2004). NK cell function is regulated by a network of inhibitory and activating receptors (Colucci et al. 2002; Lanier 2005), including the highly polymorphic killer cell immunoglobulin-like receptors (KIRs). KIR haplotypes vary in the type and number of genes present (Uhrberg et al. 1997), and some of the KIR genes show extensive allelic polymorphism to the extent that it is unlikely for any two randomly selected individuals to have identical KIR genotypes (Shilling et al. 2002a). KIR gene expression patterns vary clonally (Valiante et al. 1997), adding yet another layer of complexity to the system. HLA class I allotypes are ligands for KIR, and variability at HLA-A, HLA-B, and HLA-C is thought to be due in part to selection pressure through disease morbidity and mortality (Hill et al. 1991; Klein et al. 1993), processes that may result in part from KIR-HLA interactions. Comparisons of KIR genes across primates indicate rapid evolution of this receptor family (Khakoo and Carrington 2006; Khakoo et al. 2000; Vilches and Parham 2002), and recent data suggest that the KIR and HLA loci are indeed coevolving (Norman et al. 2007; Single et al. 2007).
The KIR locus maps to chromosome 19q13.4 within the leukocyte receptor complex (LRC), where the genes are tandemly arrayed over a segment of about 100–200 kb (Wilson et al. 2000). The KIR genes show high sequence similarity that may facilitate the occurrence of non-allelic homologous recombination (Carrington and Cullen 2004; Lupski 1998) and explain to some extent the expansion and contraction of KIR haplotypes (Martin et al. 2000; Wilson et al. 2000). The elasticity at the KIR locus has resulted in the generation of KIR haplotypes that may have two (or more) copies of the same gene on a single haplotype, a rearranged gene order, or novel hybrid genes (Gomez-Lozano et al. 2005; Martin et al. 2003; Williams et al. 2003).
Based on gene content, KIR haplotypes have been divided into two groups, termed A and B, which were originally differentiated by the presence of a 24-kb HindIII fragment on Southern blot analysis (Uhrberg et al. 1997). Haplotype A is uniform in terms of gene content (3DL3, 2DL3, 2DL1, 2DP1, 3DP1, 2DL4, 3DL1, 2DS4, and 3DL2), whereas the B haplotypes contain variable numbers and combinations of KIR genes. Human populations can vary remarkably in the frequencies of KIR genes and haplotypes, possibly a reflection of selection pressures conferred by population-specific diseases (Cook et al. 2003; Crum et al. 2000; Denis et al. 2005; Frassati et al. 2006; Gendzekhadze et al. 2006; Jiang et al. 2005; Kulkarni et al. 2008a; Middleton et al. 2007; Norman et al. 2001; Rajalingam et al. 2002; Toneva et al. 2001; Velickovic et al. 2006; Whang et al. 2005; Witt et al. 1999; Yawata et al. 2002). The frequency distribution of haplotypes A and B are roughly equal in Caucasian populations, but this balance is not maintained in some other populations, such as in Australian Aborigines where the A haplotype has a frequency of only about 13% (Toneva et al. 2001). Currently, more than 40 different B haplotypes have been described based on segregation analysis (summarized in Khakoo and Carrington 2006), and this number is likely to increase as more populations are genotyped. Given the role of KIR in both arms of the immune response, their specificity for HLA class I allotypes, and their extensive diversity, it is not surprising that variation at the KIR locus has been shown to influence resistance and susceptibility to a number of human diseases (reviewed in Kulkarni et al. 2008b). However, the diversity of the KIR genes/haplotypes and strong linkage disequilibrium (LD) between pairs of KIR loci imparts a challenge when attempting to determine the gene(s) or allelic variants that directly contribute to disease phenotypes.
In order to more fully characterize the KIR locus in Caucasians, we typed for the presence/absence status of 16 KIR genes (Martin and Carrington 2007) in members of 57 CEPH families of European descent and determined haplotypes based on segregation analysis (Fig. 1). These families included one Amish family, ten French families, and 46 families from Utah. All genotyping data will be uploaded to the Centre d’Etude Polymorphisme Humain (CEPH) database (http://www.cephb.fr/en/cephdb/), which is freely accessible to the scientific community. The framework genes KIR3DL3, KIR3DP1, KIR2DL4, and KIR3DL2 were present in all individuals, as were KIR2DL2/KIR2DL3 (alleles of the same locus) and KIR3DL1/KIR3DS1 (also alleles of the same locus). Overall, carrier frequencies were similar to those found for other Caucasian populations (Cook et al. 2003; Hsu et al. 2002; Norman et al. 2001; Uhrberg et al. 1997, 2002; Witt et al. 1999), as were gene frequencies (Table 1). We also performed allelic subtyping (Table 2) for KIR2DL4 and limited subtyping for KIR2DL5 in order to distinguish 2DL5A and 2DL5B, for KIR2DS4 to determine presence/absence of the deletion variant (Δ2DS4), and for KIR3DP1 to identify individuals with the variant allele (3DP1v) that actually contains an exon 2.
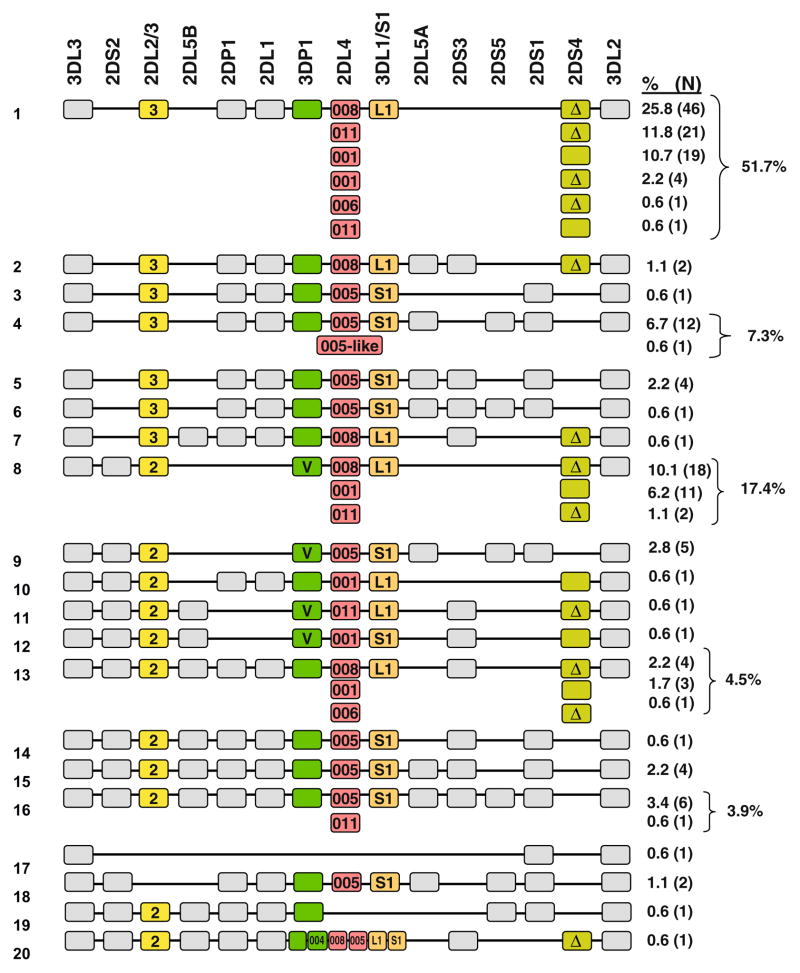
Fig. 1.
KIR haplotypes in CEPH based on gene content and allelic variation determined by segregation analysis. The gene order is based on published sequences of KIR haplotypes that were sequenced in their entirety. One exception is the extended haplotype 20 where the duplicated genes are not in their proper order, but for simplicity, they have been placed in their usual location (see Martin et al. 2003 for the exact order). Genes depicted as colored boxes have been subtyped, at least to some extent. The 2DS4 alleles containing the 22bp deletion are designated by “Δ” The variant 3DP1 alleles that contain exon 2 are designated by “v”. KIR2DS3 and 2DS5 can be found on the centromeric end between 2DL5 and 2DP1 and on the telomeric end between 2DL5 and 2DS1, but since it was not possible to determine the exact location of these genes, they were arbitrarily placed on the telomeric end in this figure
Table 1.
KIR gene frequencies in unrelated CEPH individuals
| Locus | Gene frequency (%) | Carrier frequency (%) |
|---|---|---|
| 3DL3 | 100.00 | 100.00 |
| 2DS2 | 35.39 | 60.67 |
| 2DL2 | 34.27 | 58.43 |
| 2DL3 | 64.04 | 88.76 |
| 2DL5B | 14.04 | 24.72 |
| 2DP1 | 78.09 | 96.63 |
| 2DL1 | 78.09 | 96.63 |
| 3DP1 | 99.44 | 100.00 |
| 2DL4 | 98.88 | 100.00 |
| 3DL1 | 76.97 | 94.38 |
| 3DS1 | 22.47 | 39.33 |
| 2DL5A | 21.35 | 37.08 |
| 2DS3 | 17.42 | 29.21 |
| 2DS5 | 16.29 | 30.34 |
| 2DS1 | 22.47 | 39.33 |
| 2DS4 | 77.53 | 94.38 |
| 3DL2 | 100.00 | 100.00 |
Table 2.
KIR allele frequencies in unrelated CEPH individuals
| Locus | Allele | Frequency (%) | Count |
|---|---|---|---|
| 2DL2/3 | Absent | 1.69 | 3 |
| 2DL2 | 34.27 | 61 | |
| 2DL3 | 64.04 | 114 | |
| 3DP1 | Absent | 0.56 | 1 |
| Δex2 | 77.53 | 138 | |
| Δex2+004a | 0.56 | 1 | |
| 3DP1v | 21.35 | 38 | |
| 2DL4 | Absent | 1.12 | 2 |
| 001 | 21.91 | 39 | |
| 005 | 20.22 | 36 | |
| 005-like | 0.56 | 1 | |
| 006 | 1.12 | 2 | |
| 008 | 39.89 | 71 | |
| 008+005a | 0.56 | 1 | |
| 011 | 14.61 | 26 | |
| 3DL1/S1 | Absent | 1.12 | 2 |
| 3DL1 | 76.40 | 136 | |
| 3DL1+3DS1a | 0.56 | 1 | |
| 3DS1 | 21.91 | 39 | |
| 2DS4 | Absent | 22.47 | 40 |
| Δ | 57.30 | 102 | |
| FL | 20.22 | 36 |
For 3DP1, Δex2=alleles missing exon 2; v=alleles containing exon 2. For 2DS4, Δ=deletion alleles; FL=full length alleles
Two copies present on the same haplotype
Figure 1 lists the unique haplotypes identified by segregation analysis based on presence/absence of KIR genes along with the haplotypes defined by allelic subtyping. Some of these haplotypes are not necessarily definitive, since our typing method does not determine gene copy number; that is, if both parents have a particular gene and all offspring also carry that gene, it is impossible to know by our typing method whether both parents have two copies of the gene (one on each chromosome 19) or one parent has two copies and the other parent has only one copy, as in both cases, all offspring will type positive for the gene. Therefore, to generate these haplotypes, several assumptions were made based on gene frequencies, patterns of linkage disequilibrium, and consistent observations across other studies: (1) 3DL3 and 3DL2 are present on all haplotypes; (2) if 2DS4 is present, 2DS1 is absent and vice versa; (3) if 2DL1 is present, 2DP1 is present; (4) if 3DP1v is present, 2DL1 and 2DP1 are absent; (5) 2DL5B is always assumed to be in the centromeric position, and 2DL5A is always assumed to be in the telomeric position; and (6) 2DS3 and 2DS5 were arbitrarily placed in the telomeric position, even though they can map to either half of the locus. Deduced haplotypes were further supported by the estimated haplotypes produced by the HAPLO-IHP (haplotype inference using identified haplotypes patterns) software (Yoo et al. 2007; see review by Single et al. in this issue). A total of 20 unique haplotypes based on presence/absence of KIR genes alone were identified with frequencies ranging from 51.7% to <1%. The most common haplotype was the A haplotype (51.7%), which is consistent with previous studies of Caucasians. The most common B haplotype was detected at a frequency of 17.4% overall (Fig. 1, haplotype 8) and 36% among the set of B haplotypes. The other B haplotypes were relatively rare with overall frequencies ranging from 7.3% to <1%, and of the19 B haplotypes, 13 of them were observed only once or twice.
A few of the haplotypes deduced from the analysis were unusual in that they did not follow the general characteristics of KIR haplotypes. For example, haplotype 17 appears to contain only three genes (3DL3, 2DS1, and 3DL2), and it is missing the framework genes 3DP1 and 2DL4, as well as 2DL2/3 and 3DL1/SI. Full characterization and sequence of this haplotype will be published elsewhere (Traherne et al., in preparation). Haplotype 20 on the other hand is an extended haplotype containing two copies of both 2DL4 and 3DL1/S1 as well as a 2DL5A/3DP1 hybrid, which we described previously (Martin et al. 2003). The hybrid 2DL5A/3DP1 gene has now been officially designated 3DP1*004 (Gomez-Lozano et al. 2005). Haplotype 18 is missing 2DL2/3, and haplotype 19 is missing both 2DL4 and 3DL1/S1. These unique haplotypes probably arose as a consequence of unequal crossing over.
Allelic variation of KIRs creates further diversity such that it is extremely unlikely that any two individuals will be 100% identical (Shilling et al. 2002b). Thus far, the number of alleles per KIR locus ranges from 5 to 55 (http://www.ebi.ac.uk/ipd/kir/database). Variation tends to occur throughout all regions of the gene, but many of the amino acid residues that vary among KIR allotypes are found in the extracellular domains, which might sometimes result from selection of polymorphisms that alter their interaction with ligands (Fan et al. 2001; Gardiner et al. 2001; Snyder et al. 1999) and/or expression levels (Gardiner et al. 2001; Thomas et al. 2008; Yawata et al. 2006). Subtyping of KIR2DL4, KIR2DL5 (A vs B), KIR2DS4 (Δ vs full length), and 3DP1 (3DP1v vs 3DP1Δex2) extended the number of unique haplotypes to 31 (Fig. 1). The most frequent haplotype was an A haplotype containing 2DL4*008 and Δ2DS4 (25.8% overall; 50% of the A subset of haplotypes). Interestingly, KIR2DL4*008 is not expressed on the cell surface due to a deletion of an adenine in the transmembrane domain (exon 6) that results in a frameshift leading to a premature stop codon four codons into exon 7, which along with exon 8 encodes the cytoplasmic domain of the molecule (Witt et al. 2000). Likewise, Δ2DS4 is also not expressed as a consequence of a 22-bp deletion in the D2 domain. Thus, the most common KIR haplotype contains no activating KIR that is expressed on the cell surface. 2DL4*005 was always found on a haplotype containing KIR3DS1 and is therefore not present on the A haplotype in this population. However, of the 14 haplotypes containing KIR3DS1, two did not contain 2DL4*005, but rather contained alleles very similar to 2DL4*005, both of which were probably derived from 2DL4*005. For the most part, the majority of unexpressed 2DL4 alleles (*008, *011) were found on haplotypes with Δ2DS4, while the expressed alleles (*001, *002) tended to be found with the expressed 2DS4 allotypes. 2DL4*006, which encodes an allotype that is expressed, was observed only once and was found on a Δ2DS4 haplotype.
Pairwise LD analysis was performed for the KIR loci using Cramer’s V statistic (Hedrick 1987) excluding the framework genes 3DL3, 3DP1, 2DL4, and 3DL2 (Table 3). The significance of overall LD between two loci was tested using Fisher’s exact test. In general, the LD values were similar to previously published LD values. As expected, there was a significant negative LD between 2DS2 and genes present on the A haplotype, including 2DL3 and 2DL1, and to a lesser extent 3DL1 and 2DS4. 2DL3 and 2DL1, on the other hand, were in negative LD with several activating receptors found on the B haplotypes, including 2DS3, 2DS1, and 2DL5B. Overall, LD (negative or positive) was stronger between genes located on the same half of the locus as delineated by 2DL4; that is, genes found centromeric to 2DL4 were in stronger negative or positive LD with each other than with those telomeric to 2DL4 and vice versa. One exception was 2DS3, which can be found at either end of the locus. It is possible that the framework genes might in fact impose structural constraints on recombination between the centromeric and telomeric genes, explaining at least in part the LD values observed. Table 4 shows LD values at the allelic level, which were obtained using Lewontin’s normalized measure (D′ij=Dij/Dmax), which quantifies the deviation from random association for alleles at two loci (Lewontin 1964). Complete negative or positive LD between certain pairs of alleles raises the possibility that allelic subtyping might be a useful tool for predicting haplotypes when segregation analysis is not an option. As mentioned above, for example, 2DL4*005 is almost always found on a haplotype with KIR3DS1, and consequently, it is not seen on haplotypes with KIR3DL1.
Table 3.
Linkage disequilibrium analysis of KIR genes in CEPH
| Wn
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2DL2 | 2DL3 | 2DL5B | 2DP1 | 2DL1 | 3DL1 | 3DS1 | 2DL5A | 2DS3 | 2DS5 | 2DS1 | 2DS4 | |
| 2DS2 | 0.976 | −0.988 | 0.512 | −0.687 | −0.687 | −0.181 | 0.193 | 0.130 | 0.373 | 0.151 | 0.164 | −0.164 |
| <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.025 | 0.014 | NS | <0.001 | NS | 0.038 | 0.038 | |
| 2DL2 | −0.964 | 0.526 | −0.705 | −0.705 | −0.139 | 0.150 | 0.086 | 0.386 | 0.098 | 0.122 | −0.122 | |
| <0.001 | <0.001 | <0.001 | <0.001 | NS | NS | NS | <0.001 | NS | NS | NS | ||
| 2DL3 | −0.506 | 0.707 | 0.707 | 0.202 | −0.186 | −0.124 | −0.366 | −0.145 | −0.186 | 0.186 | ||
| <0.001 | <0.001 | <0.001 | 0.009 | 0.016 | NS | <0.001 | NS | 0.016 | 0.016 | |||
| 2DL5B | 0.136 | 0.136 | −0.316 | 0.325 | 0.223 | 0.838 | 0.172 | 0.286 | −0.286 | |||
| NS | NS | <0.001 | <0.001 | 0.007 | <0.001 | 0.037 | <0.001 | <0.001 | ||||
| 2DP1 | 1.000 | −0.064 | 0.090 | 0.110 | 0.172 | 0.050 | 0.090 | −0.090 | ||||
| <0.001 | NS | NS | NS | 0.029 | NS | NS | NS | |||||
| 2DL1 | −0.064 | 0.090 | 0.110 | 0.172 | 0.050 | 0.090 | −0.090 | |||||
| NS | NS | NS | 0.029 | NS | NS | NS | ||||||
| 3DL1 | −0.952 | −0.887 | −0.382 | −0.806 | −0.984 | 0.984 | ||||||
| <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |||||||
| 3DS1 | 0.902 | 0.427 | 0.783 | 0.936 | −0.936 | |||||||
| <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | ||||||||
| 2DL5A | 0.411 | 0.810 | 0.902 | −0.902 | ||||||||
| <0.001 | <0.001 | <0.001 | <0.001 | |||||||||
| 2DS3 | 0.118 | 0.356 | −0.356 | |||||||||
| NS | <0.001 | <0.001 | ||||||||||
| 2DS5 | 0.819 | −0.819 | ||||||||||
| <0.001 | <0.001 | |||||||||||
| 2DS1 | −1.000 | |||||||||||
| <0.001 | ||||||||||||
LD values with p<0.001 in bold. 2DL4, 3DL2, 3DL3, and 3DP1 have been omitted from the table, as all three have a frequency of 100%
NS not significant
Table 4.
Linkage disequilibrium analysis at the allelic level
| 2DL2/3
|
3DP1
|
2DL4
|
3DL1/S1
|
2DS4
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 2 | 3 | Δex2 | v | 001 | 005 | 006 | 008 | 011 | 0 | 0 | L1 | S1 | 0 | Δ | FL | ||
| 2DL2/3 | 0 | −0.140 | −1.000 | −1.000 | 0.582 | −1.000 | −1.000 | −1.000 | 0.491 | 0.491 | −1.000 | 0.573 | 1.000 | −1.000 | −1.000 | |||
| 2 | −0.962 | 1.000 | 0.103 | 0.155 | 0.239 | −0.096 | −0.650 | 0.239 | 0.239 | −0.203 | 0.181 | 0.163 | −0.228 | 0.155 | ||||
| 3 | 1.000 | −1.000 | −0.079 | −0.219 | −0.219 | 0.138 | 0.666 | −1.000 | −1.000 | 0.294 | −0.239 | −0.258 | 0.264 | −0.133 | ||||
| 3DP1 | Δex2 | −0.140 | −0.962 | 1.000 | −0.107 | 0.382 | 1.000 | −0.085 | 0.644 | −0.355 | −0.355 | −0.152 | 0.315 | 0.333 | 0.040 | −0.140 | ||
| v | −1.000 | 1.000 | −1.000 | 0.124 | −0.349 | −1.000 | 0.124 | −0.625 | −1.000 | −1.000 | 0.331 | −0.279 | −0.414 | −0.036 | 0.152 | |||
| 2DL4 | 001 | −1.000 | 0.103 | −0.079 | −0.107 | 0.124 | −1.000 | 0.891 | −0.883 | −1.000 | −0.821 | 0.964 | ||||||
| 005 | 0.582 | 0.155 | −0.219 | 0.382 | −0.349 | −1.000 | −1.000 | 1.000 | 1.000 | −1.000 | −1.000 | |||||||
| 006 | −1.000 | 0.239 | −0.219 | 1.000 | −1.000 | −1.000 | 1.000 | −1.000 | −1.000 | 1.000 | −1.000 | |||||||
| 008 | −1.000 | −0.096 | 0.138 | −0.085 | 0.124 | −1.000 | 1.000 | −1.000 | −1.000 | 1.000 | −1.000 | |||||||
| 011 | −1.000 | −0.650 | 0.666 | 0.644 | −0.625 | −1.000 | 0.830 | −0.817 | −0.822 | 0.813 | −0.802 | |||||||
| 0 | 0.491 | 0.239 | −1.000 | −0.355 | −1.000 | 1.000 | −1.000 | −1.000 | 1.000 | −1.000 | −1.000 | |||||||
| 3DL1/S1 | 0 | 0.491 | 0.239 | −1.000 | −0.355 | −1.000 | −1.000 | −1.000 | −1.000 | −1.000 | −1.000 | 1.000 | 1.000 | −1.000 | −1.000 | |||
| L1 | −1.000 | −0.203 | 0.294 | −0.152 | 0.331 | 0.891 | −1.000 | 1.000 | 1.000 | 0.830 | −1.000 | −1.000 | 0.958 | 0.882 | ||||
| S1 | 0.573 | 0.181 | −0.239 | 0.315 | −0.279 | −0.883 | 1.000 | −1.000 | −1.000 | −0.817 | −1.000 | 0.967 | −1.000 | −0.873 | ||||
| 2DS4 | 0 | 1.000 | 0.163 | −0.258 | 0.333 | −0.414 | −1.000 | 1.000 | −1.000 | −1.000 | −0.822 | 1.000 | 1.000 | −1.000 | 0.967 | |||
| Δ | −1.000 | −0.228 | 0.264 | 0.040 | −0.036 | −0.821 | −1.000 | 1.000 | 1.000 | 0.813 | −1.000 | −1.000 | 0.958 | −1.000 | ||||
| FL | −1.000 | 0.155 | −0.133 | −0.140 | 0.152 | 0.964 | −1.000 | −1.000 | −1.000 | −0.802 | −1.000 | −1.000 | 0.882 | −0.873 | ||||
0=absence of gene. For 3DP1, Δex2=the alleles missing exon 2; v = the alleles containing exon 2. For 2DS4, FL=full length alleles; Δ =deletion alleles
Concluding remarks
We have shown that there is extensive variability at the KIR locus in families of Northern European descent, which likely contributes to differences across individuals in both their innate and adaptive immune responses to various pathogens. Functional consequences of KIR haplotype variation in infectious diseases, cancer, and autoimmune diseases are quite plausible, and an in depth understanding of the molecular genetic properties of the locus is essential for accurate interpretation of such disease association data.
Acknowledgments
This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under contract N01-CO-12400. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government. This Research was supported in part by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research. This publication was supported in part by NIH/NIAID contract number HHSN266200400076C, ABD N01-AI-40076 (R.M.S). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. The computational resources provided by the Vermont Advanced Computing Center which is supported by NASA (NNX 06AC88G) are gratefully acknowledged. J.T. and M.W. were supported by grants from MRC and Wellcome. We thank Arman Bashirova for her assistance with Fig. 1.
Contributor Information
M. P. Martin, Cancer and Inflammation Program, Laboratory of Experimental Immunology, SAIC-Frederick, Inc, NCI-Frederick, Frederick, MD 21702 USA
R. M. Single, Department of Mathematics and Statistics, University of Vermont, Burlington, VT 05405 USA
M. J. Wilson, CSL Limited, Bio21 Institute, Parkville, Victoria 3010, Australia
J. Trowsdale, Immunology Division, Department of Pathology, University of Cambridge, Tennis Court Road, Cambridge CB2 1QP, UK
M. Carrington, Email: carringt@ncifcrf.gov, Cancer and Inflammation Program, Laboratory of Experimental Immunology, SAIC-Frederick, Inc, NCI-Frederick, Frederick, MD 21702 USA
References
- Bancroft GJ. The role of natural killer cells in innate resistance to infection. Curr Opin Immunol. 1993;5:503–510. doi: 10.1016/0952-7915(93)90030-V. [DOI] [PubMed] [Google Scholar]
- Biron CA, Nguyen KB, Pien GC, Cousens LP, Salazar-Mather TP. Natural killer cells in antiviral defense: Function and regulation by innate cytokines. Annu Rev Immunol. 1999;17:189–220. doi: 10.1146/annurev.immunol.17.1.189. [DOI] [PubMed] [Google Scholar]
- Carrington M, Cullen M. Justified chauvinism: advances in defining meiotic recombination through sperm typing. Trends Genet. 2004;20:196–205. doi: 10.1016/j.tig.2004.02.006. [DOI] [PubMed] [Google Scholar]
- Colucci F, Di Santo JP, Leibson PJ. Natural killer cell activation in mice and men: different triggers for similar weapons? Nat Immunol. 2002;3:807–813. doi: 10.1038/ni0902-807. [DOI] [PubMed] [Google Scholar]
- Cook MA, Moss PA, Briggs DC. The distribution of 13 killer-cell immunoglobulin-like receptor loci in UK blood donors from three ethnic groups. Eur J Immunogenet. 2003;30:213–221. doi: 10.1046/j.1365–2370.2003.00394.x. [DOI] [PubMed] [Google Scholar]
- Crum KA, Logue SE, Curran MD, Middleton D. Development of a PCR-SSOP approach capable of defining the natural killer cell inhibitory receptor (KIR) gene sequence repertoires. Tissue Antigens. 2000;56:313–326. doi: 10.1034/j.1399-0039.2000.560403.x. [DOI] [PubMed] [Google Scholar]
- Denis L, Sivula J, Gourraud PA, Kerdudou N, Chout R, Ricard C, Moisan JP, Gagne K, Partanen J, Bignon JD. Genetic diversity of KIR natural killer cell markers in populations from France, Guadeloupe, Finland, Senegal and Reunion. Tissue Antigens. 2005;66:267–276. doi: 10.1111/j.1399-0039.2005.00473.x. [DOI] [PubMed] [Google Scholar]
- Fan QR, Long EO, Wiley DC. Crystal structure of the human natural killer cell inhibitory receptor KIR2DL1-HLA-Cw4 complex. Nat Immunol. 2001;2:452–460. doi: 10.1038/87766. [DOI] [PubMed] [Google Scholar]
- Frassati C, Touinssi M, Picard C, Segura M, Galicher V, Papa K, Gagne K, Vivier E, Degioanni A, Boetsch G, Mercier P, Vely F, de Micco P, Reviron D, Chiaroni J. Distribution of killer-cell immunoglobulin-like receptor (KIR) in Comoros and Southeast France. Tissue Antigens. 2006;67:356–367. doi: 10.1111/j.1399-0039.2006.00592.x. [DOI] [PubMed] [Google Scholar]
- Gardiner CM, Guethlein LA, Shilling HG, Pando M, Carr WH, Rajalingam R, Vilches C, Parham P. Different NK cell surface phenotypes defined by the DX9 antibody are due to KIR3DL1 gene polymorphism. J Immunol. 2001;166:2992–3001. doi: 10.4049/jimmunol.166.5.2992. [DOI] [PubMed] [Google Scholar]
- Gendzekhadze K, Norman PJ, Abi-Rached L, Layrisse Z, Parham P. High KIR diversity in Amerindians is maintained using few gene-content haplotypes. Immunogenetics. 2006;58:474–480. doi: 10.1007/s00251-006-0108-3. [DOI] [PubMed] [Google Scholar]
- Gomez-Lozano N, Estefania E, Williams F, Halfpenny I, Middleton D, Solis R, Vilches C. The silent KIR3DP1 gene (CD158c) is transcribed and might encode a secreted receptor in a minority of humans, in whom the KIR3DP1, KIR2DL4 and KIR3DL1/KIR3DS1 genes are duplicated. Eur J Immunol. 2005;35:16–24. doi: 10.1002/eji.200425493. [DOI] [PubMed] [Google Scholar]
- Hedrick PW. Gametic disequilibrium measures: proceed with caution. Genetics. 1987;117:331–341. doi: 10.1093/genetics/117.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill AV, Allsopp CE, Kwiatkowski D, Anstey NM, Twumasi P, Rowe PA, Bennett S, Brewster D, McMichael AJ, Greenwood BM. Common west African HLA antigens are associated with protection from severe malaria. Nature. 1991;352:595–600. doi: 10.1038/352595a0. [DOI] [PubMed] [Google Scholar]
- Hsu KC, Liu XR, Selvakumar A, Mickelson E, O’Reilly RJ, Dupont B. Killer Ig-like receptor haplotype analysis by gene content: evidence for genomic diversity with a minimum of six basic framework haplotypes, each with multiple subsets. J Immunol. 2002;169:5118–5129. doi: 10.4049/jimmunol.169.9.5118. [DOI] [PubMed] [Google Scholar]
- Jiang K, Zhu FM, Lv QF, Yan LX. Distribution of killer cell immunoglobulin-like receptor genes in the Chinese Han population. Tissue Antigens. 2005;65:556–563. doi: 10.1111/j.1399-0039.2005.00412.x. [DOI] [PubMed] [Google Scholar]
- Khakoo SI, Carrington M. KIR and disease: A model system or system of models? Immunol Rev. 2006;214:186–201. doi: 10.1111/j.1600065X.2006.00459.x. [DOI] [PubMed] [Google Scholar]
- Khakoo SI, Rajalingam R, Shum BP, Weidenbach K, Flodin L, Muir DG, Canavez F, Cooper SL, Valiante NM, Lanier LL, Parham P. Rapid evolution of NK cell receptor systems demonstrated by comparison of chimpanzees and humans. Immunity. 2000;12:687–698. doi: 10.1016/S1074-7613(00)80219-8. [DOI] [PubMed] [Google Scholar]
- Klein J, Satta Y, O’HUigin C, Takahata N. The molecular descent of the major histocompatibility complex. Annu Rev Immunol. 1993;11:269–295. doi: 10.1146/annurev.iy.11.040193.001413. [DOI] [PubMed] [Google Scholar]
- Kulkarni S, Single RM, Martin MP, Rajalingam R, Badwe R, Joshi N, Carrington M. Comparison of the rapidly evolving KIR locus in Parsis and natives of India. Immunogenetics. 2008a;60:121–129. doi: 10.1007/s00251-008-0279-1. [DOI] [PubMed] [Google Scholar]
- Kulkarni S, Martin MP, Carrington M. The Yin and Yang of HLA and KIR in human disease. Semin Immunol. 2008b doi: 10.1016/j. smim.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanier LL. NK cell recognition. Annu Rev Immunol. 2005;23:225–274. doi: 10.1146/annurev.immunol.23.021704.115526. [DOI] [PubMed] [Google Scholar]
- Lewontin RC. The interaction of selection and linkage. I. General considerations; heterotic models. Genetics. 1964;49:49–67. doi: 10.1093/genetics/49.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupski JR. Charcot–Marie–Tooth disease: lessons in genetic mechanisms. Mol Med. 1998;4:3–11. [PMC free article] [PubMed] [Google Scholar]
- Martin MP, Carrington M. KIR locus polymorphisms: genotyping and disease association analysis. In: Ewbank J, Vivier E, editors. Methods in molecular biology: innate immunity. Humana; Totowa, NJ: 2007. pp. 49–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin AM, Freitas EM, Witt CS, Christiansen FT. The genomic organization and evolution of the natural killer immunoglobulin-like receptor (KIR) gene cluster. Immunogenetics. 2000;51:268–280. doi: 10.1007/s002510050620. [DOI] [PubMed] [Google Scholar]
- Martin MP, Bashirova A, Traherne J, Trowsdale J, Carrington M. Cutting edge: expansion of the KIR locus by unequal crossing over. J Immunol. 2003;171:2192–2195. doi: 10.4049/jimmunol.171.5.2192. [DOI] [PubMed] [Google Scholar]
- Middleton D, Meenagh A, Gourraud PA. KIR haplotype content at the allele level in 77 Northern Irish families. Immunogenetics. 2007;59:145–158. doi: 10.1007/s00251-006-0181-7. [DOI] [PubMed] [Google Scholar]
- Norman PJ, Stephens HA, Verity DH, Chandanayingyong D, Vaughan RW. Distribution of natural killer cell immunoglobulin-like receptor sequences in three ethnic groups. Immunogenetics. 2001;52:195–205. doi: 10.1007/s002510000281. [DOI] [PubMed] [Google Scholar]
- Norman PJ, Abi-Rached L, Gendzekhadze K, Korbel D, Gleimer M, Rowley D, Bruno D, Carrington CV, Chandanayingyong D, Chang YH, Crespi C, Saruhan-Direskeneli G, Fraser PA, Hameed K, Kamkamidze G, Koram KA, Layrisse Z, Matamoros N, Mila J, Park MH, Pitchappan RM, Ramdath DD, Shiau MY, Stephens HA, Struik S, Verity DH, Vaughan RW, Tyan D, Davis RW, Riley EM, Ronaghi M, Parham P. Unusual selection on the KIR3DL1/S1 natural killer cell receptor in Africans. Nat Genet. 2007;39:1092–1099. doi: 10.1038/ng2111. [DOI] [PubMed] [Google Scholar]
- Rajalingam R, Krausa P, Shilling HG, Stein JB, Balamurugan A, McGinnis MD, Cheng NW, Mehra NK, Parham P. Distinctive KIR and HLA diversity in a panel of north Indian Hindus. Immunogenetics. 2002;53:1009–1019. doi: 10.1007/s00251-001-0425-5. [DOI] [PubMed] [Google Scholar]
- Raulet DH. Interplay of natural killer cells and their receptors with the adaptive immune response. Nat Immunol. 2004;5:996–1002. doi: 10.1038/ni1114. [DOI] [PubMed] [Google Scholar]
- Shilling HG, Guethlein LA, Cheng NW, Gardiner CM, Rodriguez R, Tyan D, Parham P. Allelic polymorphism synergizes with variable gene content to individualize human KIR genotype. J Immunol. 2002a;168:2307–2315. doi: 10.4049/jimmunol.168.5.2307. [DOI] [PubMed] [Google Scholar]
- Shilling HG, Young N, Guethlein LA, Cheng NW, Gardiner CM, Tyan D, Parham P. Genetic control of human NK cell repertoire. J Immunol. 2002b;169:239–247. doi: 10.4049/jimmunol.169.1.239. [DOI] [PubMed] [Google Scholar]
- Single RM, Martin MP, Gao X, Meyer D, Yeager M, Kidd JR, Kidd KK, Carrington M. Global diversity and evidence for coevolution of KIR and HLA. Nat Genet. 2007;39:1114–1119. doi: 10.1038/ng2077. [DOI] [PubMed] [Google Scholar]
- Snyder GA, Brooks AG, Sun PD. Crystal structure of the HLA-Cw3 allotype-specific killer cell inhibitory receptor KIR2DL2. Proc Natl Acad Sci U S A. 1999;96:3864–3869. doi: 10.1073/pnas.96. 7.3864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas R, Yamada E, Alter G, Martin MP, Bashirova AA, Norman PJ, Altfeld M, Parham P, Anderson SK, McVicar DW, Carrington M. Novel KIR3DL1 alleles and their expression levels on NK cells: convergent evolution of KIR3DL1 phenotype variation? J Immunol. 2008;180:6743–6750. doi: 10.4049/jimmunol.180.10.6743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toneva M, Lepage V, Lafay G, Dulphy N, Busson M, Lester S, Vu-Trien A, Michaylova A, Naumova E, McCluskey J, Charron D. Genomic diversity of natural killer cell receptor genes in three populations. Tissue Antigens. 2001;57:358–362. doi: 10.1034/j.1399-0039.2001.057004358.x. [DOI] [PubMed] [Google Scholar]
- Trinchieri G. Biology of natural killer cells. Adv Immunol. 1989;47:187–376. doi: 10.1016/S0065-2776(08)60664-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhrberg M, Valiante NM, Shum BP, Shilling HG, Lienert-Weidenbach K, Corliss B, Tyan D, Lanier LL, Parham P. Human diversity in killer cell inhibitory receptor genes. Immunity. 1997;7:753–763. doi: 10.1016/S1074-7613(00)80394-5. [DOI] [PubMed] [Google Scholar]
- Uhrberg M, Parham P, Wernet P. Definition of gene content for nine common group B haplotypes of the Caucasoid population: KIR haplotypes contain between seven and eleven KIR genes. Immunogenetics. 2002;54:221–229. doi: 10.1007/s00251-002-0463-7. [DOI] [PubMed] [Google Scholar]
- Valiante NM, Uhrberg M, Shilling HG, Lienert-Weidenbach K, Arnett KL, D’Andrea A, Phillips JH, Lanier LL, Parham P. Functionally and structurally distinct NK cell receptor repertoires in the peripheral blood of two human donors. Immunity. 1997;7:739–751. doi: 10.1016/S1074-7613(00)80393-3. [DOI] [PubMed] [Google Scholar]
- Velickovic M, Velickovic Z, Dunckley H. Diversity of killer cell immunoglobulin-like receptor genes in Pacific Islands populations. Immunogenetics. 2006;58:523–532. doi: 10.1007/s00251-006-0124-3. [DOI] [PubMed] [Google Scholar]
- Vilches C, Parham P. KIR: diverse, rapidly evolving receptors of innate and adaptive immunity. Annu Rev Immunol. 2002;20:217–251. doi: 10.1146/annurev.immunol.20.092501.134942. [DOI] [PubMed] [Google Scholar]
- Whang DH, Park H, Yoon JA, Park MH. Haplotype analysis of killer cell immunoglobulin-like receptor genes in 77 Korean families. Hum Immunol. 2005;66:146–154. doi: 10.1016/j. humimm.2004.10.013. [DOI] [PubMed] [Google Scholar]
- Williams F, Maxwell LD, Halfpenny IA, Meenagh A, Sleator C, Curran MD, Middleton D. Multiple copies of KIR 3DL/S1 and KIR 2DL4 genes identified in a number of individuals. Hum Immunol. 2003;64:729–732. doi: 10.1016/S0198-8859(03)00089-2. [DOI] [PubMed] [Google Scholar]
- Wilson MJ, Torkar M, Haude A, Milne S, Jones T, Sheer D, Beck S, Trowsdale J. Plasticity in the organization and sequences of human KIR/ILT gene families. Proc Natl Acad Sci USA. 2000;97:4778–4783. doi: 10.1073/pnas.080588597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witt CS, Dewing C, Sayer DC, Uhrberg M, Parham P, Christiansen FT. Population frequencies and putative haplotypes of the killer cell immunoglobulin-like receptor sequences and evidence for recombination. Transplantation. 1999;68:1784–1789. doi: 10.1097/00007890-199912150-00024. [DOI] [PubMed] [Google Scholar]
- Witt CS, Martin A, Christiansen FT. Detection of KIR2DL4 alleles by sequencing and SSCP reveals a common allele with a shortened cytoplasmic tail. Tissue Antigens. 2000;56:248–257. doi: 10.1034/j.1399-0039.2000.560307.x. [DOI] [PubMed] [Google Scholar]
- Yawata M, Yawata N, Abi-Rached L, Parham P. Variation within the human killer cell immunoglobulin-like receptor (KIR) gene family. Crit Rev Immunol. 2002;22:463–482. [PubMed] [Google Scholar]
- Yawata M, Yawata N, Draghi M, Little AM, Partheniou F, Parham P. Roles for HLA and KIR polymorphisms in natural killer cell repertoire selection and modulation of effector function. J Exp Med. 2006;203:633–645. doi: 10.1084/jem.20051884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo YJ, Tang J, Kaslow RA, Zhang K. Haplotype inference for present-absent genotype data using previously identified haplotypes and haplotype patterns. Bioinformatics. 2007;23:2399–2406. doi: 10.1093/bioinformatics/btm371. [DOI] [PubMed] [Google Scholar]