Abstract
Many tissues express multiple gap junction proteins, or connexins (Cx); for example, Cx43, Cx40, and Cx37 are coexpressed in vascular cells. This study was undertaken to elucidate the consequences of coexpression of Cx40 or Cx37 with Cx43 at different ratios. EcR-293 cells (which endogenously produce Cx43) were transfected with ecdysone-inducible plasmids encoding Cx37 or Cx40. Immmunoblotting showed a ponasterone dose-dependent induction of Cx37 or Cx40 while constant levels of Cx43 were maintained. The coexpressed connexins colocalized at appositional membranes. Double whole-cell patch clamp recordings showed no significant change in total junctional conductances in cells treated with 0, 0.5, or 4 µM ponasterone; however, they did show a diversity of unitary channel sizes consistent with the induced connexin expression. In cells with induced expression of either Cx40 or Cx37, intercellular transfer of microinjected Lucifer yellow was reduced, but transfer of NBD-TMA (2-(4-nitro- 2,1,3-benzoxadiol-7-yl)[aminoethyl]trimethylammonium) was not affected. In cocultures containing uninduced EcR cells together with cells induced to coexpress Cx37 or Cx40, Lucifer yellow transfer was observed only between the cells expressing Cx43 alone. These data show that induced expression of either Cx37 or Cx40 in Cx43- expressing cells can selectively alter the intercellular exchange of some molecules without affecting the transfer of others.
Keywords: Connexins, Electrophysiology, Gap junction, Gap junctions, Gap junctions/cell–cell channels
The intercellular channels in gap junctions are formed by docking of two hemichannels (connexons), each composed of six subunit proteins called connexins (Cx) (for reviews, see Harris 2001; Saez et al. 2003). Each connexin can form channels by itself (homomeric/homotypic channels), and different connexins form channels with different conductance, permeability, and gating properties. In cells coexpressing more than one connexin, heteromeric channels (containing different connexins in the same connexon) can potentially be formed.
Most cells contain multiple connexins. Cx37, Cx40, and Cx43 are abundant components of the gap junctions in various cells of the cardiovascular system, and they are coexpressed in some of these cells. In ventricular myocytes, Cx43 is the predominant connexin; however, in atrial myocytes, the abundances of Cx40 and Cx43 are approximately equal (Lin et al. 2010). In diseased myocardium, gap junctions may undergo remodeling, and the levels of Cx40 and Cx43 may be altered (reviewed by Severs et al. 2008). Endothelial cells from different sources or vascular beds contain Cx37, Cx40, and/or Cx43 (Johnstone et al. 2009). The relative abundances of these proteins and their coexpression are dynamic and may vary depending on a number of factors, including developmental stage (Delorme et al. 1997; Gabriels and Paul 1998), aging (Yeh et al. 2000), hemodynamics or shear stress (Gabriels and Paul 1998; Chang et al. 2010), and pathologies including inflammation, atherosclerosis, and hypertension (Haefliger et al. 2004; Chanson et al. 2005; Brisset et al. 2009).
Coexpression of connexins and heteromeric channel formation may have profound effects on conductance, permeability/selectivity, and regulation of gap junction channels (reviewed by Cottrell and Burt 2005). Cx43 is one of the most widely expressed gap junction proteins, and channels formed of this protein show little selectivity in the intercellular passage of positively or negatively charged ions or dye tracers (Veenstra et al. 1995; Weber et al. 2004). In contrast, despite forming channels with larger unitary conductances, both Cx37 and Cx40 form channels with substantially less permeability to some negatively charged molecules than to positive ones (Veenstra et al. 1994, 1995; Weber et al. 2004; Beblo et al. 1995). As examples, homomeric Cx43 channels exhibit substantial permeability to both Lucifer yellow (net charge = −2, molecular weight 457) and NBD-TMA (2-(4-nitro-2,1,3- benzoxadiol-7-yl)[aminoethyl]trimethylammonium; net charge = +1; molecular weight 280) (Ek-Vitorin and Burt 2005; Heyman and Burt 2008); in contrast, while homomeric Cx37 and Cx40 channels also allow permeation by NBD-TMA, the permeation of Lucifer yellow is much less (Cottrell et al. 2002; Valiunas et al. 2002; Heyman et al. 2009).
Because Cx37 and Cx40 are often coexpressed with Cx43 in different cells (especially those of the cardiovascular system), the properties of intercellular communication will be determined by the channel properties of each connexin and by their formation of heteromeric and heterotypic channels. Strong evidence suggests that coexpressed Cx37 and Cx43 mix to form many different heteromeric channels (Brink et al. 1997). Previous studies have emphasized the effects of Cx40 and Cx43 coexpression on intercellular communication (Valiunas et al. 2000, 2001; Burt et al. 2001; Cottrell et al. 2002), although there are lingering controversies about the extent or ability of these two connexins to form heterotypic channels (Valiunas et al. 2000; Cottrell and Burt 2001; Rackauskas et al. 2007). Some previous studies have examined different clones of the same cells that had been manipulated to express different relative amounts of two connexins. Burt et al. (2001) studied A7r5 cells (that naturally coexpress Cx40 and Cx43) after stable transfection with a vector coding for Cx43 in an antisense orientation; they identified different clones with different Cx43/Cx40 ratios depending on the number of copies of antisense Cx43 incorporated into genome.
The current study was designed to characterize (and contrast) the effects of induced expression of Cx37 or Cx40 with Cx43 to allow regulated expression of different ratios of the two coexpressed connexins.
Materials and Methods
Connexin Expression Plasmids, Cell Culture, and Transfections
Unless otherwise specified, plasmids, EcR293 cells (HEK- 293 cells stably transfected with the ecdysone receptor), and culture medium ingredients were obtained from Invitrogen (Carlsbad, CA). Connexin DNAs were generated by PCR methods. Human Cx37 (T1019/Ser-319 polymorphic variant) with a C-terminal FLAG epitope tag was subcloned into pIND/V5-His-A; Cx37 with a C-terminal HA tag was subcloned into pcDNA3.1/hygro; and rat Cx40 was subcloned into pIND(Sp1)/hygro. Plasmids were purified using a high-purity plasmid purification kit (Marligen Biosciences, Ijamsville, MD) and fully sequenced. EcR293 cells were grown in Dulbecco modified Eagle medium supplemented with 10 % fetal calf serum, 2 mM l-glutamine, 100 U/ml penicillin, and 100 µg/ml streptomycin. Cells were stably transfected with linearized DNA with lipofectamine 2000. Stable clones were selected by culturing in medium containing 400 µg/ml G418 or 50 µg/ ml hygromycin (EMD/Calbiochem, San Diego, CA).
Immunochemical Detection of Connexins
Cx43 was detected using a mouse monoclonal antibody (MAB 3068; Millipore/Chemicon, Billerica, MA) or rabbit antibodies (C6219, Sigma Chemical Company, St. Louis, MO). Cx40 was detected using rabbit antibodies directed against a bacterially expressed Cx40 carboxyl tail fusion protein (Kwong et al. 1998) or directed against a 19 amino acid peptide sequence within its C-terminal domain (AB 1726; Millipore/Chemicon). Epitope-tagged Cx37 was detected using anti-FLAG M2 monoclonal antibody (F3165; Sigma) or rabbit anti-HA antibodies (71-5500; Invitrogen/Zymed).
Immunoblotting was performed similarly to our previous studies (Valiunas et al. 2001) using protein extracts from cells prepared as described by Laing and Beyer (1995) resolved on 10 % polyacrylamide gels containing SDS, and blotted onto Immobilon-P (Millipore, Bedford, MA). Immunoblots were developed with ECL chemiluminescence reagents. Rainbow molecular weight marker standards (GE Healthcare, Piscataway, NJ) were used to calibrate the gels.
Immunofluorescence was performed by staining cells cultured on multiwell slides essentially as described previously (Valiunas et al. 2001; Gemel et al. 2004, 2006). Briefly, EcR293 cells were plated on slides coated for 30 min with 0.01 % poly-l-lysine (Sigma) to increase their adherence, and cells were fixed using 4 % paraformaldehyde for 30 min. For double-labeling experiments, cells were incubated simultaneously with both mouse anti-Cx43 monoclonal antibody and rabbit anti-Cx40 antibodies or with both mouse anti-FLAG monoclonal antibody and rabbit anti-Cx43 antibodies followed by Cy2- and Cy3- conjugated secondary antibodies (Jackson ImmunoResearch Laboratories, West Grove, PA).
Detergent Solubilization and Affinity Purification of Connexons
Solubilization of connexons with Triton X-100 was performed essentially as described previously (Berthoud et al. 2001; Gemel et al. 2004, 2006). Briefly, cultured cells were harvested in PBS containing protease inhibitors, pelleted, and resuspended in buffer containing 1 % Triton X-100. After incubation on ice for 30 min, samples were centrifuged at 100,000gave for 30 min. In some experiments, we examined the relative solubilization of total connexins and forms that differ in electrophoretic mobilities by immunoblotting comparable fractions of the initial homogenates, supernatants, and pellets.
The supernatant containing solubilized connexons was also used for purification of HA-tagged connexins and associated proteins. For these experiments, EcR293 cells were transiently transfected with Cx37HA (in pcDNA3.1/ hygro) using lipofectamine, and HA-tagged (and associated) proteins were purified 72 h later using the µMACS HA isolation kit (Miltenyi Biotec, Bergisch Gladbach, Germany) (Gemel et al. 2006, 2008). We have previously established this as a system in which a coexpressed connexin that forms heteromeric connexons copurifies with the tagged connexin (e.g., Cx40 and Cx43), (Valiunas et al. 2001) while a connexin that does not participate in heteromers with the tagged connexin does not copurify with it (e.g., Cx43 and Cx26) (Gemel et al. 2004).
Microinjection of Gap Junction Tracers
Cells cultured on coverslips (80–100 % confluent cultures) were impaled with a micropipette filled with 150 mM LiCl and 4 % Lucifer yellow (Sigma Chemical Company) or NBD-TMA (Bednarczyk et al. 2000). Solutions were microinjected with a picospritzer (model PLI-188, Nikon Inc) using 0.2- to 0.3-s pulses of 1–2 psi; cells were impaled for 1 min. The extent of intercellular transfer of both tracers was determined by recording the number of adjacent cells containing the tracer after visualization by epifluorescence and digital microscopy. The statistical significance of differences between treated vs. untreated cells was calculated by Student’s t test for paired data.
Some dye transfer experiments were also performed in mixed cocultures of EcR293 and EcR293-Cx37 or EcR293-Cx40 cells in which the connexin coexpressing cells were labeled with the red fluorescent dye PKH26 (Sigma) while the EcR293 cells were unlabeled. EcR293- Cx37 (or EcR293-Cx40) cells were trypsinized, counted and labeled with PKH26 for 5 min. They were plated at a 1:1 ratio with unlabeled EcR293 cells. The next day, expression of Cx37 or Cx40 was induced using 4 µM ponasterone. After 20 h, Lucifer yellow was injected into an unlabeled (EcR293) cell that neighbored both unlabeled (EcR293) and labeled (EcR293-Cx37 or EcR293-Cx40) cells; the extent of dye transfer was determined by recording the number of adjacent cells (PKH26 labeled and unlabeled) containing the tracer after visualization by epifluorescence and digital microscopy.
Electrophysiological Measurements
The dual whole cell voltage-clamp technique was used to assess both macroscopic and single-channel conductance between pairs of cells in culture as described previously (Cottrell et al. 2002). Cells were grown to confluence in a 100-mm dish, released with 0.25 % trypsin in Ca2+- and Mg2+-free buffer, and replated at low density on glass coverslips (in the presence or absence of ponasterone). At 20–28 h after plating, coverslips were mounted in a custommade chamber, and an Olympus inverted (IMT2) microscope with phase contrast optics was used to identify pairs of cells in the dish (typically only two or three pairs of cells were found on any given 25-mm coverslip). Cells were bathed in external solution containing 142.5 mM NaCl, 4 mM KCl, 1 mM MgCl2, 5 mM glucose, 2 mM sodium pyruvate, 10 mM HEPES, 15 mM CsCl, 10 mM TEACl, 1 mM BaCl2, and 1 mM CaCl2, pH 7.2, with an osmolarity of 330 mOsm. Junctional conductance was determined on all pairs within 30 min using dual whole cell voltage-clamp techniques as previously described. The pipette solution contained 124 mM KCl, 14 mM CsCl, 9 mM HEPES, 9 mMEGTA, 0.5 mMCaCl2, 5 mMglucose, 9 mMTEACl, 3 mM MgCl2, and 5 mM disodium ATP, pH 7.2 with an osmolarity of 326 mOsm. Macroscopic junctional conductance (gj) was evaluated with 10-mV transjunctional pulses. Single-channel events were studied in cell pairs that had been partially uncoupled with halothane such that only one or a few channels were active (gj < 0.5 nS). In all cell pairs, the transjunctional voltage (Vj) applied was 40 mV with pulse durations of >20 s. Records were filtered at 50–100 Hz, and transitions in current amplitude that were of equal amplitude but had opposite polarity in the two current traces and lasted longer than 0.1 s were noted. Amplitude data were binned in 10 pS bins, and the relative frequency of each bin was calculated for each cell pair. Histograms display the mean (±SEM, or range for sample sizes less than 3); the relative frequency of each bin was derived by averaging each bin’s frequency across multiple cell pairs.
Results
Generation and Immunological Characterization of EcR293-Cx37 and EcR293-Cx40 Cells that Inducibly Coexpress Cx37 or Cx40 with Cx43
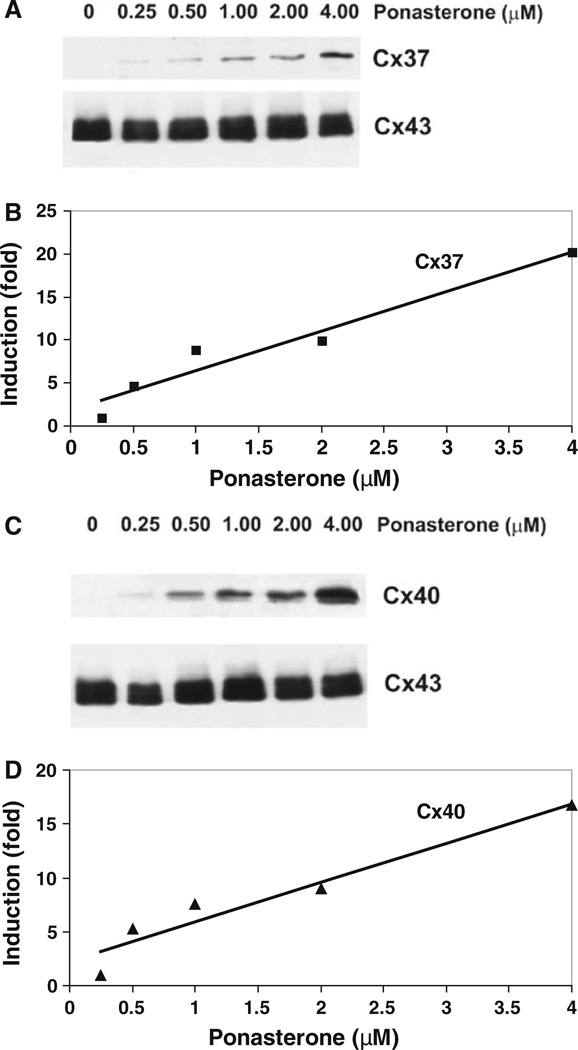
Cx37 (T1019/Ser-319 polymorphic variant) or Cx40 were introduced into EcR293 cells by stable transfection (generating inducible EcR293-Cx37 and EcR293-Cx40 cells). Production of connexin proteins was examined by immunoblotting after 20 h of induction with ponasterone (Fig. 1). The EcR293 cells abundantly produced Cx43 (Fig. 1a, c), as expected, because they are derived from HEK293 cells, which endogenously express Cx43 (Gemel et al. 2006, 2008). The levels of Cx43 were not significantly affected by treatment with different doses of ponasterone (Fig. 1a, c). No immunoreactive Cx37 or Cx40 bands were detected in untreated EcR293-Cx37 or EcR293-Cx40 cells (Fig. 1a, c, lanes marked 0 µM ponasterone). Connexin protein induction was detected after treatment with as little as 0.25 µM ponasterone (Fig. 1). Immunoblots showed that levels of Cx37 (Fig. 1a, b) and Cx40 (Fig. 1c, d) increased linearly when cells were treated with increasing concentrations of ponasterone in the range between 0.5 and 4.0 µM ponasterone.
Fig. 1.
Treatment of transfected EcR293 cells with ponasterone for 20 h induces a dose-dependent production of Cx37 or Cx40 while relatively stable levels of Cx43 are maintained. Homogenates of EcR293 cells transfected with Cx37 or Cx40 and treated with different doses of ponasterone were analyzed by immunoblotting for Cx37 and Cx43 (a) or Cx40 and Cx43 (b). Graphs show the densitometric values derived from these blots after induction of Cx37 (b) or Cx40 (d). These data were fit with lines according to the equations: y = 4.67x + 1.8 and y = 3.7x + 2.3 with R2 = 0.9443 and R2 = 0.9294 for b and d, respectively
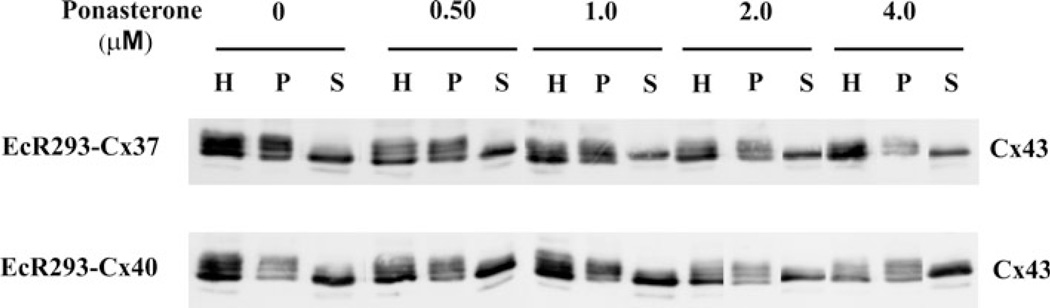
Acquisition of insolubility in 1 % Triton-X-100 has previously been associated with incorporation of Cx43 into gap junction plaques (Musil and Goodenough 1991). We tested whether induction of a second connexin affected the detergent solubility and electrophoretic mobilities of Cx43 in the EcR293-Cx37 and EcR293-Cx40 cells. Cx43 immunoblots had similar appearances in samples derived from both EcR293-Cx37 and EcR293-Cx40 cells regardless of treatment with any concentrations of ponasterone (Fig. 2). In samples prepared from total cellular homogenates or in TritonX-100-insoluble material, three immunoreactive Cx43 bands were observed (likely representing different phosphorylated forms of Cx43 (Musil et al. 1990; Musil and Goodenough 1991)) (Fig. 2, lanes labeled H and P). In contrast samples prepared from the Triton X-100 soluble material predominantly contained a single Cx43 band (likely representing the “NP” form based on its migration faster than the other Cx43 bands). The bands in these immunoblots were quantified by densitometry. This analysis showed that there was little major variation in the distribution of Cx43 between supernatant (Triton soluble) and pellet (Triton insoluble) material regardless of ponasterone treatments; each varied between 40 and 60 % (data not shown).
Fig. 2.
Induced expression of Cx37 or Cx40 in EcR293 cells does not affect the detergent solubility or electrophoretic mobilities of coexpressed Cx43. EcR293-Cx37 and EcR293-Cx40 cells untreated or treated with different concentrations of ponasterone for 20 h. Then cells were harvested; total homogenates (H) were prepared in Triton X-100 and centrifuged to separate them into material that was detergent insoluble (pellet, P) or detergent soluble (supernatant, S). Cx43 was detected by immunoblotting aliquots of the homogenates containing 30 µg protein and corresponding fractions of the supernatants and pellets
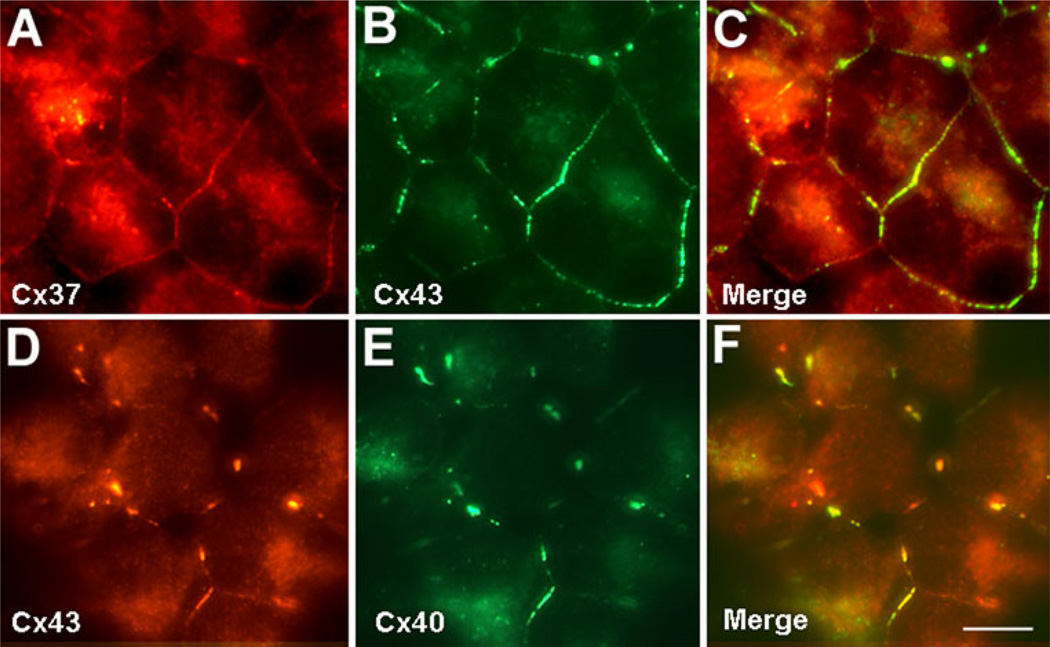
We used double label immunofluorescence microscopy to examine the localization of Cx43 and Cx37 or Cx40 in the coexpressing EcR293 cells. No immunoreactive Cx37 or Cx40 was detected in noninduced EcR293-Cx37 or EcR293-Cx40 cells (not shown). Untreated and ponaster-one treated EcR293-Cx37 and EcR293-Cx40 cells abundantly produced Cx43 which localized within the cytoplasm and at appositional membranes in a distribution consistent with that expected for gap junctions (Fig. 3b, d). After treatment with ponasterone, Cx37 was detected in EcR293-Cx37 cells (Fig. 3a) and Cx40 was detected in EcR293-Cx40 cells (Fig. 3e). Cx37 and Cx40 were both found at appositional membranes where there was substantial overlap with Cx43 (Fig. 3c, f).
Fig. 3.
Induced Cx37 or Cx40 extensively colocalizes with Cx43 at appositional membranes. EcR293-Cx37 (a–c) and EcR293-Cx40 (d–f) cells were treated for 20 h with 0.5 µM ponasterone, and Cx37 (red in a and c), Cx40 (green in e and f), and Cx43 (green in b and c; red in d and f) were detected by double label immunofluorescence. The overlap of immunoreactive Cx37 and Cx43 in EcR293-Cx37 cells or Cx40 and Cx43 in EcR293-Cx40 cells appears yellow in c and f, respectively. Bar = 10 µm
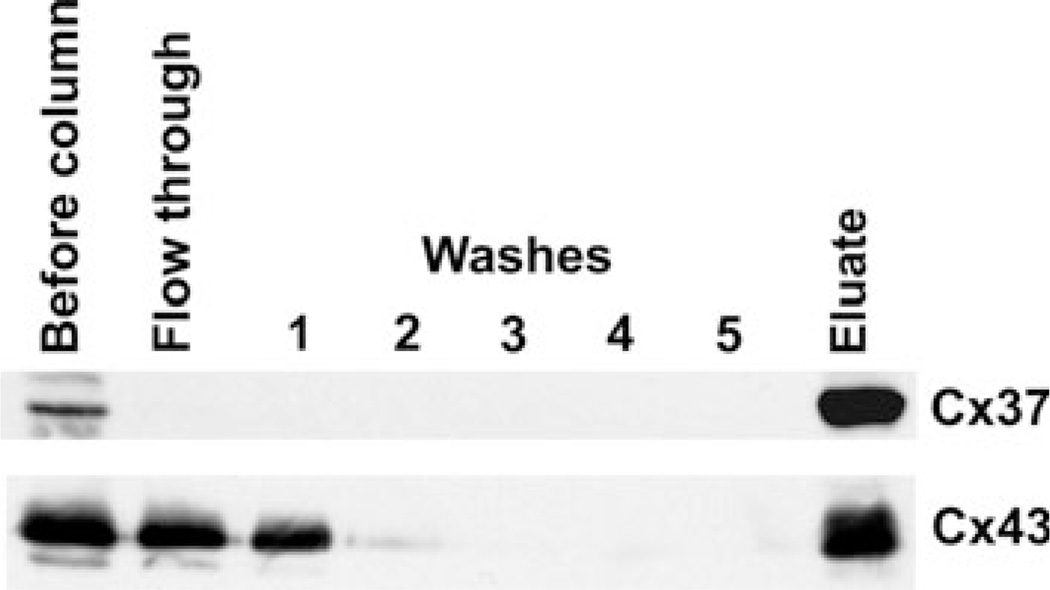
Although we have previously published biochemical data suggesting that Cx43 can form heteromeric connexons with other connexins including Cx40 (He et al. 1999; Valiunas et al. 2001), no such analysis has been conducted for the combination of Cx37 with Cx43. Therefore, we examined the potential heteromeric association of Cx37 with Cx43 using an affinity purification strategy similar to that previously used to study formation of heteromeric connexons between Cx43 and other connexins (Gemel et al. 2006, 2008). The 100,000 g supernatant was prepared from a 1 % Triton X-100 extract of EcR293 cells stably transfected with HA-tagged Cx37, and it was affinity purified using an anti-HA column, which allowed binding and elution of the tagged connexin and any tightly associated proteins. Both Cx37 and Cx43 were recovered in the eluate from this column (Fig. 4). A reasonable explanation for the copurification of Cx43 with Cx37 is that this pair of connexins can also form heteromeric associations.
Fig. 4.
Copurification of Cx43 and Cx37 suggests that they form heteromeric connexons. Triton X-100 soluble material (containing connexons) from EcR293 cells transfected with HA-tagged Cx37 was affinity purified using an anti-HA resin. Fractions of the initial extract, various column fractions, and the eluate were analyzed by immunoblotting. Even though only Cx37 contained the HA-tag, both Cx37 and Cx43 were detected in the eluate
Electrophysiological Characterization of Gap Junction Currents and Channels in EcR293-Cx37 and EcR293-Cx40 Cells
We analyzed the gap junctional currents in pairs of parental EcR293, EcR293-Cx37 and EcR293-Cx40 cells using the double whole-cell patch clamp technique. As Cx37 or Cx40 expression was controlled by an inducible promoter, analysis was performed under control conditions (no ponasterone, with or without ethanol treatment) and in cells treated with two different concentrations of ponasterone (0.5 or 4 µM with comparable ethanol concentration). All analyzed cell pairs showed substantial intercellular coupling (Table 1). Although induction of Cx37 by treatment with either 0.5 or 4 µMponasterone produced an apparent increase (33–60 %) of total conductance, the data did not differ significantly from the mean junctional conductance measured in uninduced EcR293-Cx37 cells. Similarly, mean junctional conductance in EcR293-Cx40 cells was not significantly affected by ponasterone treatment.
Table 1.
Gap junction conductance in pairs of transfected EcR293 cells
| Transfected connexin |
Ponasterone concentration (µM) |
Junctional conductance |
Single channel data |
||
|---|---|---|---|---|---|
| Mean ± SEM (nS) | n | Events | n | ||
| None | 0 (untreated) | 6 ± 3 | 8 | 265 | 6 |
| Cx37 | 0 (ethanol) | 6 ± 2 | 6 | 124 | 2 |
| 0.5 | 9 ± 2 | 17 | 356 | 8 | |
| 4.0 | 8 ± 2 | 18 | 459 | 12 | |
| Cx40 | 0 (untreated) | 5 ± 2 | 4 | 127 | 3 |
| 0 (ethanol) | 5 ± 3 | 7 | 541 | 6 | |
| 0.5 | 4 ± 1 | 16 | 663 | 10 | |
| 4.0 | 6 ± 1 | 17 | 850 | 12 | |
nS nanoSiemens
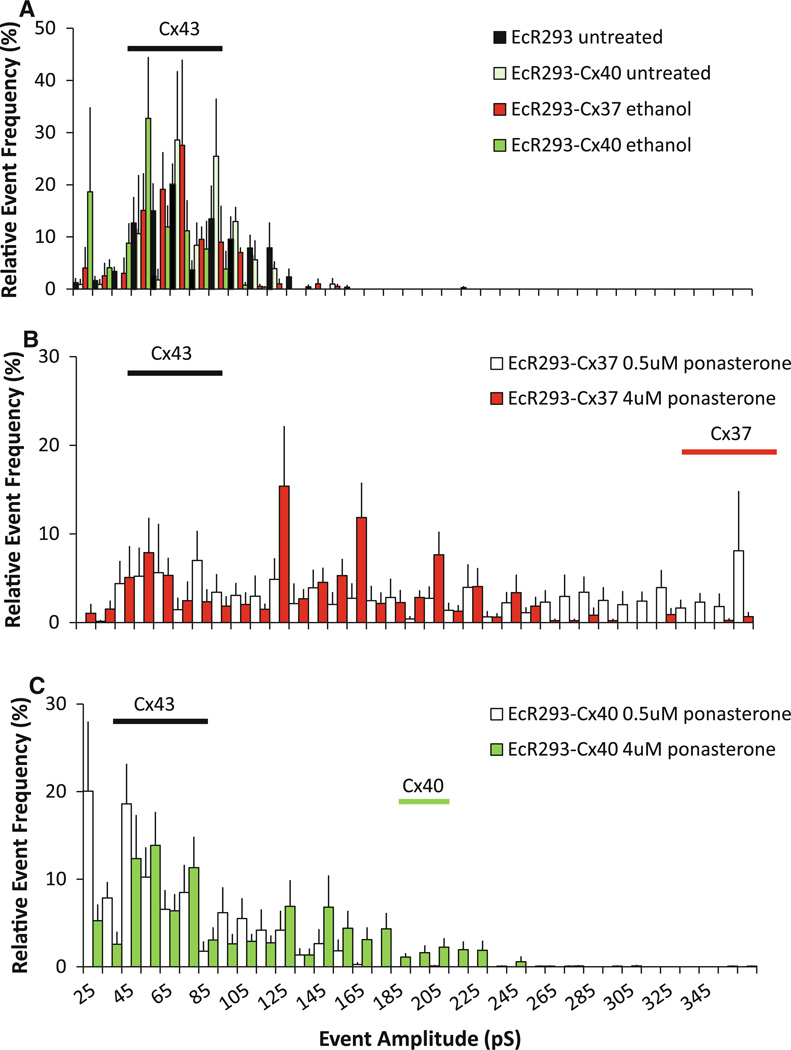
Individual channel events were observed in poorly coupled pairs of EcR293, EcR293-Cx37 or EcR293-Cx40 cells. In EcR293 cells and noninduced EcR293-Cx37 or EcR293- Cx40 cells (with or without ethanol exposure), most channel events were between 50 and 100 pS, and very few events larger than 120 pS were observed (Fig. 5a). This range is consistent with the expected conductances of Cx43 channels (approximately 50, 80 and 100 pS) recorded under these conditions (Cottrell and Burt 2001; Cottrell et al. 2002). In cells coexpressing Cx37 and Cx43 (Fig. 5b) or EcR293- Cx40 and Cx43 (Fig. 5c) a diversity of unitary channel conductances were observed many of which were much larger than observed in the absence of induced coexpression.
Fig. 5.
Induced coexpression of Cx37 or Cx40 with Cx43 alters the distribution of single channel events with a shift toward larger unitary conductances. Single gap junction channel events were recorded in pairs of EcR293 cells, EcR293-Cx37 cells, and EcR293-Cx40 cells. a Control experiments including untreated EcR293 (black bars) and EcR-Cx40 cells (light green bars) and ethanol (vehicle) treated EcR-Cx37 (red bars) and EcR-Cx40 cells (dark green bars). b EcR293-Cx37 cells exposed to 0.5 or 4 µM ponasterone (white and red bars, respectively). c EcR293-Cx40 cells exposed to 0.5 or 4 µM ponasterone (white and green bars, respectively). Horizontal bars indicate the distributions of the most common Cx43 single channel events (black bars in all panels; 50–110 pS) and the fully open homomeric/homotypic Cx37 channels (red bar in b; 330–370 pS) or Cx40 channels (green bar in c; 190–220 pS). Coexpression of either Cx37 (b) or Cx40 (c) with Cx43 resulted in many events with amplitudes greater than those common in the Cx43 expressing EcR293 cells (a), but smaller than fully open homomeric/homotypic Cx37 channels or Cx40 channels. The numbers of cell pairs (n) and events recorded in each data set are shown in Table 1
Properties of Gap Junction Channels in EcR-Cx37 or EcR-Cx40 Cells Assessed by Dye Transfer
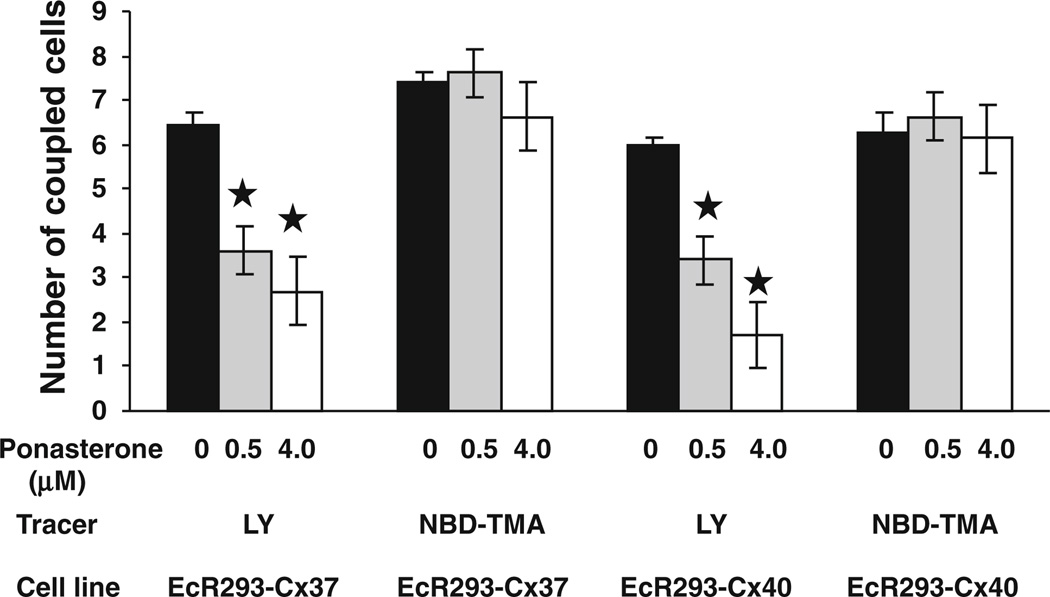
To determine how induced coexpression of Cx37 or Cx40 with Cx43 affected intercellular passage of larger molecules, we microinjected two different tracers (Lucifer yellow or NBD-TMA) into noninduced EcR293-Cx37 or EcR293-Cx40 cells or into cells that had been treated with 0.5 or 4 µM ponasterone. Dye transfer was quantified by performing multiple injections and counting dye-filled neighbors (Fig. 6). Microinjected Lucifer yellow transferred to 6.4 ± 0.3 or 6.0 ± 0.4 neighbors (mean ± SEM) for uninduced EcR-Cx37 or EcR-Cx40 cells, respectively. Induction of Cx37 or Cx40 by treatment with 0.5 µM ponasterone significantly reduced Lucifer yellow transfer by nearly 50 %. Induction of greater levels of the second connexin by treatment with 4 µM ponasterone reduced Lucifer yellow transfer to even lower levels; however, differences between dye transfer in cells induced with 0.5 and 4 µM ponasterone did not achieve statistical significance. Uninduced EcR-Cx37 and EcR-Cx40 cells exhibited robust transfer of NBD-TMA, but there was no significant change in NBD-TMA transfer among these cells in response to treatment with either 0.5 or 4 µM ponasterone (Fig. 6).
Fig. 6.
Induced coexpression of Cx37 or Cx40 with Cx43 selectively affects the intercellular transfer of two different tracers. Intercellular transfer of microinjected dyes was studied in uninduced or induced EcR293-Cx37 or EcR293-Cx40 cells. Lucifer yellow or NBD-TMA were microinjected into individual cells within a monolayer. After 1 min, dye-filled neighbors were counted. Black bars indicate untreated cells, gray bars cells induced with 0.5 µM ponasterone, and white bars cells induced with 4 µM ponasterone. Values represent the mean ± SEM (n ≥ 13 for Lucifer yellow; n = 8 for NBD-TMA). *p < 0.05 as compared to 0 µM ponasterone)
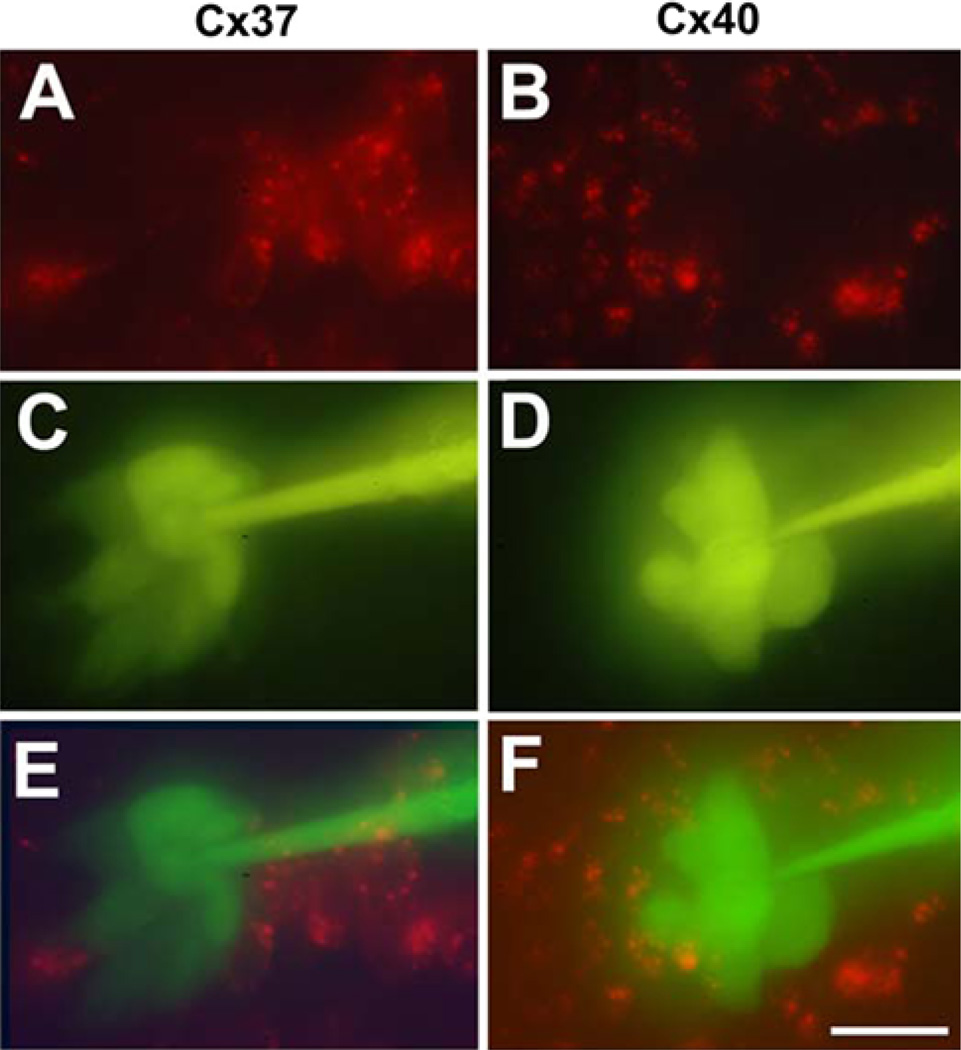
In vivo, cells expressing one connexin may border cells coexpressing multiple connexins, and these differences in connexin expression might affect intercellular communication within that tissue. We mimicked this situation by coculturing EcR293 cells with ponasterone-induced EcR293-Cx37 or EcR293-Cx40 cells. Lucifer yellow was microinjected into EcR293 cells, and we examined the intercellular transfer of to neighboring EcR293 cells (unlabeled) and to ponasterone-induced EcR293-Cx37 or EcR293-Cx40 cells (labeled with PKH26). Representative photomicrographs obtained after such injections are shown in Fig. 7. In all injections, we observed substantial dye transfer to EcR cell recipients: 7.9 ± 1.0 (n = 7) in EcR/EcR-Cx37 coculture and 8.0 ± 0.3 (n = 16) in EcR/EcR-Cx40 coculture; however, no Lucifer yellow labeled EcR-Cx37 or EcR-Cx40 cell neighbors were observed.
Fig. 7.
Coexpression of Cx37 or Cx40 with Cx43 restricts communication with cells expressing Cx43 alone. Intercellular transfer of microinjected Lucifer yellow was studied in EcR293-Cx37 (a, c, e) or EcR293-Cx40 (b, d, f) cells induced with 4 µM ponasterone and cocultured with EcR293 cells. EcR293-Cx37 and EcR293-Cx40 cells were labeled with PKH26 red fluorescent cell linker while cocultured EcR293 cells were not labeled (a, b). Lucifer yellow was injected into an EcR293 cell (unlabeled) that neighbored both other EcR293 cells and labeled EcR293-Cx37 (c) or EcR293-Cx40 (d) cells. Superimposed images e, f show dye transfer only among the EcR293 cells, but not to the (red labeled) cocultured EcR293-Cx37 or EcR293-Cx40 cells. Bar = 30 µm
Discussion
In the current paper, we have developed a system for inducible expression of connexins and used it to examine the functional consequences of their coexpression. Inducible systems have previously been exploited to examine some other aspects of the biology of connexins. Zhong et al. (2003) used a lactose-inducible/regulatable system to study effects of differing amounts of Cx43 on its gating and single channel properties in N2A cells. Burt et al. (2008) used an inducible system to express Cx37 in RIN cells and study the growth properties of Cx37-expressing cells because they were unable to isolate constitutively Cx37- expressing clones (because they would not proliferate). Ours is the first study to use inducible coexpression specifically to study effects of different relative amounts of two coexpressed connexins on the properties of channels in the coexpressing cells. Our transfections of cells derived from human embryonic kidney (HEK293) that endogenously express Cx43 with Cx37 or Cx40 allowed us to mimic the complexity of the intercellular communication present in endothelial cells or atrial myocytes that coexpress differing ratios of these connexins in vivo. Our current studies have expanded upon the data available from previous studies of coexpression of connexins by allowing regulated coexpression.
When we induced Cx37 or Cx40, these connexins extensively colocalized with Cx43, implying that the two connexins were both present in the same gap junction plaques. We have previously studied connexons that were enriched by centrifugation, and shown that Cx40 and Cx43 copurify (Valiunas et al. 2001) and can be coimmunoprecipitated (He et al. 1999) suggesting that at least some hemichannels contain both connexins. Similarly, in the current study, we found that Cx37 and Cx43 copurified; this result provides biochemical support for the extensive previous physiological data suggesting that Cx37 and Cx43 can make heteromeric channels with intermediate properties (Brink et al. 1997).
Our biochemical studies suggest that induced coexpression of Cx37 or Cx40 had relatively little effect on the endogenous Cx43. The relative amounts of Cx43 that were insoluble in Triton X-100 were little changed by induced expression of a second connexin (Fig. 2), suggesting little change in the fraction of Cx43 contained in gap junction plaques (Musil and Goodenough 1991). Similarly, the electrophoretic mobility pattern of Cx43 observed in immunoblots (a crude surrogate for phosphorylation; Musil and Goodenough 1991) also showed little change. The absolute amount of Cx43 did not show significant changes with Cx37 or Cx40 induction (Figs. 1, 2).
We observed selective effects on dye transfer after induction of Cx37 or Cx40. Some of these results may be explained by differences in the charge selectivity of channels containing the different connexins: Cx40 and Cx37 channels are cation selective, while Cx43 channels are nonselective. We observed that induced expression of Cx40 or Cx37 reduced the extent of transfer of Lucifer yellow (charge −2) but did not affect transfer of NBDTMA (charge +1). These data suggest that after induction the intercellular transfer was dominated by the most restrictive connexin. It was previously observed that the properties of a heteromeric channel may be determined by the more selective connexin (Martinez et al. 2002). Interestingly, induction of high levels of Cx37 or Cx40 by treatment with 4 µM ponasterone did not completely abolish Lucifer yellow transfer. This argues that these cells still contained a substantial number of channels that were permeable to this tracer, possibly homomeric or predominantly Cx43-containing channels, although previously published data do suggest that negatively charged molecules as large as ATP can permeate Cx37 hemichannels (and likely intercellular gap junction channels) (Wong et al. 2006; Derouette et al. 2009). Our dye transfer studies also illustrate an obvious consequence of the different molecular permeabilities of these connexins and their heteromeric combinations, boundary formation; when cells expressing only (or predominantly) Cx43 encounter a boundary with cells coexpressing Cx37 or Cx40, the intercellular passage of larger and negatively charged molecules is restricted (Fig. 7).
In both Cx37/Cx43 and Cx40/Cx43 coexpressing cells, we observed a large variety of single channel conductances. As with previous studies of cells stably coexpressing these connexins, the unitary conductances that differ from the expected sizes of homomeric/homotypic Cx43, Cx37 or Cx40, likely represent “mixed” channels containing both of the coexpressed connexins; we favor the interpretation that many of them are heteromeric Cx37/Cx43 and Cx40/Cx43 channels. The conductance of the fully open Cx37 channel is 330–370 pS (Veenstra et al. 1994; Kumari et al. 2000), and that of Cx40 is 190–220 pS (Cottrell and Burt 2001; Cottrell et al. 2002). Because these are larger than fully open Cx43 channels (~100 pS), the shift toward larger conductances with induced expression of Cx37 or Cx40 matches expectations for production of heteromeric channels (containing Cx43 and the induced connexin). Heterotypic channels represent a special case of “mixed” channels formed by the docking of homomeric connexons containing the two different connexins. Certainly, heterotypic Cx37/ Cx43 and Cx40/Cx43 channels might account for some of the diversity of unitary conductances observed after induced coexpression. Indeed, the histograms shown in Fig. 5c do not appear dramatically different from our previous studies of heterotypic Cx40-Cx43 performed using transfected HeLa or RIN cells (Valiunas et al. 2001; Cottrell et al. 2002). As previously stated, determination of the abundance and distributions of channel sizes do not permit us to distinguish unambiguously between homotypic, heterotypic, and heteromeric forms (Valiunas et al. 2001). However, we suspect limited contribution of Cx40-Cx43 heterotypic channels because our studies and those of others have shown very reduced junctional conductances produced by pairing of Cx40- and Cx43-expressing cells (Cottrell et al. 2002; Rackauskas et al. 2007).
When we induced Cx37 expression, the mean values for total junctional conductance in cell pairs appeared to be increased (Table 1) (although not statistically different from the uninduced controls). An increase in total conductance would have been expected if induction led to a greater total amount of connexins in these cells. These cells may have contained a greater number of gap junction channels; or, alternatively, the increased total conductance could be explained by a nearly constant number of total channels that were larger in individual conductance (Fig. 4). That Cx37 expression did not significantly increase electrical coupling could reflect a relatively small sample size in the face of considerable pair to pair variability. However, it could also reflect a decrease in channel permselectivity (as suggested by the parallel dye coupling data) or open probability differences (long duration events were much rarer in Cx37 coexpressing cells than in either the parental cells or Cx40 coexpressing cells). The total junctional conductance of Cx40-expressing cell pairs was certainly not different from uninduced cells. Total conductance might be unchanged if Cx43 was reduced after Cx40 induction; however, we did not observe a statistically significant change in Cx43 levels by immunoblotting. Alternatively, it might reflect the formation of some heteromeric channels with differential permselective properties (or even some that are nonfunctional). It is also possible that the 293 cells have an absolute upper limit (a fixed capacity) to the number of gap junctional channels that they can form thereby preventing an increase in channel number with expression of Cx37 or Cx40.
We have previously observed that expression of Cx37 leads to the death of endothelial cells (Seul et al. 2004) and greatly slows the proliferation of rat insulinoma cells (Burt et al. 2008). The effects of Cx37 on vascular growth require a functional channel (Good et al. 2011). However, in the current study, treatment of our cells with ecdysone (and its ethanol vehicle) and induction of either Cx37 or Cx40 had little or no observed effects on growth or cell viability (data not shown). This lack of effect may represent the brief induction period required to perform the present experiments. It may also reflect our choice of human Cx37 polymorphic variant; we studied the serine- 319 polymorphic variant. Morel et al. (2010) have observed that the proline-319 variant slows the proliferation of tumor cells, while the serine-319 variant has no effect. Finally, it may be that our incorporation of an epitope tag (FLAG) at the end of Cx37 reduced its toxicity.
There is certainly substantial biological importance of our observation that altered coexpression of two connexins in the same cell has substantial functional consequences (like alteration of the intercellular exchange of different small molecules). For example, changes in the connexin expression ratio can affect the response of cells to growth factors. Burt and Steele (2003) observed that Cx43 channels were sensitive to PDGF, while Cx40 channels were not; intermediate sensitivity could be achieved by modifying the Cx40/Cx43 expression ratio. In the current study we have generated cell lines in which the stoichiometry of connexin coexpression can be regulated; these cell lines may be useful for elucidating how intercellular exchange of small molecules that differ in charge (and consequent permeation through the endogenous vs. induced intercellular channels) affect various processes such as stimulated proliferation.
Acknowledgments
This work was supported by National Institutes of Health grants HL59199 (ECB), 5R01HL058732 (JMB), and 5R01HL077675 (JMB).
Contributor Information
Joanna Gemel, Department of Pediatrics, University of Chicago, Chicago, IL, USA.
Tasha K. Nelson, Department of Physiology, University of Arizona, Tucson, AZ, USA
Janis M. Burt, Department of Physiology, University of Arizona, Tucson, AZ, USA
Eric C. Beyer, Email: ebeyer@peds.bsd.uchicago.edu, Department of Pediatrics, University of Chicago, Chicago, IL, USA; Section of Pediatric Hematology/Oncology, University of Chicago, 900 E 57th St., KCBD 5152, Chicago, IL 60637, USA.
References
- Beblo DA, Wang HZ, Beyer EC, Westphale EM, Veenstra RD. Unique conductance, gating, and selective permeability properties of gap junction channels formed by connexin40. Circ Res. 1995;77:813–822. doi: 10.1161/01.res.77.4.813. [DOI] [PubMed] [Google Scholar]
- Bednarczyk D, Mash EA, Aavula BR, Wright SH. NBD-TMA: a novel fluorescent substrate of the peritubular organic cation transporter of renal proximal tubules. Pflugers Arch. 2000;440:184–192. doi: 10.1007/s004240000283. [DOI] [PubMed] [Google Scholar]
- Berthoud VM, Montegna EA, Atal N, Aithal NH, Brink PR, Beyer EC. Heteromeric connexons formed by the lens connexins, connexin43 and connexin56. Eur J Cell Biol. 2001;80:11–19. doi: 10.1078/0171-9335-00132. [DOI] [PubMed] [Google Scholar]
- Brink PR, Cronin K, Banach K, Peterson E, Westphale EM, Seul KH, Ramanan SV, Beyer EC. Evidence for heteromeric gap junction channels formed from rat connexin43 and human connexin37. Am J Physiol (Cell Physiol) 1997;273:C1386–C1396. doi: 10.1152/ajpcell.1997.273.4.C1386. [DOI] [PubMed] [Google Scholar]
- Brisset AC, Isakson BE, Kwak BR. Connexins in vascular physiology and pathology. Antioxid Redox Signal. 2009;11:267–282. doi: 10.1089/ars.2008.2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt JM, Steele TD. Selective effect of PDGF on connexin43 versus connexin40 comprised gap junction channels. Cell Commun Adhes. 2003;10:287–291. doi: 10.1080/cac.10.4-6.287.291. [DOI] [PubMed] [Google Scholar]
- Burt JM, Fletcher AM, Steele TD, Wu Y, Cottrell GT, Kurjiaka DT. Alteration of Cx43:Cx40 expression ratio in A7r5 cells. Am J Physiol Cell Physiol. 2001;280:C500–C508. doi: 10.1152/ajpcell.2001.280.3.C500. [DOI] [PubMed] [Google Scholar]
- Burt JM, Nelson TK, Simon A, Fang JS. Connexin 37 profoundly slows cell cycle progression in rat insulinoma cells. Am J Physiol Cell Physiol. 2008;296:C1103–C1112. doi: 10.1152/ajpcell.299.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CJ, Wu LS, Hsu LA, Chang GJ, Chen CF, Yeh HI, Ko YS. Differential endothelial gap junction expression in venous vessels exposed to different hemodynamics. J Histochem Cytochem. 2010;58:1083–1092. doi: 10.1369/jhc.2010.956425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanson M, Derouette JP, Roth I, Foglia B, Scerri I, Dudez T, Kwak BR. Gap junctional communication in tissue inflammation and repair. Biochim Biophys Acta. 2005;1711:197–207. doi: 10.1016/j.bbamem.2004.10.005. [DOI] [PubMed] [Google Scholar]
- Cottrell GT, Burt JM. Heterotypic gap junction channel formation between heteromeric and homomeric Cx40 and Cx43 connexons. Am J Physiol Cell Physiol. 2001;281:C1559–C1567. doi: 10.1152/ajpcell.2001.281.5.C1559. [DOI] [PubMed] [Google Scholar]
- Cottrell GT, Burt JM. Functional consequences of heterogeneous gap junction channel formation and its influence in health and disease. Biochim Biophys Acta. 2005;1711:126–141. doi: 10.1016/j.bbamem.2004.11.013. [DOI] [PubMed] [Google Scholar]
- Cottrell GT, Wu Y, Burt JM. Cx40 and Cx43 expression ratio influences heteromeric/heterotypic gap junction channel properties. Am J Physiol Cell Physiol. 2002;282:C1469–C1482. doi: 10.1152/ajpcell.00484.2001. [DOI] [PubMed] [Google Scholar]
- Delorme B, Dahl E, Jarry-Guichard T, Briand JP, Willecke K, Gros D, Theveniau-Ruissy M. Expression pattern of connexin gene products at the early developmental stages of the mouse cardiovascular system. Circ Res. 1997;81:423–437. doi: 10.1161/01.res.81.3.423. [DOI] [PubMed] [Google Scholar]
- Derouette JP, Desplantez T, Wong CW, Roth I, Kwak BR, Weingart R. Functional differences between human Cx37 polymorphic hemichannels. J Mol Cell Cardiol. 2009;46:499–507. doi: 10.1016/j.yjmcc.2008.12.018. [DOI] [PubMed] [Google Scholar]
- Ek-Vitorin JF, Burt JM. Quantification of gap junction selectivity. Am J Physiol Cell Physiol. 2005;289:C1535–C1546. doi: 10.1152/ajpcell.00182.2005. [DOI] [PubMed] [Google Scholar]
- Gabriels JE, Paul DL. Connexin43 is highly localized to sites of disturbed flow in rat aortic endothelium but connexin37 and connexin40 are more uniformly distributed. Circ Res. 1998;83:636–643. doi: 10.1161/01.res.83.6.636. [DOI] [PubMed] [Google Scholar]
- Gemel J, Valiunas V, Brink PR, Beyer EC. Connexin43 and connexin26 form gap junctions, but not heteromeric channels in co-expressing cells. J Cell Sci. 2004;117:2469–2480. doi: 10.1242/jcs.01084. [DOI] [PubMed] [Google Scholar]
- Gemel J, Lin X, Veenstra RD, Beyer EC. N-terminal residues in Cx43 and Cx40 determine physiological properties of gap junction channels, but do not influence heteromeric assembly with each other or with Cx26. J Cell Sci. 2006;119:2258–2268. doi: 10.1242/jcs.02953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gemel J, Lin X, Collins R, Veenstra RD, Beyer EC. Cx30.2 can form heteromeric gap junction channels with other cardiac connexins. Biochem Biophys Res Commun. 2008;369:388–394. doi: 10.1016/j.bbrc.2008.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good ME, Nelson TK, Simon AM, Burt JM. A functional channel is necessary for growth suppression by Cx37. J Cell Sci. 2011;124:2448–2456. doi: 10.1242/jcs.081695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haefliger JA, Nicod P, Meda P. Contribution of connexins to the function of the vascular wall. Cardiovasc Res. 2004;62:345–356. doi: 10.1016/j.cardiores.2003.11.015. [DOI] [PubMed] [Google Scholar]
- Harris AL. Emerging issues of connexin channels: biophysics fills the gap. Q Rev Biophys. 2001;34:325–472. doi: 10.1017/s0033583501003705. [DOI] [PubMed] [Google Scholar]
- He DS, Jiang JX, Taffet SM, Burt JM. Formation of heteromeric gap junction channels by connexins 40 and 43 in vascular smooth muscle cells. Proc Natl Acad Sci USA. 1999;96:6495–6500. doi: 10.1073/pnas.96.11.6495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyman NS, Burt JM. Hindered diffusion through an aqueous pore describes invariant dye selectivity of Cx43 junctions. Biophys J. 2008;94:840–854. doi: 10.1529/biophysj.107.115634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyman NS, Kurjiaka DT, Ek Vitorin JF, Burt JM. Regulation of gap junctional charge selectivity in cells coexpressing connexin 40 and connexin 43. Am J Physiol Heart Circ Physiol. 2009;297:H450–H459. doi: 10.1152/ajpheart.00287.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone S, Isakson B, Locke D. Biological and biophysical properties of vascular connexin channels. Int Rev Cell Mol Biol. 2009;278:69–118. doi: 10.1016/S1937-6448(09)78002-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari SS, Varadaraj K, Valiunas V, Ramanan SV, Christensen EA, Beyer EC, Brink PR. Functional expression and biophysical properties of polymorphic variants of the human gap junction protein connexin37. Biochem Biophys Res Commun. 2000;274:216–224. doi: 10.1006/bbrc.2000.3054. [DOI] [PubMed] [Google Scholar]
- Kwong KF, Schuessler RB, Green KG, Laing JG, Beyer EC, Boineau JP, Saffitz JE. Differential expression of gap junction proteins in the canine sinus node. Circ Res. 1998;82:604–612. doi: 10.1161/01.res.82.5.604. [DOI] [PubMed] [Google Scholar]
- Laing JG, Beyer EC. The gap junction protein connexin43 is degraded via the ubiquitin proteasome pathway. J Biol Chem. 1995;270:26399–26403. doi: 10.1074/jbc.270.44.26399. [DOI] [PubMed] [Google Scholar]
- Lin X, Gemel J, Glass A, Zemlin CW, Beyer EC, Veenstra RD. Connexin40 and connexin43 determine gating properties of atrial gap junction channels. J Mol Cell Cardiol. 2010;48:238–245. doi: 10.1016/j.yjmcc.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez AD, Hayrapetyan V, Moreno AP, Beyer EC. Connexin43 and connexin45 form heteromeric gap junction channels in which individual components determine permeability and regulation. Circ Res. 2002;90:1100–1107. doi: 10.1161/01.res.0000019580.64013.31. [DOI] [PubMed] [Google Scholar]
- Morel S, Burnier L, Roatti A, Chassot A, Roth I, Sutter E, Galan K, Pfenniger A, Chanson M, Kwak BR. Unexpected role for the human Cx37 C1019T polymorphism in tumour cell proliferation. Carcinogenesis. 2010;31:1922–1931. doi: 10.1093/carcin/bgq170. [DOI] [PubMed] [Google Scholar]
- Musil LS, Goodenough DA. Biochemical analysis of connexin43 intracellular transport, phosphorylation, and assembly into gap junctional plaques. J Cell Biol. 1991;115:1357–1374. doi: 10.1083/jcb.115.5.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musil LS, Cunningham BA, Edelman GM, Goodenough DA. Differential phosphorylation of the gap junction protein connexin43 in junctional communication-competent and -deficient cell lines. J Cell Biol. 1990;111:2077–2088. doi: 10.1083/jcb.111.5.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rackauskas M, Kreuzberg MM, Pranevicius M, Willecke K, Verselis VK, Bukauskas FF. Gating properties of heterotypic gap junction channels formed of connexins 40, 43, and 45. Biophys J. 2007;92:1952–1965. doi: 10.1529/biophysj.106.099358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saez JC, Berthoud VM, Branes MC, Martinez AD, Beyer EC. Plasma membrane channels formed by connexins: their regulation and functions. Physiol Rev. 2003;83:1359–1400. doi: 10.1152/physrev.00007.2003. [DOI] [PubMed] [Google Scholar]
- Seul KH, Kang KY, Lee KS, Kim SH, Beyer EC. Adenoviral delivery of human connexin37 induces endothelial cell death through apoptosis. Biochem Biophys Res Commun. 2004;319:1144–1151. doi: 10.1016/j.bbrc.2004.05.097. [DOI] [PubMed] [Google Scholar]
- Severs NJ, Bruce AF, Dupont E, Rothery S. Remodelling of gap junctions and connexin expression in diseased myocardium. Cardiovasc Res. 2008;80:9–19. doi: 10.1093/cvr/cvn133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valiunas V, Weingart R, Brink PR. Formation of heterotypic gap junction channels by connexins 40 and 43. Circ Res. 2000;86:E42–E49. doi: 10.1161/01.res.86.2.e42. [DOI] [PubMed] [Google Scholar]
- Valiunas V, Gemel J, Brink PR, Beyer EC. Gap junction channels formed by coexpressed connexin40 and connexin43. Am J Physiol Heart Circ Physiol. 2001;281:H1675–H1689. doi: 10.1152/ajpheart.2001.281.4.H1675. [DOI] [PubMed] [Google Scholar]
- Valiunas V, Beyer EC, Brink PR. Cardiac gap junction channels show quantitative differences in selectivity. Circ Res. 2002;91:104–111. doi: 10.1161/01.res.0000025638.24255.aa. [DOI] [PubMed] [Google Scholar]
- Veenstra RD, Wang HZ, Beyer EC, Ramanan SV, Brink PR. Connexin37 forms high conductance gap junction channels with subconductance state activity and selective dye and ionic permeabilities. Biophys J. 1994;66:1915–1928. doi: 10.1016/S0006-3495(94)80985-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veenstra RD, Wang HZ, Beblo DA, Chilton MG, Harris AL, Beyer EC, Brink P. Selectivity of connexin-specific gap junctions does not correlate with channel conductance. Circ Res. 1995;77:1156–1165. doi: 10.1161/01.res.77.6.1156. [DOI] [PubMed] [Google Scholar]
- Weber PA, Chang HC, Spaeth KE, Nitsche JM, Nicholson BJ. The permeability of gap junction channels to probes of different size is dependent on connexin composition and permeant-pore affinities. Biophys J. 2004;87:958–973. doi: 10.1529/biophysj.103.036350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong CW, Christen T, Roth I, Chadjichristos CE, Derouette JP, Foglia BF, Chanson M, Goodenough DA, Kwak BR. Connexin37 protects against atherosclerosis by regulating monocyte adhesion. Nat Med. 2006;12:950–954. doi: 10.1038/nm1441. [DOI] [PubMed] [Google Scholar]
- Yeh HI, Chang HM, Lu WW, Lee YN, Ko YS, Severs NJ, Tsai CH. Age-related alteration of gap junction distribution and connexin expression in rat aortic endothelium. J Histochem Cytochem. 2000;48:1377–1389. doi: 10.1177/002215540004801008. [DOI] [PubMed] [Google Scholar]
- Zhong G, Mantel PL, Jiang X, Jarry-Guichard T, Gros D, Labarrere C, Moreno AP. LacSwitch II regulation of connexin43 cDNA expression enables gap-junction single-channel analysis. Biotechniques. 2003;34:1034–1046. doi: 10.2144/03345rr03. [DOI] [PubMed] [Google Scholar]