Abstract
Low temperatures at the initial stages of rice development prevent fast germination and seedling establishment and may cause significant productivity losses. In order to develop rice cultivars exhibiting cold tolerance, it is necessary to investigate genetic resources, providing basic knowledge to allow the introduction of genes involved in low temperature germination ability from accessions into elite cultivars. Japanese rice accessions were evaluated at the germination under two conditions: 13°C for 28 days (cold stress) and 28°C for seven days (optimal temperature). The traits studied were coleoptile and radicle length under optimal temperature, coleoptile and radicle length under cold and percentage of the reduction in coleptile and radicle length due to low temperature. Among the accessions studied, genetic variation for traits related to germination under low temperatures was observed and accessions exhibiting adequate performance for all investigated traits were identified. The use of multivariate analysis allowed the identification of the genotypes displaying cold tolerance by smaller reductions in coleoptile and radicle lenght in the presence of cold and high vigour, by higher coleoptile and radicle growth under cold.
Keywords: cold tolerance, rice, germplasm, genetic variation, germination, low temperature
Introduction
Oryza sativa is a worldwide spread crop, being cultivated in tropical, subtropical and temperate areas, as well as at high and low altitude regions. Temperatures lower than the optimal for rice cultivation occur in several regions during one or more growing stages and may cause significant productivity losses. Low temperatures during rice growing season constitute a serious problem in countries such as Australia, China, Indonesia, Japan, South Korea, Nepal, the United States (Yoshida 1981) and Southern Latin America, mainly Chile (Castillo and Alvarado 2002) and Brazil (Cruz and Milach 2004).
Intraspecific genetic variation to cold tolerance has been identified in rice. Japonica cultivars are, in general, more tolerant than those from the indica group (Andaya and Mackil 2003). Generally, tropical japonica genotypes show characteristics intermediate between indica and temperate japonica cultivars, however, no significant differences in cold tolerance are observed between tropical and temperate japonica groups. Although tropical japonica cultivars are adapted to tropical regions, they exhibit traits of low temperature adaptation as those found in temperate japonica cultivars (Mackill and Lei 1997). Low temperature adaptation is a trait under strong selective pressure for rice grown under temperate climates, mainly in the northeast of Asia (China, Korea and Japan). Japonica cultivars grown in high altitudes in Southeastern Asia may have also acquired the traits underlying low temperature adaptation. Genetic variation for cold tolerance is associated to the genotype geographical distribution, indicating that the genetic differentiation for cold tolerance is the result of local adaptation (Baruah et al. 2009, Mackill and Lei 1997).
Due to the negative effects of low temperature on rice growth, cold tolerance is an important feature for both, temperate and high altitude, regions. In rice breeding programs, cold tolerance is mainly evaluated at germination, seedling and reproductive stages. In early developmental stages, low temperatures can impair and delay germination, as well as have negative impacts on seedling growth, causing poor stand establishment and non-uniform crop maturation.
Genetic variability for cold tolerance at the initial development stages in rice has been reported for some cultivars, mainly from the japonica subspecies (Sthapit and Witcombre 1998). High variability for germination under low temperatures has been observed in more than 700 varieties from Japan, Europe, China, Russia and other regions (Kotaka and Abe 1988). It has also been reported that cultivars originated from high latitudes germinate faster than those from low latitudes (Takahashi 1997) and that cultivars native from Hokkaido, Japan and South Korea show high germination rates under low temperatures, such as 8°C, whereas indica cultivars exhibit lower germination frequencies under cold stress (Takahashi 1997).
In order to develop rice cultivars exhibiting adequate seedling establishment under low temperatures, it is necessary to investigate genetic resources originated from various regions of the world, providing basic knowledge to allow the introduction of genes involved in low temperature germination ability from landraces and traditional varieties into elite cultivars (Miura et al. 2001).
Due to the difficulties in field selection for cold tolerance, it is necessary to develop strategies for controlled condition experiments to phenotype the material. Tests based on seedling length performed under controlled conditions are adequate indicators of field performance for the number of days to emergence, emergence percentage and emergence index (Jones and Peterson 1976), whereas coleoptile length is well-correlated to low temperature seedling establishment under field conditions (Ogiwara and Terashima 2001).
Phenotyping methods for cold tolerance at the germination stage under controlled conditions were developed and have allowed the characterization of tolerant and susceptible plant genotypes (Cruz and Milach 2004, Jones and Peterson 1976). These methods consist in evaluating traits such as germination index, radicle and coleoptile length in seedlings germinated under low temperatures. The approach has been used in studies for cold tolerance inheritance (Cruz et al. 2006, Sharifi 2008, Sthapit and Witcombre 1998), QTL mapping (Fujino et al. 2004, Miura et al. 2001) and characterization and selection of cold tolerance gene sources in germplasm banks (Castillo and Alvarado 2002, Sharifi 2010, Sharifi and Aminpanah 2010).
The rice germplasm bank at the Genetics Department of ESALQ/USP has approximately 450 cultivated rice accessions, being 192 from Japanese sources and introduced in Brazil by Dr. Akihiko Ando. High germinability under low temperatures in rice seeds from various regions of Japan has been reported (Takahashi 1997), suggesting that, among the Japanese accessions maintained by the germplam bank at ESALQ cold tolerance sources for breeding programs are likely to be present. Thus, the current work aimed to evaluate the cold tolerance of the Japanese rice accessions at the germination stage and to study cold tolerance-related traits to establish selection criteria using multivariate analyses.
Materials and Methods
Plant material
A total of 195 rice genotypes were investigated in this study; 192 Japanese accessions of unknown response to cold at germination (Table 1) and three cold tolerant control genotypes (CT 6748-8CA-17P, L 201 e QUILLA 66304). For each genotype, seeds harvested in the same cropping season were selected based on size uniformity and absence of spots. After harvesting, seeds were stored at 15°C and relative humidity of 40% for 6 months before being used in the germination study.
Table 1.
Rice germplasm accessions evaluated for cold tolerance at the germination stage. All accessions are of Japanese origin and are maintained at the Seeds Bank of the Genetics Department–ESALQ/USP
| Number | Name |
|---|---|
| 1 | Kunihikari Mochi |
| 2 | Senshou |
| 3 | Fukuton |
| 4 | Ezo Wase |
| 5 | Shin Hakaburi |
| 6 | Senshou Ibaragi 1 |
| 7 | Yamanoi |
| 8 | Namekata Mochi |
| 9 | Sonobe Mochi |
| 10 | Wase Mochi |
| 11 | Seion Uruchi |
| 12 | Gaisen Mochi |
| 13 | Shiro Hige |
| 14 | Kinkabou |
| 15 | Nakaahara Mochi |
| 16 | Nourin Mochi |
| 17 | Toukyo Hirayama |
| 18 | Iwata Hata Mochi |
| 19 | Susono Mochi |
| 20 | Mitsukasane |
| 21 | Mie |
| 22 | Wase Esoshima Mochi |
| 23 | Mizuhoshi |
| 24 | Kyuushuu |
| 25 | Oohata Mochi |
| 26 | Miyako |
| 27 | Yoridashi |
| 28 | Nourin 24 |
| 29 | Saitama Senshou |
| 30 | Kirishima |
| 31 | Aichi Rikutou 1 |
| 32 | Yonoyuki Mochi |
| 33 | Dango Mochi |
| 34 | Sangoku |
| 35 | Terenzu |
| 36 | Iwate Kurumi Wase 1 |
| 37 | Eika Ine |
| 38 | Araki |
| 39 | Oiran |
| 40 | Mikuni No Homare |
| 41 | Japan 1 (unknown) |
| 42 | Kyuushuu |
| 43 | Gaisen Ibaragi 1 |
| 44 | Kurombo |
| 45 | Shirotsuka Wase |
| 46 | Nourin 5 |
| 47 | Chiyoda Wase |
| 48 | Touzan Mochi |
| 49 | Hakamuri 20 |
| 50 | Esojima Mochi |
| 51 | Esojima |
| 52 | Mino |
| 53 | Nourin Mochi |
| 54 | Suzume Shirazu |
| 55 | Yamato Nishiki |
| 56 | Nourin Mochi 1 |
| 57 | Nourin Mochi 17 |
| 58 | Miyamae Okute |
| 59 | Fukutomi |
| 60 | Atoshirazu |
| 61 | Col/Fukui/1965 |
| 62 | Tachiminori |
| 63 | Minami Hata Mochi |
| 64 | Gaisen Mochi |
| 65 | No Mochi |
| 66 | Takasago Wase |
| 67 | Tanaka Yakan |
| 68 | Oosumi |
| 69 | Oohataho |
| 70 | Nourin 11 |
| 71 | Col/Miyazaki/1963 |
| 72 | Tomoe Mochi |
| 73 | Shinshuu Wase |
| 74 | Nourin 16 |
| 75 | Urasan 1 |
| 76 | Yashino Mochi |
| 77 | Kirishima |
| 78 | Chiba Senshou |
| 79 | Okabo |
| 80 | Col/Fukui/1965 |
| 81 | Toga |
| 82 | Kahee |
| 83 | Hitachi Nishiki |
| 84 | Ooba Kirishima |
| 85 | Horarin |
| 86 | Matsuyama |
| 87 | Taishou Mochi |
| 88 | Kangyouho |
| 89 | Japan 2 (unknown) |
| 90 | Kaneko Mochi |
| 91 | Iwate Kurumi Wase 1 |
| 92 | Col/Miyazaki/1963 |
| 93 | Toukyo Kaneko |
| 94 | Gaisen (4X) |
| 95 | Ishikawa |
| 96 | Tariu Saku Mochi |
| 97 | Col/Tokushima/1967 |
| 98 | Miyanishiki |
| 99 | Hatamurasaki |
| 100 | Toga 1 |
| 101 | Rikutou Shinriki 1 |
| 102 | Col/Miyazaki/1963 |
| 103 | Ishiwari Mochi |
| 104 | Mizugirai Mochi |
| 105 | Col/Tokushima/1967 |
| 106 | Jouon |
| 107 | Oota Wase |
| 108 | Shizuoka |
| 109 | Hiderishirazu |
| 110 | Iwate Ryoon 1 |
| 111 | Kozo |
| 112 | Kahei |
| 113 | Tamasari 3 |
| 114 | Miyakonojoo Mochi |
| 115 | Ookuma Nishiki |
| 116 | Nourin Mochi 6 |
| 117 | Taishou Nishiki |
| 118 | Shina Mochi |
| 119 | Nagae Wase |
| 120 | Arabiya Mochi |
| 121 | Tozo Mochi |
| 122 | Urasar |
| 123 | Ootama |
| 124 | Okabo Mochi |
| 125 | Furuwase |
| 126 | Hirakawa Okute |
| 127 | Nourin 7 |
| 128 | Oiran |
| 129 | Shinhoku Daiou Mochi |
| 130 | Riku Araki |
| 131 | Suzume Shirazu |
| 132 | Col/Miyazaki/1963 |
| 133 | Hideshirazu Mochi |
| 134 | Japan 3 (unknown) |
| 135 | Nourin Mochi 4 |
| 136 | Kazusa Wase |
| 137 | Shinkuko Mochi |
| 138 | Hikouki Gome |
| 139 | Senshou |
| 140 | Shindai Okoshi |
| 141 | Col/Ooita/1964 |
| 142 | Taiwan Mochi |
| 143 | Urasan |
| 144 | Owari Mochi |
| 145 | Nourin Mochi 2 |
| 146 | Senshou |
| 147 | Gaisen Mochi 909 |
| 148 | Japan 4 (unknown) |
| 149 | Rikuu |
| 150 | Rikuu 23 |
| 151 | Ohata Wase |
| 152 | Japan 5 (unknown) |
| 153 | Japan 6 (unknown) |
| 154 | Owari 79 |
| 155 | Ouu 22 |
| 156 | Rikuu 15 |
| 157 | Col/Miyazaki/1963 |
| 158 | Rikuu 13 |
| 159 | Rikuu 22 |
| 160 | Fujimizu Bansei |
| 161 | Iwate Kinsen 1 |
| 162 | Bansei Tarou |
| 163 | Shiro Uzura |
| 164 | Japan 7 (unknown) |
| 165 | Japan 8 (unknown) |
| 166 | Mogami Uruchi 1 |
| 167 | Gaisen |
| 168 | Okka Modoshi |
| 169 | Mino Senshutsu |
| 170 | Japan 9 (unknown) |
| 171 | Kurohige |
| 172 | Mogami Chikanari 1 |
| 173 | Kounoso Rikutou 2 |
| 174 | Minami Hata Mochi |
| 175 | Wase Shinshuu |
| 176 | Igisu Mochi |
| 177 | Kurumi Wase 43 |
| 178 | Hiderishirazu |
| 179 | Susono Mochi |
| 180 | Seta Gaisen |
| 181 | Korotou Mochi |
| 182 | Owari Hata Mochi |
| 183 | Ishiyakushi Mochi |
| 184 | Shiro Hige |
| 185 | Edogawa |
| 186 | Gose Yonkoku |
| 187 | Mie |
| 188 | Shizouka |
| 189 | Chousen |
| 190 | Tosa Mochi |
| 191 | Aogara |
| 192 | Japan 10 (unknown) |
Cold tolerance evaluation
For cold tolerance evaluation, seeds of 195 rice genotypes were germinated under two conditions: 13°C for 28 days (cold) and 28°C for seven days (control), as described by Cruz and Milach (2004) with slight modifications. Seeds of each genotype were sown in wet rolled paper towels (Germitest) moist with a water volume equivalent to 2.5 times the weight of the paper. Paper rolls containing the seeds were placed into perforated plastic transparent bags (thickness of 0.05 mm) and transferred to a BOD germinator regulated to 28°C for the control and 13°C for the cold treatment.
The experiment was conducted in a randomized block design with three replicates. Each paper roll contained 20 seeds and the mean value of the investigated trait for the 20 seeds was considered one replicate. The measured parameters were coleoptile (CL 13) and radicle length (RL 13) at 28 days from the beginning of the experiment for seeds germinated at 13°C and at the seventh day for the seeds germinated at 28°C.
The percentage of the reduction in coleptile (RCL) and radicle (RRL) length due to low temperatures was evaluated according to Cruz and Milach (2004), comparing the lengths of the seedlings germinated under 13°C and 28°C.
RCL or RRL = [(CL or RL under 13°C temperature × 100)/CL or RL under control] − 100, where coleoptile length is the mean of 20 seedlings evaluated per replicate per genotype.
Statistical analyses
The residual analysis was carried out to verify normality and variance homogeneity, while regression analysis was used to test whether data transformation was necessary. The regression analysis demonstrated the necessity of RCL and RRL data transformation to arcsin [square root (×/100)]. Data for the remaining evaluated traits were not transformed. Data were submitted to analyses of variance and mean comparison by the Scott-Knott test (P = 0.01).
Estimates of phenotypic variance (σp2), environmental variance (σe2), genotypic variance (σg2), broad sense heritability (h2), genotypic coefficient of variation (GCV) and genotypic and environmental coefficient of variation ratio (GCV/ECV) were calculated.
Pearson’s coefficient correlation was used to establish relationships among the measured characteristics, while principal component analysis was performed to identify cold tolerance relationship among genotypes. Analyses were carried out using SAS (SAS Institute, Cary, NC, USA) and Genes (Cruz 2006).
Results
Trait variation associated to cold tolerance
The investigated genotypes exhibit variation for cold tolerance at the germination stage. Variance analysis detected a significant difference for all evaluated parameters at 13°C and at 28°C, as well as for traits determined by comparisons between cold and control treatments (Table 2). The partition of genotypes in accessions and controls identified significant differences among accessions for all evaluated traits. The significant differences among the controls were detected for two investigated characters (RL13 and RRL). Mean contrast between accessions and control genotypes detected significant differences between the groups for RCL and RRL due to the application of cold stress, CL and RL at 13°C and CL at 28°C. The control genotypes exhibited higher values of CL and RL and lower values of RCL and RR.
Table 2.
Variance Analysis of the traits measured under optimal growth and cold stress conditions, and by comparison between cold stress and optimal growth temperatures for 195 rice genotypes (192 Japanese accessions and three cold tolerant controls)
| MS | |||||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| Source of variation | Df | Optimal (28°C) | Cold (13 °C) | Cold/Optimal | |||
|
|
|
|
|||||
| CL28 | RL28 | CL13 | RL13 | RCL | RRL | ||
| Block | 2 | 330.888 | 903.004 | 12.626 | 34.349 | 2324.314 | 1523.322 |
| Genotypes | 194 | 1.702** | 8.761** | 0.365** | 0.782** | 65.147** | 33.803** |
| Accessions | 191 | 1.472** | 8.784** | 0.29** | 0.691** | 65.290** | 31.147** |
| Cold-tolerant controls | 2 | 0.0751 | 6.662 | 0.39 | 4.6** | 22.521 | 101.171** |
| Accessions vs Checks | 1 | 48.669** | 8.493 | 14.544** | 10.356** | 123.186* | 406.287** |
| Error | 388 | 0.316 | 4.408 | 0.191 | 0.489 | 30.772 | 17.710 |
| CV (%) | 11.045 | 14.905 | 31.49 | 42.19 | 9.467 | 5.983 | |
|
| |||||||
| Mean | 5.094 | 14.086 | 1.388 | 1.657 | 58.592 | 70.328 | |
| Accessions mean | 5.050 | 14.105 | 1.368 | 1.641 | 58.650 | 70.432 | |
| Cold-tolerant controls mean | 7.920 | 12.906 | 2.649 | 2.722 | 54.921 | 63.661 | |
| Minimum value | 3.125 (81) | 6.604 (50) | 0.558 (189) | 0.436 (189) | 46.764 (141) | 59.935 (CT6748) | |
| Maximum value | 9.901 (42) | 18.447 (39) | 2.921 (QUILLA) | 3.884 (QUILLA) | 73.443 (189) | 79.807 (189) | |
Significant at 1%
CL28, coleoptile length under optimal temperature; RL28, radicle length under optimal temperature; CL13, coleoptile length under cold stress; RL13, radicle length under cold stress; RCL, percentage of reduction in coleoptile length; RRL, percentage of reduction in radicle length.
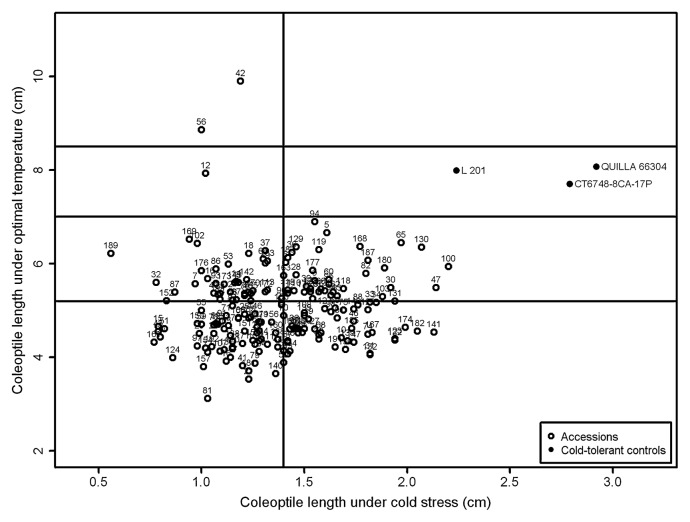
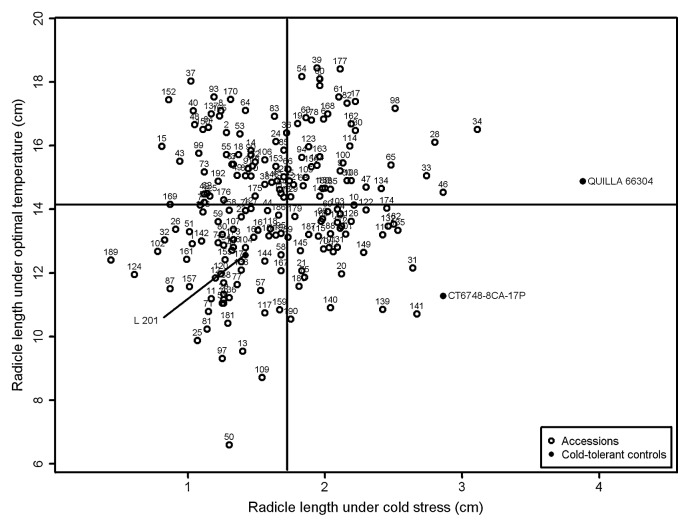
The minimum and maximum values for coleoptile and radicle length were observed for different genotypes at temperatures of 13°C and 28°C (Figs. 1, 2). At 28°C, accession Kyuushuu showed the highest value for coleoptiles and Oiran showed the highest value for radicle length, whereas accessions Toga and Esojima Mochi showed the lowest values. At 13°C, the highest values for both traits were observed for the control genotype QUILLA 66304. Among the accessions, the highest value for CL13 was observed for Toga 1, whereas Sangoku displayed the highest value for RL13. The lowest values for both CL13 and RL13 were observed for accession Chousen.
Fig. 1.
Means of coleoptile length under cold stress (13°C) and optimal temperature (28°C) of 192 Japanese rice accessions and three cold-tolerant controls evaluated for cold tolerance at the germination stage. Vertical and horizontal lines represent the separation of the average groups identified by Scott-Knott test.
Fig. 2.
Means of radicle length under cold stress (13°C) and optimal temperature (28°C) of 192 Japanese rice accessions and three cold-tolerant controls evaluated for cold tolerance at the germination stage. Vertical and horizontal lines represent the separation of the average groups identified by Scott-Knott test.
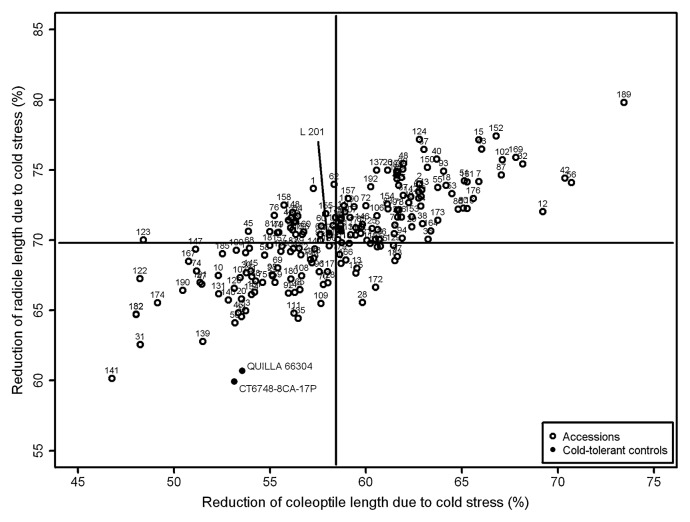
The control genotype CT 6748-8CA-17P showed the lowest value for RRL, while accession Col/Ooita/1964 exhibited the lowest value for RCL. The highest values for both traits were observed for accession Chousen (Fig. 3).
Fig. 3.
Percentage coleoptile and radicule length reduction due to cold stress, obtained by comparison between lengths under cold stress (13°C) and optimal temperature conditions (28°C) of 192 Japanese rice accessions and three cold-tolerant controls. Vertical and horizontal lines represent the separation of the average groups identified by Scott-Knott test.
Genotypes were divided by Scott-Knott analysis into two groups for coleoptile length evaluated under cold treatment and into four groups under optimal temperature (Fig. 1) whereas for radicle length they were divided into two groups under both cold treatment and optimal temperature (Fig. 2). When comparing the coleoptile and radicle lengths of the seedlings germinated under 13°C and 28°C by percentage of reduction of coleoptile and radicle length due to cold, genotypes also were divided into two groups (Fig. 3). All controls clustered with higher values for CL13 and lower values for RCL. A total of 71 accessions were grouped with the controls for CL13 and a total of 90 accessions exhibited a performance similar to the cold-tolerant controls, displaying lower values for RCL.
The control genotypes CT6748-8CA-17P and QUILLA 66304 clustered with genotypes exhibiting higher values for RL13 and lower values for RRL. A total of 80 accessions presented similar features, namely, higher values for RL13 and a total of 111 accessions with lower values of RRL. The cold-tolerant control L 201 clustered with materials exhibiting lower RL13 and higher RRL values.
Genetic parameters estimates
Estimates of phenotypic variance (σp2), environmental variance (σe2), genotypic variance (σg2), broad sense heritability (h2), genotypic coefficient of variation (GCV) and genotypic and environmental coefficient of variation ratio (GCV/ECV) are presented in Table 3. GCV was higher for RL13°C and lower for RRL. GCV/ECV ratio was higher for the traits evaluated under the control conditions than for those evaluated under cold treatment.
Table 3.
Estimates of phenotypic variance (σp2), environmental variance (σe2), genotypic variance (σg2), broad sense heritability (h2), genotypic coefficient of variation (GCV) and genotypic and environmental coefficient of variation ratio (GCV/ECV)
| Trait | σp2 | σe2 | σg2 | h2 | GCV(%) | GCV/ECV |
|---|---|---|---|---|---|---|
| RCL | 21.715 | 10.257 | 11.458 | 52.764 | 5.777 | 0.61 |
| RRL | 11.267 | 5.903 | 5.364 | 47.606 | 3.293 | 0.55 |
| CL13 | 0.121 | 0.064 | 0.058 | 47.629 | 17.338 | 0.55 |
| RL13 | 0.261 | 0.163 | 0.098 | 37.432 | 18.842 | 0.446 |
| CL28 | 0.851 | 0.158 | 0.693 | 81.395 | 16.336 | 1.479 |
| RL28 | 4.380 | 2.204 | 2.176 | 49.680 | 10.472 | 0.702 |
RCL, percentage of reduction in coleoptile length; RRL, percentage of reduction in radicle length; CL13, coleoptile length under cold stress; RL13, radicle length under cold stress; CL28, coleoptile length under optimal temperature; RL28, radicle length under optimal temperature.
Heritability ranged from 37.43% for RL13 to 81.39% for CL28. Although the heritability was high for coleoptile length at 28°C, for coleoptile length at 13°C its value was intermediate (47.63%). For radicle length, the heritability value at 13°C was also lower than at 28°C. Among the traits evaluated under cold treatment and in comparison between cold and control conditions, RCL showed the highest heritability.
Correlation coefficient among investigated traits
The traits evaluated under cold stress showed significant correlation with those evaluated by comparisons between cold and optimal temperature (Table 4). Coleoptile length exhibited significant correlation with radicle length at both temperatures 13°C and 28°C, but the correlation value for temperature at 13°C was higher than at 28°C.
Table 4.
Correlation coefficients between the following traits: percentage of reduction in coleoptile length (RCL), percentage of reduction in radicle length (RRL), coleoptile length under cold stress (CL13), radicle length under cold stress (RL13), coleoptile length under optimal temperature (CL28) and radicle length under optimal temperature (RL28) evaluated in 195 rice genotypes
| Trait | RCL | RRL | CL13 | RL13 | CL28 | RL28 |
|---|---|---|---|---|---|---|
| RCL | 1.00 | 0.770** | −0.768** | −0.684** | 0.434** | 0.209** |
| RRL | 1.00 | −0.737** | −0.869** | 0.1266 | 0.332** | |
| CL13 | 1.00 | 0.807** | 0.2098** | 0.054 | ||
| RL13 | 1.00 | 0.0746 | 0.145* | |||
| CL28 | 1.00 | 0.366** | ||||
| RL28 | 1.00 |
Significant at 5%;
Significant at 1%
Correlation between coleoptile length and radicle length at 13°C and their respective measures at 28°C were low. RCL and RRL were highly correlated and highly negatively correlated to CL and RL at 13°C. RCL showed significant correlation with CL28 and RL28, while RRL was significantly correlated only with RL28.
Principal component analysis
Principal component analysis of four traits associated to the genotypes performance under low temperature revealed that the first three principal components (PCs) accounted for 97.8% of the total cold tolerance variation (Table 5). Approximately 83% of the total variation was explained by the first principal component, which was a contrast between the evaluated traits under 13°C (CL13 and RL13) and the features obtained by comparing the genotypes performance under cold and optimal temperature (RCL and RRL).
Table 5.
Principal components of four traits used to evaluate cold tolerance in 192 Japanese rice accessions and three cold-tolerant controls
| Trait | Eigenvectors | ||
|---|---|---|---|
|
| |||
| CP1 | CP2 | CP3 | |
| RCL | 0.4841 | 0.7480 | 0.3186 |
| RRL | 0.5094 | −0.3048 | 0.5439 |
| CL13 | −0.4988 | −0.1619 | 0.7708 |
| RL13 | −0.5073 | 0.5669 | 0.0922 |
|
| |||
| Eigenvalue | 3.318 | 0.333 | 0.258 |
| Total variance explained (%) | 82.968 | 8.346 | 6.473 |
| Cumulative total variance explained (%) | 82.968 | 91.315 | 97.789 |
RCL, percentage of reduction in coleoptile length; RRL, percentage of reduction in radicle length; CL13, coleoptile length under cold stress; RL13, radicle length under cold stress.
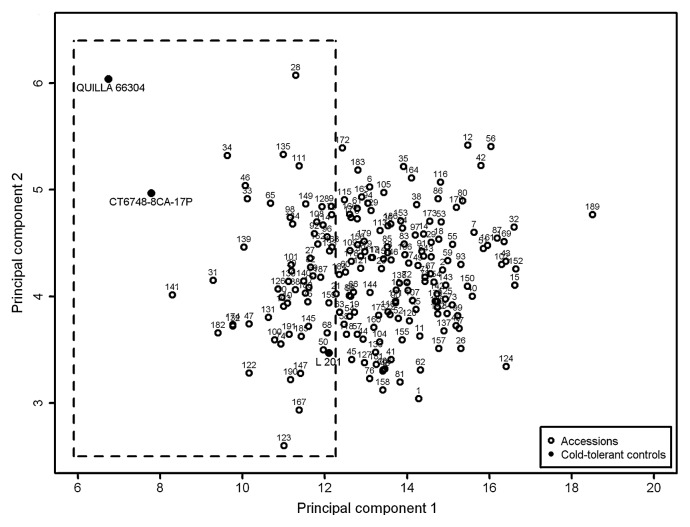
Using the genotypes scores of the first principal component (PC1), the contrast enabled the identification and selection of accessions with higher values of CL13 and RL13 and also lower values of RCL and RRL. Genotypes showing high means for RCL and RRL and low for CL13 and RL13, received higher scores for PC1 and are cold intolerant. In contrast, genotypes presenting low means for RCL and RRL and high for CL and RL, received lower scores for PC1 and are cold tolerant. As demonstrated in Fig. 4, some accessions had scores close to those found for the cold-tolerant controls. A total of 50 accessions presented intermediate scores, between the control genotypes QUILLA 66304 and L201; QUILLA 66304 showed the lowest score among all genotypes evaluated, while L 201 displayed the highest score among controls. The variance accounted to PC2 was explained mainly by RCL, whereas CL under cold stress had the highest eigenvector to PC3.
Fig. 4.
Cold tolerance relation among 195 rice genotypes for the first two principal components (PC) as revealed by two-dimensional plots. The dotted lines identify accessions that are considered cold-tolerant according to their scores for PC1.
Discussion
Low temperatures at the initial stages of rice development prevent fast germination and seedling establishment, essential conditions for homogeneous vegetative growth and uniform maturity. The use of cold tolerant cultivars is considered the best option to avoid losses caused by low temperatures. The first step to obtain cold tolerant cultivars is to identify sources of tolerance in germplasm banks (Miura et al. 2001). Among the Japanese accessions studied, genetic variation for traits related to germination under low temperatures was observed (Table 2) and accessions exhibiting adequate performance for all investigated traits were identified (Fig. 4). The performance of the identified cold-tolerant accessions is similar to those of known cold-tolerant genotypes, indicating their potential to be used in breeding programs as source of cold tolerance at the germination stage.
Control genotypes showed higher means than the tested accessions for CL13 (Fig. 1) and RL13 (Fig. 2), and lower values for RCL and RRL (Fig. 3), but 18 accessions exhibited RCL values lower than those from the control genotypes used. Although all controls demonstrated better performances than accessions for CL13, those 18 accessions showed better performance for RCL due to the confusing effects of low temperature and seedling vigour. In fact, evaluating germination under more than one temperature condition is essential to clearly distinguish cold tolerance from vigour-associated traits (Massardo et al. 2000). In the evaluation of cold tolerance at the germination stage in rice, a comparison of the genotypes performance under cold stress and optimal temperature was proposed. The percentage of coleoptile length reduction due to low temperatures was the characteristic enabling the precise identification of genotypes previously known as cold tolerant or susceptible (Cruz and Milach 2004). In the current study, the reduction in coleoptile length due to cold stress was evaluated, and our results have demonstrated a high negative correlation to CL13 (Table 4), indicating that genotypes with increased growth at 13 °C are able to sustain normal growth under cold stress. However, due to the presence of vigour differences among genotypes, plant performance at 13°C is not exclusively dependent on the genotype cold tolerance. For this reason, some authors agree that it is important to evaluate performance under more than one temperature, enabling the separation of vigour from cold tolerance effects (Cruz and Milach 2004, Massardo et al. 2000).
The correlation between coleoptile length under low and optimal temperatures was low, indicating that there is a differential performance of the genotypes under different temperatures.
The heritability of the traits evaluated under cold conditions was lower than that found for the same traits under optimal temperature (Table 3). The observed lower heritability can be explained by the polygenic control of the responses under low temperature at the germination stage, as suggested by inheritance studies of cold tolerance (Cruz et al. 2006, Sharifi 2008, Sthapit and Witcombe 1998), and as supported by studies involving QTL analysis for low temperature germinability (Fujino et al. 2004, Miura et al. 2001) and cold tolerance at the early growth stages (Baruah et al. 2009, Zhang et al. 2005).
GCV/ECV ratio demonstrated a favorable situation for selection (values higher than 1) exclusively for CL at 28°C, providing further evidences of the difficulties involved in the selection of cold-tolerance traits under low temperatures. Among the traits measured to detect the genotypes performance under cold, the highest values for GCV/ECV ratio and heritability were observed for RCL, indicating that it is the most reliable trait to identify cold tolerance at the germination stage for rice genotypes, among the characteristics evaluated. Cruz and Milach (2004) observed that reduction in coleptile length due to the cold also permits the precise identification of tolerant and susceptible genotypes.
Under field conditions, the genotypes good performance under low temperatures is dependent on cold tolerance and high vigour, which enable fast and homogeneous seedling establishment. As RCL was a trait that allowed the identification of cold tolerant genotypes (Cruz and Milach 2004) and varietal differences for coleoptile length are correlated with seedling establishment under low temperature (Miura et al. 2004, Ogiwara and Terashima 2001), we have analyzed the genotypes performance using principal component analysis as a multivariate approach to study cold tolerance of Japanese rice accessions.
Principal component analysis enabled us to summarize the information obtained for the four traits related to cold tolerance in one principal component (Table 5). PC1 allowed the identification of the genotypes displaying cold tolerance by smaller reductions in coleoptile and radicle lenght in the presence of cold and high vigour, by higher coleoptile and radicle growth under cold.
Genotype scores for the first principal component allowed us to identify accessions with intermediate values, between cold-tolerant controls QUILLA 66304 and CT 6748-8CA-17P showing the lowest values and the control L 201, showing the highest values among the cold-tolerant genotypes. A total of 50 accessions exhibited intermediate values in comparison to the controls, showing a good performance for low temperature germination.
Acknowledgements
The first author, Fátima Bosetti, acknowledges Conselho Nacional de Pesquisa e Desenvolvimento Tecnológico–CNPq and Pioneer Hi-Bred International, for the financial grant. This work was supported by a research grant from Fundação de Amparo à Pesquisa do Estado de São Paulo–FAPESP (project no 2007/06615-6).
Literature Cited
- Andaya V.C., Mackill D.J. (2003) Mapping of QTLs associated with cold tolerance during the vegetative stage in rice. J. Exp. Bot. 54: 2579–2585 [DOI] [PubMed] [Google Scholar]
- Baruah A.R., Ishigo-Oka N., Adachi M., Oguma Y., Tokizono Y., Onishi K., Sano Y. (2009) Cold tolerance at the early growth stage in wild and cultivated rice. Euphytica 165: 459–470 [Google Scholar]
- Castillo D., Alvarado R. (2002) Caracterización de germoplasma de arroz para tolerancia a frío en la etapa de germinación. Agricultura Técnica 62: 596–605 [Google Scholar]
- Cruz C.D. (2006) Genes Program: Multivariate Analysis and Simulation. UFV, Viçosa, MG, Brazil [Google Scholar]
- Cruz R.P., Milach S.C.K. (2004) Cold tolerance at the germination stage of rice: methods of evaluation and characterization of genotypes. Sci. Agri. 61: 1–8 [Google Scholar]
- Cruz R.P., Milach S.C.K., Federizzi L.C. (2006) Inheritance of rice cold tolerance at the germination stage. Genet. Molec. Biol. 29: 314–320 [Google Scholar]
- Fujino K., Sekiguchi H, Sato T, Kiuchi H, Nonoue Y, Takeuchi Y, Ando T, Lin S.Y., Yano M. (2004) Mapping of quantitative trait loci controlling low-temperature germinability in rice (Oryza Sativa L.). Theor. Appl. Genet. 108: 794–799 [DOI] [PubMed] [Google Scholar]
- Jones D.B., Peterson M.L. (1976) Rice seedling vigor at suboptimal temperatures. Crop Sci. 16: 102–105 [Google Scholar]
- Kotaka S., Abe N. (1988) The varietal difference of germinability at low-temperature in rice varieties and the testing method for the percentage establishment of seedlings. J. Agri. Sci. Tokyo 43: 165–168 [Google Scholar]
- Mackill D.J., Lei X. (1997) Genetic variation for traits related to temperate adaptation of rice cultivars. Crop Sci. 37: 1340–1346 [Google Scholar]
- Massardo F., Corcuera L, Alberdi M. (2000) Embryo physiological responses to cold by two cultivars of oat during germination. Crop Sci. 40: 1694–1701 [Google Scholar]
- Miura K., Lin S.Y., Yano M, Nagamine T. (2001) Mapping quantitative trait loci controlling low-temperature germinability in rice (Oryza sativa L.). Breed. Sci. 51: 293–299 [Google Scholar]
- Ogiwara H, Terashima K. (2001) A varietal difference in coleoptile growth is correlated with seedling establishment of direct seeded rice in submerged field under low temperature conditions. Plant Prod. Sci. 4: 166–172 [Google Scholar]
- Sharifi P. (2008) Inheritance of cold tolerance in rice at the germination stage. Asian J. Plant Sci. 7: 485–489 [Google Scholar]
- Sharifi P. (2010) Evaluation on sixty-eight rice germplasms in cold tolerance at germination stage. Rice Sci. 17: 77–81 [Google Scholar]
- Sharifi P, Aminpanah H. (2010) Evaluation eighteen rice genotypes in cold tolerance at germination stage. World Appl. Sci. J. 11: 1476–1480 [Google Scholar]
- Sthapit B.R., Witcombre J.R. (1998) Inheritance of tolerance to chilling stress in rice during germination and plumule greening. Crop Sci. 38: 660–665 [Google Scholar]
- Takahashi N. (1997) Inheritance of seed germination and dormancy. In: Matsuo T., Futsuhara Y., Kikuchi F., Yamaguchi H. (eds.) The Science of the Rice Plant, Food and Agriculture Policy Research Center, Tokyo, pp. 348–359 [Google Scholar]
- Yoshida S. (1981) Growth and development of the rice plant. Fundamentals of Rice Crop Science, International Rice Research Institute, Los Banos, Philippines, pp. 1–63 [Google Scholar]
- Zhang Z.H., Su L, Li W, Chen W, Zhu Y.G. (2005) A major QTL conferring cold tolerance at the early seedling stage using recombinant inbred lines of rice (Oryza sativa L.). Plant Sci. 168: 527–534 [Google Scholar]