Abstract
Tremor in Parkinson's disease has several mysterious features. Clinically, tremor is seen in only three out of four patients with Parkinson's disease, and tremor-dominant patients generally follow a more benign disease course than non-tremor patients. Pathophysiologically, tremor is linked to altered activity in not one, but two distinct circuits: the basal ganglia, which are primarily affected by dopamine depletion in Parkinson's disease, and the cerebello-thalamo-cortical circuit, which is also involved in many other tremors. The purpose of this review is to integrate these clinical and pathophysiological features of tremor in Parkinson's disease. We first describe clinical and pathological differences between tremor-dominant and non-tremor Parkinson's disease subtypes, and then summarize recent studies on the pathophysiology of tremor. We also discuss a newly proposed ‘dimmer-switch model’ that explains tremor as resulting from the combined actions of two circuits: the basal ganglia that trigger tremor episodes and the cerebello-thalamo-cortical circuit that produces the tremor. Finally, we address several important open questions: why resting tremor stops during voluntary movements, why it has a variable response to dopaminergic treatment, why it indicates a benign Parkinson's disease subtype and why its expression decreases with disease progression.
Keywords: Parkinson's disease; tremor; basal ganglia; cerebellum, thalamus
Introduction
Parkinson's disease is a surprisingly heterogeneous neurodegenerative disorder. The classical triad of motor symptoms includes resting tremor, akinesia and rigidity (Lees et al., 2009). However, the expression of these cardinal motor symptoms varies markedly between patients. This review focuses on the occurrence of tremor, a striking example of this phenotypic heterogeneity. While some patients with Parkinson's disease have a prominent and disabling tremor, others never develop this symptom (Hoehn and Yahr, 1967). This observation formed the basis for classifying patients with Parkinson's disease into tremor-dominant and non-tremor subtypes (Zetusky et al., 1985; Jankovic et al., 1990; Rajput et al., 1993; Lewis et al., 2005; Burn et al., 2006). Moreover, patients with Parkinson's disease can manifest different types of tremor: at rest, with action or postural, including orthostatic. The reason for this marked phenotypic heterogeneity remains unclear. A better understanding of the mechanisms underlying the expression of tremor could help clarify tremor pathophysiology, and as such offer potential new avenues for symptomatic treatment. Here, we will review some salient clinical features of tremor in Parkinson's disease, discuss recent studies on the pathophysiology of tremor and review a newly proposed model (Helmich et al., 2011b) to explain the cerebral mechanisms involved in generating resting tremor in Parkinson's disease.
The tremors of Parkinson's disease
Tremor is characterized clinically by involuntary, rhythmic and alternating movements of one or more body parts (Abdo et al., 2010). Parkinson's disease harbours many different tremors. These tremors can vary according to the circumstances under which they occur, the body part that is involved and the frequency at which the tremor occurs. For example, tremor may occur at rest, during postural holding or during voluntary movements; it can be seen in the hands, feet or other body parts; and tremor frequency can vary from low (4–5 Hz) to high (8–10 Hz). A consensus statement of the Movement Disorder Society includes a parallel classification scheme that categorizes three tremor syndromes associated with Parkinson's disease (Deuschl et al., 1998). This classification is still used widely today (Fahn, 2011). First, the most common or classical Parkinson's disease tremor is defined as a resting tremor, or rest and postural/kinetic tremor with the same frequency. This tremor is inhibited during movement and may reoccur with the same frequency when adopting a posture or even when moving. When recurring with posture, it has been called re-emergent tremor. Second, some patients with Parkinson's disease develop rest and postural/kinetic tremors of different frequencies, with the postural/kinetic tremor displaying a higher (>1.5 Hz) and non-harmonically related frequency to the resting tremor. This form occurs in <10% of patients with Parkinson's disease. Some consider it to be an incidental combination of an essential tremor with Parkinson's disease (Louis and Frucht, 2007), but it appears more plausible that postural tremor is a manifestation of Parkinson's disease. Third, isolated postural and kinetic tremors do occur in Parkinson's disease. The frequency of these tremors may vary between 4 and 9 Hz. A specific form of (position-dependent) postural tremor is orthostatic tremor, which may occur in Parkinson's disease at different frequencies (4–6, 8–9 or 13–18 Hz), with or without co-existent resting tremor (Leu-Semenescu et al., 2007). Since this tremor type occurs at a higher age of onset than primary orthostatic tremor, and since it may respond to dopaminergic treatment, it has been argued that it is a manifestation of Parkinson's disease rather than a chance association of two tremor syndromes (Leu-Semenescu et al., 2007).
The distinction between these different tremors is not always visible to the naked eye. For example, resting tremor can re-emerge during postural holding, making it difficult to clinically distinguish it from essential tremor. This distinction can be made by focusing on the delay between adopting a posture and the emergence of tremor: in essential tremor there is no delay, while Parkinson's disease resting tremor re-emerges after a few seconds (on average ± 10 s) (Jankovic et al., 1999). Since the frequency of re-emergent and resting tremor can be similar, it has been hypothesized that both tremors share a similar pathophysiological mechanism. One interesting patient with Parkinson's disease had no resting tremor, but a marked 3–6 Hz postural tremor that occurred after a delay of 2–4 s following postural holding (Louis et al., 2008), thus resembling re-emergent tremor. Such observations point to heterogeneity in the circumstances under which the classical Parkinson's disease ‘resting’ tremor occurs.
In the following sections, we will mainly focus on the classic resting tremor in Parkinson's disease. We will first describe the clinical and cerebral differences between patients with tremor-dominant and non-tremor Parkinson's disease. Then we will detail how these differences may inform us about the causes and consequences of Parkinson's disease resting tremor.
The clinical phenotype of tremor-dominant Parkinson's disease
The classification of patients with Parkinson's disease into tremor-dominant and non-tremor subtypes is well established. Different taxonomies have been used to define these two subtypes. First, tremor-dominant Parkinson's disease has been contrasted with a form of Parkinson's disease dominated by axial symptoms, i.e. postural instability and gait disability (the PIGD subtype) (Zetusky et al., 1985; Jankovic et al., 1990). This distinction is based on the relative expression of tremor and PIGD, using subscores of the Unified Parkinson's Disease Rating Scale (UPDRS). Second, tremor-dominant Parkinson's disease has been contrasted with a Parkinson's disease subtype dominated by bradykinesia and rigidity (Rajput et al., 1993; Schiess et al., 2000), again using UPDRS subscores. A third, data-driven approach has identified Parkinson's disease subtypes by applying clustering algorithms to several clinical parameters such as symptom severity, disease onset and clinical progression (Lewis et al., 2005). The latter approach again produced tremor-dominant and non-tremor clusters of patients, together with a young-onset form and a rapid progression group.
Tremor is an independent symptom
Tremor may have a pathophysiology different from most other Parkinson's disease symptoms. First, tremor does not progress at the same rate as bradykinesia, rigidity, gait and balance (Louis et al., 1999). Second, tremor severity does not correlate with other motor symptoms (Louis et al., 2001). Third, tremor can occur on the body side contralaterally to the otherwise most affected side, i.e. where bradykinesia and rigidity are most prominent. This so-called wrong-sided tremor is seen in ∼4% of patients with Parkinson's disease (Koh et al., 2010). Finally, tremor responds less well to dopaminergic treatment than bradykinesia and rigidity (Koller et al., 1994; Fishman, 2008).
Tremor is a marker of benign Parkinson's disease
Clinical and behavioural differences between patients with tremor-dominant and non-tremor Parkinson's disease suggest that tremor is a marker of benign Parkinson's disease. For instance, there are indications that patients with tremor-dominant Parkinson's disease have a relatively slow disease progression. This idea was first proposed by Hoehn and Yahr (1967), who found a greater proportion of death and disability in patients with Parkinson's disease with a non-tremor onset mode, at least during the first 10 years of the disease. Other studies confirmed this: compared to patients with Parkinson's disease with a tremor-dominant subtype, patients with Parkinson's disease with a PIGD subtype had a larger annual increase in symptom severity (Jankovic and Kapadia, 2001) and a shorter survival (Lo et al., 2009; Forsaa et al., 2010). Furthermore, a post-mortem study showed that patients with tremor-dominant Parkinson's disease progressed more slowly to Hoehn and Yahr grade 4 than patients with akinetic-rigid dominant Parkinson's disease (Rajput et al., 2009), using the subtyping scheme by Rajput et al. (1993). Another post-mortem study found that patients with tremor-dominant Parkinson's disease had a lower degree of disability (Hoehn and Yahr grade) than tremor-dominant patients at 5 and 8 years (Selikhova et al., 2009), using the subtyping scheme discussed earlier (Lewis et al., 2005). However, tremor-dominant and non-tremor patients with Parkinson's disease had similar disease duration at the time of death. This led to their conclusion that tremor alone does not predict a significantly longer survival: patients with tremor-dominant Parkinson's disease progress more slowly during the initial course of the disease, but lose this relative advantage later on (Selikhova et al., 2009). Finally, patients with tremor-dominant Parkinson's disease have better cognitive performance than non-tremor patients with Parkinson's disease (Vakil and Herishanu-Naaman, 1998; Lewis et al., 2005; Burn et al., 2006) and are less likely to develop dementia (Aarsland et al., 2003; Williams-Gray et al., 2007). To distinguish between tremor-dominant and non-tremor Parkinson's disease subtypes, all of the studies reviewed above used a combined resting and postural/action tremor score on the UPDRS. Therefore, it remains unclear which of these tremors best predicts the clinical advantages associated with the tremor-dominant subtype. Since postural and action tremors frequently occur in the akinetic-rigid subtype (Deuschl et al., 2000; Helmich et al., 2011b), it could be argued that the presence of resting tremor is the best predictor of clinical progression. This remains to be tested.
The neurochemical basis of parkinsonian resting tremor
The core pathological process in Parkinson's disease involves dopaminergic cell loss in the substantia nigra pars compacta, particularly the lateral ventral tier (Fearnley and Lees, 1991). This leads to dopamine depletion in the striatum, particularly in the dorsolateral putamen (Kish et al., 1988). These changes are strongly linked to bradykinesia (Albin et al., 1989), but their relevance to resting tremor remains unclear (Rodriguez-Oroz et al., 2009). Here, we will discuss data from post-mortem and nuclear imaging studies that examined whether resting tremor has a dopaminergic basis.
Post-mortem studies
Patients with tremor-dominant Parkinson's disease have milder cell loss in the substantia nigra pars compacta (particularly in the lateral portion) and in the locus coeruleus than patients with non-tremor Parkinson's disease (Paulus and Jellinger, 1991; Jellinger, 1999). This suggests that patients with tremor-dominant Parkinson's disease have less dopaminergic (and possibly also less nor-adrenergic) dysfunction than non-tremor patients. This could explain some of the clinical advantages that are associated with tremor. On the other hand, patients with tremor-dominant Parkinson's disease have considerably more cell loss in the retrorubral area of the midbrain, as assessed in a post-mortem study comparing six patients with tremulous Parkinson's disease to five patients with non-tremor Parkinson's disease (Hirsch et al., 1992). The small sample size in this study warrants caution although confirmatory evidence came from animal work. In non-human primates, the retrorubral area contains ∼10% of the mesencephalic dopaminergic neurons, compared with 76% in the substantia nigra pars compacta and 14% in the ventral tegmental area (Francois et al., 1999). Injection of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) destroys these dopaminergic neurons (Burns et al., 1983), but with marked differences between species. Clinically, rhesus (Macaca mulatta) monkeys develop infrequent and brief episodes of high-frequency tremor, whereas vervet (African green) monkeys frequently have prolonged episodes of low-frequency resting tremor (Bergman et al., 1998b). Both species develop akinesia, rigidity and severe postural instability. Thus, vervet monkeys resemble the tremulous Parkinson's disease subtype, while rhesus monkeys resemble the non-tremor Parkinson's disease subtype (Rivlin-Etzion et al., 2010). There are no studies directly comparing the spatial topography of MPTP-induced neural damage between these species, but in vervet monkeys, the retrorubral area (A8) is preferentially damaged (Deutch et al., 1986), while in rhesus monkeys, the substantia nigra pars compacta (A9) is more affected (German et al., 1988; Oiwa et al., 2003). Although circumstantial, these observations lend further support to the idea that tremor is related to—and possibly caused by—dopaminergic cell loss in the retrorubral area (Jellinger, 1999). The retrorubral area could produce tremor via its dopaminergic projections to, among other regions, the subthalamic region (Francois et al., 2000) and the pallidum (Jan et al., 2000). Accordingly, a study in parkinsonian vervet monkeys found that tremor severity correlated exclusively with dopaminergic fibres in the external globus pallidus (Mounayar et al., 2007). Similarly, a post-mortem study in patients with Parkinson's disease showed that clinical tremor severity was correlated exclusively with concentrations of the dopamine metabolite homovanillic acid in the pallidum (Bernheimer et al., 1973). Taken together, these data suggest that tremor might result from pallidal dysfunction, triggered by a specific loss of dopaminergic projections from the retrorubral area. Note that not all findings consistently point in this direction: a recent post-mortem study showed higher dopamine levels in the ventral internal globus pallidus of patients with tremor-dominant than non-tremor Parkinson's disease (Rajput et al., 2008). However, only few data points were measured (a single hemisphere of two tremor-dominant patients), which limit the interpretation of these results. New imaging techniques may confirm the role of the retrorubral area in tremor in vivo, for example by measuring N-acetyl-aspartate (a marker of neuronal viability) in the retrorubral area with magnetic resonance spectroscopy or by measuring connectivity between the retrorubral area and the basal ganglia using diffusion tensor imaging and functional MRI.
In vivo dopaminergic and serotonergic imaging
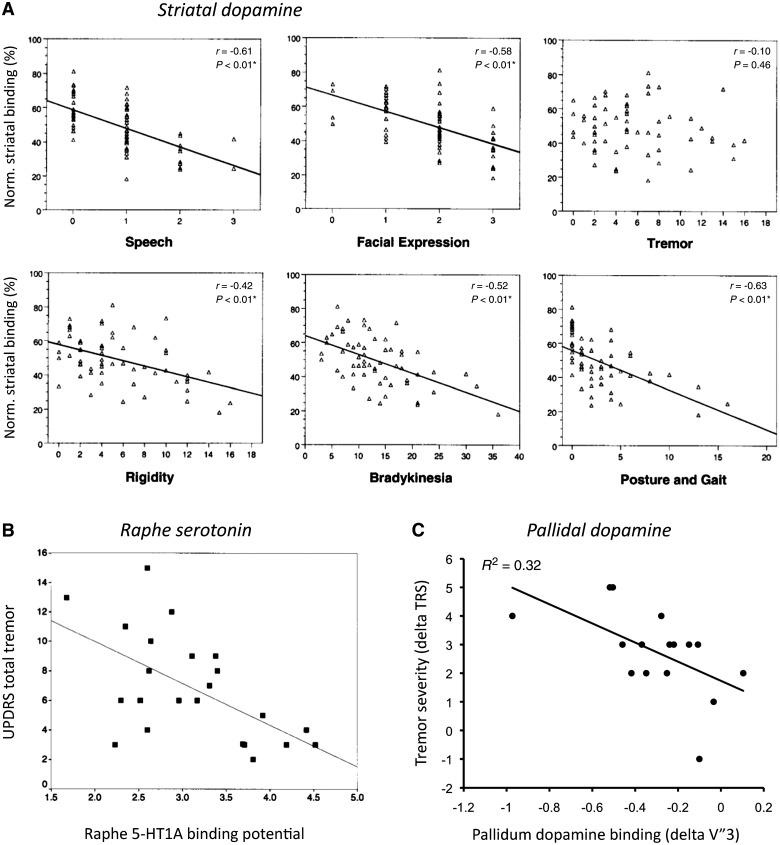
Five [123I]FP-CIT SPECT studies have described neurochemical differences between patients with tremor-dominant and non-tremor Parkinson's disease (Fig. 1). Three of these found that patients with tremor-dominant Parkinson's disease had less striatal dopamine depletion than those with non-tremor Parkinson's disease (Spiegel et al., 2007; Rossi et al., 2010; Helmich et al., 2011b). These differences were spatially localized to the putamen in one report (Rossi et al., 2010) and extended to the caudate in the other studies (Spiegel et al., 2007; Helmich et al., 2011b). These findings fit with the milder nigral pathology of tremor-dominant patients noted earlier (Paulus and Jellinger, 1991; Jellinger, 1999). The fourth SPECT study found the opposite pattern (Isaias et al., 2007), perhaps because of a different definition of Parkinson's disease subgroups, or because only 10 tremor-dominant patients with Parkinson's disease were included, whereas the other studies included 16–24 tremor-dominant patients (Spiegel et al., 2007; Rossi et al., 2010; Helmich et al., 2011b). The fifth study found different spatial distributions of dopamine depletion in tremor-dominant and non-tremor Parkinson's disease subtypes. Specifically, non-tremor patients showed pronounced dopamine depletion in the posterior striatum, while tremor-dominant patients had severe dopamine depletion in the lateral putamen (Eggers et al., 2011). Finally, several SPECT studies have correlated the amount of striatal dopamine depletion with the severity of individual symptoms. These studies consistently show that, unlike other Parkinson's disease symptoms, resting tremor does not correlate with nigrostriatal dopamine depletion (Benamer et al., 2000, 2003; Pirker, 2003; Isaias et al., 2007; Spiegel et al., 2007). The weight of the evidence therefore suggests milder nigral pathology in tremor-dominant patients, possibly with a different spatial distribution as well. This raises the question whether other neurotransmitters could be involved in generating tremor. Two imaging studies indicate a role for serotonin: one study found an association between reduced midbrain raphe 5-HT1A binding and increased tremor severity (Doder et al., 2003), and another study found that patients with tremor-dominant Parkinson's disease had lower levels of thalamic serotonin transporters than non-tremor patients (Caretti et al., 2008). This opens the possibility that abnormalities in the serotonergic system are involved in generating resting tremor in Parkinson's disease although this hypothesis remains to be tested in post-mortem studies.
Figure 1.
Neurochemical correlates of Parkinson's disease tremor. (A) Correlation between age-normalized striatal [123] I beta-CIT binding and UPDRS motor subscores for speech, facial expression, tremor (rest and action tremor), rigidity, bradykinesia, and posture and gait (n = 59). Reprinted from Pirker (2003), with permission from John Wiley and Sons. (B) Correlation between 11C-WAY 100635 PET in the raphe and total UPDRS tremor score (r = −0529; P < 0.01; n = 23). Reprinted from Doder et al. (2003), with permission from Wolters Kluwer. (C) Correlation between pallidal [I-123] FP-CIT binding and resting tremor severity [tremor rating scale (TRS); r = −0.57; P = 0.023], using within-patient difference scores (between most-affected and least-affected sides). This procedure controls for non-specific differences between patients. Reprinted from Helmich et al. (2011b), with permission from John Wiley and Sons. These data show that tremor severity is correlated with dopamine depletion in the pallidum (C), but not the striatum (A), and also with serotonin depletion in the raphe (B).
These findings suggest that tremor severity does not depend on the amount of nigrostriatal dopamine depletion. On the other hand, it has been argued that the expression of resting tremor is conditional upon dopaminergic denervation in the midbrain (Deuschl et al., 2000). Indeed, many disorders with resting tremor show a form of nigrostriatal dopamine depletion, e.g. mono-symptomatic resting tremor (Ghaemi et al., 2002), dystonic resting tremor after mesencephalic lesions (Vidailhet et al., 1999) and Holmes' tremor (formerly called rubral or midbrain tremor) (Remy et al., 1995; Seidel et al., 2009; Sung et al., 2009). Thus, we are left with the paradox that nigrostriatal dopamine depletion may be a prerequisite for developing resting tremor, but the level of tremor expression is independent of nigrostriatal degeneration. How these findings can be reconciled will be discussed later in this paper.
Thalamic nomenclature
The thalamus is one of the key regions involved in tremor. The nomenclature of the thalamic subregions is diverse and often confusing. Here we will follow the nomenclature proposed by Jones (Hirai and Jones, 1989). Using acetylcholinesterase and Nissl staining on post-mortem thalamic sections in humans, Jones divided the ventral lateral (VL) thalamus into anterior and posterior nuclei. These two nuclei have clearly corresponding areas in the primate thalamus, where information about the connectivity of these areas is available. Thus, according to Jones, the anterior VL receives input from the pallidum, and the posterior VL receives input from the cerebellum. Many clinicians, on the other hand, use the thalamic nomenclature by Hassler, because of its widespread use in the deep brain stimulation (DBS) literature. The most common thalamic target for tremor relief is Hassler's ventral intermediate nucleus, which is localized during surgery based on the presence of tremor-synchronous bursts and kinaesthetic cells, anterior to cutaneous receptive cells (Krack et al., 2002). It is generally agreed that interference with this region relieves tremor by deafferenting the thalamus from cerebellar projections (Percheron et al., 1996; Krack et al., 2002). Therefore, the neurosurgeon's ventral intermediate nucleus is thought to correspond to (part of) Jones' posterior VL (Krack et al., 2002). To maintain a uniform nomenclature throughout this paper, we will refer to the ventral intermediate nucleus as posterior VL although it must be borne in mind that this analogy is not a strict one.
Neural mechanisms underlying parkinsonian resting tremor
Parkinsonian tremor is caused mainly by central, rather than peripheral mechanisms (Elble, 1996; Deuschl et al., 2000; McAuley and Marsden, 2000). Evidence for this view comes from work showing that peripheral deafferentation (Pollock and Davis, 1930), peripheral anaesthesia of tremulous muscles (Walshe, 1924) and mechanical perturbations (Lee and Stein, 1981; Homberg et al., 1987) have little effect on Parkinson's disease tremor although peripheral reflexes (muscle stretch) may interact with central oscillations (Rack and Ross, 1986).
Several electrophysiological and metabolic investigations have studied the cerebral basis of tremor production. Methodological differences between these studies must be considered for interpreting their results. First, different experimental designs have been used: (i) an ‘event-related’ design, where tremor characteristics (such as fluctuations in tremor amplitude) were directly related to cerebral activity and (ii) a ‘trait’ design, where baseline characteristics (such as cerebral perfusion or grey matter density) were compared between tremor-dominant and non-tremor Parkinson's disease subgroups. With this latter approach, differences between subgroups might be related to tremor, but also to other factors. Second, different recording techniques have been used: (i) electrophysiological studies tried to relate cycle-to-cycle oscillations in tremor (typically ∼4 Hz) to neural oscillations with the same frequency as the tremor. The spatial resolution of such studies was limited to a group of neurons (e.g. studies in patients with deep electrodes) or to a shallow portion of the cortical mantle (e.g. studies using magnetoencephalography) and (ii) metabolic imaging methods such as PET and functional MRI are blind to the rapid cycle-to-cycle changes in neural activity, but are sensitive to episodic changes in tremor output, and offer a view of the whole brain. In the following section, we briefly review these studies, taking these methodological considerations into account.
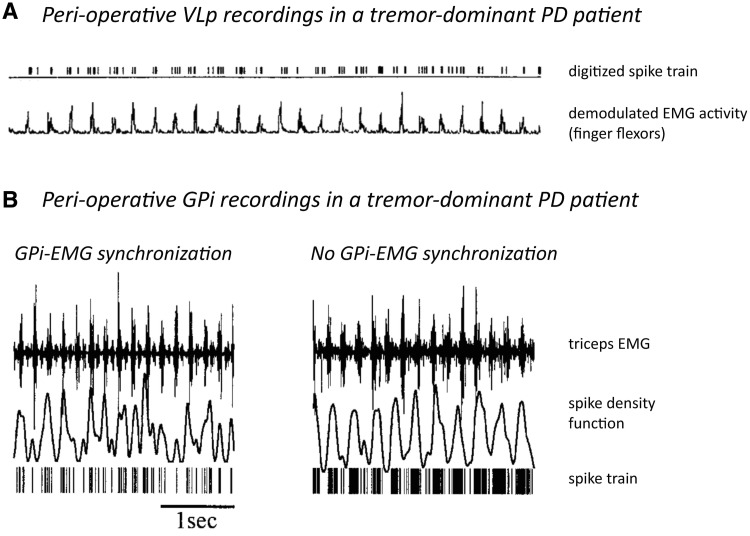
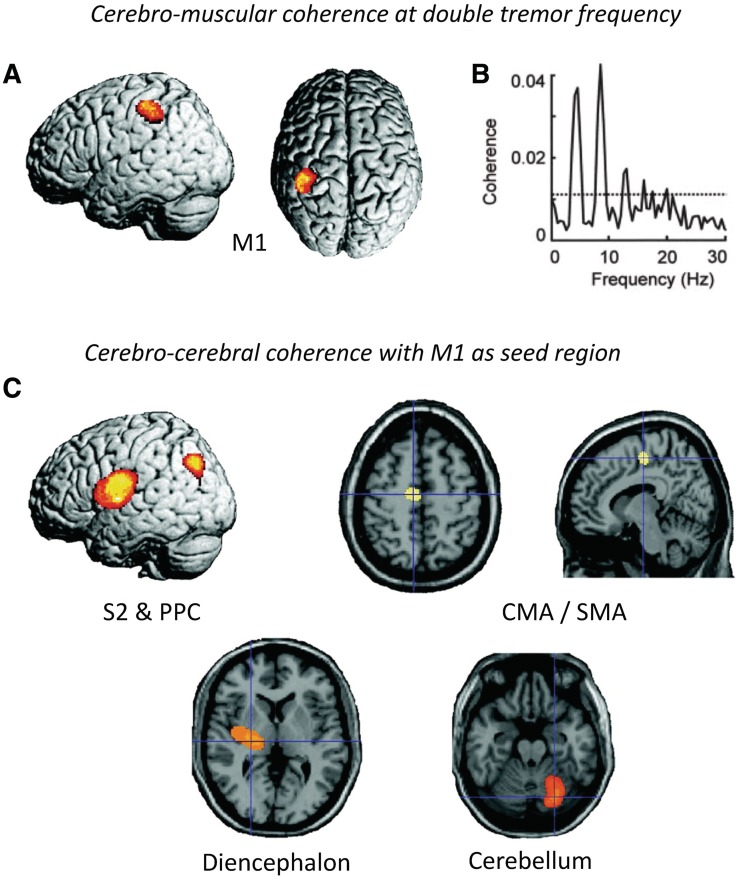
Electrophysiological studies have identified cells firing at tremor frequency both in the basal ganglia [subthalamic nucleus (STN) and pallidum (Levy et al., 2000; Raz et al., 2000)] and the posterior VL (Lenz et al., 1994; Magnin et al., 2000). Within the basal ganglia-cortical circuit, tremor activity is organized in parallel and partly segregated subloops: intra-operative recording of local field potentials in the STN of patients with tremor-dominant Parkinson's disease revealed clusters of tremor-associated coupling between STN and tremor EMG that were spatially distinct for different muscles (Reck et al., 2009). Another study using intra-operative STN recordings in patients with tremor-dominant Parkinson's disease found that neurons (episodically) oscillating at tremor frequency were locally surrounded by non-oscillating or out-of-phase neurons, while larger populations of neurons continuously oscillated at 8–20 Hz (Moran et al., 2008). Given the known somatotopic organization of the posterior VL (Ohye et al., 1989), a similar organization of tremor in subloops may apply to the cerebello-thalamo-cortical circuit. These findings explain why tremor in different limbs is generally not coherent with each other (Hurtado et al., 2000; Raethjen et al., 2000). Importantly, the synchronicity of basal ganglia and thalamic activity to tremor varies between regions. Pallidal neurons are only transiently and inconsistently coherent with tremor (Hurtado et al., 1999; Raz et al., 2000), while posterior VL neurons are highly synchronous with tremor (Lenz et al., 1994) (Fig. 2). These findings suggest that the basal ganglia cannot be the driving force behind resting tremor (Zaidel et al., 2009). Magnetoencephalography work supports this view, showing that a cerebello-thalamo-cortical circuit, rather than the basal ganglia, fires in synchrony with ongoing resting tremor in Parkinson's disease (Timmermann et al., 2003) (Fig. 3).
Figure 2.
Neuronal correlates of Parkinson's disease tremor. (A) Simultaneous recording of thalamic posterior VL (VLp) single-unit activity and peripheral EMG during tremor in a parkinsonian patient. These data show continuous synchronization between internal globus pallidus activity and peripheral EMG. Reprinted from Lenz et al. (1988), with permission from the Society for Neuroscience. (B) Simultaneous recording of internal globus pallidus (GPi) multi-unit activity and peripheral EMG during tremor in a patient with Parkinson's disease (PD). The two plots illustrate the raw signals of two epochs of data sampled 5 min apart. Note that in the left trace the peaks in the spike density function coincide with the EMG bursts, whereas in the right trace the oscillations in the spike density function occur at a lower frequency than the EMG. These data show that synchronization between neuronal activity in internal globus pallidus and peripheral EMG is transient in nature. Reprinted from Hurtado et al. (1999). Copyright (2011) National Academy of Sciences, USA.
Figure 3.
Oscillatory correlates of Parkinson's disease tremor. This figure shows statistical parametric (SPM) maps of spatially normalized cerebro-muscular and cerebro-cerebral coherence of four patients with right-sided rest tremor. Cerebral activity was measured with magnetoencephalography and muscular activity with EMG. (A) Cerebro-muscular coherence at double tremor frequency is located in the contralateral primary motor cortex (M1). (B) This plot shows the coherence between M1 activity and the tremor EMG for one patient with Parkinson's disease. The dashed line indicates the 99% confidence level. (C) Cerebro-cerebral coherence was computed with the reference region in M1 and averaged for all four patients. Areas of consistent coupling with M1 were found in the secondary somatosensory cortex (S2), posterior parietal cortex (PPC), cingulate motor area (CMA)/supplementary motor area (SMA), contralateral diencephalon and ipsilateral cerebellum. Due to the poor coverage by and the large distance to the magnetoencephalography sensors, localization in the latter two areas is not as precise as at the cortical level. These data show a cerebello-thalamo-cortical circuit coupled with tremor on a cycle-by-cycle basis. Reprinted from Timmermann et al. (2003), by permission of Oxford University Press.
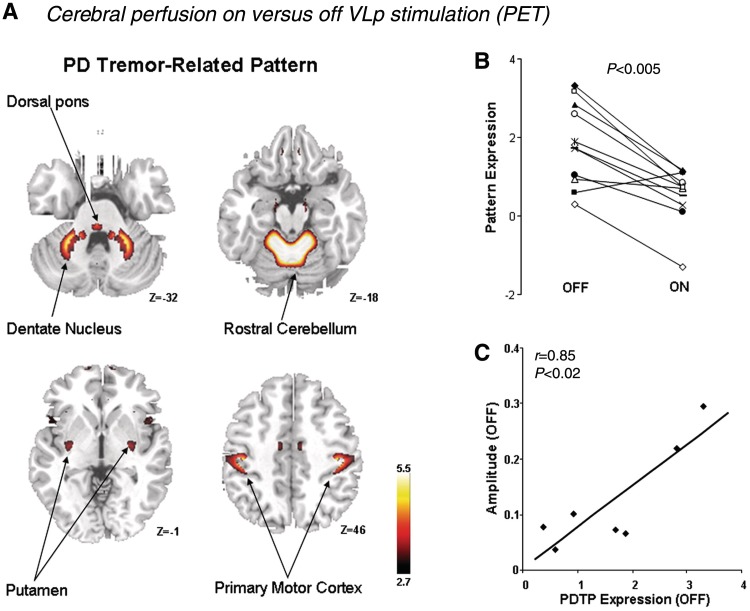
Several metabolic imaging studies (PET) investigated how ‘event-related’ cerebral activity changed after posterior VL-DBS, which reduces tremor amplitude. These studies found that posterior VL stimulation was associated with reduced activity in the cerebellum (Deiber et al., 1993; Fukuda et al., 2004), motor cortex (Fukuda et al., 2004) and medial frontal cortex (Fukuda et al., 2004). Another approach is to localize cerebral activity that co-varied with differences (between-subjects) in tremor amplitude. This identified similar areas, namely tremor-related activity in the cerebellum, ventrolateral thalamus and motor cortex (Kassubek et al., 2001). Other PET studies compared baseline cerebral perfusion (a ‘trait’) between tremor-dominant and non-tremor patients with Parkinson's disease. They found that tremor-dominant patients had relatively increased perfusion of the thalamus, pons and premotor cortex, compared to non-tremor patients (Antonini et al., 1998). Compared to healthy controls, both Parkinson's disease groups had increased pallido–thalamic and pontine activity, and reduced activity in premotor cortex, supplementary motor area and parietal cortex (Antonini et al., 1998). Accordingly, bradykinesia and rigidity were found to correlate with activity in the Parkinson's disease-wide network, but tremor did not (Eidelberg et al., 1994). A recent PET study combined these approaches, describing a tremor-related network consisting of sensorimotor cortex, cerebellum (lobules IV/V and dentate), cingulate cortex and—to a lesser extent—putamen (Mure et al., 2011). Activity in this network was correlated with clinical tremor scores, it was higher in patients with tremor-dominant than non-tremor Parkinson's disease, it increased with disease progression and it was suppressed by both posterior VL and STN-DBS (Fig. 4). Importantly, the metabolic effects of posterior VL and STN-DBS overlapped in a single region, the motor cortex. This suggests that the basal ganglia and cerebellar tremor circuits converge in the motor cortex.
Figure 4.
Metabolic correlates of Parkinson's disease tremor. (A) Spatial covariance pattern identified by ordinal trends canonical variate analysis of FDG PET data from 11 hemispheres of nine patients with tremor-dominant Parkinson's disease (PD) scanned on and off posterior VL stimulation (labelled ventral intermediate nucleus in the original manuscript). Posterior VL stimulation improved tremor severity and reduced metabolic activity in the primary motor cortex, anterior cerebellum/dorsal pons, and the caudate/putamen. (B) The expression of this Parkinson's disease tremor-related metabolic pattern (PDTP) was reduced by posterior VL stimulation in 10 of the 11 treated hemispheres. (C) Baseline PDTP expression (i.e. off-stimulation pattern scores) correlated (r = 0.85, P < 0.02) with tremor amplitude, measured with concurrent accelerometry. These data show that metabolic activity in both the cortico-cerebellar circuit and the basal ganglia is related to tremor severity. Reprinted from Mure et al. (2011), with permission from Elsevier.
Structural imaging
Two MRI studies used voxel-based morphometry to test for structural changes (‘traits’) in tremor-dominant Parkinson's disease. These studies compared tremor-dominant Parkinson's disease with either non-tremor patients with Parkinson's disease (Benninger et al., 2009) or with controls (Kassubek et al., 2002). Tremor-dominant patients had reduced grey matter volume in the right cerebellum (Benninger et al., 2009) and increased grey matter volume in the posterior VL (Kassubek et al., 2002). These findings fit with the involvement of the cerebello-thalamo-cortical network in tremor, as shown with functional imaging (Deiber et al., 1993; Fukuda et al., 2004). It is unclear how increased activation of these areas can translate in both reduced and increased grey matter volume. This divergence might be explained by differences in neuroplasticity between brain regions, which—in the context of skill acquisition—can produce opposite volumetric changes in the cortex and basal ganglia (Kloppel et al., 2010).
Taken together, these findings provide evidence for the involvement of both the cerebello-thalamo-cortical circuit and the basal ganglia in Parkinson's disease resting tremor. The arrest of tremor by focused interventions in either of these circuits further confirms that both are causally related to tremor. That is, DBS targeted towards either the basal ganglia [pallidum or STN (Lozano et al., 1995; Krack et al., 1997)] or the cerebellar loop [posterior VL (Benabid et al., 1991)] reduces tremor severity in Parkinson's disease. The efficacy of posterior VL and STN-DBS on Parkinson's disease tremor appears to be comparable (Pollak et al., 2002): UPDRS tremor scores (items 20 and 21, OFF medication) decreased by 75–84% with STN-DBS (Kumar et al., 1998; Krack et al., 2003; Kim et al., 2010) and by 77–88% with posterior VL-DBS (Rehncrona et al., 2003; Hariz et al., 2008). Earlier studies found that 58–88% of patients with Parkinson's disease had a total suppression of tremor 3–6 months after posterior VL-DBS (Benabid et al., 1991; Koller et al., 1994). There are no studies directly comparing the effects of these two targets, but one study reported an additional reduction of tremor scores after STN-DBS in patients with Parkinson's disease with previous thalamic surgery (Fraix et al., 2005).
Models explaining the occurrence of parkinsonian resting tremor
Several hypotheses have been put forward to explain the occurrence of resting tremor in Parkinson's disease. As outlined above, there is evidence that both the basal ganglia and the cerebello-thalamo-cortical circuit are implicated in tremor. However, most models are based on detailed recordings in a limited set of neurons (e.g. ex vivo preparations) or a limited set of structures (e.g. electrophysiological recordings). Therefore, most models focus on a node in a single circuit and interpret the changes in other circuits as secondary. Here we will place concurrent changes in two separate circuits into perspective. This section also updates and elaborates on earlier reviews about the pathophysiology of parkinsonian tremor (Elble, 1996; Deuschl et al., 2000; Rodriguez-Oroz et al., 2009).
Model 1: the thalamic pacemaker hypothesis
This hypothesis (Jahnsen and Llinas, 1984) is based on in vitro preparations of guinea pig thalamic neurons, where it was found that the intrinsic biophysical properties of thalamic neurons allow them to serve as relay systems and as single cell oscillators at two distinct frequencies, 9–10 and 5–6 Hz. Specifically, slightly depolarized thalamic cells tend to oscillate at 10 Hz, while hyperpolarized cells oscillate at 6 Hz (Llinas, 1988). These two frequencies coincide with the frequency of physiological tremor and Parkinson's disease tremor, respectively. The key assumption of this model is that (single) thalamic neurons, not the basal ganglia circuitry, form the tremor pacemaker. However, in vivo measurements in the thalamus of patients with Parkinson's disease have questioned the presence of these thalamic pacemaker cells. That is, while the 6 Hz oscillatory mode in the animal model is associated with low threshold calcium spike bursts, this pattern was not observed (with rare exception) in the thalamus of patients with Parkinson's disease with tremor (Zirh et al., 1998). In contrast, a second study found a convincing low threshold calcium spike bursts pattern in a larger number of cells in the thalamus (both the anterior and posterior VL) of patients with Parkinson's disease (Magnin et al., 2000), possibly due to more sensitive processing techniques. However, low-threshold calcium spike bursts were present both in patients with tremor-dominant and akinetic Parkinson's disease, and the bursts were not coherent with peripheral tremor recordings. Patients with tremor-dominant Parkinson's disease showed distinct tremor-locked bursts without low-threshold calcium spike bursts characteristics in the posterior VL, but not in the anterior VL. These findings could be taken as evidence that pathological low-threshold calcium spike bursting is not related to tremor. Alternatively, thalamic low-threshold calcium spike bursts might be transformed into tremor-locked bursts by re-entry properties of the thalamo-cortical circuit (Magnin et al., 2000).
Another issue concerns the mechanisms that drive thalamic cells into an oscillatory mode in Parkinson's disease. In theory, any mechanism that engenders membrane hyperpolarization, whether through reduction of excitatory drive (dysfacilitation) or excess inhibition, will trigger low-frequency rhythmicity of thalamic neurons (Llinas et al., 2005). Several different mechanisms have been suggested. First, according to the classical model of Parkinson's disease (Albin et al., 1989), the internal globus pallidus sends increased (inhibitory) output to the thalamus, which may hyperpolarize thalamic neurons and thus trigger oscillations at 5–6 Hz (Llinas et al., 2005). However, this mechanism would predict a predominant role for the pallidal thalamus, anterior VL, in tremor genesis. This does not fit with findings from DBS, which show that interference with the cerebellar thalamus, posterior VL, is superior for treating tremor (Atkinson et al., 2002), or with the finding that there are more tremor cells in the posterior VL than anterior VL (Magnin et al., 2000). Second, it has recently been proposed that increased inhibitory input from the zona incerta towards the posterior VL could hyperpolarize these thalamic neurons (Plaha et al., 2008). The zona incerta is located in the subthalamic region, medially with respect to the STN. In non-human primates, the zona incerta receives projections from the internal globus pallidus [although mainly the cognitive division (Sidibe et al., 1997)], and it sends GABA-ergic projections to the posterior VL (Bartho et al., 2002). This makes the zona incerta an interface between the basal ganglia and the cerebello-thalamo-cortical circuits, and its involvement in tremor may explain why both circuits are related to tremor. In line with this hypothesis, DBS of the zona incerta can reduce tremor (Plaha et al., 2006) and low-frequency stimulation of the zona incerta can induce tremor in patients with previously non-tremulous Parkinson's disease (Plaha et al., 2008). Third, thalamic posterior VL neurons may be hyperpolarized through the cerebellum. Moreover, the finding of disynaptic projections from the STN to the cerebellar cortex in non-human primates (Bostan et al., 2010) opens the possibility that pathological activity in the basal ganglia produces downstream changes in the cerebellum and cerebellar thalamus (posterior VL). Fourth, it is possible that hyperpolarization of thalamic posterior VL neurons is related to the degeneration of dopaminergic projections from the midbrain to the posterior VL. Specifically, both the retrorubral area [that is degenerated in tremor-dominant Parkinson's disease (Hirsch et al., 1992)] and the substantia nigra pars compacta send sparse dopaminergic projections to the whole thalamus, including the posterior VL (Sanchez-Gonzalez et al., 2005). According to this hypothesis, basal ganglia dysfunction would not be required for the hyperpolarization of posterior VL neurons.
Model 2: the thalamic filter hypothesis
This hypothesis (Pare et al., 1990) is also based on in vitro data and proposes that parkinsonian resting tremor emerges when high-frequency (12–15 Hz) oscillations in the basal ganglia are transformed into a 4–6 Hz pattern by thalamic anterior VL neurons. The key feature of this hypothesis is that the tremor pacemaker is primarily located in the basal ganglia (pallidum), with pallido-thalamic interactions determining the net frequency of the tremor. This hypothesis seems to fit with recent data in non-human primates, where it was found that 10 Hz pallidal oscillations were only present in tremor-dominant vervet monkeys but not in non-tremor macaques (Rivlin-Etzion et al., 2010). In contrast, pallidal oscillations at 5 Hz were present in both species. However, high-frequency stimulation of the pallidum did not spread to the motor cortex (Rivlin-Etzion et al., 2008). This makes it unlikely that high-frequency oscillations in the basal ganglia drive Parkinson's disease resting tremor (Rivlin-Etzion et al., 2006; Zaidel et al., 2009). Other work also questions the specific role of high-frequency basal ganglia oscillations in the generation of tremor. That is, dopaminergic treatment reduced the pathologically enhanced 8–35 Hz rhythms in the STN of patients with Parkinson's disease, but this improved only akinesia and rigidity, not tremor (Kuhn et al., 2006). Therefore, most authors relate the increased high-frequency (8–35 Hz) oscillations in the basal ganglia of patients with Parkinson's disease to akinesia, but not to tremor (Rivlin-Etzion et al., 2006; Hammond et al., 2007).
Model 3: the subthalamic nucleus-external globus pallidus pacemaker hypothesis
This hypothesis (Plenz and Kital, 1999) is again based on in vitro data and proposes that the STN and external globus pallidus constitute a central pacemaker that is modulated by striatal inhibition of external globus pallidus neurons. This pacemaker could be responsible for synchronized oscillatory activity in the normal and pathological basal ganglia. However, these oscillations occurred at frequencies between 0.4 and 1.8 Hz, and it is unclear whether they have any relationship with parkinsonian tremor, given the lack of in vivo measurements. Thus, it is not possible to test whether these oscillations are consistently coherent with tremor, and hence this hypothesis suffers from the same critique as Model 2.
Model 4: the loss-of-segregation hypothesis
This hypothesis (Bergman et al., 1998a) is based on the finding that—in normal primates—the activity of neighbouring pallidal neurons is completely uncorrelated (Bar-Gad et al., 2003), while parkinsonian primates develop markedly increased correlations between remotely situated pallidal neurons (Bergman et al., 1998a). This could lead to excessive synchronization in the basal ganglia, possibly because inhibitory collaterals in the pallidum are affected by dopamine depletion (Bevan et al., 1998), resulting in tremor (Deuschl et al., 2000). The key feature of this model is that the basal ganglia circuitry, not the thalamus, forms the tremor pacemaker. In line with this hypothesis, parkinsonian primates show less selective neuronal responses to proprioceptive stimulation in the entire pallido-thalamo-cortical loop (Filion et al., 1988; Goldberg et al., 2002; Pessiglione et al., 2005). These changes were found to be larger in the pallidum of patients with tremor-dominant than non-tremor Parkinson's disease (Levy et al., 2002), and hence related to tremor. However, as already mentioned, the inconsistent coherence between basal ganglia oscillations and tremor (Hurtado et al., 1999; Raz et al., 2000) complicates a causal link between these phenomena (Zaidel et al., 2009).
Taken together, the different models discussed above position the tremor pacemaker either in the thalamus (Model 1) or in the basal ganglia (Models 2–4). Since the cerebellar (not pallidal) thalamus is primarily involved in parkinsonian tremor, the thalamic pacemaker hypothesis does not account for the mechanisms that trigger thalamic oscillations, and it remains unclear whether these mechanisms have any relationship with basal ganglia dysfunction. On the other hand, the basal ganglia pacemaker hypotheses are more directly linked to the core pathophysiological substrate of Parkinson's disease, but these models struggle with the fact that tremor-related oscillations in the basal ganglia are only transient and inconsistent in nature.
The ‘dimmer-switch’ model of parkinsonian resting tremor
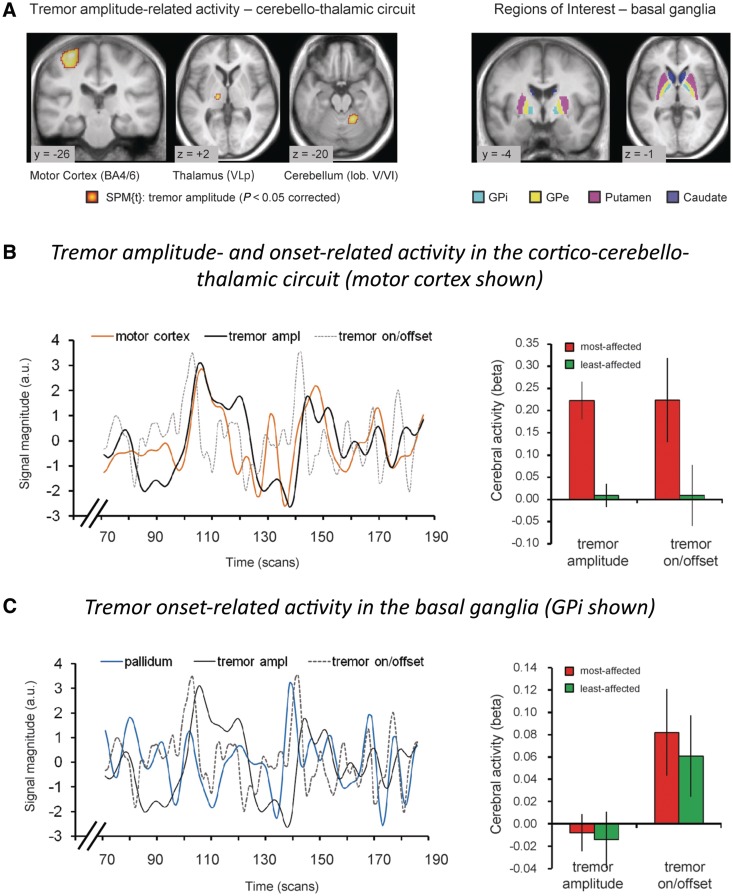
Given the well-known role of both the basal ganglia and the cerebello-thalamic circuits in tremor, we aimed to construct a model that specifies and integrates the role of both circuits in tremor (Helmich et al., 2011b). To test this systems-level view on tremor, we used functional MRI to identify cerebral responses that co-fluctuated with spontaneous variations in tremor amplitude, as measured with EMG during scanning (van Duinen et al., 2005; van Rootselaar et al., 2008; Helmich et al., 2011b). These abrupt amplitude fluctuations are very characteristic of parkinsonian tremor, and they do not occur, for example, in essential tremor (Elble and Koller, 1990; Gao, 2004; Ropper and Samuels, 2009). This method also allowed us to quantify functional interactions between the basal ganglia and the cerebello-thalamo-cortical circuit. We obtained the following results (Fig. 5): (i) tremor amplitude-related activity was localized to the cerebello-thalamo-cortical circuit (posterior VL, cerebellum and motor cortex); (ii) cerebral activity time-locked to the onset of high-amplitude tremor episodes was localized to the basal ganglia and the cerebello-thalamo-cortical circuit; and (iii) tremor-dominant patients with Parkinson's disease had increased functional connectivity between the basal ganglia and the cerebello-thalamo-cortical circuit, compared with non-tremor Parkinson's disease and healthy controls. On the basis of these data, we suggest the following model, which is also illustrated in Fig. 6: (i) activity in the basal ganglia triggers tremor-related responses in the cerebello-thalamo-cortical circuit, which produces the tremor; and (ii) these interactions occur in the motor cortex, where both circuits converge (Hoover and Strick, 1999). The novel aspect of this model is that it offers a mechanism explaining how the basal ganglia and the cerebello-thalamo-cortical circuits interact with each other. Given the emphasis on a combination of basal ganglia contributions (that trigger tremor on/offset, analogous to a light switch) and cerebello-thalamo-cortical contributions (that modulate tremor intensity, analogous to a light dimmer), we call this model the ‘dimmer-switch model’ of Parkinson's disease resting tremor (Fig. 6).
Figure 5.
Tremor amplitude- and onset-related cerebral activity in Parkinson's disease. (A) Left: Cerebral regions where activity co-fluctuated with tremor amplitude (19 tremor-dominant patients, P < 0.05 whole-brain corrected). Activity was localized to the motor cortex, thalamus (posterior VL; VLp) and cerebellum (left side = side contralateral to tremor). Right: Regions of interest in the basal ganglia are shown. (B) In the cerebello-thalamo-cortical circuit, we found two separate effects: (i) cerebral activity related to tremor amplitude and (ii) cerebral activity related to changes in tremor amplitude (tremor on/offset). Left: These two tremor-related effects are illustrated for the motor cortex of one patient. Right: These two tremor-related effects are shown for the motor cortex across the whole group (19 tremor-dominant patients), separately for the most- and least-affected hemisphere. Similar effects were found in the posterior VL and cerebellum (not shown). (C) In the basal ganglia, we found cerebral activity related to changes in tremor amplitude (tremor on/offset), but not cerebral activity related to tremor amplitude. Left: This effect is illustrated for the internal globus pallidus of one patient. Right: This effect is shown for the internal globus pallidus (GPi) across the whole group (19 tremor-dominant patients), separately for the most- and least-affected hemisphere. Similar effects were found for the putamen, but not for the caudate (not shown). The line graphs in B and C show three relevant time courses: (i) brain activity (motor cortex in orange, internal globus pallidus in blue); (ii) tremor amplitude of the contralateral hand (in black; EMG regressor convolved with the haemodynamic response function); and (iii) tremor on/offset (in dotted grey, first temporal derivative of the tremor amplitude regressor, convolved with the haemodynamic response function). These data suggest distinct contributions of two circuits to tremor: the cerebello-thalamo-cortical circuit controls tremor amplitude, and the striato-pallidal circuit produces changes in tremor amplitude. Reprinted from Helmich et al. (2011b), with permission from John Wiley and Sons. GPe = external globus pallidus.
Figure 6.
The dimmer-switch model of parkinsonian resting tremor. In tremor-dominant Parkinson's disease, dopaminergic cell death in the retrorubral area A8 causes dopamine depletion in the pallidum (in red). Pallidal dopamine depletion leads to emergence of pathological activity in the striato-pallidal circuit, which triggers activity in the cerebello-thalamo-cortical circuit (in blue) through the primary motor cortex (red line between pallidum and primary motor cortex). Thus, the striato-pallidal circuit triggers tremor episodes (analogues to a light switch), while the cerebello-thalamo-cortical circuit produces the tremor and controls its amplitude (analogous to a light dimmer). This model is based on Helmich et al. (2011b). VLp = posterior VL.
To identify the dopaminergic mechanisms underlying these changes, we compared striato-pallidal DAT binding between tremor-dominant and non-tremor patients with Parkinson's disease, using [123I]FP-CIT SPECT (Helmich et al., 2011b). This revealed that pallidal, but not striatal, dopamine depletion correlates with the severity of resting tremor. This finding could solve the dopaminergic paradox of Parkinson's disease resting tremor. Specifically, the pallidum receives distinct dopaminergic projections from the substantia nigra pars compacta (Smith et al., 1989) and the retrorubral area (Jan et al., 2000), but these same mesencephalic areas also send separate projections to the striatum [substantia nigra pars compacta (Anden et al., 1964); retrorubral area (Francois et al., 1999)]. This pattern of divergence and convergence makes it unlikely that midbrain pathology can produce either pure striatal or pure pallidal dopamine depletion although the degree of dopamine depletion in each area may vary between patients. Thus, patients with Parkinson's disease with resting tremor will generally have some degree of striatal dopamine depletion, explaining why the presence of striatal dopamine depletion appears required for developing resting tremor (Deuschl et al., 2000). Indeed, if pallidal (but not striatal) dopamine depletion is involved in tremor genesis, this could also explain why striatal DAT signal is not correlated with tremor severity.
The dimmer-switch model combines several features of the previous hypotheses into a larger explanatory framework. First, loss of segregation in the dopamine-depleted pallidum may be a mechanism that explains both the emergence of pathological activity in the basal ganglia, and the increased connectivity between basal ganglia and motor cortex (Rivlin-Etzion et al., 2008). Second, altered basal ganglia output may influence neurons in the cerebellar thalamus (posterior VL) via the motor cortex. That is, excitatory cortico-thalamic projections from motor cortex to ventrolateral thalamus (Fonnum et al., 1981; Rouiller et al., 1998; Kultas-Ilinsky et al., 2003) can activate inhibitory intra-thalamic circuits (Ando et al., 1995; Landisman and Connors, 2007; Cruikshank et al., 2010), leading to low-frequency oscillations within the thalamo-cortical network (Blumenfeld and McCormick, 2000). This model would explain why basal ganglia oscillations are only transient and inconsistent, why thalamic oscillations are highly synchronous with the tremor, and thus why both basal ganglia and the cerebello-thalamo-cortical circuit are causally related to tremor. It remains to be shown why posterior VL neurons are more prone to develop tremor oscillations than anterior VL neurons, since both regions receive cortico-thalamic projections from the motor cortex. Hypothetically, the connections between the posterior VL and the cerebellum are a prerequisite for the development of tremor oscillations.
Limitations of the model and future research
Role of the subthalamic nucleus
With the methods (functional MRI) employed in our previous paper (Helmich et al., 2011b), we were not able to reliably detect cerebral activity in a small nucleus such as the STN. The presence of tremor oscillations in the STN that are coherent with peripheral tremor activity (Levy et al., 2000) and the ability of STN-DBS to reduce tremor (Kumar et al., 1998; Krack et al., 2003; Kim et al., 2010) suggest that this nucleus has an important pathophysiological role in tremor. In contrast to the pallidum, the STN receives direct anatomical projections from the motor cortex (Nambu et al., 2000), and functional connectivity between the motor cortex and the STN is increased in Parkinson's disease (Baudrexel et al., 2011; Moran et al., 2011). The STN also sends disynaptic anatomical projections to the cerebellar cortex (Bostan et al., 2010). Therefore, the STN has both afferent and efferent connections with the cerebello-thalamo-cortical tremor circuit. Whether the STN is part of the basal ganglia trigger, or whether the STN is involved in the cerebello-thalamo-cortical circuit producing the tremor, remains to be investigated in future studies using high-resolution MRI in combination with connectivity analyses.
Tremor oscillator
Although our methods (functional MRI) enabled a systems-level view on tremor, we could not detect oscillatory activity at tremor frequency. Therefore, it remains an open question which brain region(s) determine the tremor frequency. The cerebello-thalamo-cortical tremor network we identified matches closely the network identified in studies that have directly tracked cerebral changes occurring at resting-tremor frequency (using magneto-encephalography; Fig. 3). Thus, in our view, it is the cerebello-thalamo-cortical network that is the ultimate tremor generator, but as influenced and triggered by the coupled basal ganglia network. Accordingly, a novel DBS paradigm that takes these network properties into account was found to be more successful than standard DBS in reducing both Parkinson's disease symptoms and tremor oscillations in the internal globus pallidus (Rosin et al., 2011). This new paradigm, termed closed-loop DBS, uses a trigger detected in a reference structure (M1) as the input to deliver DBS trains to the stimulated structure (internal globus pallidus). These data show that the interconnectivity between various participating brain areas plays a crucial role in the emergence of pathological oscillations and clinical symptoms.
Relationship between metabolic and oscillatory activity
Based on our model, we suspect that the intermittent oscillations in the basal ganglia (Fig. 1) could be related to tremor dynamics such as abrupt changes in amplitude, but this remains to be tested. Most previous studies did not take tremor amplitude fluctuations into account, but focused on cycle-by-cycle coherence between neural and muscular activity, i.e. signals occurring at ∼4 Hz. These approaches are blind to neural changes at the onset of high-amplitude tremor episodes, because neuromuscular coherence at 4 Hz is similar across low- and high-tremor amplitude (Reck et al., 2009). In a preliminary study in one patient with Parkinson's disease, the authors recorded local field potentials from the STN and related them to changes in tremor amplitude, as measured with EMG (Wang et al., 2005). They found that beta suppression in the STN preceded the onset of tremor episodes and made way for oscillations at tremor frequency in the STN. If activity in the beta band is a way by which the sensorimotor system maintains the status quo (Gilbertson et al., 2005; Engel and Fries, 2010; Jenkinson and Brown, 2011), then beta suppression in the STN before tremor onset could indicate a removal of neural inhibition to establish tremor onset-related activity (trigger). On the other hand, since beta suppression in the cortex (Crone et al., 1998) and STN (Kuhn et al., 2004) is known to precede voluntary movements, the finding of beta suppression prior to tremor could just reflect a more general phenomenon preceding any movement.
Role of dopamine in tremor dynamics
In our model, pallidal activity was related to changes in tremor amplitude, rather than the amplitude of the tremor itself (Fig. 5). This raises the question how the severity of pallidal dopamine depletion could predict clinical tremor severity (Fig. 1C). This likely depends on the effect of dopamine depletion on pallidal activity. For example, dopamine depletion may increase the amplitude of tremor onset-related activity in the pallidum. This should lead to more abrupt tremor changes, but not to increased tremor amplitude. Second, dopamine depletion may increase the rate of onset-related activity in the pallidum. More frequent episodes of pallidal activity could lead to more frequent tremor episodes, but also, if the bursts of pallidal activity occur shortly after each other, to amplified activity in the cerebello-thalamo-cortical circuit (and hence to increased tremor amplitude). Finally, more severe pallidal dopamine depletion may lead to enhanced connectivity between the basal ganglia and the cerebello-thalamo-cortical systems. This would make the cerebello-thalamo-cortical circuit more susceptible to perturbing signals from the basal ganglia, and the increased input–output relationship may lead to more severe tremor. To investigate these possibilities, we are currently testing tremor-dominant Parkinson patients ON and OFF dopaminergic medication using functional MRI.
Causality
Using metabolic imaging, several groups have found a correlation between tremor amplitude and cerebral activity in the cerebellum, motor cortex and posterior VL (e.g. Deiber et al., 1993; Helmich et al., 2011b; Mure et al., 2011). One interpretational problem is that the limited temporal resolution of these techniques makes it difficult to determine whether the cerebral effects are causal or reactive to the tremor. Electrophysiological studies, which have a much higher temporal resolution, partly suffer from the same problem. That is, single-cell recordings in the thalamus, STN and internal globus pallidus of patients with Parkinson's disease show that many neurons with tremor-related activity also respond to somatosensory stimulation (Lenz et al., 1994; Magnin et al., 2000). Therefore, neural activity that leads peripheral tremor activity might also relate to the preceding tremor beat. Conduction times are not helpful to disentangle cause from effect, given the diverse pathways through which afferent input can reach the basal ganglia and thalamus. Nevertheless, there are also thalamic cells that do not respond to somatosensory stimulation, and that show tremulous activity preceding muscular activity (Lenz et al., 1994). This suggests that the thalamus has a causal role in tremor. Other electrophysiological studies have calculated the oscillatory activity (at tremor or double tremor frequency) of single neurons or larger groups of neurons, for example using subcortical DBS electrodes or cortical magnetoencephalography recordings (Levy et al., 2000; Timmermann et al., 2003). Since oscillations are defined over longer temporal windows, this procedure makes it difficult to determine whether neural oscillations drive the tremor or vice versa. Analytical methods including the phase of coherence or Granger causality might help to solve this problem (Timmermann et al., 2003; Van Quyen and Bragin, 2007) although these methods are susceptible to noise contamination and volume conduction (Albo et al., 2004; Rivlin-Etzion et al., 2006).
To reliably disentangle cause from effect, interference studies are helpful. Lesion studies provide strong evidence that activity in the posterior VL is causally linked to tremor: thalamotomy, posterior VL-DBS and thalamic stroke lead to immediate tremor arrest (Benabid et al., 1991; Atkinson et al., 2002; Probst-Cousin et al., 2003; Choi et al., 2008). The motor cortex is the only region of the cerebello-thalamo-cortical circuit that has a direct access to the spinal cord, and therefore it seems plausible that activity in this area drives the tremor. Accordingly, interference with M1 activity using transcranial magnetic stimulation can reset resting tremor in Parkinson's disease (Ni et al., 2010). In contrast, transcranial magnetic stimulation over the cerebellum did not reset tremor, suggesting that this region does not directly drive the tremor. Lesion studies support this idea: cerebellar stroke (Kim et al., 2009) and cerebellectomy (Deuschl et al., 1999) did not remove ipsilateral resting tremor, but transformed it into a Holmes tremor (i.e. a slow-frequency and combined resting, intention and postural tremor). Therefore, the role of the cerebellum in tremor may be modulatory rather than causal. Accordingly, a previous magnetoencephalography study showed that oscillatory activity in the cerebellum is coherent with thalamic and motor activity, but not with the tremor itself (Timmermann et al., 2003). This suggests that the cerebellum does not have a direct efferent or afferent relationship with peripheral tremor. This relationship could be different for other tremor pathologies. For example, other than in Parkinson's disease, cerebellar stroke can ameliorate ipsilateral essential tremor (Dupuis et al., 2010). Finally, an approach to gain mechanistic insights into the role of altered oscillations in Parkinson's disease has been to stimulate the basal ganglia or thalamus at precisely these frequencies, using implanted DBS electrodes. For example, 5–40 Hz stimulation of the STN, zona incerta and ventrolateral thalamus induced tremor in patients with Parkinson's disease (Plaha et al., 2008), suggesting that oscillatory activity in these regions is causally linked to tremor.
Why does resting tremor stop during voluntary movements?
A characteristic feature of Parkinson's disease resting tremor is its decrease during voluntary movements (Deuschl et al., 2000). This feature is routinely used in clinical practice to distinguish resting tremor from other tremor forms (Deuschl et al., 1998), for example from dystonic tremor where a decrease with movement is typically absent (Schneider et al., 2007). However, the neural mechanisms underlying the interaction between voluntary movements and resting tremor remain unclear. As outlined above, parkinsonian tremor results from altered responses in both the basal ganglia and the cerebello-thalamo-cortical circuit. This indicates that voluntary movements may interact with resting tremor in either or both of these circuits.
Movement–tremor interactions in the cerebello-thalamo-cortical circuit
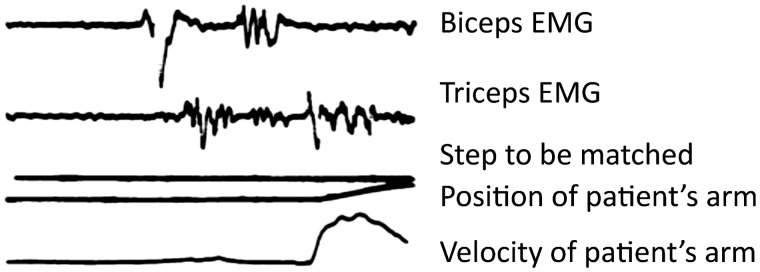
We recently investigated the cerebral interactions between motor planning and Parkinson's disease resting tremor. To this end, we used a motor imagery paradigm (as a quantifiable proxy of motor planning) while measuring tremor-related activity during functional MRI scanning. This procedure avoids the confounding effects of somatosensory reafference associated with the production of voluntary movements. There were two main findings: (i) planning- and tremor-related responses overlapped in the posterior VL, but not in the cerebellum or in the motor cortex and (ii) tremor amplitude was unaffected by motor imagery (Helmich et al., 2011a). This indicates that motor planning-related activity in the posterior VL does not remove tremor-related responses in this region, possibly because both processes involve (partly) different neuronal populations (Lenz et al., 1994; Magnin et al., 2000). Another study directly assessed the electrophysiological interactions between motor execution and Parkinson's disease resting tremor (Hallett et al., 1977). The pattern of alternating activity in agonist and antagonist muscles seen during Parkinson's disease resting tremor strongly resembled the activity seen during voluntary flexion of the arm. Furthermore, in many patients with Parkinson's disease, a single ‘beat of tremor’ preceded voluntary movements, even when there was no clinically noticeable tremor (Fig. 7). This suggests that resting tremor and voluntary movement execution arise from similar oscillations in the motor cortex, which may explain why they do not occur simultaneously. Finally, the observation that resting tremor at movement onset is no longer inhibited when the cerebellum is absent (Deuschl et al., 1999) or malfunctioning (Kim et al., 2009) suggests that the cortico-cerebellar activation during voluntary movements suppresses the tremor rhythm. Re-emergent tremor can then be explained by reinitiating the cerebello-thalamo-cortical tremor circuit through the basal ganglia.
Figure 7.
A beat of tremor preceding movement in Parkinson's disease. Fast flexion patterns in patients with Parkinson's disease. In many patients with Parkinson's disease (17 arms of 15 patients), although resting tremor was not continuously present, a single ‘beat of tremor’ occasionally occurred before the pattern that moved the limb. This is illustrated for one patient. Adapted from Hallett et al. (1977) with permission from BMJ Publishing Group Ltd.
Movement–tremor interactions in the basal ganglia
According to our model, transient activity in the pallidum and putamen can trigger tremor-related activity in the cerebello-thalamo-cortical circuit. The pallidum is also activated during voluntary movement planning (Owen et al., 1998; Helmich et al., 2009). Movement-related activity may replace tremor-related activity in the pallidum, and this could interfere with tremor in two ways. First, the absence of intermittent ‘triggers’ from the pallidum could cause the tremor to fade out. However, this mechanism does not explain why tremor is immediately reduced at the onset of voluntary movements, which suggests an active (instead of a passive) disturbance of the cerebello-thalamo-cortical tremor circuit. Second, the pallidum may actively inhibit the motor cortex during voluntary movements. The pallidum supports action selection by exciting desired motor programmes, while inhibiting all others [centre-surround inhibition (Mink, 1996; Beck and Hallett, 2011)]. Inhibition of motor representations in the motor cortex during voluntary movement selection could actively interfere with tremor-related firing in the cerebello-thalamo-cortical circuit, causing an immediate arrest of the tremor. This concept may also explain why resting tremor re-emerges during fixed postural holding (Jankovic et al., 1999). That is, while the basal ganglia are strongly involved in changing movement set, they are not involved in maintaining a fixed posture (Cools et al., 1984; Hayes et al., 1998; Helmich et al., 2009). Thus, Parkinson's disease tremor may emerge not necessarily in the absence of movement (rest), but rather in the absence of selection demands (including maintaining a posture when no other posture needs to be selected to satisfy the current task context).
Why does tremor have a variable response to dopaminergic treatment?
Previous work has extensively reviewed the response of tremor to different pharmacological preparations (Elble, 2002; Fishman, 2008), and we will not repeat this here. Levodopa and other dopaminergic drugs are generally less efficacious against tremor than other key features of Parkinson's disease (Fishman, 2008; Rodriguez-Oroz et al., 2009). This failure to respond to dopaminergic treatment is difficult to reconcile with most models, which assume that tremor is triggered by dopamine depletion. One possibility is that non-dopaminergic neurotransmitters play a role. For example, patients with Parkinson's disease have 27% lower serotonin binding in the raphe than controls, and tremor was the only symptom that correlated with the amount of serotonin depletion (Doder et al., 2003). In humans, there are serotonergic projections from the raphe to the basal ganglia, including the pallidum (Wallman et al., 2011). If both serotonergic and dopaminergic changes can produce tremor, then this may explain why some patients fail to respond to dopaminergic therapy. Another speculative possibility is that there are crucial temporal windows during disease progression in which the tremor is responsive to dopaminergic treatment. For example, basal ganglia signals might be required for driving the cerebello-thalamo-cortical circuit into tremor only during the early phases of the disease. Later in the disease, perhaps due to depletion of inhibitory neurotransmitters in the tremor circuit, oscillations in the cerebello-thalamo-cortical circuit might not need to be triggered by basal ganglia signals. This would predict that the response of tremor to dopaminergic therapy is modulated by disease duration. Finally, one report investigated the effect of dopaminergic treatment on the oscillatory tremor network in Parkinson's disease (Pollok et al., 2009). They found that levodopa specifically reduced thalamo-cortical coupling. This suggests that the thalamo-cortical axis has a central role in tremor genesis, but that dopaminergic areas (such as the basal ganglia) control the emergence of tremor-related oscillations in this circuit.
Why does tremor indicate a benign Parkinson's disease subtype?
We have reviewed and discussed several clinical and pathophysiological differences between tremor-dominant and non-tremor Parkinson's disease subtypes. We suggest that pallidal dopamine depletion is related to tremor, while other pathophysiological markers could explain a more benign disease course. First, there is converging evidence from post-mortem and nuclear imaging studies that patients with tremor-dominant Parkinson's disease have relatively benign nigrostriatal degeneration. This may explain why other features of Parkinson's disease also take a more benign course in tremor-dominant patients. Second, there is post-mortem evidence that patients with non-tremor Parkinson's disease have more cortical lesions than patients with tremor-dominant Parkinson's disease, and this may explain the worse cognitive dysfunction of patients with non-tremor Parkinson's disease. Appearance of such cortical lesions with advancing disease may also explain why tremor can diminish or even disappear after several years in some patients, because the cerebello-thalamo-cortical circuit now becomes damaged. Finally, resting tremor may emerge as a collateral effect of cerebral mechanisms that compensate for pathophysiological changes producing akinesia (Hallett and Khoshbin, 1980; Rivlin-Etzion et al., 2006). Voluntary movement arises in phase with the tremor, suggesting that the tremor may facilitate the ability to initiate movement in the face of akinesia (Hallett et al., 1977). This possibility finds support in the fact that—in MPTP primate models of Parkinson's disease—tremor usually appears several days after akinesia and rigidity (Zaidel et al., 2009), i.e. its appearance coincides with the time when compensatory mechanisms are presumably being activated. This does not yet prove any causal relationship, but one possibility is that such compensatory changes—for example in the motor cortex and cerebellum—may render these regions more susceptible to pathological influences (tremor triggers) from the basal ganglia. For example, a study that used transcranial magnetic stimulation pulses to probe the excitability of the primary motor cortex showed that, when tested at rest, the slope of the input–output relationship between stimulus intensity and response size is steeper in patients with Parkinson's disease than in controls (Valls-Sole et al., 1994). Although this could be the result of a primary basal ganglia deficit (loss of normal inhibition), it could also reflect an attempt to cortically compensate for the slow recruitment of commands to move, by making it easier to recruit activity from a resting state (Berardelli et al., 2001). Similarly, increased activity of the cerebellum during movements has been observed frequently in Parkinson's disease—perhaps to compensate for dysfunction of dopamine-dependent circuits (Rascol et al., 1997; Yu et al., 2007; Wu et al., 2010b). The increased cerebellar activity may sensitize the cerebello-thalamo-cortical circuit to perturbing influences from the basal ganglia, resulting in tremor. This suggests that a combination of basal ganglia pathology (i.e. pallidal dopamine depletion) and compensation in the cerebello-thalamo-cortical circuit leads to tremor, explaining why tremor and a benign disease course are seen in the same patients.
Why does resting tremor decrease with disease progression?
Parkinsonian resting tremor has a puzzling feature that distinguishes it from other Parkinson's disease symptoms: in some patients, tremor severity tends to decrease instead of worsen during disease progression (Toth et al., 2004; Lees, 2007). One study found that tremor was lost in 9% of patients late in the disease (Hughes et al., 1993). Accordingly, patients with Parkinson's disease of tremor-dominant subtype in the early phases of their disease can convert to a non-tremor subtype later on, with PIGD symptoms replacing the tremor (Alves et al., 2006). This suggests that the progression of cerebral dysfunction in Parkinson's disease may at some point disrupt the ability of brain regions to produce tremor. Post-mortem work has shown that the primary motor cortex becomes affected in later stages of Parkinson's disease (Braak et al., 2003). Furthermore, post-mortem work revealed an association between a non-tremor Parkinson's disease phenotype, cognitive disability and pathological lesions including cortical Lewy bodies, cortical amyloid-beta plaques, and cerebral amyloid angiopathy (Selikhova et al., 2009). Also, the cognitive deterioration occurring in non-tremor Parkinson's disease [i.e. in the PIGD subtype (Williams-Gray et al., 2007)] was associated with cortical Lewy body pathology instead of cortico-striatal dysfunction (Williams-Gray et al., 2009). These studies indicate that progressing cortical Lewy body pathology may stop tremor and introduce dementia-like cognitive dysfunction. Genetic variations between patients, for instance in the tau gene (microtubule-associated protein tau; MAPT), may determine whether patients develop these pathologies or not (Williams-Gray et al., 2009). Finally, as outlined in the previous paragraph, patients with tremor-dominant Parkinson's disease might have increased cerebral compensation. This concept would offer an explanation how failure of compensatory mechanisms in later stages of the disease will lead to gradual disappearance of tremor, possibly because the (previously healthy) brain areas involved in compensation now become affected by neurodegeneration as well.
Conclusion
We propose that Parkinson's disease resting tremor involves both the basal ganglia and the cerebello-thalamo-cortical circuit. Previous models of tremor have largely focused on localizing the tremor pacemaker in either one of these two distinct circuits. These models have provided valuable information about the neural mechanisms underlying tremor oscillations in these circuits, but they were unable to solve one crucial paradox of Parkinson's disease resting tremor: why is tremor produced by the cerebello-thalamo-cortical circuit, but only in the presence of striato-pallidal dopaminergic dysfunction? A systems-level view on tremor is necessary to answer this question. We have suggested a new ‘dimmer-switch model’ of parkinsonian tremor (Helmich et al., 2011b): depletion of pallidal dopamine (and possibly serotonin) causes pathological activity in the striato-pallidal circuit that triggers—through the motor cortex—tremor-related activity in the cerebello-thalamo-cortical circuit. This striato-pallidal activity can only emerge under motorically static conditions when the basal ganglia are not involved in voluntary motor behaviour, explaining why classical Parkinson's disease tremor is seen both at rest and during fixed postural holding (re-emergent tremor). Future electrophysiological studies may validate this model by focusing on oscillatory phenomena time-locked to changes in tremor amplitude.
Our model may have clinical implications for the treatment of tremor: if the basal ganglia are only transiently involved at the onset of tremor episodes, then DBS of the STN or pallidum may be applied more selectively in an ‘event-related’ manner, interrupting activity in these regions only when required. For this approach to work, one has to be able to accurately predict the onset of tremor episodes. This may be done by using the DBS electrodes to identify tremor-related signals that typically precede tremor episodes (Wu et al., 2010a), for example, desynchronization in the beta band (Wang et al., 2005). Demand-based DBS may also prolong the life span of implanted batteries. Finally, a similar systems-level approach as adopted here could be used to investigate other tremors occurring in Parkinson's disease, as well as different tremor pathologies such as essential tremor.
Funding
The Alkemade-Keuls Foundation (to B.R.B.); the Netherlands Organisation for Scientific Research (NWO; VIDI grant No. 016.076.352 to B.R.B.; VIDI grant No. 452-03-339 to I.T.; Brain & Cognition grant No. 433-09-248 to I.T.); the German Research Council (SFB 855 to G.D.) and the NIH Intramural Program (to M.H.).
Glossary
Abbreviations
- DBS
deep brain stimulation
- PIGD
postural instability and gait disability
- STN
subthalamic nucleus
- UPDRS
Unified Parkinson's Disease Rating Scale
- VL
ventral lateral thalamus
References
- Aarsland D, Andersen K, Larsen JP, Lolk A, Kragh-Sorensen P. Prevalence and characteristics of dementia in Parkinson disease: an 8-year prospective study. Arch Neurol. 2003;60:387–92. doi: 10.1001/archneur.60.3.387. [DOI] [PubMed] [Google Scholar]
- Abdo WF, van de Warrenburg BP, Burn DJ, Quinn NP, Bloem BR. The clinical approach to movement disorders. Nat Rev Neurol. 2010;6:29–37. doi: 10.1038/nrneurol.2009.196. [DOI] [PubMed] [Google Scholar]
- Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989;12:366–75. doi: 10.1016/0166-2236(89)90074-x. [DOI] [PubMed] [Google Scholar]
- Albo Z, Di Prisco GV, Chen YH, Rangarajan G, Truccolo W, Feng JF, Vertes RP, Ding MZ. Is partial coherence a viable technique for identifying generators of neural oscillations? Biol Cybernet. 2004;90:318–26. doi: 10.1007/s00422-004-0475-5. [DOI] [PubMed] [Google Scholar]
- Alves G, Larsen JP, Emre M, Wentzel-Larsen T, Aarsland D. Changes in motor subtype and risk for incident dementia in Parkinson's disease. Mov Disord. 2006;21:1123–30. doi: 10.1002/mds.20897. [DOI] [PubMed] [Google Scholar]
- Anden NE, Carlsson A, Dahlstroem A, Fuxe K, Hillarp NA, Larsson K. Demonstration and mapping out of nigro-neostriatal dopamine neurons. Life Sci. 1964;3:523–30. doi: 10.1016/0024-3205(64)90161-4. [DOI] [PubMed] [Google Scholar]
- Ando N, Izawa Y, Shinoda Y. Relative contributions of thalamic reticular nucleus neurons and intrinsic interneurons to inhibition of thalamic neurons projecting to the motor cortex. J Neurophysiol. 1995;73:2470–85. doi: 10.1152/jn.1995.73.6.2470. [DOI] [PubMed] [Google Scholar]
- Antonini A, Moeller JR, Nakamura T, Spetsieris P, Dhawan V, Eidelberg D. The metabolic anatomy of tremor in Parkinson's disease. Neurology. 1998;51:803–10. doi: 10.1212/wnl.51.3.803. [DOI] [PubMed] [Google Scholar]
- Atkinson JD, Collins DL, Bertrand G, Peters TM, Pike GB, Sadikot AF. Optimal location of thalamotomy lesions for tremor associated with Parkinson disease: a probabilistic analysis based on postoperative magnetic resonance imaging and an integrated digital atlas. J Neurosurg. 2002;96:854–66. doi: 10.3171/jns.2002.96.5.0854. [DOI] [PubMed] [Google Scholar]
- Bar-Gad I, Heimer G, Ritov Y, Bergman H. Functional correlations between neighboring neurons in the primate globus pallidus are weak or nonexistent. J Neurosci. 2003;23:4012–6. doi: 10.1523/JNEUROSCI.23-10-04012.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartho P, Freund TF, Acsady L. Selective GABAergic innervation of thalamic nuclei from zona incerta. Eur J Neurosci. 2002;16:999–1014. doi: 10.1046/j.1460-9568.2002.02157.x. [DOI] [PubMed] [Google Scholar]
- Baudrexel S, Witte T, Seifried C, von Wegner F, Beissner F, Klein JC, Steinmetz H, Deichmann R, Roeper J, Hilker R. Resting state fMRI reveals increased subthalamic nucleus-motor cortex connectivity in Parkinson's disease. Neuroimage. 2011;55:1728–38. doi: 10.1016/j.neuroimage.2011.01.017. [DOI] [PubMed] [Google Scholar]
- Beck S, Hallett M. Surround inhibition in the motor system. Exp Brain Res. 2011;210:165–72. doi: 10.1007/s00221-011-2610-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benabid AL, Pollak P, Gervason C, Hoffmann D, Gao DM, Hommel M, Perret JE, de Rougemont J. Long-term suppression of tremor by chronic stimulation of the ventral intermediate thalamic nucleus. Lancet. 1991;337:403–6. doi: 10.1016/0140-6736(91)91175-t. [DOI] [PubMed] [Google Scholar]
- Benamer HT, Oertel WH, Patterson J, Hadley DM, Pogarell O, Hoffken H, Gerstner A, Grosset DG. Prospective study of presynaptic dopaminergic imaging in patients with mild parkinsonism and tremor disorders: part 1. Baseline and 3-month observations. Mov Disord. 2003;18:977–84. doi: 10.1002/mds.10482. [DOI] [PubMed] [Google Scholar]
- Benamer HT, Patterson J, Wyper DJ, Hadley DM, Macphee GJ, Grosset DG. Correlation of Parkinson's disease severity and duration with 123I-FP-CIT SPECT striatal uptake. Mov Disord. 2000;15:692–8. doi: 10.1002/1531-8257(200007)15:4<692::aid-mds1014>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Benninger DH, Thees S, Kollias SS, Bassetti CL, Waldvogel D. Morphological differences in Parkinson's disease with and without rest tremor. J Neurol. 2009;256:256–63. doi: 10.1007/s00415-009-0092-2. [DOI] [PubMed] [Google Scholar]
- Berardelli A, Rothwell JC, Thompson PD, Hallett M. Pathophysiology of bradykinesia in Parkinson's disease. Brain. 2001;124:2131–46. doi: 10.1093/brain/124.11.2131. [DOI] [PubMed] [Google Scholar]
- Bergman H, Feingold A, Nini A, Raz A, Slovin H, Abeles M, Vaadia E. Physiological aspects of information processing in the basal ganglia of normal and parkinsonian primates. Trends Neurosci. 1998a;21:32–8. doi: 10.1016/s0166-2236(97)01151-x. [DOI] [PubMed] [Google Scholar]
- Bergman H, Raz A, Feingold A, Nini A, Nelken I, Hansel D, Ben Pazi H, Reches A. Physiology of MPTP tremor. Mov Disord. 1998b;13(Suppl 3):29–34. doi: 10.1002/mds.870131305. [DOI] [PubMed] [Google Scholar]
- Bernheimer H, Birkmayer W, Hornykiewicz O, Jellinger K, Seitelberger F. Brain dopamine and the syndromes of Parkinson and Huntington. Clinical, morphological and neurochemical correlations. J Neurol Sci. 1973;20:415–55. doi: 10.1016/0022-510x(73)90175-5. [DOI] [PubMed] [Google Scholar]
- Bevan MD, Booth PA, Eaton SA, Bolam JP. Selective innervation of neostriatal interneurons by a subclass of neuron in the globus pallidus of the rat. J Neurosci. 1998;18:9438–52. doi: 10.1523/JNEUROSCI.18-22-09438.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenfeld H, McCormick DA. Corticothalamic inputs control the pattern of activity generated in thalamocortical networks. J Neurosci. 2000;20:5153–62. doi: 10.1523/JNEUROSCI.20-13-05153.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostan AC, Dum RP, Strick PL. The basal ganglia communicate with the cerebellum. Proc Natl Acad Sci U S A. 2010;107:8452–56. doi: 10.1073/pnas.1000496107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- Burn DJ, Rowan EN, Allan LM, Molloy S, O'Brien JT, McKeith IG. Motor subtype and cognitive decline in Parkinson's disease, Parkinson's disease with dementia, and dementia with Lewy bodies. J Neurol Neurosurg Psychiatry. 2006;77:585–9. doi: 10.1136/jnnp.2005.081711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns RS, Chiueh CC, Markey SP, Ebert MH, Jacobowitz DM, Kopin IJ. A primate model of parkinsonism: selective destruction of dopaminergic neurons in the pars compacta of the substantia nigra by N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Proc Natl Acad Sci U S A. 1983;80:4546–50. doi: 10.1073/pnas.80.14.4546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caretti V, Stoffers D, Winogrodzka A, Isaias IU, Costantino G, Pezzoli G, Ferrarese C, Antonini A, Wolters EC, Booij J. Loss of thalamic serotonin transporters in early drug-naïve Parkinson’s disease patients is associated with tremor: an [123I]β-CIT SPECT study. J Neural Transm. 2008;115:721–9. doi: 10.1007/s00702-007-0015-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SM, Lee SH, Park MS, Kim BC, Kim MK, Cho KH. Disappearance of resting tremor after thalamic stroke involving the territory of the tuberothalamic artery. Parkinsonism Relat Disord. 2008;14:373–5. doi: 10.1016/j.parkreldis.2007.06.016. [DOI] [PubMed] [Google Scholar]
- Cools AR, van den Bercken JH, Horstink MW, van Spaendonck KP, Berger HJ. Cognitive and motor shifting aptitude disorder in Parkinson's disease. J Neurol Neurosurg Psychiatry. 1984;47:443–53. doi: 10.1136/jnnp.47.5.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone NE, Miglioretti DL, Gordon B, Sieracki JM, Wilson MT, Uematsu S, Lesser RP. Functional mapping of human sensorimotor cortex with electrocorticographic spectral analysis - I. Alpha and beta event-related desynchronization. Brain. 1998;121:2271–99. doi: 10.1093/brain/121.12.2271. [DOI] [PubMed] [Google Scholar]
- Cruikshank SJ, Urabe H, Nurmikko AV, Connors BW. Pathway-specific feedforward circuits between thalamus and neocortex revealed by selective optical stimulation of axons. Neuron. 2010;65:230–45. doi: 10.1016/j.neuron.2009.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deiber MP, Pollak P, Passingham R, Landais P, Gervason C, Cinotti L, Friston K, Frackowiak R, Mauguiere F, Benabid AL. Thalamic stimulation and suppression of parkinsonian tremor. Evidence of a cerebellar deactivation using positron emission tomography. Brain. 1993;116(Pt 1):267–79. doi: 10.1093/brain/116.1.267. [DOI] [PubMed] [Google Scholar]
- Deuschl G, Bain P, Brin M. Consensus statement of the Movement Disorder Society on Tremor. Ad Hoc Scientific Committee. Mov Disord. 1998;13(Suppl 3):2–23. doi: 10.1002/mds.870131303. [DOI] [PubMed] [Google Scholar]
- Deuschl G, Raethjen J, Baron R, Lindemann M, Wilms H, Krack P. The pathophysiology of parkinsonian tremor: a review. J Neurol. 2000;247(Suppl 5):V33–V48. doi: 10.1007/pl00007781. [DOI] [PubMed] [Google Scholar]
- Deuschl G, Wilms H, Krack P, Wurker M, Heiss WD. Function of the cerebellum in Parkinsonian rest tremor and Holmes' tremor. Ann Neurol. 1999;46:126–8. doi: 10.1002/1531-8249(199907)46:1<126::aid-ana20>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Deutch AY, Elsworth JD, Goldstein M, FUXE K, Redmond DE, Jr, Sladek JR, Jr, Roth RH. Preferential vulnerability of A8 dopamine neurons in the primate to the neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Neurosci Lett. 1986;68:51–6. doi: 10.1016/0304-3940(86)90228-4. [DOI] [PubMed] [Google Scholar]
- Doder M, Rabiner EA, Turjanski N, Lees AJ, Brooks DJ. Tremor in Parkinson's disease and serotonergic dysfunction: an 11C-WAY 100635 PET study. Neurology. 2003;60:601–5. doi: 10.1212/01.wnl.0000031424.51127.2b. [DOI] [PubMed] [Google Scholar]
- Dupuis MJ, Evrard FL, Jacquerye PG, Picard GR, Lermen OG. Disappearance of essential tremor after stroke. Mov Disord. 2010;25:2884–2887. doi: 10.1002/mds.23328. [DOI] [PubMed] [Google Scholar]
- Eggers C, Kahraman D, Fink GR, Schmidt M, Timmermann L. Akinetic-rigid and tremor-dominant Parkinson's disease patients show different patterns of FP-CIT single photon emission computed tomography. Mov Disord. 2011;26:416–23. doi: 10.1002/mds.23468. [DOI] [PubMed] [Google Scholar]
- Eidelberg D, Moeller JR, Dhawan V, Spetsieris P, Takikawa S, Ishikawa T, Chaly T, Robeson W, Margouleff D, Przedborski S. The metabolic topography of parkinsonism. J Cereb Blood Flow Metab. 1994;14:783–801. doi: 10.1038/jcbfm.1994.99. [DOI] [PubMed] [Google Scholar]
- Elble R, Koller W. Baltimore, United States: Johns Hopkins University Press; 1990. Tremor. [Google Scholar]
- Elble RJ. Central mechanisms of tremor. J Clin Neurophysiol. 1996;13:133–44. doi: 10.1097/00004691-199603000-00004. [DOI] [PubMed] [Google Scholar]
- Elble RJ. Tremor and dopamine agonists. Neurology. 2002;58:S57–S62. doi: 10.1212/wnl.58.suppl_1.s57. [DOI] [PubMed] [Google Scholar]
- Engel AK, Fries P. Beta-band oscillations - signalling the status quo? Curr Opin Neurobiol. 2010;20:156–65. doi: 10.1016/j.conb.2010.02.015. [DOI] [PubMed] [Google Scholar]
- Fahn S. Classification of movement disorders. Mov Disord. 2011;26:947–57. doi: 10.1002/mds.23759. [DOI] [PubMed] [Google Scholar]
- Fearnley JM, Lees AJ. Ageing and Parkinson's disease: substantia nigra regional selectivity. Brain. 1991;114(Pt 5):2283–301. doi: 10.1093/brain/114.5.2283. [DOI] [PubMed] [Google Scholar]
- Filion M, Tremblay L, Bedard PJ. Abnormal influences of passive limb movement on the activity of globus pallidus neurons in parkinsonian monkeys. Brain Res. 1988;444:165–76. doi: 10.1016/0006-8993(88)90924-9. [DOI] [PubMed] [Google Scholar]
- Fishman PS. Paradoxical aspects of parkinsonian tremor. Mov Disord. 2008;23:168–73. doi: 10.1002/mds.21736. [DOI] [PubMed] [Google Scholar]
- Fonnum F, Stormmathisen J, Divac I. Biochemical-evidence for glutamate as neurotransmitter in corticostriatal and corticothalamic fibers in rat-brain. Neuroscience. 1981;6:863–73. doi: 10.1016/0306-4522(81)90168-8. [DOI] [PubMed] [Google Scholar]
- Forsaa EB, Larsen JP, Wentzel-Larsen T, Alves G. What predicts mortality in Parkinson disease?: a prospective population-based long-term study. Neurology. 2010;75:1270–76. doi: 10.1212/WNL.0b013e3181f61311. [DOI] [PubMed] [Google Scholar]
- Fraix V, Pollak P, Moro E, Chabardes S, Xie J, Ardouin C, Benabid AL. Subthalamic nucleus stimulation in tremor dominant parkinsonian patients with previous thalamic surgery. J Neurol Neurosurg Psychiatry. 2005;76:246–8. doi: 10.1136/jnnp.2003.022707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francois C, Savy C, Jan C, Tande D, Hirsch EC, Yelnik J. Dopaminergic innervation of the subthalamic nucleus in the normal state, in MPTP-treated monkeys, and in Parkinson's disease patients. J Comp Neurol. 2000;425:121–9. doi: 10.1002/1096-9861(20000911)425:1<121::aid-cne10>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Francois C, Yelnik J, Tande D, Agid Y, Hirsch EC. Dopaminergic cell group A8 in the monkey: anatomical organization and projections to the striatum. J Comp Neurol. 1999;414:334–47. [PubMed] [Google Scholar]
- Fukuda M, Barnes A, Simon ES, Holmes A, Dhawan V, Giladi N, Fodstad H, Ma Y, Eidelberg D. Thalamic stimulation for parkinsonian tremor: correlation between regional cerebral blood flow and physiological tremor characteristics. Neuroimage. 2004;21:608–15. doi: 10.1016/j.neuroimage.2003.09.068. [DOI] [PubMed] [Google Scholar]
- Gao JB. Analysis of amplitude and frequency variations of essential and Parkinsonian tremors. Med Biol Eng Comput. 2004;42:345–9. doi: 10.1007/BF02344710. [DOI] [PubMed] [Google Scholar]
- German DC, Dubach M, Askari S, Speciale SG, Bowden DM. 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced parkinsonian syndrome in Macaca fascicularis: which midbrain dopaminergic neurons are lost? Neuroscience. 1988;24:161–74. doi: 10.1016/0306-4522(88)90320-x. [DOI] [PubMed] [Google Scholar]
- Ghaemi M, Raethjen J, Hilker R, Rudolf J, Sobesky J, Deuschl G, Heiss WD. Monosymptomatic resting tremor and Parkinson's disease: a multitracer positron emission tomographic study. Mov Disord. 2002;17:782–8. doi: 10.1002/mds.10125. [DOI] [PubMed] [Google Scholar]
- Gilbertson T, Lalo E, Doyle L, Di Lazzaro V, Cioni B, Brown P. Existing motor state is favored at the expense of new movement during 13-35 Hz oscillatory synchrony in the human corticospinal system. J Neurosci. 2005;25:7771–9. doi: 10.1523/JNEUROSCI.1762-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg JA, Boraud T, Maraton S, Haber SN, Vaadia E, Bergman H. Enhanced synchrony among primary motor cortex neurons in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine primate model of Parkinson's disease. J Neurosci. 2002;22:4639–53. doi: 10.1523/JNEUROSCI.22-11-04639.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallett M, Khoshbin S. A physiological mechanism of bradykinesia. Brain. 1980;103:301–14. doi: 10.1093/brain/103.2.301. [DOI] [PubMed] [Google Scholar]
- Hallett M, Shahani BT, Young RR. Analysis of stereotyped voluntary movements at the elbow in patients with Parkinson's disease. J Neurol Neurosurg Psychiatry. 1977;40:1129–35. doi: 10.1136/jnnp.40.12.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond C, Bergman H, Brown P. Pathological synchronization in Parkinson's disease: networks, models and treatments. Trends Neurosci. 2007;30:357–64. doi: 10.1016/j.tins.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Hariz MI, Krack P, Alesch F, Augustinsson LE, Bosch A, Ekberg R, Johansson F, Johnels B, Meyerson BA, N'Guyen JP, Pinter M, Pollak P, von Raison F, Rehncrona S, Speelman JD, Sydow O, Benabid AL. Multicentre European study of thalamic stimulation for parkinsonian tremor: a 6 year follow-up. J Neurol Neurosurg Psychiatry. 2008;79:694–9. doi: 10.1136/jnnp.2007.118653. [DOI] [PubMed] [Google Scholar]
- Hayes AE, Davidson MC, Keele SW, Rafal RD. Toward a functional analysis of the basal ganglia. J Cogn Neurosci. 1998;10:178–98. doi: 10.1162/089892998562645. [DOI] [PubMed] [Google Scholar]
- Helmich RC, Aarts E, de Lange FP, Bloem BR, Toni I. Increased dependence of action selection on recent motor history in Parkinson's disease. J Neurosci. 2009;29:6105–13. doi: 10.1523/JNEUROSCI.0704-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmich RC, Bloem BR, Toni I. Motor imagery evokes increased somatosensory activity in parkinson's disease patients with tremor. Hum Brain Mapp. 2011a doi: 10.1002/hbm.21318. Advance Access published on June 14, 2011, doi:10.1002/hbm.21318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmich RC, Janssen MJ, Oyen WJ, Bloem BR, Toni I. Pallidal dysfunction drives a cerebellothalamic circuit into Parkinson tremor. Ann Neurol. 2011b;69:269–81. doi: 10.1002/ana.22361. [DOI] [PubMed] [Google Scholar]
- Hirai T, Jones EG. A new parcellation of the human thalamus on the basis of histochemical staining. Brain Res Brain Res Rev. 1989;14:1–34. doi: 10.1016/0165-0173(89)90007-6. [DOI] [PubMed] [Google Scholar]
- Hirsch EC, Mouatt A, Faucheux B, Bonnet AM, Javoy-Agid F, Graybiel AM, Agid Y. Dopamine, tremor, and Parkinson's disease. Lancet. 1992;340:125–6. doi: 10.1016/0140-6736(92)90457-e. [DOI] [PubMed] [Google Scholar]
- Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology. 1967;17:427–42. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- Homberg V, Hefter H, Reiners K, Freund HJ. Differential effects of changes in mechanical limb properties on physiological and pathological tremor. J Neurol Neurosurg Psychiatry. 1987;50:568–79. doi: 10.1136/jnnp.50.5.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover JE, Strick PL. The organization of cerebellar and basal ganglia outputs to primary motor cortex as revealed by retrograde transneuronal transport of herpes simplex virus type 1. J Neurosci. 1999;19:1446–163. doi: 10.1523/JNEUROSCI.19-04-01446.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes AJ, Daniel SE, Blankson S, Lees AJ. A clinicopathologic study of 100 cases of Parkinson's disease. Arch Neurol. 1993;50:140–8. doi: 10.1001/archneur.1993.00540020018011. [DOI] [PubMed] [Google Scholar]
- Hurtado JM, Gray CM, Tamas LB, Sigvardt KA. Dynamics of tremor-related oscillations in the human globus pallidus: a single case study. Proc Natl Acad Sci U S A. 1999;96:1674–79. doi: 10.1073/pnas.96.4.1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurtado JM, Lachaux JP, Beckley DJ, Gray CM, Sigvardt KA. Inter- and intralimb oscillator coupling in parkinsonian tremor. Mov Disord. 2000;15:683–91. doi: 10.1002/1531-8257(200007)15:4<683::aid-mds1013>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Isaias IU, Benti R, Cilia R, Canesi M, Marotta G, Gerundini P, Pezzoli G, Antonini A. [123I]FP-CIT striatal binding in early Parkinson's disease patients with tremor vs. akinetic-rigid onset. Neuroreport. 2007;18:1499–502. doi: 10.1097/WNR.0b013e3282ef69f9. [DOI] [PubMed] [Google Scholar]
- Jahnsen H, Llinas R. Ionic basis for the electro-responsiveness and oscillatory properties of guinea-pig thalamic neurones in vitro. J Physiol. 1984;349:227–47. doi: 10.1113/jphysiol.1984.sp015154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan C, Francois C, Tande D, Yelnik J, Tremblay L, Agid Y, Hirsch E. Dopaminergic innervation of the pallidum in the normal state, in MPTP-treated monkeys and in parkinsonian patients. Eur J Neurosci. 2000;12:4525–35. [PubMed] [Google Scholar]
- Jankovic J, Kapadia AS. Functional decline in Parkinson disease. Arch Neurol. 2001;58:1611–5. doi: 10.1001/archneur.58.10.1611. [DOI] [PubMed] [Google Scholar]
- Jankovic J, McDermott M, Carter J, Gauthier S, Goetz C, Golbe L, Huber S, Koller W, Olanow C, Shoulson I. Variable expression of Parkinson's disease: a base-line analysis of the DATATOP cohort. The Parkinson Study Group. Neurology. 1990;40:1529–34. doi: 10.1212/wnl.40.10.1529. [DOI] [PubMed] [Google Scholar]
- Jankovic J, Schwartz KS, Ondo W. Re-emergent tremor of Parkinson's disease. J Neurol Neurosurg Psychiatry. 1999;67:646–50. doi: 10.1136/jnnp.67.5.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jellinger KA. Post mortem studies in Parkinson's disease—is it possible to detect brain areas for specific symptoms? J Neural Transm. 1999;56(Suppl):1–29. doi: 10.1007/978-3-7091-6360-3_1. [DOI] [PubMed] [Google Scholar]
- Jenkinson N, Brown P. New insights into the relationship between dopamine, beta oscillations and motor function. Trends Neurosci. 2011;34(12):611–8. doi: 10.1016/j.tins.2011.09.003. [DOI] [PubMed] [Google Scholar]
- Kassubek J, Juengling FD, Hellwig B, Knauff M, Spreer J, Lucking CH. Hypermetabolism in the ventrolateral thalamus in unilateral Parkinsonian resting tremor: a positron emission tomography study. Neurosci Lett. 2001;304:17–20. doi: 10.1016/s0304-3940(01)01737-2. [DOI] [PubMed] [Google Scholar]
- Kassubek J, Juengling FD, Hellwig B, Spreer J, Lucking CH. Thalamic gray matter changes in unilateral Parkinsonian resting tremor: a voxel-based morphometric analysis of 3-dimensional magnetic resonance imaging. Neurosci Lett. 2002;323:29–32. doi: 10.1016/s0304-3940(02)00111-8. [DOI] [PubMed] [Google Scholar]
- Kim DG, Koo YH, Kim OJ, Oh SH. Development of Holmes' tremor in a patient with Parkinson's disease following acute cerebellar infarction. Mov Disord. 2009;24:463–4. doi: 10.1002/mds.22394. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Jeon BS, Paek SH, Lee JY, Kim HJ, Kim CK, Kim DG. Bilateral subthalamic deep brain stimulation in Parkinson disease patients with severe tremor. Neurosurgery. 2010;67:626–32. doi: 10.1227/01.NEU.0000374850.98949.D4. [DOI] [PubMed] [Google Scholar]
- Kish SJ, Shannak K, Hornykiewicz O. Uneven pattern of dopamine loss in the striatum of patients with idiopathic Parkinson's disease. Pathophysiologic and clinical implications. N Engl J Med. 1988;318:876–80. doi: 10.1056/NEJM198804073181402. [DOI] [PubMed] [Google Scholar]
- Kloppel S, Mangin JF, Vongerichten A, Frackowiak RSJ, Siebner HR. Nurture versus nature: long-term impact of forced right-handedness on structure of pericentral cortex and basal ganglia. J Neurosci. 2010;30:3271–75. doi: 10.1523/JNEUROSCI.4394-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh SB, Kwon DY, Seo WK, Kim JH, Kim JH, Lee SH, Oh K, Kim BJ, Park KW. Dissociation of cardinal motor signs in Parkinson's disease patients. Eur Neurol. 2010;63:307–10. doi: 10.1159/000314179. [DOI] [PubMed] [Google Scholar]
- Koller WC, Busenbark K, Miner K. The relationship of essential tremor to other movement disorders: report on 678 patients. Essential Tremor Study Group. Ann Neurol. 1994;35:717–23. doi: 10.1002/ana.410350613. [DOI] [PubMed] [Google Scholar]
- Krack P, Batir A, Van Blercom N, Chabardes S, Fraix V, Ardouin C, Koudsie A, Limousin PD, Benazzouz A, LeBas JF, Benabid AL, Pollak P. Five-year follow-up of bilateral stimulation of the subthalamic nucleus in advanced Parkinson's disease. N Engl J Med. 2003;349:1925–34. doi: 10.1056/NEJMoa035275. [DOI] [PubMed] [Google Scholar]
- Krack P, Dostrovsky J, Ilinsky I, Kultas-Ilinsky K, Lenz F, Lozano A, Vitek J. Surgery of the motor thalamus: problems with the present nomenclatures. Mov Disord. 2002;17(Suppl 3):S2–S8. doi: 10.1002/mds.10136. [DOI] [PubMed] [Google Scholar]
- Krack P, Pollak P, Limousin P, Benazzouz A, Benabid AL. Stimulation of subthalamic nucleus alleviates tremor in Parkinson's disease. Lancet. 1997;350:1675. doi: 10.1016/s0140-6736(97)24049-3. [DOI] [PubMed] [Google Scholar]
- Kuhn AA, Kupsch A, Schneider GH, Brown P. Reduction in subthalamic 8-35 Hz oscillatory activity correlates with clinical improvement in Parkinson's disease. Eur J Neurosci. 2006;23:1956–60. doi: 10.1111/j.1460-9568.2006.04717.x. [DOI] [PubMed] [Google Scholar]
- Kuhn AA, Williams D, Kupsch A, Limousin P, Hariz M, Schneider GH, Yarrow K, Brown P. Event-related beta desynchronization in human subthalamic nucleus correlates with motor performance. Brain. 2004;127:735–46. doi: 10.1093/brain/awh106. [DOI] [PubMed] [Google Scholar]
- Kultas-Ilinsky K, Sivan-Loukianova E, Ilinsky IA. Reevaluation of the primary motor cortex connections with the thalamus in primates. J Comp Neurol. 2003;457:133–58. doi: 10.1002/cne.10539. [DOI] [PubMed] [Google Scholar]
- Kumar R, Lozano AM, Kim YJ, Hutchison WD, Sime E, Halket E, Lang AE. Double-blind evaluation of subthalamic nucleus deep brain stimulation in advanced Parkinson's disease. Neurology. 1998;51:850–5. doi: 10.1212/wnl.51.3.850. [DOI] [PubMed] [Google Scholar]
- Landisman CE, Connors BW. VPM and PoM nuclei of the rat somatosensory thalamus: intrinsic neuronal properties and corticothalamic feedback. Cereb Cortex. 2007;17:2853–65. doi: 10.1093/cercor/bhm025. [DOI] [PubMed] [Google Scholar]
- Lee RG, Stein RB. Resetting of tremor by mechanical perturbations: a comparison of essential tremor and parkinsonian tremor. Ann Neurol. 1981;10:523–31. doi: 10.1002/ana.410100606. [DOI] [PubMed] [Google Scholar]
- Lees AJ. Unresolved issues relating to the shaking palsy on the celebration of James Parkinson's 250th birthday. Mov Disord. 2007;22(Suppl 17):S327–34. doi: 10.1002/mds.21684. [DOI] [PubMed] [Google Scholar]
- Lees AJ, Hardy J, Revesz T. Parkinson's disease. Lancet. 2009;373:2055–66. doi: 10.1016/S0140-6736(09)60492-X. [DOI] [PubMed] [Google Scholar]
- Lenz FA, Kwan HC, Martin RL, Tasker RR, Dostrovsky JO, Lenz YE. Single unit analysis of the human ventral thalamic nuclear group. Tremor-related activity in functionally identified cells. Brain. 1994;117(Pt 3):531–43. doi: 10.1093/brain/117.3.531. [DOI] [PubMed] [Google Scholar]
- Lenz FA, Tasker RR, Kwan HC, Schnider S, Kwong R, Murayama Y, Dostrovsky JO, Murphy JT. Single unit analysis of the human ventral thalamic nuclear group: correlation of thalamic "tremor cells" with the 3-6 Hz component of parkinsonian tremor. J Neurosci. 1988;8:754–64. doi: 10.1523/JNEUROSCI.08-03-00754.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leu-Semenescu S, Roze E, Vidailhet M, Legrand AP, Trocello JM, Cochen V, Sangla S, Apartis E. Myoclonus or tremor in orthostatism: an under-recognized cause of unsteadiness in Parkinson's disease. Mov Disord. 2007;22:2063–9. doi: 10.1002/mds.21651. [DOI] [PubMed] [Google Scholar]
- Levy R, Hutchison WD, Lozano AM, Dostrovsky JO. High-frequency synchronization of neuronal activity in the subthalamic nucleus of parkinsonian patients with limb tremor. J Neurosci. 2000;20:7766–75. doi: 10.1523/JNEUROSCI.20-20-07766.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy R, Hutchison WD, Lozano AM, Dostrovsky JO. Synchronized neuronal discharge in the basal ganglia of parkinsonian patients is limited to oscillatory activity. J Neurosci. 2002;22:2855–61. doi: 10.1523/JNEUROSCI.22-07-02855.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis SJ, Foltynie T, Blackwell AD, Robbins TW, Owen AM, Barker RA. Heterogeneity of Parkinson's disease in the early clinical stages using a data driven approach. J Neurol Neurosurg Psychiatry. 2005;76:343–8. doi: 10.1136/jnnp.2003.033530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinas R, Urbano FJ, Leznik E, Ramirez RR, van Marle HJ. Rhythmic and dysrhythmic thalamocortical dynamics: GABA systems and the edge effect. Trends Neurosci. 2005;28:325–33. doi: 10.1016/j.tins.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Llinas RR. The intrinsic electrophysiological properties of mammalian neurons: insights into central nervous system function. Science. 1988;242:1654–64. doi: 10.1126/science.3059497. [DOI] [PubMed] [Google Scholar]
- Lo RY, Tanner CM, Albers KB, Leimpeter AD, Fross RD, Bernstein AL, McGuire V, Quesenberry CP, Nelson LM, Van Den Eeden SK. Clinical features in early Parkinson disease and survival. Arch Neurol. 2009;66:1353–8. doi: 10.1001/archneurol.2009.221. [DOI] [PubMed] [Google Scholar]
- Louis ED, Frucht SJ. Prevalence of essential tremor in patients with Parkinson's disease vs. Parkinson-plus syndromes. Mov Disord. 2007;22:1402–7. doi: 10.1002/mds.21383. [DOI] [PubMed] [Google Scholar]
- Louis ED, Levy G, Cote LJ, Mejia H, Fahn S, Marder K. Clinical correlates of action tremor in Parkinson disease. Arch Neurol. 2001;58:1630–4. doi: 10.1001/archneur.58.10.1630. [DOI] [PubMed] [Google Scholar]
- Louis ED, Pullman SL, Eidelberg D, Dhawan V. Re-emergent tremor without accompanying rest tremor in Parkinson's disease. Can J Neurol Sci. 2008;35:513–5. doi: 10.1017/s0317167100009239. [DOI] [PubMed] [Google Scholar]
- Louis ED, Tang MX, Cote L, Alfaro B, Mejia H, Marder K. Progression of parkinsonian signs in Parkinson disease. Arch Neurol. 1999;56:334–7. doi: 10.1001/archneur.56.3.334. [DOI] [PubMed] [Google Scholar]
- Lozano AM, Lang AE, Galvez-Jimenez N, Miyasaki J, Duff J, Hutchinson WD, Dostrovsky JO. Effect of GPi pallidotomy on motor function in Parkinson's disease. Lancet. 1995;346:1383–7. doi: 10.1016/s0140-6736(95)92404-3. [DOI] [PubMed] [Google Scholar]
- Magnin M, Morel A, Jeanmonod D. Single-unit analysis of the pallidum, thalamus and subthalamic nucleus in parkinsonian patients. Neuroscience. 2000;96:549–64. doi: 10.1016/s0306-4522(99)00583-7. [DOI] [PubMed] [Google Scholar]
- McAuley JH, Marsden CD. Physiological and pathological tremors and rhythmic central motor control. Brain. 2000;123(Pt 8):1545–67. doi: 10.1093/brain/123.8.1545. [DOI] [PubMed] [Google Scholar]
- Mink JW. The basal ganglia: focused selection and inhibition of competing motor programs. Prog Neurobiol. 1996;50:381–425. doi: 10.1016/s0301-0082(96)00042-1. [DOI] [PubMed] [Google Scholar]
- Moran A, Bergman H, Israel Z, Bar-Gad I. Subthalamic nucleus functional organization revealed by parkinsonian neuronal oscillations and synchrony. Brain. 2008;131:3395–409. doi: 10.1093/brain/awn270. [DOI] [PubMed] [Google Scholar]
- Moran RJ, Mallet N, Litvak V, Dolan RJ, Magill PJ, Friston KJ, Brown P. Alterations in brain connectivity underlying beta oscillations in Parkinsonism. Plos Comput Biol. 2011;7:e1002124. doi: 10.1371/journal.pcbi.1002124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mounayar S, Boulet S, Tande D, Jan C, Pessiglione M, Hirsch EC, Feger J, Savasta M, Francois C, Tremblay L. A new model to study compensatory mechanisms in MPTP-treated monkeys exhibiting recovery. Brain. 2007;130:2898–914. doi: 10.1093/brain/awm208. [DOI] [PubMed] [Google Scholar]
- Mure H, Hirano S, Tang CC, Isaias IU, Antonini A, Ma Y, Dhawan V, Eidelberg D. Parkinson's disease tremor-related metabolic network: characterization, progression, and treatment effects. Neuroimage. 2011;54:1244–53. doi: 10.1016/j.neuroimage.2010.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nambu A, Tokuno H, Hamada I, Kita H, Imanishi M, Akazawa T, Ikeuchi Y, Hasegawa N. Excitatory cortical inputs to pallidal neurons via the subthalamic nucleus in the monkey. J Neurophysiol. 2000;84:289–300. doi: 10.1152/jn.2000.84.1.289. [DOI] [PubMed] [Google Scholar]
- Ni Z, Pinto AD, Lang AE, Chen R. Involvement of the cerebellothalamocortical pathway in Parkinson disease. Ann Neurol. 2010;68:816–24. doi: 10.1002/ana.22221. [DOI] [PubMed] [Google Scholar]
- Ohye C, Shibazaki T, Hirai T, Wada H, Hirato M, Kawashima Y. Further Physiological observations on the ventralis intermedius neurons in the human thalamus. J Neurophysiol. 1989;61:488–500. doi: 10.1152/jn.1989.61.3.488. [DOI] [PubMed] [Google Scholar]
- Oiwa Y, Eberling JL, Nagy D, Pivirotto P, Emborg ME, Bankiewicz KS. Overlesioned hemiparkinsonian non human primate model: correlation between clinical, neurochemical and histochemical changes. Front Biosci. 2003;8:a155–66. doi: 10.2741/1104. [DOI] [PubMed] [Google Scholar]
- Owen AM, Doyon J, Dagher A, Sadikot A, Evans AC. Abnormal basal ganglia outflow in Parkinson's disease identified with PET. Implications for higher cortical functions. Brain. 1998;121(Pt 5):949–65. doi: 10.1093/brain/121.5.949. [DOI] [PubMed] [Google Scholar]
- Pare D, Curro'Dossi R, Steriade M. Neuronal basis of the parkinsonian resting tremor: a hypothesis and its implications for treatment. Neuroscience. 1990;35:217–26. doi: 10.1016/0306-4522(90)90077-h. [DOI] [PubMed] [Google Scholar]
- Paulus W, Jellinger K. The neuropathologic basis of different clinical subgroups of Parkinson's disease. J Neuropathol Exp Neurol. 1991;50:743–55. doi: 10.1097/00005072-199111000-00006. [DOI] [PubMed] [Google Scholar]
- Percheron G, Francois C, Talbi B, Yelnik J, Fenelon G. The primate motor thalamus. Brain Res Brain Res Rev. 1996;22:93–181. [PubMed] [Google Scholar]
- Pessiglione M, Guehl D, Rolland AS, Francois C, Hirsch EC, Feger J, Tremblay L. Thalamic neuronal activity in dopamine-depleted primates: evidence for a loss of functional segregation within basal ganglia circuits. J Neurosci. 2005;25:1523–31. doi: 10.1523/JNEUROSCI.4056-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirker W. Correlation of dopamine transporter imaging with parkinsonian motor handicap: how close is it? Mov Disord. 2003;18(Suppl 7):S43–S51. doi: 10.1002/mds.10579. [DOI] [PubMed] [Google Scholar]
- Plaha P, Ben Shlomo Y, Patel NK, Gill SS. Stimulation of the caudal zona incerta is superior to stimulation of the subthalamic nucleus in improving contralateral parkinsonism. Brain. 2006;129:1732–47. doi: 10.1093/brain/awl127. [DOI] [PubMed] [Google Scholar]
- Plaha P, Filipovic S, Gill SS. Induction of parkinsonian resting tremor by stimulation of the caudal zona incerta nucleus: a clinical study. J Neurol Neurosurg Psychiatry. 2008;79:514–21. doi: 10.1136/jnnp.2006.112342. [DOI] [PubMed] [Google Scholar]
- Plenz D, Kital ST. A basal ganglia pacemaker formed by the subthalamic nucleus and external globus pallidus. Nature. 1999;400:677–82. doi: 10.1038/23281. [DOI] [PubMed] [Google Scholar]
- Pollak P, Krack P, Fraix V, Mendes A, Moro E, Chabardes S, Benabid AL. Intraoperative micro- and macrostimulation of the subthalamic nucleus in Parkinson's disease. Mov Disord. 2002;17(Suppl 3):S155–61. doi: 10.1002/mds.10158. [DOI] [PubMed] [Google Scholar]
- Pollock LJ, Davis L. Muscle tone in parkinsonian states. Arch Neurol Psychiatry. 1930;23:303–19. [Google Scholar]
- Pollok B, Makhloufi H, Butz M, Gross J, Timmermann L, Wojtecki L, Schnitzler A. Levodopa affects functional brain networks in parkinsonian resting tremor. Mov Disord. 2009;24:91–8. doi: 10.1002/mds.22318. [DOI] [PubMed] [Google Scholar]
- Probst-Cousin S, Druschky A, Neundorfer B. Disappearance of resting tremor after "stereotaxic" thalamic stroke. Neurology. 2003;61:1013–4. doi: 10.1212/01.wnl.0000086810.14643.fc. [DOI] [PubMed] [Google Scholar]
- Rack PM, Ross HF. The role of reflexes in the resting tremor of Parkinson's disease. Brain. 1986;109(Pt 1):115–41. doi: 10.1093/brain/109.1.115. [DOI] [PubMed] [Google Scholar]
- Raethjen J, Lindemann M, Schmaljohann H, Wenzelburger R, Pfister G, Deuschl G. Multiple oscillators are causing parkinsonian and essential tremor. Mov Disord. 2000;15:84–94. doi: 10.1002/1531-8257(200001)15:1<84::aid-mds1014>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Rajput AH, Pahwa R, Pahwa P, Rajput A. Prognostic significance of the onset mode in parkinsonism. Neurology. 1993;43:829–30. doi: 10.1212/wnl.43.4.829. [DOI] [PubMed] [Google Scholar]
- Rajput AH, Sitte HH, Rajput A, Fenton ME, Pifl C, Hornykiewicz O. Globus pallidus dopamine and Parkinson motor subtypes: clinical and brain biochemical correlation. Neurology. 2008;70:1403–10. doi: 10.1212/01.wnl.0000285082.18969.3a. [DOI] [PubMed] [Google Scholar]
- Rajput AH, Voll A, Rajput ML, Robinson CA, Rajput A. Course in Parkinson disease subtypes: a 39-year clinicopathologic study. Neurology. 2009;73:206–12. doi: 10.1212/WNL.0b013e3181ae7af1. [DOI] [PubMed] [Google Scholar]
- Rascol O, Sabatini U, Fabre N, Brefel C, Loubinoux I, Celsis P, Senard JM, Montastruc JL, Chollet F. The ipsilateral cerebellar hemisphere is overactive during hand movements in akinetic parkinsonian patients. Brain. 1997;120:103–10. doi: 10.1093/brain/120.1.103. [DOI] [PubMed] [Google Scholar]
- Raz A, Vaadia E, Bergman H. Firing patterns and correlations of spontaneous discharge of pallidal neurons in the normal and the tremulous 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine vervet model of parkinsonism. J Neurosci. 2000;20:8559–71. doi: 10.1523/JNEUROSCI.20-22-08559.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reck C, Florin E, Wojtecki L, Krause H, Groiss S, Voges J, Maarouf M, Sturm V, Schnitzler A, Timmermann L. Characterisation of tremor-associated local field potentials in the subthalamic nucleus in Parkinson's disease. Eur J Neurosci. 2009;29:599–612. doi: 10.1111/j.1460-9568.2008.06597.x. [DOI] [PubMed] [Google Scholar]
- Rehncrona S, Johnels B, Widner H, Tornqvist AL, Hariz M, Sydow O. Long-term efficacy of thalamic deep brain stimulation for tremor: double-blind assessments. Mov Disord. 2003;18:163–70. doi: 10.1002/mds.10309. [DOI] [PubMed] [Google Scholar]
- Remy P, de Recondo A, Defer G, Loc'h C, Amarenco P, Plante-Bordeneuve V, Dao-Castellana MH, Bendriem B, Crouzel C, Clanet M. Peduncular 'rubral' tremor and dopaminergic denervation: a PET study. Neurology. 1995;45:472–7. doi: 10.1212/wnl.45.3.472. [DOI] [PubMed] [Google Scholar]
- Rivlin-Etzion M, Elias S, Heimer G, Bergman H. Computational physiology of the basal ganglia in Parkinson's disease. Prog Brain Res. 2010;183:259–73. doi: 10.1016/S0079-6123(10)83013-4. [DOI] [PubMed] [Google Scholar]
- Rivlin-Etzion M, Marmor O, Heimer G, Raz A, Nini A, Bergman H. Basal ganglia oscillations and pathophysiology of movement disorders. Curr Opin Neurobiol. 2006;16:629–37. doi: 10.1016/j.conb.2006.10.002. [DOI] [PubMed] [Google Scholar]
- Rivlin-Etzion M, Marmor O, Saban G, Rosin B, Haber SN, Vaadia E, Prut Y, Bergman H. Low-pass filter properties of basal ganglia cortical muscle loops in the normal and MPTP primate model of parkinsonism. J Neurosci. 2008;28:633–49. doi: 10.1523/JNEUROSCI.3388-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Oroz MC, Jahanshahi M, Krack P, Litvan I, Macias R, Bezard E, Obeso JA. Initial clinical manifestations of Parkinson's disease: features and pathophysiological mechanisms. Lancet Neurol. 2009;8:1128–39. doi: 10.1016/S1474-4422(09)70293-5. [DOI] [PubMed] [Google Scholar]
- Ropper AH, Samuels MA. Adams & Victor's principles of neurology. 9 edn. McGraw-Hill Medical; 2009. p. 93. [Google Scholar]
- Rosin B, Slovik M, Mitelman R, Rivlin-Etzion M, Haber SN, Israel Z, Vaadia E, Bergman H. Closed-loop deep brain stimulation is superior in ameliorating parkinsonism. Neuron. 2011;72:370–84. doi: 10.1016/j.neuron.2011.08.023. [DOI] [PubMed] [Google Scholar]
- Rossi C, Frosini D, Volterrani D, De Feo P, Unti E, Nicoletti V, Kiferle L, Bonuccelli U, Ceravolo R. Differences in nigro-striatal impairment in clinical variants of early Parkinson's disease: evidence from a FP-CIT SPECT study. Eur J Neurol. 2010;17:626–30. doi: 10.1111/j.1468-1331.2009.02898.x. [DOI] [PubMed] [Google Scholar]
- Rouiller EM, Tanne J, Moret V, Kermadi I, Boussaoud D, Welker E. Dual morphology and topography of the corticothalamic terminals originating from the primary, supplementary motor, and dorsal premotor cortical areas in macaque monkeys. J Comparative Neurol. 1998;396:169–85. doi: 10.1002/(sici)1096-9861(19980629)396:2<169::aid-cne3>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Sanchez-Gonzalez MA, Garcia-Cabezas MA, Rico B, Cavada C. The primate thalamus is a key target for brain dopamine. J Neurosci. 2005;25:6076–83. doi: 10.1523/JNEUROSCI.0968-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiess MC, Zheng H, Soukup VM, Bonnen JG, Nauta HJ. Parkinson's disease subtypes: clinical classification and ventricular cerebrospinal fluid analysis. Parkinsonism Relat Disord. 2000;6:69–76. doi: 10.1016/s1353-8020(99)00051-6. [DOI] [PubMed] [Google Scholar]
- Schneider SA, Edwards MJ, Mir P, Cordivari C, Hooker J, Dickson J, Quinn N, Bhatia KP. Patients with adult-onset dystonic tremor resembling parkinsonian tremor have scans without evidence of dopaminergic deficit (SWEDDs) Mov Disord. 2007;22:2210–5. doi: 10.1002/mds.21685. [DOI] [PubMed] [Google Scholar]
- Seidel S, Kasprian G, Leutmezer F, Prayer D, Auff E. Disruption of nigrostriatal and cerebellothalamic pathways in dopamine responsive Holmes' tremor. J Neurol Neurosurg Psychiatry. 2009;80:921–3. doi: 10.1136/jnnp.2008.146324. [DOI] [PubMed] [Google Scholar]
- Selikhova M, Williams DR, Kempster PA, Holton JL, Revesz T, Lees AJ. A clinico-pathological study of subtypes in Parkinson's disease. Brain. 2009;132:2947–57. doi: 10.1093/brain/awp234. [DOI] [PubMed] [Google Scholar]
- Sidibe M, Bevan MD, Bolam JP, Smith Y. Efferent connections of the internal globus pallidus in the squirrel monkey: I. Topography and synaptic organization of the pallidothalamic projection. J Comp Neurol. 1997;382:323–47. [PubMed] [Google Scholar]
- Smith Y, Lavoie B, Dumas J, Parent A. Evidence for a distinct nigropallidal dopaminergic projection in the squirrel monkey. Brain Res. 1989;482:381–6. doi: 10.1016/0006-8993(89)91205-5. [DOI] [PubMed] [Google Scholar]
- Spiegel J, Hellwig D, Samnick S, Jost W, Mollers MO, Fassbender K, Kirsch CM, Dillmann U. Striatal FP-CIT uptake differs in the subtypes of early Parkinson's disease. J Neural Transm. 2007;114:331–5. doi: 10.1007/s00702-006-0518-2. [DOI] [PubMed] [Google Scholar]
- Sung YF, Hsu YD, Huang WS. (99m)Tc-TRODAT-1 SPECT study in evaluation of Holmes tremor after thalamic hemorrhage. Ann Nucl Med. 2009;23:605–8. doi: 10.1007/s12149-009-0271-3. [DOI] [PubMed] [Google Scholar]
- Timmermann L, Gross J, Dirks M, Volkmann J, Freund HJ, Schnitzler A. The cerebral oscillatory network of parkinsonian resting tremor. Brain. 2003;126:199–212. doi: 10.1093/brain/awg022. [DOI] [PubMed] [Google Scholar]
- Toth C, Rajput M, Rajput AH. Anomalies of asymmetry of clinical signs in parkinsonism. Mov Disord. 2004;19:151–7. doi: 10.1002/mds.10685. [DOI] [PubMed] [Google Scholar]
- Vakil E, Herishanu-Naaman S. Declarative and procedural learning in Parkinson's disease patients having tremor or bradykinesia as the predominant symptom. Cortex. 1998;34:611–20. doi: 10.1016/s0010-9452(08)70518-5. [DOI] [PubMed] [Google Scholar]
- Valls-Sole J, Pascualleone A, Brasilneto JP, Cammarota A, Mcshane L, Hallett M. Abnormal facilitation of the response to transcranial magnetic stimulation in patients with Parkinsons-disease. Neurology. 1994;44:735–41. doi: 10.1212/wnl.44.4.735. [DOI] [PubMed] [Google Scholar]
- van Duinen H, Zijdewind I, Hoogduin H, Maurits N. Surface EMG measurements during fMRI at 3T: accurate EMG recordings after artifact correction. Neuroimage. 2005;27:240–6. doi: 10.1016/j.neuroimage.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Van Quyen M, Bragin A. Analysis of dynamic brain oscillations: methodological advances. Trends Neurosci. 2007;30:365–73. doi: 10.1016/j.tins.2007.05.006. [DOI] [PubMed] [Google Scholar]
- van Rootselaar AF, Maurits NM, Renken R, Koelman JH, Hoogduin JM, Leenders KL, Tijssen MA. Simultaneous EMG-functional MRI recordings can directly relate hyperkinetic movements to brain activity. Hum Brain Mapp. 2008;29:1430–41. doi: 10.1002/hbm.20477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidailhet M, Dupel C, Lehericy S, Remy P, Dormont D, Serdaru M, Jedynak P, Veber H, Samson Y, Marsault C, Agid Y. Dopaminergic dysfunction in midbrain dystonia: anatomoclinical study using 3-dimensional magnetic resonance imaging and fluorodopa F 18 positron emission tomography. Arch Neurol. 1999;56:982–9. doi: 10.1001/archneur.56.8.982. [DOI] [PubMed] [Google Scholar]
- Wallman MJ, Gagnon D, Parent M. Serotonin innervation of human basal ganglia. Eur J Neurosci. 2011;33:1519–32. doi: 10.1111/j.1460-9568.2011.07621.x. [DOI] [PubMed] [Google Scholar]
- Walshe FMR. Observations on the nature of muscular rigidity of paralysis agitans, and on its relationship to tremor. Brain. 1924;47:159–77. [Google Scholar]
- Wang SY, Aziz TZ, Stein JF, Liu X. Time-frequency analysis of transient neuromuscular events: dynamic changes in activity of the subthalamic nucleus and forearm muscles related to the intermittent resting tremor. J Neurosci Methods. 2005;145:151–8. doi: 10.1016/j.jneumeth.2004.12.009. [DOI] [PubMed] [Google Scholar]
- Williams-Gray CH, Evans JR, Goris A, Foltynie T, Ban M, Robbins TW, Brayne C, Kolachana BS, Weinberger DR, Sawcer SJ, Barker RA. The distinct cognitive syndromes of Parkinson's disease: 5 year follow-up of the CamPaIGN cohort. Brain. 2009;132:2958–69. doi: 10.1093/brain/awp245. [DOI] [PubMed] [Google Scholar]
- Williams-Gray CH, Foltynie T, Brayne CE, Robbins TW, Barker RA. Evolution of cognitive dysfunction in an incident Parkinson's disease cohort. Brain. 2007;130:1787–98. doi: 10.1093/brain/awm111. [DOI] [PubMed] [Google Scholar]
- Wu D, Warwick K, Ma Z, Gasson MN, Burgess JG, Pan S, Aziz TZ. Prediction of Parkinson's disease tremor onset using a radial basis function neural network based on particle swarm optimization. Int J Neural Syst. 2010a;20:109–116. doi: 10.1142/S0129065710002292. [DOI] [PubMed] [Google Scholar]
- Wu T, Wang LA, Hallett M, Li KC, Chan P. Neural correlates of bimanual anti-phase and in-phase movements in Parkinson's disease. Brain. 2010b;133:2394–409. doi: 10.1093/brain/awq151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Sternad D, Corcos DM, Vaillancourt DE. Role of hyperactive cerebellum and motor cortex in Parkinson's disease. Neuroimage. 2007;35:222–33. doi: 10.1016/j.neuroimage.2006.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaidel A, Arkadir D, Israel Z, Bergman H. Akineto-rigid vs. tremor syndromes in Parkinsonism. Curr Opin Neurol. 2009;22:387–93. doi: 10.1097/WCO.0b013e32832d9d67. [DOI] [PubMed] [Google Scholar]
- Zetusky WJ, Jankovic J, Pirozzolo FJ. The heterogeneity of Parkinson's disease: clinical and prognostic implications. Neurology. 1985;35:522–6. doi: 10.1212/wnl.35.4.522. [DOI] [PubMed] [Google Scholar]
- Zirh TA, Lenz FA, Reich SG, Dougherty PM. Patterns of bursting occurring in thalamic cells during parkinsonian tremor. Neuroscience. 1998;83:107–21. doi: 10.1016/s0306-4522(97)00295-9. [DOI] [PubMed] [Google Scholar]