Abstract
This article provides a neurobiological account of symptoms that have been called ‘hysterical’, ‘psychogenic’ or ‘medically unexplained’, which we will call functional motor and sensory symptoms. We use a neurobiologically informed model of hierarchical Bayesian inference in the brain to explain functional motor and sensory symptoms in terms of perception and action arising from inference based on prior beliefs and sensory information. This explanation exploits the key balance between prior beliefs and sensory evidence that is mediated by (body focused) attention, symptom expectations, physical and emotional experiences and beliefs about illness. Crucially, this furnishes an explanation at three different levels: (i) underlying neuromodulatory (synaptic) mechanisms; (ii) cognitive and experiential processes (attention and attribution of agency); and (iii) formal computations that underlie perceptual inference (representation of uncertainty or precision). Our explanation involves primary and secondary failures of inference; the primary failure is the (autonomous) emergence of a percept or belief that is held with undue certainty (precision) following top-down attentional modulation of synaptic gain. This belief can constitute a sensory percept (or its absence) or induce movement (or its absence). The secondary failure of inference is when the ensuing percept (and any somatosensory consequences) is falsely inferred to be a symptom to explain why its content was not predicted by the source of attentional modulation. This account accommodates several fundamental observations about functional motor and sensory symptoms, including: (i) their induction and maintenance by attention; (ii) their modification by expectation, prior experience and cultural beliefs and (iii) their involuntary and symptomatic nature.
Keywords: attention, sensorimotor information processing, cognitive neuroscience
Introduction
For thousands of years, a core pursuit of medical science has been the careful observation of physical symptoms and signs. Through these observations, supplemented more recently by investigative techniques, an understanding of how symptoms and signs are generated by disease has developed. However, there is a group of patients with symptoms and signs that, from the earliest medical records to the present day, elude a diagnosis with a typical ‘organic’ disease. This is not simply because of an absence of pathology after sufficient investigation, rather that symptoms themselves are inconsistent with those occurring in typical disease. In times past, these symptoms were said to be ‘hysterical’, a term now replaced by the less pejorative but no more enlightening labels: ‘medically unexplained’, ‘psychogenic’, ‘conversion’, ‘non-organic’ and ‘functional’.
There are numerous historical examples of patients identified as having hysteria who would now be diagnosed with an organic medical disorder. Some have assumed that this process of salvaging patients from (mis)diagnosis with hysteria would continue inexorably until a ‘proper’ medical diagnosis was achieved. Slater (1965), in his influential paper on the topic, described the diagnosis of hysteria as ‘a disguise for ignorance and a fertile source of clinical error’. In other words, with increasing medical knowledge, all patients would be rescued from a diagnostic category that did little more than assert that they were ‘too difficult’.
This has not come to pass (Stone et al., 2005). Recent epidemiological work has demonstrated that neurologists continue to diagnose a ‘non-organic’ disorder in ∼16% of their patients, making this the second most common diagnosis of neurological outpatients (Stone et al., 2010a). In contrast to Slater’s study (1965), long-term follow-up of such patients shows that they only rarely receive an alternative ‘organic’ diagnosis for their symptoms (0.4% of a cohort of 1030 patients in the largest such study; Stone et al., 2009a). Patients with these symptoms are disabled and generate major costs to health and social services, with UK estimates for annual costs associated with working-age patients with ‘medically unexplained symptoms’ of around £18 billion (Bermingham et al., 2010), slightly more than the cost associated with dementia for patients of all ages in the UK (Knapp et al., 2007). But despite the common occurrence of these symptoms, their associated disability, impact on quality of life and cost to health and social care systems, they remain without clear explanation.
Here, we propose a neurobiological framework to explain the pathophysiology of (the subset of) sensory and motor symptoms, which comprise the absence of normal or presence of abnormal sensations or movements. Examples include: anaesthesia, blindness, deafness, pain, sensorimotor aspects of fatigue, weakness, aphonia, abnormal gait, tremor, dystonia and seizures. We choose the term ‘functional motor and sensory symptoms’ (FMSS) because the term ‘functional’ is accommodating in its theoretical implications and is rated by patients as inoffensive compared with other terms (Stone et al., 2002).
Within FMSS we do not include functional symptoms involving autonomic dysfunction and/or arousal, which may undergo psychological elaboration (such as diarrhoea, constipation and bloating in irritable bowel syndrome, palpitations in cardiac neurosis, etc.). Neither do we cover normal sensations that are misinterpreted as evidence of serious illness, as occurs in hypochondriasis, normal bodily appearances which are felt to be ugly, as in body dysmorphic disorder, nor dissociative amnesia, stupor or fugue. The FMSS we refer to comprise sensations and movements, as it is the genesis of these abnormal phenomena that we seek to explain. That said, our framework may be relevant to understanding aspects of many of the disorders we have excluded.
A unified theory for functional motor and sensory symptoms
Our premise is that all FMSS are created by attentional and belief-driven processes. These processes might involve subcortical affective factors in many patients, but we believe such factors are not always necessary and are certainly not sufficient to produce FMSS. Below, we explain how a hierarchical Bayesian formulation of brain function can account for the generation of FMSS by attentional and belief-driven processes, and how such processes—operating within the normal functional anatomy of perception and voluntary movement—might generate symptoms that are interpreted by patients as involuntary and unwilled.
The hierarchical Bayesian formulation of brain function
To understand the failures of perceptual inference that may underpin FMSS, it is necessary to consider the precise neurobiological mechanisms that underlie perception and attention. In turn, this requires a formal understanding of these processes in computational terms. In what follows, we appeal to recent advances in theoretical neurobiology that link perceptual inference, at the representational level, to functional anatomy and synaptic processes.
Given the extensive evidence for a hierarchical structure in the brain (Felleman and van Essen, 1991), it is reasonable to suggest that any generative model of the brain will also have hierarchical structure where the outputs of one level provide inputs to the next. Our neurobiological explanation for FMSS rests on hierarchical Bayesian models of the brain underwritten by a theory of nervous system function called the ‘free-energy principle’ (Friston et al., 2006, 2010; Feldman and Friston, 2010). The free-energy principle asserts that any adaptive change made by a biological system or organism must minimize its long-term average surprise. Surprise in this context means unexpected (i.e. unpredicted) sensations. Because the long-term average of surprise corresponds to the entropy (dispersion) of sensations, a failure to minimize surprise would lead to a progressive increase in entropy (sensory disorder) and violate the principles of self-organization and homoeostasis that are characteristic of biological systems. Organisms can minimize their sensory surprise by constructing a hierarchical model of how sensations (exteroceptive, interoceptive and proprioceptive) are caused. Sensory surprise can then be minimized by reducing prediction errors, based on the predictions of the model; either by changing sensory samples through action or by changing the predictions through perception. In this framework, perception corresponds to optimizing the model by changing synaptic activity and connection strengths to minimize prediction errors. This is known as predictive coding in the computational literature and the model is said to be generative because it generates sensory predictions given probabilistic beliefs about their causes.
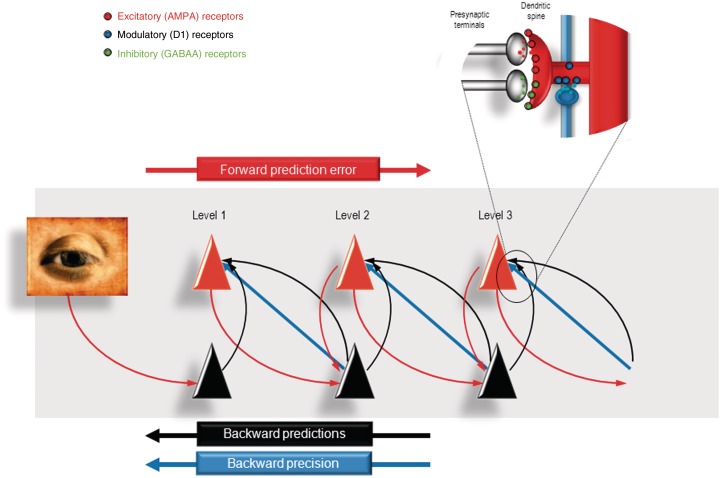
In predictive coding, surprise or free energy is minimized at each level of the cortical hierarchy by changing levels of activity in neuronal populations encoding predictions and prediction errors, namely prediction units and prediction error units. Prediction units represent the most likely causes of sensations (posterior expectations or beliefs) and attempt to predict beliefs in the level below. Prediction error units receive top-down predictions from the level above and compare them to beliefs at that level; the discrepancy between the prediction and the current belief at that level constitutes a prediction error, which is projected back up to the prediction unit in the level above. This forward projection drives the prediction units to provide a better prediction and thereby suppress the prediction error it receives; minimizing prediction error is the fundamental aim of the network. In doing so, prediction units at all levels in the hierarchy come to represent the (hidden) causes of sensory input at multiple levels of description. This surprise or free energy minimization process—inferring the likely causes of sensory data—is perception. Figure 1 gives a schematic summary and further detail.
Figure 1.
This figure provides a schematic overview of the message passing scheme usually presented as a neurobiologically plausible implementation of predictive coding. In these schemes, neurons are divided into prediction (black) and prediction error (red) units that pass messages to each other, within and between hierarchical levels in the cortex. Superficial pyramidal cells (red) send forward prediction errors to deep pyramidal cells (black), which reciprocate with predictions that are conveyed by (polysynaptic) backward extrinsic connections. These are functions of conditional expectations encoded by the activity of the prediction units. This process continues until the amplitude of prediction error has been minimized and the predictions are optimized in a Bayesian sense. The prediction errors are the (precision weighted) difference between conditional expectations encoded at any level and top down or lateral predictions. Note that there are prediction errors at every level of the hierarchy. Crucially, the potency of prediction errors at any level of the hierarchy depends upon their precision (blue arrows), which effectively modulates or weights the prediction error. The synaptic infrastructure proposed to mediate this comparison and subsequent modulation is shown in the insert, in terms of a doubly innervated synapse that is gated by dopamine (blue). Here, dopamine is delivered by en passant synaptic boutons and postsynaptic D1 receptors have been located on a dendritic spine expressing asymmetric (excitatory) and symmetric (inhibitory) synaptic connections.
Why is this scheme ‘Bayesian’? Bayesian probability theory evaluates the (posterior) probability of a hypothesis given prior beliefs about its probability and the likelihood of relevant data. The model is Bayesian because its hypotheses regarding the causes of sensory input at any hierarchical level—i.e. its posterior beliefs—are derived from both prior beliefs about the world and current sensory evidence. In this context, (empirical) prior beliefs about the world are conveyed by the (top-down) predictive backward connections between hierarchical levels, and sensory evidence by the (bottom-up) forward connections that pass prediction errors up the hierarchy until they are ‘explained away’ by changes in predictions. Empirical prior beliefs are associated with, and unique to, hierarchical statistical models. In the present context, this means that the posterior beliefs (expectations) at any level of the hierarchy constitute prior beliefs (expectations) for the level below. In short, by minimizing prediction errors or surprise, the brain is trying to maximize the evidence for its generative model of the world.
Note that we use the terms ‘prior beliefs’, ‘expectations’ and ‘predictions’ interchangeably—‘beliefs’ in this sense are not necessarily ‘beliefs’ in the sense that philosophers might use the term, i.e. consciously held and reportable propositions; they are probabilistic representations (encoded by neuronal activity) in a hierarchical Bayesian network, and their contribution to inference on the causes of incoming sense data is the key issue, whether or not one is conscious of their content. These beliefs or expectations can range from the velocity of an object causing visual sensations to the goals of another person whose behaviour is being witnessed.
We suggest that a common mechanism underlies the complete range of FMSS, both motor and sensory, and it is therefore of importance that the hierarchical Bayesian model we are discussing can act as well as perceive (this is a précis of arguments explored in detail by Friston et al., 2010). In the motor system, as in all systems, backward projections mediate predictions of sensory input, in this case, proprioceptive input. The major difference between the perceptual and motor systems is that in the former the only way to change prediction errors is to change predictions; however, in the latter, predictions of sensory input can be ‘fulfilled’ by movement; in other words, prediction errors can also be altered by changing the signals that are predicted. Descending projections from the motor system can therefore be regarded as proprioceptive predictions that play the role of motor commands, which, when compared with muscle spindle afferent signals at the spinal level, generate sensory prediction errors that are resolved by activation of motor neurons and movement. The key point here is that movements can be induced by top-down prior expectation of their sensory consequences, as the motor system automatically moves sensory organs in order to fulfil proprioceptive predictions. In other words, a movement is specified in terms of ‘what we want to see [or feel], rather than what we want to do’ (Friston et al., 2010).
Whether a movement will be emitted in response to top-down predictions about the proprioceptive and exteroceptive consequences of that movement clearly depends on the precision of prediction errors at different levels in the sensorimotor hierarchy. If the precision of high-level representations supervenes, then proprioceptive prediction errors will be resolved through classical reflex arcs and movement will ensue. However, if proprioceptive precision is higher, then proprioceptive prediction errors may well be resolved by changing top-down predictions to accommodate the fact that no movement is sensed. In short, not only does precision determine the delicate balance between sensory evidence and prior beliefs in perception, through exactly the same mechanisms, it can also determine whether we act or not.
How conflicting sensory data and prior beliefs are combined: the role of attention in modulating precision
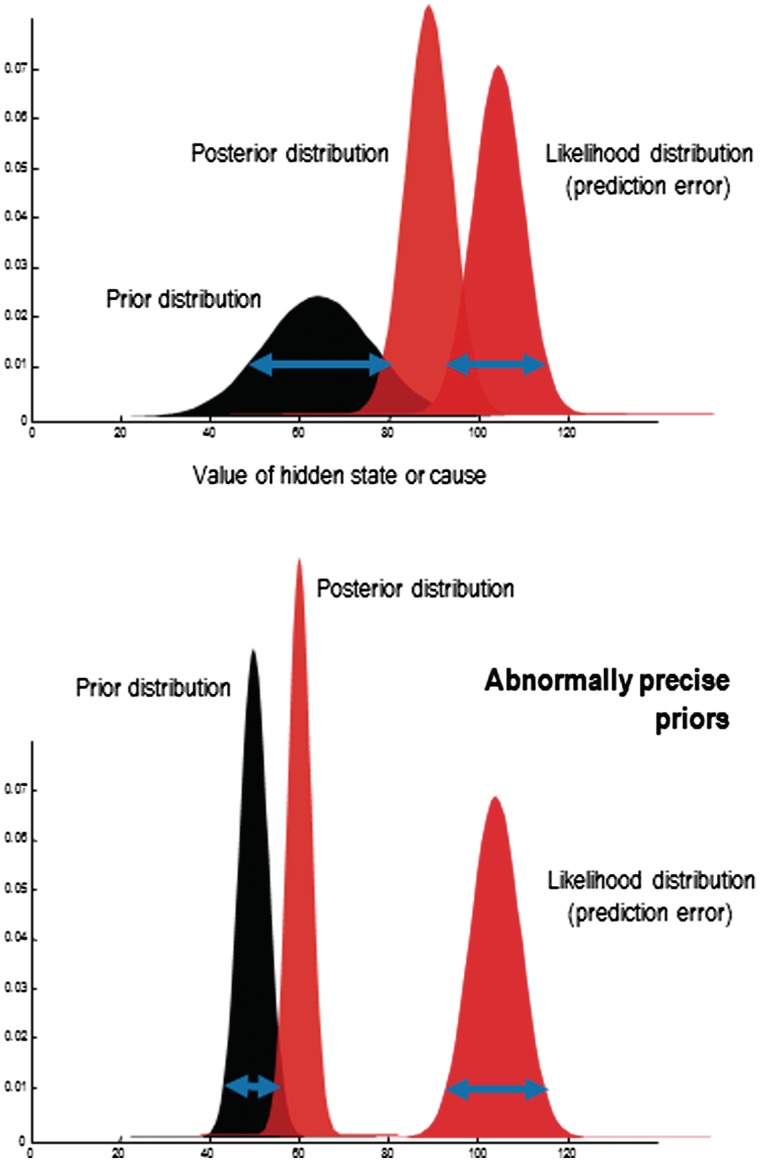
A crucial question for us in considering the application of this model to FMSS is: how are conflicting sensory evidence and prior expectations resolved? If there is a discrepancy, which one should win out? In predictive coding, prior beliefs and sensory data are represented as probability distributions, with a mean value (expectation) and a precision (inverse variance). Their precision determines the relative weights that they are given when optimizing the posterior expectation—the brain’s final estimate of the most likely cause—which is perceived. In the case of a prediction error arising from the comparison of precise sensory data and a relatively imprecise prior belief, the mean of the posterior will be closer to the mean of the sensory data. Conversely, with relatively imprecise sensory information, posterior beliefs will be much closer to prior beliefs (Fig. 2).
Figure 2.
A heuristic illustration of Bayesian inference in terms of a likelihood distribution, a prior distribution and the resulting posterior distribution. All these distributions are functions of some hidden state or cause of observed data, where the likelihood and prior distributions constitute a generative model. The important issue to observe here is that as the precision (certainty) of the prior increases, it draws the posterior estimate towards it; and away from the likelihood distribution. Here, precision corresponds to the inverse variance or dispersion (width) of the distributions, indicated with the blue arrows. Under models with additive Gaussian noise, the precision of the likelihood corresponds to the inverse amplitude of the noise (the signal-to-noise ratio).
The precision of sensory data (or prediction errors at any level of the hierarchy) and prior beliefs are not fixed: they can be optimized by attention to best reflect uncertainty about their contribution. In neurobiological implementations of predictive coding, the precision of prediction errors is encoded by the synaptic gain of the cells that encode them, the superficial pyramidal cells, in the upper layers of the cortex (Mumford, 1992). Their gain or postsynaptic responsiveness can be altered by several factors including fast synchronous oscillatory activity and/or neuromodulators such as acetylcholine and dopamine, both of which are implicated in attentional mechanisms (Feldman and Friston, 2010). In short, the potency of top-down prior beliefs in relation to bottom-up sensory evidence is controlled by the relative precision of prediction errors as each level of the cortical hierarchy. Neurobiologically, this profoundly important control may be mediated by the classical modulatory neurotransmitter systems of the sort implicated in Parkinson’s disease and schizophrenia.
The effect of changing the content and precision of prior beliefs can be seen experimentally. For example, changing sensory expectations in healthy subjects affects both stimulus perception and its electrophysiological correlates. Lorenz et al. (2005) gave subjects painful laser stimuli that were preceded by cues predicting how intense the pain would be. These cues were sometimes inaccurate: a high intensity cue could be followed by a low intensity stimulus. Subjects’ pain ratings were affected by the cues—pain perception was biased towards the predicted outcome—as was the amplitude of the magnetoencephalogram signal in contralateral secondary somatosensory cortex. Altering expectations about visual stimuli has also been shown to change the perceptual threshold and associated electrophysiological responses (Melloni et al., 2011). These expectations correspond to prior beliefs established by the experimental context or task instructions about the level or magnitude of some sensory attribute. Similar contextual beliefs can also be induced about the precision or uncertainty of an attribute (e.g. where a visual target might appear). This provides a formal account of the interaction between attention and expectation, where attention optimizes the precision of various processing channels given appropriate cues for expectations. For example, the effect of attention on the time to respond to, and EEG correlates of, stimulus presentation in the Posner task (a pre-cued reaction time task) has successfully been modelled with a hierarchical Bayesian network by Feldman and Friston (2010), reproducing previous empirical findings with respect to reaction times (Posner et al., 1978) and EEG signals (Mangun and Hillyard, 1991).
For some like James (1890), attention means the (voluntary) direction of consciousness. This Jamesian view describes the top-down, conscious control of an attentional ‘spotlight’ (LaBerge, 1983) that optimizes synaptic gain where it is ‘directed’. In the Bayesian brain, attention means the process of optimizing synaptic gain to represent the relative precisions of prior expectations and sensory information during inference (Feldman and Friston, 2010) and provides a formal basis for the Jamesian view of attention. The optimization of synaptic gain by attention can come about by (Jamesian) voluntary direction of attention through top-down task-relevant cues or bottom-up ‘pop-out’ mechanisms that occur outside conscious control (Hommel et al., 2001), and even in spite of it (Munoz and Everling, 2004). Indeed, much of the theory behind free energy minimization is based upon Helmholtz’s notion of unconscious inference.
Functional motor and sensory symptoms as a disorder of abnormal expectations and misdirected attention
In our model of hierarchal Bayesian inference, we have emphasized how the interaction between the ‘nature and precision’ of prior expectations and the nature and precision of prediction errors caused by sensory data generates what we perceive or whether and how we move. In this scheme, the precision (weight) attached to prediction errors is modulated by attention, and the content of the eventual percept depends on the hierarchical level and domain of that modulation (e.g. visual domain, somatosensory domain).
Given this framework, we propose that the common abnormality that produces FMSS is the emergence of abnormal prior beliefs that are afforded excessive precision by attention. Note that we do not distinguish between a primary pathology in neuronal populations encoding prior beliefs that could misappropriate attention or a primary pathology of attention that produces prior beliefs held with undue conviction (precision). Both are plausible candidates and both call on the pathology of neuromodulation at the synaptic level.
The consequences of endowing beliefs about sensations or movements with undue precision (certainty) are two-fold. First, there will be false perceptual inference as top-down prior beliefs overwhelm bottom-up sensory evidence from lower levels. Second, higher levels now have to explain the emergence of the belief, leading to a misattribution of agency in the sense that top-down attentional processes induced the belief but did not predict its content. In what follows, we deconstruct this broad proposal, showing how aberrant priors and attentional misdirection might arise, how sensory and motor symptoms might be generated and why these are not experienced as voluntary by patients.
How pathological expectations and attention might originate in patients with functional motor and sensory symptoms
There is a significant literature describing the importance of attention in the development and maintenance of somatic symptoms, reviewed by Brown (2004) and Kirmayer and Taillefer (1997). Patients with FMSS have a body-focused attentional bias (Robbins and Kirmayer, 1991), and introspective people are more likely to experience somatic symptoms (Pennebaker, 1982; Hansell and Mechanic, 1986; Robbins and Kirmayer, 1986). Directing attention towards the self tends to increase reports of physical symptoms (Pennebaker and Brittingham, 1982), whether during exercise (Pennebaker and Lightner, 1980; Fillingim and Fine, 1986) or not (Schmidt et al., 1994).
Such work has led Brown (2004) to propose that ‘all somatoform conditions with the exception of those involving observable physical phenomena are governed by the same basic mechanism, namely, the repetitive reallocation of high-level attention on to symptoms’. We agree with this proposal but suggest it should encompass symptoms involving observable physical phenomena, such as paralysis or tremor too, as (conscious) attention is clearly very important for their maintenance. Patients with functional tremor show changes in tremor frequency when asked to tap at a different rhythm with another limb, even to the extent of complete entrainment with the frequency of tapping (Schwingenschuh et al., 2011). Likewise, if a patient with functional leg weakness is asked to flex their unaffected hip, their unattended ‘paralysed’ hip will automatically extend; this is known as Hoover’s sign (Ziv et al., 1998). Inconsistencies between clinical signs based on muscle power or movement speed and performance of the same movements in different (more implicit) contexts are also commonly seen. These features are all consistent with a need for explicit attention towards the movement for impairment to manifest, and a normalization of movement when attention is diverted. These features are the reverse of those seen in patients with organic neurological signs. In Parkinson’s disease, for example, tremor worsens with distraction. It is much more difficult to gauge whether distraction improves functional sensory symptoms such as anaesthesia, because assessing the symptom—asking, ‘Can you feel this?’—inevitably draws attention to it. Were precise clinical assessment of sensory symptoms possible, we hypothesize that they would improve with distraction.
Some FMSS are influenced by personal and cultural beliefs that are at odds with the constraints of anatomy and physiology. One example is of a ‘tubular’ visual field defect, where patients with a functional loss of their central visual field report a defect of the same diameter, whether it is mapped close to them or far away. This defies the laws of optics, but may fit with (lay) beliefs about the nature of vision. Other examples include the triggering of symptoms by non-physiological manoeuvres (e.g. application of a vibrating tuning fork to the limb) and memory abnormalities (e.g. forgetting one’s name), which might be assumed to occur in people with memory loss, but in fact are rare even in those with severe dementia.
There are also disorders—thought by many to be wholly or at least partly functional—on whose incidence there appear to be strong cultural influences. One example is ‘whiplash injury’, a syndrome dominated by chronic neck pain after an apparently minor rear-end traffic collision. The likelihood of developing this syndrome is highly culturally biased; it is much rarer in countries where the concept of whiplash injury is not known (Ferrari et al., 2001). Importantly, the expectation in population surveys of the medical consequences of minor traffic accidents mirrors the incidence of whiplash symptoms. In a related study of low back pain after minor injury in Australia, a state-wide campaign to change expectations regarding the consequences of such injury led to a sustained and significant reduction in the incidence and severity of chronic back pain (Buchbinder and Jolley, 2005).
Further evidence for the fundamental role of beliefs or expectations in causing FMSS is a curative response to placebo. It is important to differentiate such dramatic responses from the minor to moderate benefits of placebo treatment commonly seen in patients with organic disorders. Patients with functional dystonia can obtain immediate and total resolution of their muscle spasm following a botulinum toxin injection (Edwards et al., 2011), despite the fact that botulinum toxin takes at least 24–48 h to relax muscles at the level of neuromuscular transmission. The active component of the treatment here is clearly the patient’s expectation of success (in the CNS), rather than the pharmacological action of the drug (in the PNS). Dramatic resolution of symptoms following non-physiological interventions is frequently reported in patients with FMSS, and provides the highest level of diagnostic certainty in some diagnostic classification systems (Fahn and Williams, 1988; Gupta and Lang, 2009).
This evidence highlights the importance of attention and beliefs about symptoms in the phenomenology of FMSS. This relationship was noted at least as far back as the mid-19th century (Reynolds, 1869). Abnormal expectations about illness could be generated by a mixture of factors highlighted in previous models of somatization, such as physical illness in the patient him or herself (Stone et al., 2009b), by exposure to illness in the family (Hotopf et al., 1999) or while in a medical or paramedical job (Crimlisk et al., 1998), over concern with children’s symptoms or reinforcement of children’s illness behaviour (Benjamin and Eminson, 1992), health scares in the media (Stewart, 1990) or within colleagues (Ismail and Lewis, 2006) or myriad other socio-cultural means.
These predisposing factors might all lead to abnormal beliefs about illness, and therefore could be both a source of increased attention towards symptoms, and also an influence on the content of inferences made about symptoms (e.g. that they are due to illness and not ‘normal’ phenomena), but they alone do not provide an explanation for the development or phenomenology of the particular FMSS that occurs. From where might the content of the abnormal prior expectation arise?
We wish to highlight here the notion of physical precipitating factors in the generation of FMSS, something highlighted by others, for example Reynolds (1869). It is notable that a physical precipitating event is commonly reported close to the onset of FMSS; and we believe that this provides an important explanation as to why particular FMSS develop. For example, viral infections commonly precede chronic fatigue syndrome or neurasthenia (Wessely et al., 1998), somatic symptoms associated with panic attacks are commonly reported prior to onset of non-epileptic seizures (Rusch et al., 2001) and physical injury to a limb (causing pain and immobilization) is commonly reported at the onset of fixed abnormal limb postures or limb paralysis (Schrag et al., 2004; Stone et al., 2009b, 2012a).
We suggest that salient sensory data arising from these precipitating events are afforded excessive precision (weight), and that this instantiates an abnormal prior belief at an intermediate level in the cortical hierarchy trying to explain or predict those sensations—and that abnormal belief or expectation is rendered resistant to extinction through the unusually high levels of precision (synaptic gain) enjoyed during its formation. For example, for sensory data from a triggering event signalling pain, the abnormal prior belief may reside in the insular cortex, an intermediate-level cortical level relevant to pain perception (Wiech et al., 2008). The excessive precision afforded to the novel sensory data could have a variety of causes in addition to the predisposing factors mentioned above, which include affective and cognitive biases and their interactions. Negative effects such as anxiety and depression themselves cause somatic symptoms, as well as increasing self-focused attention (Kolk et al., 2003) both through general arousal (Wegner and Giuliano, 1980) and ruminations (Vassend, 1989). In healthy subjects, Berna et al. (2010) demonstrated that inducing sadness increased the unpleasantness of painful stimuli with those experiencing the greatest unpleasantness showing the highest activations in the amygdala and inferior frontal gyrus. Negative affect is very common in patients with personality disorders, who in turn are at a higher risk of developing FMSS (Binzer et al., 1997; Crimlisk et al., 1998). It has also been proposed that somatically focused attention occurs in the setting of traumatic events in order to avoid a potentially overwhelming affect (Brown, 2004). Indeed, patients with non-epileptic seizures—which have been associated in some patients with childhood sexual abuse (Sharpe and Faye, 2006)—commonly report somatic symptoms associated with panic attacks (palpitations, sweating, hyperventilation) at the onset of attacks, but fail to report associated affective symptoms (panic without panic). This process could produce physical symptoms that the patient interprets as being due to physical illness as he or she is not aware of the affective content of the panic episode. Somatic symptoms of panic occurring in conjunction with a physical trigger such as injury are also reported in patients who go on to develop functional weakness (Stone et al., 2012a). This might be an important mechanism that adds additional physical symptoms and arousal to a physical precipitant such as an injury, increasing the salience of the resultant sensory data and facilitating the formation and retention of an abnormal prior belief. A predisposition to learning, in the context of salient information, is due to the fact that precision in Bayes optimal schemes plays the role of a ‘learning rate’ in classical reinforcement learning schemes. This means a high level of precision promotes fast associative learning.
Cognitive biases might also be important for the way in which sensory information from a physical triggering event is weighted during perceptual inference. Patients with somatization disorder have been shown to have cognitive biases towards retaining information relating to illness (Martin et al., 2007) and catastrophic thinking about symptoms (Crombez et al., 1998). The ‘jumping to conclusions’ bias is a well-known tendency of patients with delusions, illustrated by their deciding after fewer draws than most control subjects whether a hidden urn contains a majority of one ball colour or another (Garety and Freeman, 1999). The Bayesian perspective provides a unifying account of these failures to represent uncertainty and the key role of attention—this is because both rest on optimizing the precision in hierarchical models. We have recently demonstrated that the ‘jumping to conclusions’ bias is also present in patients with functional motor symptoms, consistent with a tendency to overweigh evidence; a tendency that could lead to abnormal inferences about sensations during a physical triggering event (Pareés et al., 2012a).
This discussion demonstrates the rich and varied potential causative factors behind the development of FMSS, which contrasts with the simplistic concept of them being caused by a single emotional traumatic event (e.g. childhood sexual abuse). Epidemiological studies have not found childhood trauma, or recent emotional life events, to be necessary for FMSS (Roelofs et al., 2002; Sharpe and Faye, 2006; Kranick et al., 2011); indeed, Sharpe and Faye (2006) comment, ‘the association with psychological issues is much less prominent than expected’. The emphasis on emotional triggering events, particularly childhood sexual abuse, is, arguably, based on a specific (and perhaps simplistic) interpretation of the concept of conversion disorder introduced by Breuer and Freud in 1893–95 (Breuer and Freud, 1991) and later extended by Freud alone. In this theory, the role of a psychological conflict is paramount. Freud believed that a psychologically challenging situation, replete with emotional conflicts, could reawaken memories of an earlier (childhood) situation containing similar, unresolved conflicts between biological drives and social demands or childhood experiences. These (unconscious) memories would give rise to unpleasant thoughts or emotions, which were repressed in order to keep them from awareness and hence from causing further distress or conscious recollection. The ‘psychic energy’ of the repressed negative memories had to find another method of discharging itself—so it was converted into a somatic symptom, which generally had some symbolic relation to the memories or wishes being repressed. This protection of consciousness from conflict and distress was the ‘primary gain’ of the production of hysterical symptoms, although Freud noted the patient might then derive a ‘secondary gain’ from their elevated status as an invalid. Although many of the constructs and the symbolism proposed by Freud have been discarded, the idea that FMSS are an unconsciously generated expression of (otherwise uncommunicated) psychological conflict retains considerable popularity. We suggest, however, that this provides a rather one-dimensional approach to causation that may not be appropriate for many patients with FMSS. Indeed, overemphasis of the importance of childhood sexual abuse and other specific life events in causing FMSS by treating physicians may directly harm patients for whom these factors are unimportant.
How expectations and attention could create perceptions de novo in patients with functional motor and sensory symptoms
Kirmayer and Taillefer’s (1997) model of somatization rests on attentional capture by bodily sensations that ‘arise from everyday physiological disturbances or common illness, such as viral infections, or from emotional arousal or major mood or anxiety disorders’, after which ‘even mild sensations can become magnified once attention is focused [upon them]’, and so even ‘neutral sensations may be re-evaluated as uncomfortable and threatening’. This account, in common with Briquet’s (1859) original description of chronically affected polysymptomatic patients, rests upon the misinterpretation (false inference) and magnification of existing sensations, but does not address how such sensations could be created de novo [a problem also identified by Brown (2004)]. Though we have highlighted the presence of physical triggering events above, which could provide such sensory information in many patients with FMSS, there are those who do not report such events, and those whose symptoms seem far removed from any possible physiological or illness-related trigger.
The hierarchical Bayesian model provides a straightforward solution to this problem, by showing that there might only be a quantitative—not qualitative—difference between ‘somatic amplification’ and the generation of completely false perceptions. No sensory system is perfectly noiseless; even in the absence of stimuli there will be random discharges of sensory receptors and neurons. Given sensory data from other sources suggesting the absence of a stimulus, or a prior expectation that no stimulus is present, this noise will be explained as such by the predictive coding scheme and will not be perceived. However, if a prior expectation regarding the presence of a stimulus is sufficiently precise, random fluctuations become perceived as a stimulus; perception will then reinforce the strength of the prior, exacerbating the problem. The false percept would be a hallucination (Friston, 2005a). This process has been studied experimentally. Pennebaker and Skelton (1981) showed that giving healthy subjects expectations about a somatic symptom (that a noise would change body temperature) caused them selectively to monitor somatic information that was consistent with the expectation and to disregard information that was inconsistent. This was despite the fact that, objectively, body temperature was simply undergoing physiological random fluctuations around a mean point. In the somatic signal detection task (Lloyd et al., 2008), a tactile stimulus was paired with a visual stimulus (light), such that if the light alone was presented, the tactile stimulus was sometimes perceived; the perception was created de novo because of the increased anticipation of its presence. The rate of false perception of the tactile stimulus has been correlated with a tendency towards reports of somatic symptoms in a non-clinical population (Brown et al., 2010).
Functional sensory systems
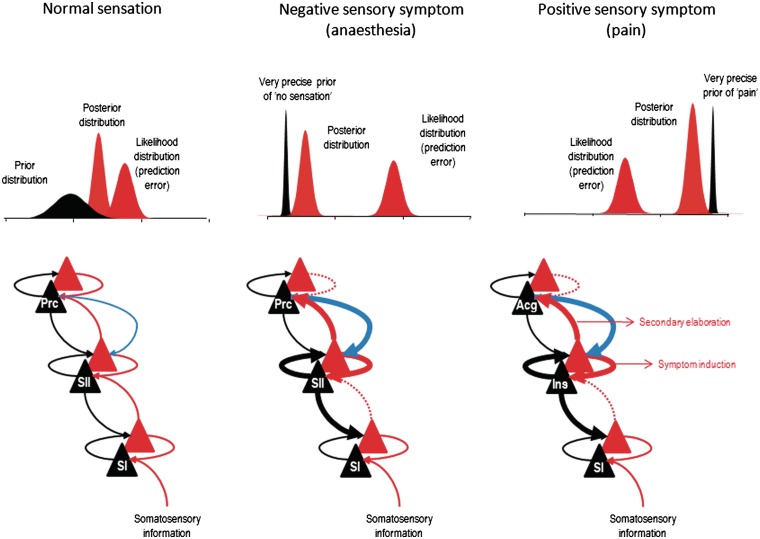
Figure 3 shows that hierarchical inference might be implemented to explain the production of functional sensory symptoms. We propose, through the mechanisms suggested above, the creation of an abnormal prior belief (false inference) within a particular (sensory) domain. We make no distinction in mechanism between functional sensory loss and positive functional sensory phenomena such as pain or paraesthesia, simply that the prior belief is different.
Figure 3.
Here we illustrate schematically our proposal with regard to generation of functional sensory symptoms. In healthy subjects, we suggest that an intermediate hierarchical level involved in sensation (e.g. secondary sensory cortex) integrates bottom-up prediction error related to incoming sensory data (red arrows) and top-down priors about the causes of sensory data (black arrows) in an optimal way. Top: The interaction between the likelihood of sensory data and the prior beliefs over those data at this intermediate hierarchical level, which results in a posterior distribution corresponding to the percept or posterior belief. By this simplification, we do not mean that the percept occurs only at the intermediate level; in reality, its physical representations are likely to be distributed across several levels. The x-axes of the graphs in the top panels denote ‘the amount of sensation’; from none to a maximum. In FMSS, we propose that an abnormal prior belief related to sensation in the relevant domain (here illustrated as the insular cortex for pain and the secondary sensory cortex for non-painful sensation) is afforded too much precision via misdirected attentional gain from higher hierarchical levels (thick blue arrow). This increase in precision (synaptic gain) causes a shift in the posterior distribution towards the prior expectation, overwhelming the influence of bottom-up prediction errors (dotted red arrow). This results in a percept that matches the prior beliefs encoded by the intermediate level, which is impervious to bottom-up prediction errors. At the same time, a precise prediction error is returned to higher levels to excite higher-level representations or explanations for the abnormal percept—again these pathologically boosted prediction errors dominate over prediction errors at the higher level when competing to influence high-level beliefs. These beliefs may include perceptual attributes—like agency. Note that we are not proposing that increased attention to secondary sensory cortex (SII; for example) per se causes anaesthesia; the crucial factor is the pre-existence of an abnormal prior belief predicting anaesthesia, whose precision is then increased by attention to that area. The existence of a different prior belief in secondary sensory cortex would lead to a different percept. Forward connections conveying prediction error are in red, backward connections conveying predictions are in black and the descending attentional modulatory connections are in blue. Superficial pyramidal cell populations encoding prediction error are shown as red triangles while deep pyramidal cells encoding posterior expectations are depicted as black triangles. Acg = anterior cingulate; Ins = insula; Prc = precuneus; SI = primary sensory cortex; SII = secondary sensory cortex.
This formulation deviates from previous theories proposing a loss of the normal ability to attend to a body part underlies functional sensory loss. The concept of loss of attentional control (dissociation) with regard to FMSS has its roots in Janet’s (1907) model for generation of functional sensory symptoms. Here, in individuals with a pathologically weak mental state, psychological stressors were proposed to lead to a narrowing of attentional focus and a resulting disregard of certain sensory input. This provided an explanation for hysterical sensory loss, a symptom that both Janet and Charcot proposed was an essential feature of hysteria. The narrowing of attentional focus proposed by Janet was also thought to prevent certain memories (for example those relating to physical sensations occurring in the traumatic event) from being properly incorporated within episodic memory. This could lead to the spontaneous and unconscious activation of these memories (perhaps triggered by salient internal or external cues), which, because of their dissociation from normal personal memory, might be experienced as perceptions rather than recollections. The attentional dysfunction proposed by Janet’s (1907) theory has influenced more modern models of functional symptoms, which have conceptualized dissociation as a normal phenomenon that becomes disruptive in those with functional symptoms. Thus in Hilgard’s (1977) neodissociation theory, dissociation is a normal and adaptive mode of cognitive processing that allows intentional activity to continue in an automatic, non-attended fashion, while the attentional resources of the executive ego are directed elsewhere. However, in hypnosis, for example, patterns of automatic activity (e.g. inhibition of limb movement) can be triggered unconsciously, and, if dissociated from the executive ego, can operate outside voluntary control. Kihlstrom (1992) has proposed that the same mechanisms of hypnotic symptom generation outlined by Hilgard (1977) contribute to neurological FMSS. Inspired in part by these ideas and Norman and Shallice’s (1986) theory of supervisory attentional systems, Brown (2004) put forward a sophisticated model of FMSS whose fundamental mechanism is the automatic (i) selection of perceptual hypotheses or (ii) triggering of actions (or action inhibition) by a primary attentional system that lies hierarchically below the self-aware, voluntarily operated secondary attentional system. A ‘horizontal dissociation’ between this conscious system and the automatic, unconscious primary attentional system is proposed to account for a subject’s loss of control of their perceptions or actions.
However, whatever form the theory takes, there is an important problem: how can one account for the need for (conscious) attention to maintain certain FMSS if one’s model proposes that symptoms result from a loss of attentional control? One would surely predict that symptoms generated by a loss of attentional control would get worse with distraction, not better. Brown (2004) proposes one solution: that in the case of FMSS—like tremor—which require (conscious) attention, ‘schemata (i.e. motor responses) may be subject to repeated reactivation through the operation of the SAS (the hierarchically higher, conscious secondary attentional system)’. However, this seems incommensurate with the idea that patients with FMSS are unable to control their cognition or action because their secondary attentional system is dissociated from their automatic primary attentional system.
The hierarchical Bayesian account is straightforward: an overly precise expectation of ‘no sensation’ is not fundamentally different to an overly precise expectation of ‘pain’; the pathologically elevated precision of the expectation of ‘no sensation’ can override any bottom-up (relatively imprecise nocioceptive) sensory data. The strength of the Bayesian account over earlier dissociative explanations of sensory loss is that the latter rely on a loss of the ability to attend to a given part of the sensorium, despite the evidence suggesting that many FMSS require attention for their maintenance. If prior beliefs about sensory input include a pathologically elevated precision, then attending to the part of the sensorium in which they are expressed will change the synaptic gain of prediction error units in order to represent this increased precision of the priors, i.e. attention to the relevant body part amplifies (if not causes) the symptom.
At this point, a sceptic might point out that the FMSS that seem to require attention involve the presence of abnormal movements or pain, not sensory loss. Therefore, the sceptic says, sensory (and indeed motor) loss must involve a dissociative mechanism, i.e. loss of attentional control. Our view, however, is that the co-occurrence of functional pain and sensory loss in the same part of the same patient (e.g. the patients described by Mailis-Gagnon et al., 2003) demands a simpler, unifying explanation. This view is supported by Brown et al. (2010), who looked at illusory perception in normal subjects who scored highly on the SDQ-20 (Nijenhuis et al., 1996), a questionnaire that mainly measures conversion/dissociation experiences (e.g. sensory and motor losses). The experimenters asked subjects whether they could detect a vibration stimulus presented at sensory threshold. Subjects scoring highly on the SDQ-20 had a more liberal response criterion, attributable not to greater sensitivity but to more false alarms. This demonstrates that a group who are more likely to experience losses of sensation (conversion/dissociation symptoms) are also more likely to show the presence of abnormal sensations (false alarms): we would explain both as being due to aberrant attentional bias towards prior expectations; and a consequent signal detection failure due to a suboptimal representation of the relative precision of sensory noise.
A related study by Horvath et al. (1980) looked at habituation of the galvanic skin response to repeated loud tones in patients with conversion symptoms, mostly involving sensory or motor losses and excluding positive symptoms, such as pain, dystonia and tremor. They showed that these patients exhibited less habituation to tones (unrelated to arousal), and interpreted this as evidence of a selective attention deficit, or loss of attentional control. As we have already emphasized, however, symptoms such as paralysis (and, we argue, sensory loss) require attention for their maintenance. This may be why Janet (1907) found that his patients had such difficulty concentrating on other things. We would reinterpret the data from Horvath et al. (1980) as further evidence of an abnormal attentional bias towards prior expectations that can both amplify and reduce sensory signals in different contexts.
The idea that abnormal ‘top-down’ influences on afferent information might be important in generating functional symptoms is also found in a previous neurobiological approach—that of Ludwig (1972). Our theory recapitulates Ludwig’s in the sense that his theory was based on corticofugal, top-down, connections that could amplify or inhibit afferent sensory signals beyond the level of primary sensory cortex, and also that attentional dysfunction is key. There is a crucial difference between us though, Ludwig proposed that ‘inhibition of afferent stimulation and attention directed towards this source of afferent stimulation are mutually incompatible’. In other words, although attention to a symptom could amplify it in hypochondriasis, the loss of sensory function in hysteria must involve ‘diversion of attention to non-symptom related areas’. We propose the opposite: namely, symptoms require attentional gain for their expression.
Functional motor systems
Our underlying assumption about functional motor symptoms is that they are fundamentally similar to other FMSS, in that they involve a pathology of precision (in this case, the synaptic gain of prediction error units in coding the proprioceptive consequences of movement), where, in the motor system, the attentional optimization of precision can be cast in terms of affordances (i.e. action possibilities).
Previous explanations for functional paralysis have proposed that it might be due to an inhibition of willed movement (Marshall et al., 1997), perhaps facilitated by dissociation from the limb and mediated by a loss of attentional control over it. It follows logically from this explanation that the pathophysiology of functional paralysis would be different from that of positive functional motor symptoms such as tremor or jerks that are clearly not due to inhibition of movement and where the attentional contribution towards the generation of symptoms is clinically obvious. However, as with the distinction in previous theories between functional sensory loss and functional pain phenomena, we believe that there is no need for different theoretical explanations for paralysis and positive functional motor symptoms. As with sensory symptoms, these motor symptoms commonly co-occur, arguing for a unified theoretical approach. Attention is also clearly important clinically for generation of functional weakness; distraction often produces a normalization of symptoms. It is also essential to note that flaccid complete paralysis is extremely rare in those with functional paralysis (Stone et al., 2010b; J. Stone, personal communication). Most patients instead have difficulty in generating movement of the required quality (power, direction or speed). We would therefore propose that the only difference between negative and positive functional motor symptoms is the content of the abnormal prior beliefs about proprioception, and in what way it specifies the scaling and dynamics of movement.
Where anatomically might abnormal prior beliefs related to (the sensory consequences of) movement reside? We propose they would be in intermediate-level motor areas, such as premotor cortex or the supplementary motor area. There are several reasons for this prediction. First, somatomotor representations of functional motor symptoms would necessarily be complex, and—if they originate within the normal sensorimotor hierarchy, which this hypothesis contends they should—they therefore must be at a sufficiently high level within the hierarchy, where movements are coded in a more abstract form (e.g. in an intrinsic frame of reference). Second, although somatomotor representations of functional motor symptoms must be at a high level, they should not be at a level associated with conscious intentions to move. Numerous functional MRI experiments have shown intention-related pre-supplementary motor area activations (Nachev et al., 2005), increasing when subjects attend to their intention rather than their movement (Lau et al., 2004), so one might conjecture that pre-supplementary motor area has either normal or reduced precision in patients with functional motor symptoms, relative to other cortical areas encoding the movement per se.
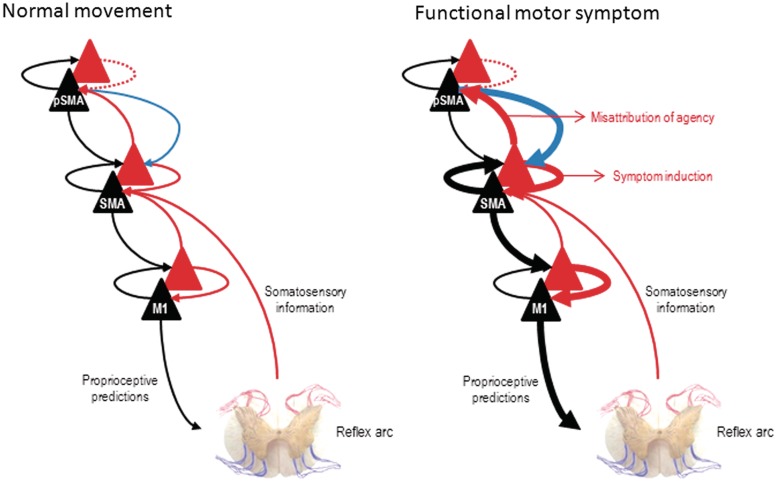
Figure 4 provides a schematic illustration of the functional anatomy implied by this hypothesis. The key idea here is that an intermediate representation of movement is afforded too much precision, relative to higher (intentional) levels of the hierarchy. The consequences of this pathologically high precision are that it will induce autonomous activity (attractor dynamics or central pattern generators) generating somatosensory and proprioceptive predictions that elicit a movement by classical reflex arcs. This rests on precise prediction errors, which entrain predictions that are propagated down the hierarchy to the spinal cord or cranial nerve nuclei. At the same time, overly precise prediction errors are passed forward to higher levels, signalling movement that was not predicted by higher hierarchical levels and, presumably, cause it to be interpreted as a symptom. This scenario is consistent with attentional exacerbation of a hierarchical imbalance in precision and the dissociation between the representation of a symptom (intermediate level) and the intentional context in which that symptom is perceived (higher level). This explanation for FMSS makes no fundamental distinction between functional motor symptoms involving weakness and those involving involuntary movements such as tremor; the movement produced simply reflects the content of the abnormal prior recruited by attention-dependent changes in precision (synaptic gain). It is notable that prefrontal hyperactivation is a common finding across functional MRI studies of sensorimotor FMSS, and that this is also the case for hypnotically induced paralysis (Bell et al., 2011). While these activations are often interpreted as inhibiting motor or sensory cortices (Broome, 2004), we suggest that they could instead reflect attentional release of abnormal priors in lower hierarchical tiers.
Figure 4.
This schematic illustrates the hierarchical anatomy we presume underlies false inference in patients with functional motor symptoms (both weakness and ‘positive’ phenomena such as tremor). In normal movement, we propose that predictions regarding the sensory consequences of intended movement arise at a high hierarchical level (here pre-supplementary motor area) and are propagated down the motor hierarchy, producing a proprioceptive prediction error (peripherally) that is fulfilled by movement. In functional motor symptoms we propose that an abnormal prior expectation related to the dynamics/scaling of movement is formed within an intermediate motor area (here the supplementary motor area). This prior is afforded abnormal precision by attentional processes (thick blue arrow) that cause intermediate level motor predictions (thick black arrow) to elicit movement and prediction errors (thick red arrow) to report the unpredicted content of that movement to higher cortical areas (here, pre-supplementary motor area). The secondary consequence of these prediction errors is that prefrontal regions will try to explain them away in terms of a symptomatic interpretation or misattribution of agency to external causes; in short, a failure to realize the movement was intended. Forward connections convey prediction error (red), backward connections convey predictions (black) and descending attentional modulatory connections (blue). pSMA = pre-supplementary motor area; M1 = primary motor cortex; SMA = supplementary motor area.
Functional imaging studies provide further data that can be reinterpreted in the light of our hypothesis. In a recent study (Cojan et al., 2009), a single patient with functional paralysis (versus 24 controls and six feigners of paralysis) underwent functional MRI during a GO-NOGO task—encompassing preparation, execution and inhibition of movement—which was performed with both the affected and functioning hands. A contrast between all trial types (preparation, execution and inhibition) in the affected (left) versus unaffected (right) hands showed activations in the precuneus and ventromedial prefrontal cortex in the patient, whereas in healthy controls it showed right motor cortical activation and in feigners activations in bilateral inferior frontal gyrus and parietal areas. The authors speculated whether ventromedial prefrontal cortex and precuneus could ‘have a more direct role in the modulation of motor activity’ in conversion, as previous authors have suggested that ventromedial prefrontal cortex could inhibit the motor system (Marshall et al., 1997; Halligan et al., 2000; Vuilleumier, 2005), but they conclude that ventromedial prefrontal cortex ‘may not be directly responsible for motor inhibition’ because it was not activated in any NOGO trials in controls, or the healthy hand in the patient and feigners. Their ultimate conclusion was that in the context of left sided motor conversion symptoms, ventromedial prefrontal cortex and precuneus activations may imply ‘some increases in self-monitoring processes that could control right motor activity … based on internal representations and memories related to the self’.
A reinterpretation of these data in the light of our model (namely abnormally high synaptic gain mediated by attention) yields a more specific conclusion. The region of ventromedial prefrontal cortex active in this study corresponds precisely to that region proposed by Burgess et al. (2007) to allow sustained, self-maintained attention to amplify higher sensory input and strengthen its relationship to established behavioural routines. We would therefore interpret its activation in conversion paralysis as an indication of the subject’s attentional mediation (exacerbation) of their abnormal symptom-related priors. Under our hypothesis, we would expect a higher sensory area such as the precuneus to be the site of strong prediction errors stemming from a mismatch between the actual and intended (i.e. predicted by pre-supplementary motor area but not by lower motor levels) sensory consequences of movement, hence its activation as a main effect of paralysis is unsurprising.
Not all imaging studies of motor symptoms find evidence of increased prediction errors (contrasting actual movement versus intended movement) in higher parietal sensory areas. One exception is Voon et al. (2010a), who found lower activity in the right temporoparietal junction in patients with conversion tremor, compared with activations elicited when asked to stimulate their tremor voluntarily. As the authors acknowledge, this finding goes against numerous studies that demonstrate increased activity around this area in conditions when movements feel involuntary, in both normal subjects (Farrer and Frith, 2002; Blakemore and Sirigu, 2003) and people with schizophrenia (Spence et al., 1997; Schnell et al., 2008). The explanation for this anomaly may be the decreased functional connectivity observed between the right temporoparietal junction and sensorimotor areas in the conversion tremor condition—if predictions do not reach the right temporoparietal junction, they cannot generate prediction errors.
If attending to motor expectations brings them about, then one would expect that patients with functional tremor would think it were there almost all of the time, because whenever they attend to it, it is manifested (unlike organic tremors). We have recently shown this to be the case using ambulatory tremor recordings of patients with functional and organic tremor and comparing these with self-completed diary ratings of tremor (Pareés et al., 2012b). All patients overestimated the duration of tremor they had but this happened to a far greater degree in patients with functional tremor, who had less than 30 min of tremor a day, but rated tremor to be present at least 80% of the waking day. Patients were fully aware of the purpose of the study, making malingering an unlikely explanation for these results.
Expectations, attention and the misattribution of agency: the problem of voluntariness
The issue of what is voluntary about FMSS is one of the most difficult questions to be addressed by any pathophysiological theory. This issue is difficult because FMSS and feigned symptoms have almost identical objective characteristics: Maruff and Velakoulis (2000) showed that even the imagined movements of a subject with functional paralysis had similar timing and duration as those of subjects feigning paralysis (as did the actual movements). Although functional brain imaging studies do show different patterns of activation in feigners and patients with functional motor symptoms, these studies are invariably underpowered and the differences are hard to interpret (Spence et al., 2000; Stone et al., 2007); in short, functional imaging is very far from being able to stipulate criteria that distinguish FMSS from feigning.
While not entirely solving this difficult issue, we argue that our model of FMSS, based as it is on expectations and attention, fundamentally alters the way in which one frames any question regarding voluntariness of symptom generation in patients with FMSS. Given this model, the question ‘Are these symptoms voluntary or involuntary?’ can be reframed as two questions which cast voluntariness in a different light: ‘How voluntary are these expectations?’, and ‘How voluntary is the patient’s attention to their symptoms?’
With regard to the formation of abnormal priors, we would point to important parallels between abnormal prior expectations we hypothesize to be at the root of FMSS, and very similar phenomena that are thought to underlie delusional beliefs (Corlett et al., 2009, 2010). One would not generally question a patient with delusions about the voluntariness of their beliefs, and we suggest that the same reasoning should apply to patients with FMSS. We have discussed above the range of predisposing and precipitating factors that might be relevant in patients with FMSS. The influence of these factors hardly amounts to intentional creation of abnormal priors.
The situation with regard to volition and attention is more complex, and, perhaps, truly gets to the heart of the problem of voluntariness in FMSS. First, we have described above how in addition to a (conscious) allocation of attentional resources—the Jamesian ‘attentional spotlight’—attention can be attracted onto parts of the sensorium via contextual cues or ‘bottom-up’ mechanisms that act outside of, or even in spite of, conscious control. This might be a mechanism whereby attentional misdirection towards abnormal symptom-related priors can occur without intention in patients with FMSS, and provides an explanation for the description of intrusive symptoms by some patients, for example those with non-epileptic attacks, which can occur when the patient is relaxing and not consciously attending to anything in particular.
Second, we wish to point out the normal phenomenon that when attention is self-focused (for example towards the mechanics of movement rather than the goal of the movement), performance is often impaired (Jueptner et al., 1997). This is a common occurrence in situations accompanied by high performance anxiety, and is proposed as the mechanism behind ‘choking’ in professional sport (Beilock and Carr, 2001). Functional imaging studies suggest recruitment of prefrontal areas when healthy subjects are asked to concentrate on producing over learned movements (Jueptner et al., 1997), something that produces deterioration in task performance. It is hypothesized that the deterioration in performance caused by self-focus is due to recruitment of slow, sequential, explicit processing that uses declarative rules that are inadequate to control complex movement patterns that are usually produced through implicit mechanisms (Haggard et al., 1994). We suggest that this phenomenon could lie behind the emergence of ‘blocking’ phenomena reported commonly by patients with FMSS, for example a sudden transient inability to move, speak or swallow, often occurring during examination or other time of heightened attention to symptoms, also consistent with some previous experimental findings in FMSS (Roelofs et al., 2003, 2006). As with the occurrence of this phenomenon in healthy people, one would not accuse patients with FMSS who experience symptoms through this mechanism of deliberately producing symptoms; in fact it is as if they are trying too hard to perform normally.
However, as discussed in more detail above, we suggest that the majority of symptoms are associated with the (conscious) direction of attention towards abnormal symptom-related prior beliefs. This attentional focus limits the attentional resources available to other tasks, and could explain common complaints of poor concentration, memory and mental fatigue in patients with FMSS. Although a patient might voluntarily attend to his or her symptoms when they could have done otherwise, this is very different from proposing that the symptoms themselves are voluntary. To what extent a patient might focus his attention on his symptom expectations because of his illness beliefs, or because they are troubling to him, or because he derives secondary gain from his illness, is an interesting question, which doubtless has different answers in every patient. However the critical question is: if attentional allocation is voluntary, why do the resultant symptoms not feel so? Our account proposes that FMSS arise when the precision of abnormal intermediate-level prior beliefs is enhanced by misdirected, self-focused attention; however, it is important to realize that the top-down effect of attention only changes the precision of these prior beliefs—it does not predict their content. We can attend, for example, to a portion of the visual field, without predicting what will appear in it. This way, aberrant attentional biasing can ignite or maintain autonomous neuronal activity encoding percepts or movements, without any top-down predictions about the content or nature of the ensuing percept. Clearly patients will have reportable experiences with regard to their symptoms: ‘when I try to move my arm it shakes/won’t move’, ‘the left side of my body is numb/painful’ and self-focused attention combined with these beliefs will tend to enhance the precision of abnormal beliefs about sensations and movement. However, the resulting percepts are not predicted because the top-down attentional effect only amplifies their precision—it does not predict or explain away the beliefs that are made more precise. This would be like having attention drawn to some part of the sensorium with no idea (prediction) about what attracted attention—any subsequent percepts would have to be explained from scratch. In our framework, these explanations become symptoms due to a failure of inference by the patient—a failure to predict or recognize that they caused the percept (prior belief) by endowing it with too much precision. This failure can be seen as a rational attempt to explain percepts generated at intermediate levels in sensorimotor hierarchies that were enabled but not predicted by higher hierarchical levels.
If patients with functional motor symptoms over-attend to the sensory consequences of movement, relative to the motor planning that underwrites conscious intentions, one would expect that their sense of intention for other movements might also be abnormal, where this tendency a trait-like phenomenon. We have previously (Edwards et al., 2011) demonstrated this to be the case, using the well-known paradigm for timing conscious intentions first developed by Libet et al. (1983). We found that patients with a functional tremor judged the timing of their intention to move as occurring at the same time as their actual movement, in contrast to the perception of intention occurring significantly before actual movement in healthy subjects. In other words, patients with FMSS may misattribute agency in a post hoc fashion and fail to exploit high-level posterior beliefs that they are the agents of their own actions.
Influences from outside the somatomotor network
In proposing the above, we are not saying that no other brain area outside the normal somatomotor network can make a contribution to functional motor symptoms. Our aim is to describe the final common pathway for these activations, when they result in FMSS, and to explain the universal importance of precision (synaptic gain or sensitivity) and attention in this pathway. This can render a cortical area population inappropriately sensitive to afferents from many other systems and, indeed, increase the amplitude of its efferents to other regions—in other words, lead to increased connectivity with other regions. There is certainly evidence for FMSS-related activations in the amygdala, orbitofrontal cortices and posterior cingulate cortices. In a functional MRI study, Voon et al. (2010b) looked for and found a greater functional connectivity between the right amygdala and right supplementary motor area in patients with functional motor symptoms when they were exposed to stimuli of high emotional valence. Similarly, in a case study of one patient with a right sided functional paralysis, Kanaan et al. (2007) found that recall of an emotionally salient (but clinically repressed) memory, when compared with recall of a similarly emotional but unrepressed memory, was associated with activation of the amygdala, cingulate gyrus and premotor areas (amongst others), and relative deactivation of left primary motor cortex. In an earlier single photon emission computed tomography study, Vuilleumier et al. (2001) found decreased activation of the caudate nucleus and thalamus in patients with conversion sensorimotor loss, which correlated with the duration of their symptoms. They noted that both the caudate nucleus and thalamus receive limbic projections (e.g. from the amygdala).
Predictions and conclusions
Predictions
The hierarchical Bayesian model we have used is grounded in the fundamental mathematical and computational imperatives for adaptive biological systems like the brain (Friston et al., 2006), and also makes very specific predictions about both its functional anatomy and the kinds of measurable responses it should generate (Friston, 2005b; Garrido et al., 2009). Using this model, one can make quantitative empirical predictions about how the encoding of uncertainty or precision and attention can affect perception and its neurophysiological correlates (Feldman and Friston, 2010), which should mean we can generate testable predictions about our model of FMSS.
The framework for FMSS proposed here posits a single mechanism for the generation of a wide variety of symptoms: if this is correct, certain features can be expected to generalize from one symptom type to another. Particularly important, in the context of the framework proposed here, is the role of attention in symptom generation. Attention is known to be important for the maintenance of functional motor symptoms (Pareés et al., 2012b), but its role in the generation or perpetuation of sensory loss has not been examined. Given the common causal mechanism for FMSS outlined above, we would expect attention to have an exacerbating effect on the severity of all FMSS, including sensory loss; given the difficulty of using self-reports of sensation in patients with anaesthesia, proxies such as sensory evoked potentials (Levy and Mushin 1973; Kenntner-Mabiela et al., 2008) could be used instead. In controls, attention has been shown to increase the magnitude of somatosensory evoked potentials; we would expect opposite responses in patients with functional sensory loss.
Patients with FMSS may be hard to differentiate from those malingering symptoms and it is almost impossible to ascertain with standard diagnostic techniques whether a functional symptom is generated consciously or not. However, recent studies have demonstrated differences in EEG responses to stimuli when they are consciously perceived compared to when they are not (Babiloni et al., 2006; Schubert et al., 2006). Investigating such correlates of conscious perception might pave the way towards more sophisticated diagnoses for patients with FMSS.
We have proposed that the fundamental pathology in FMSS is the presence of overly precise priors at intermediate levels of the hierarchy, which may lead to the overweighting of bottom-up inputs that accord with those priors. Abnormalities of precision, or uncertainty, in Bayesian processing in the brain have also been proposed to underlie the overweighting of sensory data, which is thought to lead to positive symptoms of schizophrenia (Corlett et al., 2010). Based on this commonality in the proposed causative mechanisms, we would predict that patients with FMSS will perform better than normal subjects in a force-matching task that measures the attenuation of proprioceptive prediction errors in voluntary movement (Shergill et al., 2003). Schizophrenic subjects also perform better than controls on this task (Shergill et al., 2005), as they do in other illusory perception paradigms (Dakin et al., 2005), because they are unable to generate predictions that will suppress proprioceptive prediction errors; FMSS patients’ abnormal priors may lead to a similar overweighting of sensory data.
Conclusions
We have employed a Bayesian hierarchical model to explain FMSS. Figure 5 provides a general schematic. In this formulation, symptoms across the somatization-conversion spectrum can all be understood as pathologically precise prior beliefs, mediated by attentional processes, which result in perceptual and/or motor symptoms (precise posterior beliefs) that are experienced by the patient as involuntary. This unified approach to diverse FMSS is consistent with the common co-occurrence of these symptoms; patients with a diagnosis of somatization disorder have, by definition, suffered from at least eight FMSS, and careful history taking will reveal that many patients with conversion disorder have suffered or are suffering from other FMSS (Stone et al., 2010).
Figure 5.
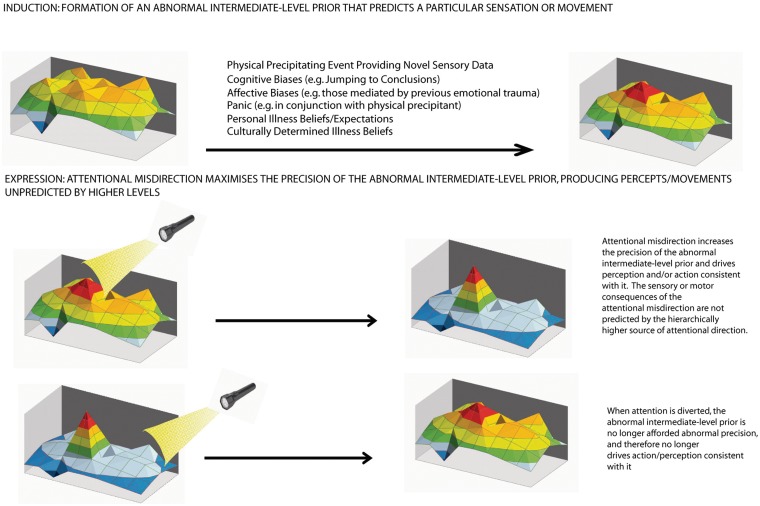
Here we provide a schematic that summarizes our theory. The 3D coloured plot represents an intermediate level within the nervous system, e.g. secondary sensory cortex, supplementary motor area or insular cortex. The plot represents the probability over different hidden states, arranged on a grid, where certain states are predicted to be more probable (peaks/red tones) and some are less probable (troughs/blue tones). We propose that the primary problem is the formation of an abnormal prior at this level originally instantiated via a number of interacting factors (not all of which are mentioned here and which will be different for different patients). The formation of this abnormal prior belief (peak) is mediated by the misdirection of attention (illustrated by a self-directed attentional ‘spotlight’), which affords it abnormal precision. This changes the strength of the abnormal prior, so that it drives perception or action consistent with it through top-down effects and calls for a higher-level explanation (e.g. agency) through bottom-up effects. Bottom: When attention is diverted, the precision of the abnormal prior is reduced so that it no longer drives perception or action. A crucial point is that the hierarchically higher sources of attention do not predict the precise dynamics of the percepts they induce. The resulting prediction error is explained (rationally and within the cognitive/affective framework of the individual) as an involuntary symptom of illness.
The picture that emerges here is of a primary problem with the attentional control of synaptic gain at intermediate (domain-specific) levels of sensorimotor hierarchies. Functionally, when considered in the context of formal theories of hierarchical neuronal computations, this abnormal gain control translates into an abnormally high precision or conditional confidence in probabilistic representations at that level in the hierarchy. The primary functional consequence of precise posterior beliefs is, in the sensory domain, the presence (or absence) of a well formed percept. If the cortical areas involve motor systems, then abnormally precise top-down predictions will elicit motor behaviour (or even its absence) through classical motor reflex arcs. The resulting percepts or motor phenomena become symptoms when the patient infers them to be caused by some pathology or illness. This can be regarded as a secondary false inference by higher levels that are trying to explain percepts that they did not predict. The result is a misattribution of agency, where experiences that are usually generated in a voluntary way are perceived as involuntary.
Despite the unusual and often severe nature of symptoms, FMSS can be seen as an extreme instance of a common phenomenon in predictive coding networks—the overweighting of prior beliefs over sensory data—which underlies normal experiences from optical illusions to placebo effects. Psychological stressors including childhood adversity or recent stressful events may well influence expectations and the deployment of attentional modulation (or the content of secondary false inferences about the causes of sensations), but this need not be the exclusive route to the production of functional symptoms. We propose a flexible approach to causation, which neither dismisses the relevance of traumatic events nor requires them to be present in all patients.
We have highlighted how common and disabling FMSS are, particularly in neurology practice, but there is an even wider relevance for understanding the origin of and how best to treat these symptoms. Most practising physicians would recognize that different patients suffering from the same organic disorder of apparently similar severity can experience vastly different levels of symptoms and consequent disability. This phenomenon, often known as functional overlay, is very common in neurology practice (Stone et al., 2012b) and, we suggest, is often considered more of an annoyance to the delivery of proper treatment for the organic disorder rather than something of real significance in itself. However, the model we have presented here—with its emphasis on triggering by physical illness and injury, and a range of predisposing factors related to illness beliefs and personal experience of illness—provides a principled explanation for the common co-occurrence of functional and organic symptoms. These functional symptoms may in many cases be more amenable to treatment than the underlying neurological disease.
We close by acknowledging that this approach represents a refinement and synthesis of ideas that have been developing over centuries. The statistical framework upon which our model is based and the theoretical underpinnings of active inference can be traced back to the work by Bayes (1763) and Helmholtz (1860), respectively. With regard to ‘hysteria’, it is sobering to read Russell Reynold’s brilliant report of patients from the 1860s (Reynolds, 1869) to appreciate that, while the neurobiological implementation may have moved on, the fundamental approach we are advocating may not be very different from a concept proposed a century and a half ago. Despite the undoubted excesses and some negative consequences, it is interesting that the explosion of clinical and academic interest in hysteria in the latter part of the 19th century came at a time when neurology and psychiatry were not seen as separate disciplines. The schism that developed in the 20th century between neurology and psychiatry may be a major reason for the subsequent lack of clinical and academic interest in these patients, and the rather static nature of theoretical approaches. The re-emergence of ‘functional’ disorders as a suitable topic for scientific study, in the past 15 years, parallels an ever closer union between clinical and academic work in neurology and psychiatry and allows a reappraisal of the subject with the benefit of a century of neuroscientific progress, and a wealth of new experimental techniques. More than a century since William James wrote ‘Poor hysterics … first they were treated as victims of sexual trouble … then of moral perversity and mediocrity … then of imagination’, and despite the frequency of this disorder and its impact on quality of life and healthcare economics, scientific and therapeutic progress has been limited. The theoretical approach to FMSS we have presented here provides a biologically plausible model and testable experimental predictions which, we hope, will aid hypothesis-driven pathophysiological and therapeutic research in this area.
Funding
National Institute for Health Research Clinician Scientist Fellowship (to M.J.E.) and Wellcome trust (to K.F. and R.A.A.).
Acknowledgements
The authors would like to thank Dr Aikaterina Fotopoulou for very helpful comments on the manuscript.
Glossary
Abbreviation
- FMSS
functional motor and sensory symptoms
References
- Babiloni C, Vecchio F, Bultrini A, Luca Romani G, Rossini PM. Pre- and poststimulus alpha rhythms are related to conscious visual perception: a high-resolution EEG study. Cereb Cortex. 2006;16:1690–700. doi: 10.1093/cercor/bhj104. [DOI] [PubMed] [Google Scholar]
- Bayes TR. An essay towards solving a problem in the doctrine of chances. Philos Trans R Soc Lond. 1763;53:370–418. [Google Scholar]
- Beilock SL, Carr TH. On the fragility of skilled performance: what governs choking under pressure? J Exp Psychol Gen. 2001;130:701–25. [PubMed] [Google Scholar]
- Bell V, Oakley DA, Halligan PW, Deeley Q. Dissociation in hysteria and hypnosis: evidence from cognitive neuroscience. J Neurol Neurosurg Psychiatry. 2011;82:332–9. doi: 10.1136/jnnp.2009.199158. [DOI] [PubMed] [Google Scholar]
- Benjamin S, Eminson DM. Abnormal illness behaviour: childhood experiences and long-term consequences. Int Rev Psychiatry. 1992;4:55–69. [Google Scholar]
- Bermingham SL, Cohen A, Hague J, Parsonage M. The cost of somatisation among the working-age population in England for the year 2008/2009. Mental Health Fam Med. 2010;7:71–84. [PMC free article] [PubMed] [Google Scholar]
- Berna C, Leknes S, Holmes EA, Edwards RR, Goodwin GM, Tracey I. Induction of depressed mood disrupts emotion regulation neurocircuitry and enhances pain unpleasantness. Biol Psychiatry. 2010;67:1083–90. doi: 10.1016/j.biopsych.2010.01.014. [DOI] [PubMed] [Google Scholar]
- Binzer M, Andersen PM, Kullgren G. Clinical characteristics of patients with motor disability due to conversion disorder: a prospective control group study. J Neurol Neurosurg Psychiatry. 1997;63:83–8. doi: 10.1136/jnnp.63.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore SJ, Sirigu A. Action prediction in the cerebellum and in the parietal lobe. Exp Brain Res. 2003;153:239–45. doi: 10.1007/s00221-003-1597-z. [DOI] [PubMed] [Google Scholar]
- Breuer J, Freud S. Studies on hysteria. In: Strachey J, Strachey A, editors and Translators. The Penguin Freud library, Vol. 3. London: Penguin; 1991. [Google Scholar]
- Briquet P. Traité clinique et thérapeutique de l’hystérie. Paris: J.-B. Baillière et Fils; 1859. [Google Scholar]
- Broome M. A neuroscience of hysteria? Curr Opin Psychiatry. 2004;17:465–9. [Google Scholar]
- Brown RJ. Psychological mechanisms of medically unexplained symptoms: an integrative conceptual model. Psychol Bull. 2004;130:793–812. doi: 10.1037/0033-2909.130.5.793. [DOI] [PubMed] [Google Scholar]
- Brown RJ, Brunt N, Poliakoff E, Lloyd DM. Illusory touch and tactile perception in somatoform dissociators. J Psychosom Res. 2010;69:241–8. doi: 10.1016/j.jpsychores.2009.11.010. [DOI] [PubMed] [Google Scholar]
- Buchbinder R, Jolley D. Effects of a media campaign on back beliefs is sustained 3 years after its cessation. Spine. 2005;30:1323–30. doi: 10.1097/01.brs.0000164121.77862.4b. [DOI] [PubMed] [Google Scholar]
- Burgess PW, Dumontheil I, Gilbert SJ. The gateway hypothesis of rostral prefrontal cortex (area 10) function. Trends Cogn Sci. 2007;11:290–8. doi: 10.1016/j.tics.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Cojan Y, Waber L, Carruzzo A, Vuilleumier P. Motor inhibition in hysterical conversion paralysis. Neuroimage. 2009;47:1026–37. doi: 10.1016/j.neuroimage.2009.05.023. [DOI] [PubMed] [Google Scholar]
- Corlett PR, Krystal JH, Taylor JR, Fletcher PC. Why do delusions persist? Front Hum Neurosci. 2009;3:12. doi: 10.3389/neuro.09.012.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corlett PR, Taylor JR, Wang X-J, Fletcher PC, Krystal JH. Toward a neurobiology of delusions. Prog Neurobiol. 2010;92:345–69. doi: 10.1016/j.pneurobio.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crimlisk HL, Bhatia K, Cope H, David A, Marsden CD, Ron MA. Slater revisited: 6 year follow up study of patients with medically unexplained motor symptoms. BMJ. 1998;316:582–6. doi: 10.1136/bmj.316.7131.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crombez G, Eccleston C, Baeyens F, Eelen P. When somatic information threatens, catastrophic thinking enhances attentional interference. Pain. 1998;75:187–98. doi: 10.1016/s0304-3959(97)00219-4. [DOI] [PubMed] [Google Scholar]
- Dakin S, Carlin P, Hemsley D. Weak suppression of visual context in chronic schizophrenia. Curr Biol. 2005;15:822–4. doi: 10.1016/j.cub.2005.10.015. [DOI] [PubMed] [Google Scholar]
- Edwards MJ, Bhatia KP, Cordivari C. Immediate response to botulinum toxin injections in patients with fixed dystonia. Mov Disord. 2011;26:917–18. doi: 10.1002/mds.23562. [DOI] [PubMed] [Google Scholar]
- Edwards MJ, Moretto G, Schwingenschuh P, Katschnig P, Bhatia KP, Haggard P. Abnormal sense of intention preceding voluntary movement in patients with psychogenic tremor. Neuropsychologia. 2011;49:2791–3. doi: 10.1016/j.neuropsychologia.2011.05.021. [DOI] [PubMed] [Google Scholar]
- Fahn S, Williams DT. Psychogenic dystonia. Adv Neurol. 1988;50:431–55. [PubMed] [Google Scholar]
- Farrer C, Frith CD. Experiencing oneself vs. another person as being the cause of an action: the neural correlates of the experience of agency. Neuroimage. 2002;15:596–603. doi: 10.1006/nimg.2001.1009. [DOI] [PubMed] [Google Scholar]
- Feldman H, Friston KJ. Attention, uncertainty, and free-energy. Front Hum Neurosci. 2010;4:215. doi: 10.3389/fnhum.2010.00215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felleman DJ, Van Essen DC. Distributed hierarchical processing in the primate cerebral cortex. Cereb Cortex. 1991;1:1–47. doi: 10.1093/cercor/1.1.1-a. [DOI] [PubMed] [Google Scholar]
- Ferrari R, Obelieniene D, Russell AS, Darlington P, Gervais R, Green P. Symptom expectation after minor head injury. A comparative study between Canada and Lithuania. Clin Neurol Neurosurg. 2001;103:184–90. doi: 10.1016/s0303-8467(01)00143-3. [DOI] [PubMed] [Google Scholar]
- Fillingim RB, Fine MA. The effects of internal versus external information processing on symptom perception in an exercise setting. Health Psychol. 1986;5:115–23. doi: 10.1037//0278-6133.5.2.115. [DOI] [PubMed] [Google Scholar]
- Friston KJ. Hallucinations and perceptual inference. Behav Brain Sci. 2005a;28:764–6. [Google Scholar]
- Friston K. A theory of cortical responses. Philos Trans R Soc Lond B Biol Sci. 2005b;360:815–36. doi: 10.1098/rstb.2005.1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Daunizeau J, Kilner J, Kiebel SJ. Action and behavior: a free-energy formulation. Biol Cybern. 2010;102:227–60. doi: 10.1007/s00422-010-0364-z. [DOI] [PubMed] [Google Scholar]
- Friston K, Kilner J, Harrison L. A free energy principle for the brain. J Physiol Paris. 2006;100:70–87. doi: 10.1016/j.jphysparis.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Garety PA, Freeman D. Cognitive approaches to delusions: a critical review of theories and evidence. Br J Clin Psychol. 1999;38:113–54. doi: 10.1348/014466599162700. [DOI] [PubMed] [Google Scholar]
- Garrido MI, Kilner JM, Stephan KE, Friston KJ. The mismatch negativity: a review of underlying mechanisms. Clin Neurophysiol. 2009;120:453–63. doi: 10.1016/j.clinph.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A, Lang AE. Psychogenic movement disorders. Curr Opin Neurol. 2009;22:430–6. doi: 10.1097/WCO.0b013e32832dc169. [DOI] [PubMed] [Google Scholar]
- Haggard P, Jenner J, Wing A. Coordination of aimed movements in a case of unilateral cerebellar damage. Neuropsychologia. 1994;32:827–46. doi: 10.1016/0028-3932(94)90021-3. [DOI] [PubMed] [Google Scholar]
- Halligan PW, Athwal BS, Oakley DA, Frackowiak RS. Imaging hypnotic paralysis: implications for conversion hysteria. Lancet. 2000;355:986–7. doi: 10.1016/S0140-6736(00)99019-6. [DOI] [PubMed] [Google Scholar]
- Hansell S, Mechanic D. The socialization of introspection and illness behavior. In: McHugh S, Vallis TM, editors. Illness behavior: a multidisciplinary model. New York: Plenum Press; 1986. pp. 253–60. [Google Scholar]
- Helmholtz H. Handbuch der physiologischen Optik. 3 (English Trans: Southall JPC, editor). New York: Dover; 1860/1962. [Google Scholar]
- Hilgard ER. Divided consciousness: Multiple controls in human thought and action. New York: Wiley; 1977. [Google Scholar]
- Hommel B, Pratt J, Colzato L, Godijn R. Symbolic control of visual attention. Psychol Sci. 2001;12:360–5. doi: 10.1111/1467-9280.00367. [DOI] [PubMed] [Google Scholar]
- Horvath T, Friedman J, Meares R. Attention in hysteria: a study of Janet’s hypothesis by means of habituation and arousal measures. Am J Psychiatry. 1980;137:217–20. doi: 10.1176/ajp.137.2.217. [DOI] [PubMed] [Google Scholar]
- Hotopf M, Mayou R, Wadsworth M, Wessely S. Childhood risk factors for adults with medically unexplained symptoms: results from a national birth cohort study. Am J Psychiatry. 1999;156:1796–800. doi: 10.1176/ajp.156.11.1796. [DOI] [PubMed] [Google Scholar]
- Ismail K, Lewis G. Multi-symptom illnesses, unexplained illness and Gulf War Syndrome. Philos Trans R Soc Lond B Biol Sci. 2006;361:543–51. doi: 10.1098/rstb.2006.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James W. The principles of psychology. Vol. 1. New York: Henry Holt; 1890. [Google Scholar]
- Janet P. The major symptoms of hysteria. New York, NY, US: Macmillan Publishing; 1907. [Google Scholar]
- Jueptner M, Stephan KM, Frith CD, Brooks DJ, Frackowiak RS, Passingham RE. Anatomy of motor learning. I. Frontal cortex and attention to action. J Neurophysiol. 1997;77:1313–24. doi: 10.1152/jn.1997.77.3.1313. [DOI] [PubMed] [Google Scholar]
- Kanaan RA, Craig TK, Wessely SC, David AS. Imaging repressed memories in motor conversion disorder. Psychosom Med. 2007;69:202–5. doi: 10.1097/PSY.0b013e31802e4297. [DOI] [PubMed] [Google Scholar]
- Kenntner-Mabiala R, Andreatta M, Wieser MJ, Mühlberger A, Pauli P. Distinct effects of attention and affect on pain perception and somatosensory evoked potentials. Biol Psychol. 2008;78:114–22. doi: 10.1016/j.biopsycho.2008.01.007. [DOI] [PubMed] [Google Scholar]
- Kihlstrom JF. Hypnosis: a sesquicentennial essay. Int J Clin Exp Hypn. 1992;40:301–14. doi: 10.1080/00207149208409663. [DOI] [PubMed] [Google Scholar]
- Kirmayer LJ, Taillefer S. Somatoform disorders. In: Turner SM, Hersen M, editors. Adult psychopathology and diagnosis. 3rd edn. New York: Wiley; 1997. pp. 333–83. [Google Scholar]
- Knapp M, Prince M. Dementia UK: a report into the prevalence and cost of dementia. London: Alzheimer’s Society; 2007. [Google Scholar]
- Kolk AM, Hanewald GJFP, Schagen S, Gijsbers van Wijk CMT. A symptom perception approach to common physical symptoms. Soc Sci Med. 2003;57:2343–54. doi: 10.1016/s0277-9536(02)00451-3. [DOI] [PubMed] [Google Scholar]
- Kranick S, Ekanayake V, Martinez V, Ameli R, Hallett M, Voon V. Psychopathology and psychogenic movement disorders. Mov Disord. 2011;26:1844–50. doi: 10.1002/mds.23830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBerge D. Spatial extent of attention to letters and words. J Exp Psychol Hum Percept Perform. 1983;9:371–9. doi: 10.1037//0096-1523.9.3.371. [DOI] [PubMed] [Google Scholar]
- Lau HC, Rogers RD, Haggard P, Passingham RE. Attention to intention. Science. 2004;303:1208–10. doi: 10.1126/science.1090973. [DOI] [PubMed] [Google Scholar]
- Levy R, Mushin J. The somatosensory evoked response in patients with hysterical anaesthesia. J Psychosom Res. 1973;17:81–4. doi: 10.1016/0022-3999(73)90007-x. [DOI] [PubMed] [Google Scholar]
- Libet B, Gleason CA, Wright EW, Pearl DK. Time of conscious intention to act in relation to onset of cerebral activity (readiness-potential). The unconscious initiation of a freely voluntary act. Brain. 1983;106:623–42. doi: 10.1093/brain/106.3.623. [DOI] [PubMed] [Google Scholar]
- Lloyd DM, Mason L, Brown RJ, Poliakoff E. Development of a paradigm for measuring somatic disturbance in clinical populations with medically unexplained symptoms. J Psychosom Res. 2008;64:21–4. doi: 10.1016/j.jpsychores.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Lorenz J, Hauck M, Paur RC, Nakamura Y, Zimmermann R, Bromm B, et al. Cortical correlates of false expectations during pain intensity judgments—a possible manifestation of placebo/nocebo cognitions. Brain Behav Immun. 2005;19:283–95. doi: 10.1016/j.bbi.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Ludwig AM. Hysteria. A neurobiological theory. Arch Gen Psychiatry. 1972;27:771–7. doi: 10.1001/archpsyc.1972.01750300043007. [DOI] [PubMed] [Google Scholar]
- Mailis-Gagnon A, Giannoylis I, Downar J, Kwan CL, Mikulis DJ, Crawley AP, et al. Altered central somatosensory processing in chronic pain patients with ‘hysterical’ anesthesia. Neurology. 2003;60:1501–7. doi: 10.1212/wnl.60.9.1501. [DOI] [PubMed] [Google Scholar]
- Mangun GR, Hillyard SA. Modulations of sensory-evoked brain potentials indicate changes in perceptual processing during visual-spatial priming. J Exp Psychol Hum Percept Perform. 1991;17:1057–74. doi: 10.1037//0096-1523.17.4.1057. [DOI] [PubMed] [Google Scholar]
- Marshall JC, Halligan PW, Fink GR, Wade DT, Frackowiak RS. The functional anatomy of a hysterical paralysis. Cognition. 1997;64:B1–B8. doi: 10.1016/s0010-0277(97)00020-6. [DOI] [PubMed] [Google Scholar]
- Martin A, Buech A, Schwenk C, Rief W. Memory bias for health-related information in somatoform disorders. J Psychosom Res. 2007;63:663–71. doi: 10.1016/j.jpsychores.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Maruff P, Velakoulis D. The voluntary control of motor imagery. Imagined movements in individuals with feigned motor impairment and conversion disorder. Neuropsychologia. 2000;38:1251–60. doi: 10.1016/s0028-3932(00)00031-2. [DOI] [PubMed] [Google Scholar]
- Melloni L, Schwiedrzik CM, Müller N, Rodriguez E, Singer W. Expectations change the signatures and timing of electrophysiological correlates of perceptual awareness. J Neurosci. 2011;31:1386–96. doi: 10.1523/JNEUROSCI.4570-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumford D. On the computational architecture of the neocortex. II. The role of cortico-cortical loops. Biol Cybern. 1992;66:241–51. doi: 10.1007/BF00198477. [DOI] [PubMed] [Google Scholar]
- Munoz DP, Everling S. Look away: the anti-saccade task and the voluntary control of eye movement. Nat Rev Neurosci. 2004;5:218–28. doi: 10.1038/nrn1345. [DOI] [PubMed] [Google Scholar]
- Nachev P, Rees G, Parton A, Kennard C, Husain M. Volition and conflict in human medial frontal cortex. Curr Biol. 2005;15:122–8. doi: 10.1016/j.cub.2005.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nijenhuis ER, Spinhoven P, Van Dyck R, Van der Hart O, Vanderlinden J. The development and psychometric characteristics of the Somatoform Dissociation Questionnaire (SDQ-20) J Nerv Ment Dis. 1996;184:688–94. doi: 10.1097/00005053-199611000-00006. [DOI] [PubMed] [Google Scholar]
- Norman D, Shallice T. Attention to action: willed and automatic control of behavior. In: Davidson R, Schwartz R, Shapiro D, editors. Consciousness and self-regulation: advances in research and theory IV. New York: Plenum Press; 1986. pp. 1–14. [Google Scholar]
- Pareés I, Kassavetis P, Saifee TA, Sadnicka A, Bhatia KP, Fotopoulou A, et al. ‘Jumping to Conclusions’ bias in functional movement disorders. J Neurol Neurosurg Psychiatry. 2012a;83:460–3. doi: 10.1136/jnnp-2011-300982. [DOI] [PubMed] [Google Scholar]
- Pareés I, Saifee TA, Kassavetis P, Kojovic M, Rubio-Agusti I, Rothwell JC, et al. Believing is perceiving: mismatch between self-report and actigraphy in psychogenic tremor. Brain. 2012b;135:117–23. doi: 10.1093/brain/awr292. [DOI] [PubMed] [Google Scholar]
- Pennebaker JW. The psychology of physical symptoms. New York: Springer-Verlag; 1982. [Google Scholar]
- Pennebaker JW, Brittingham GL. Environmental and sensory cues affecting the perception of physical symptoms. In: Baum A, Singer J, editors. Advances in environmental psychology. Vol. 4. Hillsdale, NJ: Erlbaum; 1982. pp. 115–36. [Google Scholar]
- Pennebaker JW, Lightner JM. Competition of internal and external information in an exercise setting. J Pers Soc Psychol. 1980;39:165–74. doi: 10.1037//0022-3514.39.1.165. [DOI] [PubMed] [Google Scholar]
- Pennebaker JW, Skelton JA. Selective monitoring of physical sensations. J Pers Soc Psy. 1981;41:213–23. doi: 10.1037//0022-3514.41.2.213. [DOI] [PubMed] [Google Scholar]
- Posner MI, Nissen MJ, Ogden WC. Attended and unattended processing modes: the role of set for spatial location. In: Pick HL, Saltzman EJ, editors. Modes of perceiving and processing information. Hillsdale, NJ: Erlbaum; 1978. pp. 137–57. [Google Scholar]
- Reynolds JR. Paralysis and other disorders of motion and sensation dependent on idea. BMJ. 1869;i:483–5. doi: 10.1136/bmj.2.462.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins JM, Kirmayer LJ. Illness cognition, symptom reporting, and somatisation in primary care. In: McHugh S, Vallis TM, editors. Illness behavior: a multidisciplinary model. New York: Plenum Press; 1986. pp. 283–302. [Google Scholar]
- Robbins JM, Kirmayer LJ. Cognitive and social factors in somatisation. In: Kirmayer LJ, Robbins JM, editors. Current concepts of somatisation: research and clinical perspectives. Washington, DC: American Psychiatric Press; 1991. pp. 107–41. [Google Scholar]
- Roelofs K, de Bruijn ERA, Van Galen GP. Hyperactive action monitoring during motor-initiation in conversion paralysis: an event-related potential study. Biol Psychol. 2006;71:316–25. doi: 10.1016/j.biopsycho.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Roelofs K, Keijsers GPJ, Hoogduin KAL, Näring GWB, Moene FC. Childhood abuse in patients with conversion disorder. Am J Psychiatry. 2002;159:1908–13. doi: 10.1176/appi.ajp.159.11.1908. [DOI] [PubMed] [Google Scholar]
- Roelofs K, van Galen GP, Eling P, Keijsers GPJ, Hoogduin CAL. Endogenous and exogenous attention in patients with conversion paresis. Cogn Neuropsychol. 2003;20:733–45. doi: 10.1080/02643290342000069. [DOI] [PubMed] [Google Scholar]
- Rusch MD, Morris GL, Allen L, Lathrop L. Psychological treatment of nonepileptic events. Epilepsy Behav. 2001;2:277–83. doi: 10.1006/ebeh.2001.0180. [DOI] [PubMed] [Google Scholar]
- Schmidt AJ, Wolfs-Takens DJ, Oosterlaan J, van den Hout MA. Psychological mechanisms in hypochondriasis: attention-induced physical symptoms without sensory stimulation. Psychother Psychosom. 1994;61:117–20. doi: 10.1159/000288876. [DOI] [PubMed] [Google Scholar]
- Schnell K, Heekeren K, Daumann J, Schnell T, Schnitker R, Moller-Hartmann W, et al. Correlation of passivity symptoms and dysfunctional visuomotor action monitoring in psychosis. Brain. 2008;131:2783–97. doi: 10.1093/brain/awn184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrag A, Trimble M, Quinn N, Bhatia K. The syndrome of fixed dystonia: an evaluation of 103 patients. Brain. 2004;127:2360–72. doi: 10.1093/brain/awh262. [DOI] [PubMed] [Google Scholar]
- Schubert R, Blankenburg F, Lemm S, Villringer A, Curio G. Now you feel it now you don’t: ERP correlates of somatosensory awareness. Psychophysiology. 2006;43:31–40. doi: 10.1111/j.1469-8986.2006.00379.x. [DOI] [PubMed] [Google Scholar]
- Schwingenschuh P, Katschnig P, Seiler S, Saifee TA, Aguirregomozcorta M, Cordivari C, et al. Moving toward ‘laboratory-supported’ criteria for psychogenic tremor. Mov Disord. 2011;26:2509–15. doi: 10.1002/mds.23922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe D, Faye C. Non-epileptic seizures and child sexual abuse: a critical review of the literature. Clin Psychol Rev. 2006;26:1020–1040. doi: 10.1016/j.cpr.2005.11.011. [DOI] [PubMed] [Google Scholar]
- Shergill SS, Bays PM, Frith Chris D, Wolpert DM. Two eyes for an eye: the neuroscience of force escalation. Science. 2003;301:187. doi: 10.1126/science.1085327. [DOI] [PubMed] [Google Scholar]
- Shergill SS, Samson G, Bays PM, Frith Chris D, Wolpert DM. Evidence for sensory prediction deficits in schizophrenia. Am J Psychiatry. 2005;162:2384–6. doi: 10.1176/appi.ajp.162.12.2384. [DOI] [PubMed] [Google Scholar]
- Slater E. Diagnosis of ‘hysteria. Br Med J. 1965;1:1395–9. doi: 10.1136/bmj.1.5447.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence SA, Brooks DJ, Hirsch SR, Liddle PF, Meehan J, Grasby PM. A PET study of voluntary movement in schizophrenic patients experiencing passivity phenomena (delusions of alien control) Brain. 1997;120:1997–2011. doi: 10.1093/brain/120.11.1997. [DOI] [PubMed] [Google Scholar]
- Spence SA, Crimlisk HL, Cope H, Ron MA, Grasby PM. Discrete neurophysiological correlates in prefrontal cortex during hysterical and feigned disorder of movement. Lancet. 2000;355:1243–4. doi: 10.1016/S0140-6736(00)02096-1. [DOI] [PubMed] [Google Scholar]
- Stewart DE. The changing faces of somatization. Psychosomatics. 1990;31:153–8. doi: 10.1016/S0033-3182(90)72188-3. [DOI] [PubMed] [Google Scholar]
- Stone J, Carson A, Duncan R, Coleman R, Roberts R, Warlow C, et al. Symptoms ‘unexplained by organic disease’ in 1144 new neurology out-patients: how often does the diagnosis change at follow-up? Brain. 2009a;132:2878–88. doi: 10.1093/brain/awp220. [DOI] [PubMed] [Google Scholar]
- Stone J, Carson A, Aditya H, Prescott R, Zaubi M, Warlow C, et al. The role of physical injury in motor and sensory conversion symptoms: a systematic and narrative review. J Psychosom Res. 2009b;66:383–90. doi: 10.1016/j.jpsychores.2008.07.010. [DOI] [PubMed] [Google Scholar]
- Stone J, Warlow C, Sharpe M. Functional weakness: clues to mechanism from the nature of onset. J Neurol Neurosurg Psychiatry. 2012a;83:67–9. doi: 10.1136/jnnp-2011-300125. [DOI] [PubMed] [Google Scholar]
- Stone J, Carson A, Duncan R, Roberts R, Coleman R, Warlow C, et al. Which neurological diseases are most likely to be associated with ‘symptoms unexplained by organic disease’. J Neurol. 2012b;259:33–8. doi: 10.1007/s00415-011-6111-0. [DOI] [PubMed] [Google Scholar]
- Stone J, Warlow C, Carson A, Sharpe M. Eliot Slater’s myth of the non-existence of hysteria. J R Soc Med. 2005;98:547–8. doi: 10.1258/jrsm.98.12.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone J, Carson A, Duncan R, Roberts R, Warlow C, Hibberd C, et al. Who is referred to neurology clinics?—the diagnoses made in 3781 new patients. Clin Neurol Neurosurg. 2010a;112:747–51. doi: 10.1016/j.clineuro.2010.05.011. [DOI] [PubMed] [Google Scholar]
- Stone J, Warlow C, Sharpe M. The symptom of functional weakness: a controlled study of 107 patients. Brain. 2010b;133:1537–51. doi: 10.1093/brain/awq068. [DOI] [PubMed] [Google Scholar]
- Stone J, Wojcik W, Durrance D, Carson A, Lewis S, MacKenzie L, et al. What should we say to patients with symptoms unexplained by disease? The ‘number needed to offend’. BMJ. 2002;325:1449–50. doi: 10.1136/bmj.325.7378.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone J, Zeman A, Simonotto E, Meyer M, Azuma R, Flett S, et al. FMRI in patients with motor conversion symptoms and controls with simulated weakness. Psychosom Med. 2007;69:961–9. doi: 10.1097/PSY.0b013e31815b6c14. [DOI] [PubMed] [Google Scholar]
- Vassend O. Dimensions of negative affectivity, self-reported somatic symptoms, and health-related behaviors. Soc Sci Med. 1989;28:29–36. doi: 10.1016/0277-9536(89)90303-1. [DOI] [PubMed] [Google Scholar]
- Voon V, Gallea C, Hattori N, Bruno M, Ekanayake V, Hallett M. The involuntary nature of conversion disorder. Neurology. 2010a;74:223–8. doi: 10.1212/WNL.0b013e3181ca00e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voon V, Brezing C, Gallea C, Ameli R, Roelofs K, LaFrance WC, et al. Emotional stimuli and motor conversion disorder. Brain. 2010b;133:1526–36. doi: 10.1093/brain/awq054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuilleumier P, Chicherio C, Assal F, Schwartz S, Slosman D, Landis T. Functional neuroanatomical correlates of hysterical sensorimotor loss. Brain. 2001;124:1077–90. doi: 10.1093/brain/124.6.1077. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P. Hysterical conversion and brain function. Prog Brain Res. 2005;150:309–29. doi: 10.1016/S0079-6123(05)50023-2. [DOI] [PubMed] [Google Scholar]
- Wegner DM, Giuliano T. Arousal-induced attention to self. J Pers Soc Psychol. 1980;38:719–26. [Google Scholar]
- Wessely S, Sharpe M, Hotopf M. Chronic fatigue and its syndromes. Oxford: Oxford University Press; 1998. [Google Scholar]
- Wiech K, Ploner M, Tracey I. Neurocognitive aspects of pain perception. Trends Cogn Sci. 2008;12:306–13. doi: 10.1016/j.tics.2008.05.005. [DOI] [PubMed] [Google Scholar]
- Ziv I, Djaldetti R, Zoldan Y, Avraham M, Melamed E. Diagnosis of ‘non-organic’ limb paresis by a novel objective motor assessment: the quantitative Hoover’s test. J Neurol. 1998;245:797–802. doi: 10.1007/s004150050289. [DOI] [PubMed] [Google Scholar]