Abstract
Parkinson’s disease is a neurodegenerative disorder that can, at least partly, be mimicked by the neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. S100B is a calcium-binding protein expressed in, and secreted by, astrocytes. There is increasing evidence that S100B acts as a cytokine or damage-associated molecular pattern protein not only in inflammatory but also in neurodegenerative diseases. In this study, we show that S100B protein levels were higher in post-mortem substantia nigra of patients with Parkinson’s disease compared with control tissue, and cerebrospinal fluid S100B levels were higher in a large cohort of patients with Parkinson’s disease compared with controls. Correspondingly, mice treated with 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine showed upregulated S100B messenger RNA and protein levels. In turn, ablation of S100B resulted in neuroprotection, reduced microgliosis and reduced expression of both the receptor for advanced glycation endproducts and tumour necrosis factor-α. Our results demonstrate a role of S100B in the pathophysiology of Parkinson’s disease. Targeting S100B may emerge as a potential treatment strategy in this disorder.
Keywords: calcium-binding protein, MPTP, Parkinson’s disease, S100B
Introduction
Parkinson’s disease is a common progressive neurodegenerative disorder characterized by the accumulation of aggregated α-synuclein in neurons of particular brain regions, including the brainstem and cortical regions, and by severe loss of dopaminergic neurons (Lees et al., 2009). There is increasing evidence that glial cells are crucially involved in the initiation and progression of the disease (Halliday and Stevens, 2011). For example, astrocytes have recently been shown to effectively endocytose α-synuclein proteins secreted from neurons, and to produce glial inclusions and inflammatory responses (Lee et al., 2011). The distribution of α-synuclein-positive astrocytes parallels the distribution of Lewy bodies (Braak et al., 2007). In addition, animal models suggest that microglia are involved early in Parkinson’s disease (Halliday and Stevens, 2011). In turn, specific inhibition of microglial cells led to a significant attenuation of the Parkinson’s disease-like disease process caused by the neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) (Wu et al., 2002). MPTP is a neurotoxin that causes Parkinson’s disease in humans and primates, which is clinically almost indistinguishable from sporadic Parkinson’s disease (Langston and Irwin, 1986).
S100B, a calcium-binding protein, is synthesized in, and constitutively secreted by, astrocytes (Van Eldik and Zimmer, 1987; Sorci et al., 2010). S100B levels are positively associated with age, and increased S100B concentrations have been detected in various neurodegenerative and neuroinflammatory diseases (Rothermundt et al., 2003; Sorci et al., 2010). An increase of S100B-positive astroglia 1 day after MPTP administration (Muramatsu et al., 2003) gives rise to the hypothesis that this protein may also be involved in Parkinson’s disease pathogenesis. This finding, however, cannot rule out the alternative hypothesis that the increase in S100B might merely present an unspecific secondary effect, without playing a causal role in Parkinson’s disease-related neurodegeneration. Indirect evidence for an involvement of S100B in dopaminergic dysfunction comes from genetic studies. An association of the single-nucleotide polymorphism rs3788266 of the S100B gene with schizophrenia and psychosis in bipolar affective disorders was recently reported (Liu et al., 2005; Roche et al., 2007).
Most interestingly, S100B at low (nanomolar) concentrations acts as a neurotrophic factor promoting neuronal survival and neurite growth during development and under stress (Bianchi et al., 2007). Yet, at high (micromolar) concentrations, S100B causes neuronal apoptosis both by direct action on neurons and activation of microglia (Hu et al., 1997; Li et al., 2000; Sorci et al., 2010). These effects may, to some extent, be mediated by activation of inducible nitric oxide synthase (an enzyme responsible for the production of nitric oxide), and by inducing elevated levels of intracellular calcium and caspase-3 activation (Hu et al., 1997; Iuvone et al., 2007). Another mechanism includes the receptor for advanced glycation endproducts (RAGE) pathways (Kerkhoff et al., 1998; Donato, 1999; Hofmann et al., 1999; Bianchi et al., 2011; Villarreal et al., 2011). RAGE is a member of the immunoglobulin superfamily and can be bound by several ligands, including S100B. Both the pro-survival (at low concentrations) and the toxic effect (at high concentrations) of S100B appear, at least partly, to be because of RAGE-mediated nuclear factor-κB activation (Huttunen et al., 2000; Villarreal et al., 2011). S100B can also upregulate neuronal RAGE expression (Kogel et al., 2004; Businaro et al., 2006; Villarreal et al., 2011). Based on these findings and the hypothesis that S100B is involved in Parkinson’s disease pathogenesis, we set out to study S100B levels in patients with Parkinson’s disease and MPTP-treated mice, and to explore the consequences of S100B in S100B−/− mice. Here, we report that S100B is upregulated in the substantia nigra and CSF of patients with Parkinson’s disease, as well as in MPTP-treated mice. Reduced gliosis and decreased expression and activation of both RAGE and the pro-inflammatory cytokine tumour necrosis factor (TNF)-α in S100B−/− mice treated with MPTP indicate that ablation of S100B is neuroprotective in Parkinson’s disease-like processes.
Materials and methods
Human samples
Six post-mortem human midbrain samples (frozen tissue samples and prepared slices) from patients with Parkinson’s disease and five control samples matched for age at death and interval from death to tissue processing (see Supplementary material) were obtained from the Parkinson’s UK Brain Bank at Imperial College, London, UK. Human serum, CSF and DNA samples of 82 patients with Parkinson’s disease and 64 controls were collected under fasting conditions between 8.00 and 10.00 a.m. at the ward and outpatient clinic of the Neurodegenerative Department of the University of Tübingen according to the highest international standards on biobanking. For details see Maetzler et al. (2011a). Demographics and clinical data are shown in Table 1.
Table 1.
Demographic and clinical data, including serum and CSF S100B levels, of subjects with Parkinson’s disease and control subjects
| Demographic and clinical features | Parkinson’s disease | Controls | P-value |
|---|---|---|---|
| Subjects (female) | 82 (42) | 64 (41) | 0.18 |
| Age at examination (years), mean (SD) | 69 (8) | 67 (9) | 0.11 |
| Age at onset of parkinsonism (years), mean (SD) | 61 (9) | ||
| Disease duration (years), mean (SD) | 8 (6) | ||
| Hoehn and Yahr stage median (range) | 2 (1–4) | ||
| CSF S100B (µg/l), mean (SD) | 3.1 (1.0) | 2.2 (1.1) | <0.0001 |
| Serum S100B (µg/l), mean (SD) | 0.09 (0.06) | 0.08 (0.04) | 0.18 |
Hoehn and Yahr staging was performed in an OFF medication state.
Determination of S100B levels in cerebrospinal fluid and serum, and genotyping
S100B protein levels were measured blinded to the subject’s state (i.e. patient or control) using an automated immunoassay (LIAISON®, DiaSorin). The assay is based on a non-competitive ligand-binding method (sandwich principle). Genotyping of single-nucleotide polymorphism rs3788266 of the S100B gene was performed as described elsewhere (Maetzler et al., 2011b).
Animals and treatment
Wild-type C56/BL7 mice were obtained from Charles River Laboratories. S100B−/− mice were obtained from the Shigeyoshi Itohara Laboratory, Behavioural Genetics, Brain Science Institute, RIKEN, Japan, and genotyping was performed as published previously (Nishiyama et al., 2002). Animals received five injections of 30 mg/kg MPTP intraperitoneally for 5 consecutive days and were sacrificed at selected time points after the last injection (0, 2, 4, 7, 14 and 21 days). This protocol was in accordance with the Home Office regulations.
RNA extraction and reverse transcription polymerase chain reaction
Total RNA was extracted from selected mouse brain regions as described earlier (Teismann et al., 2003). Complementary DNA was amplified using the Roche LightCycler® 480. The primer mouse sequences used were as follows: RAGE, 5′-CAGCATCAGGGTCACAGAAA-3′ (forward) and 5′-CTGGTTGGAGAAGGAAGTGC-3′ (reverse); S100B, 5′-GCTGACCACCATGCCCCTGTAG-3′ (forward) and 5′-CTGGCCATTCCCTCCTCTGTC-3′ (reverse). As internal control, β-actin complementary DNA was co-amplified using the primer sequences 5′-TGTGATGGTGGAATGGGTCAG-3′ (forward) and 5′-TTTGATGTCACGCACGATTTCC-3′ (reverse).
Immunoblots
Mouse and human brain extracts were prepared and western blot analyses performed as described previously (Teismann et al., 2003). Primary antibodies were as follows: S100B (1:1000, monoclonal, Sigma), RAGE (1:1000, Sigma), cyclooxygenase (COX)-2 (1:250, BD Bioscience), inducible nitric oxide synthase (1:500, BD Bioscience), TNF-α (1:1000, Abcam), β-actin (1:25 000, Sigma), ionized calcium-binding adaptor molecule 1 (1:2000, Wako Chemicals, Neuss) and glial fibrillary acid protein (GFAP; 1:1000, DAKO). Horseradish peroxidise-conjugated secondary antibodies (anti-rabbit or anti-mouse 1:10000, Amersham) and enhanced chemiluminescence solution (Luminol sodium salt in 0.1 mM Tris HCl and para-hydroxycoumarin in dimethyl sulphoxide) were used for chemiluminescence detection. Bands were quantified using the FluorChem 8800 digital image system (Alpha Innotech).
S100B immunohistochemistry
All immunohistochemistry analyses were performed according to our standard protocols for immunostaining (Teismann et al., 2003). Primary antibodies (in phosphate buffered saline (PBS)–Triton–normal goat serum) were as follows: S100B (1:1000, monoclonal, Sigma Aldrich), GFAP (1:1000, DAKO), tyrosine hydroxylase (TH, 1:1000, Millipore UK) and ionized calcium-binding adaptor molecule 1 (1:500, Wako Chemicals, Neuss). The sections were incubated in anti-rabbit (1:200, Cy3, Jackson ImmunoResearch) or anti-mouse (1:300, AlexaFluor® 480, Molecular Probes) fluorescing antibodies for visualization using confocal microscopy. Tyrosine hydroxylase immunohistochemistry was carried out as described previously (Teismann et al., 2003), and the sections were counterstained with Nissl. The tyrosine hydroxylase- and Nissl-positive substantia nigra pars compacta neurons were counted using the optical fractionators method with the examiner being blinded to genotype and treatment. Striatal density of tyrosine hydroxylase immunoreactivity was measured as described elsewhere (Teismann et al., 2003).
High-performance liquid chromatography
Levels of dopamine and its metabolites, 3,4-dihydroxyphenylacetic acid and homovanillic acid, were measured in striatal samples (Teismann et al., 2003).
Immunocytochemistry
SH-SY5Y cells were grown on coverslips in 24-well plates and plated at a density of 40 000 cells/well. After 48 h, the medium was replaced with serum-free Dulbecco’s modified Eagle’s medium and RAGE antibody, at a concentration of 25 μg/ml, or PBS was added. S100B antibody was added 24 h later at a concentration of 10 μg/ml. 1-Methyl-4-phenylpyridinium (MPP+) was also added at this stage, to give a final concentration of 1.5 mM. After further 48 h, cells were fixed in 4% paraformaldehyde for 15 min. Cells were extensively washed with PBS between each step. Cells were permeabilized for 10 min in PBS containing 0.1% Triton®X (PBS-T). Unspecific binding was blocked with 10% normal goat serum (Vector Laboratories) in PBS-T containing 0.3 M glycine. Cells were first incubated in primary antibodies [mouse tyrosine hydroxylase (1:200; Millipore), rabbit TNF-α (1:200; Abcam) and rabbit COX-2 (1:500; Abcam)] overnight at 4°C before incubation in 1% normal goat serum in PBS-T with secondary antibodies [goat anti-mouse Cy3 (1:200; Jackson ImmunoResearch) and goat anti-rabbit AlexaFluor® 488 (1:300; Molecular Probes)] for 1 h at room temperature. The coverslips were mounted, sealed and imaged by fluorescent microscopy at the same setting.
Statistical analysis
All values are expressed as means ± SEM unless stated otherwise. Differences between means were analysed using Student’s t-test (two groups) or the one-way ANOVA (more than two groups). When ANOVA showed significant differences, pair-wise comparisons between means were assessed using the Newman–Keuls post hoc test. Pearson correlation coefficient was used for correlation analyses, and the Pearson’s chi-square test for comparison of categorical data (>2 × 2 table). Concerning CSF results, a receiver-operating characteristic curve was used to calculate the relationship between sensitivity and specificity for Parkinson’s disease versus healthy controls. Null hypothesis was rejected at a 0.05 level. All analyses were performed using SPSS for Windows® software.
Results
S100B is upregulated in substantia nigra of patients with Parkinson’s disease
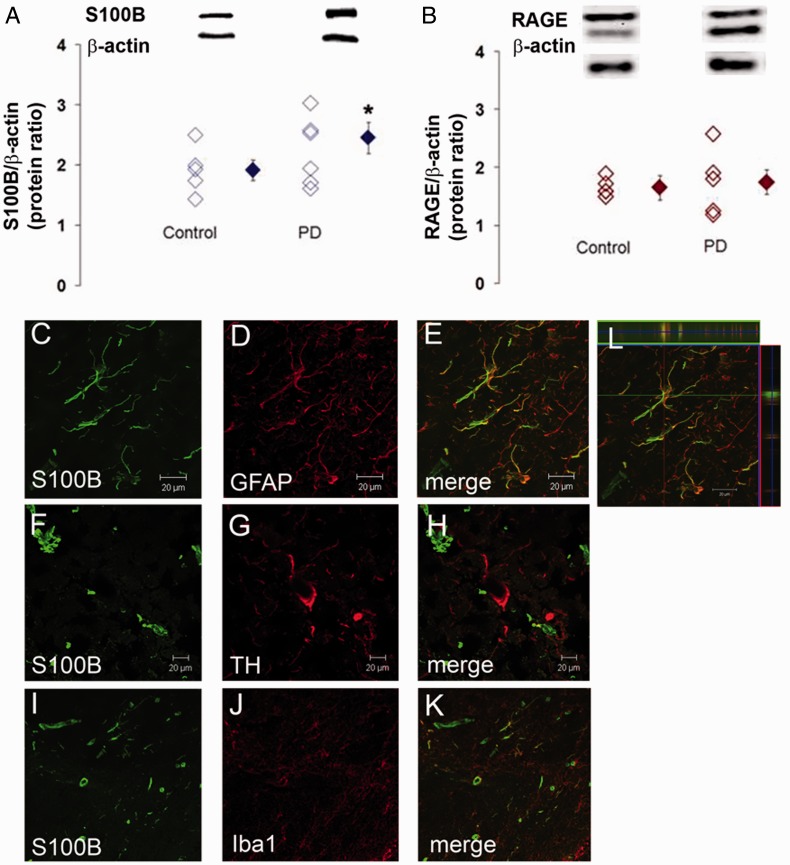
Immunostaining for S100B and RAGE protein in post-mortem midbrain slices of Parkinson’s disease and control subjects revealed significantly higher S100B protein levels in Parkinson’s disease (Fig. 1A). S100B was mainly localized in GFAP-positive astrocytes (Fig. 1C–E and L). We found S100B-positive structures neither in tyrosine hydroxylase-positive/Nissl-stained neurons (Fig. 1F–H) nor in microglia (Fig. 1I–K). RAGE protein levels were not significantly different between Parkinson’s disease and control cases (Fig. 1B).
Figure 1.
S100B and RAGE protein in the substantia nigra of patients with Parkinson’s disease. Using western blotting, substantia nigra S100B protein levels are, as a mean, higher in six samples of Parkinson’s disease (PD), compared with five control samples (A), whereas RAGE protein levels are comparable (B). Double immunofluorescence studies reveal that S100B (green) co-localizes with GFAP, an astrocytic marker (red; C–E and a 3D inset, L). S100B is not abundantly expressed in tyrosine hydroxylase neurons (red; F–H) but can be found in single calcium-binding adaptor molecule 1 (Iba1)-positive cells (red; I–K).
S100B levels are increased in cerebrospinal fluid of patients with Parkinson’s disease
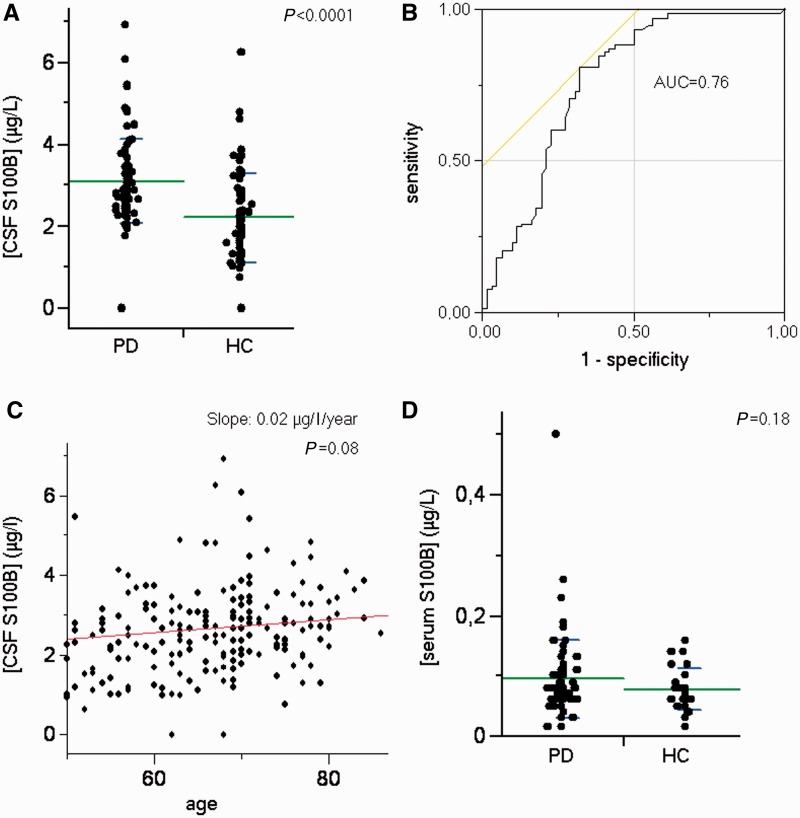
Measurement of S100B CSF and serum levels revealed that in CSF S100B levels were higher in patients with Parkinson’s disease compared with controls (3.10 versus 2.20 µg/l; P < 0.0001; Table 1 and Fig. 2A). The area under the receiver operating characteristic curve as a measure for the discrimination between Parkinson’s disease and controls equalled 0.76 (Fig. 2B), indicating a moderate discriminative effect. CSF S100B levels tended to be higher with higher age (0.02 µg/l/year; P = 0.08; Fig. 2C). Serum S100B levels were not significantly different between patients with Parkinson’s disease and controls (Fig. 2D). CSF and serum S100B levels were neither associated with age at disease onset nor with Hoehn and Yahr scores (a measure of disease stage). Gender was neither associated with CSF (P = 0.67) nor with serum S100B levels (P = 0.14). Genotype analysis of single-nucleotide polymorphism rs3788266 showed neither an association with CSF (P = 0.59) or serum S100B levels (P = 0.71), nor with occurrence of Parkinson’s disease diagnosis (P = 0.73).
Figure 2.
S100B levels in CSF and serum of patients with Parkinson’s disease. In the immunoassay, significantly higher S100B levels are detectable in CSF of 84 patients with Parkinson’s disease (PD) compared with 62 neurodegeneratively healthy subjects (HC) (A). The area under the curve (AUC) equals 0.76, indicating a moderate accuracy of the parameter in differentiating the groups (B). CSF S100B levels of patients with Parkinson’s disease and healthy subjects tend to be higher with higher age (C). In serum, S100B levels do not significantly differentiate between Parkinson’s disease and healthy subjects (D). Note also the substantially lower S100B levels in serum compared with CSF (A and D).
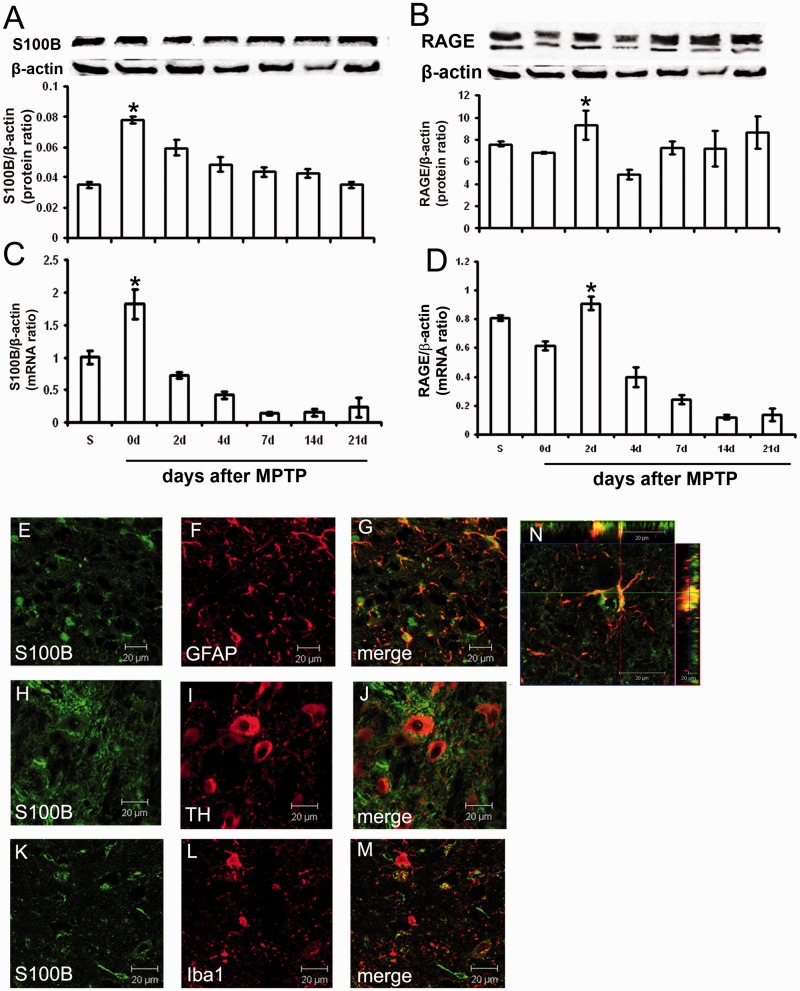
S100B and RAGE RNA and protein levels are increased in the ventral midbrain area containing the substantia nigra pars compacta of MPTP-treated mice
Analysis of messenger RNA and protein expression of S100B and RAGE in the ventral midbrain (the brain region that contains the substantia nigra pars compacta) demonstrated that S100B messenger RNA and protein levels increased immediately after MPTP administration and then returned gradually back to baseline levels (Fig. 3A and C). S100B messenger RNA and protein levels were increased at Day 0 after MPTP administration and RAGE messenger RNA and protein levels at Day 2 after MPTP administration. This indicates that induction of RAGE expression occurs later than S100B expression (Fig. 3B and D). During the following days, S100B and RAGE messenger RNA levels dropped below baseline levels, and protein levels returned to baseline levels.
Figure 3.
S100B and RAGE messenger RNA and protein in mouse ventral midbrain area containing the substantia nigra after MPTP. In mice, real-time polymerase chain reaction and western blot tests indicate that S100B messenger RNA and protein levels are increased directly at Day 0 after MPTP treatment. Within 3 weeks, S100B protein levels returned back to base levels, whereas messenger RNA levels dropped even below base levels (A and C). RAGE protein levels are increased 2 days after MPTP treatment and then drop back to base levels, whereas RAGE messenger RNA levels drop below base levels (B and D). Double immunofluorescence reveals that S100B protein (green) co-localizes with GFAP, an astrocytic marker (red; E–G and an enlarged 3D inset, N). S100B is not detectable in tyrosine hydroxylase neurons (red; H–J) but can be found in single ionized calcium-binding adaptor molecule 1 (Iba1)-positive cells (red; K–M). Data are mean ± SEM for four to six mice per group. *P < 0.05 compared with saline (Newman–Keuls post hoc test).
Immunohistochemistry revealed that S100B was mainly localized in astrocytes (Fig. 3E–G, 3D image in Fig. 3N), confirming previously published results (Van Eldik and Zimmer, 1987; Sorci et al., 2010). S100B was rarely found in microglia in our studies (Fig. 3K–M) and was absent in tyrosine hydroxylase-positive neurons (Fig. 3H–J).
Toxicity of MPTP is reduced in S100B-ablated mice
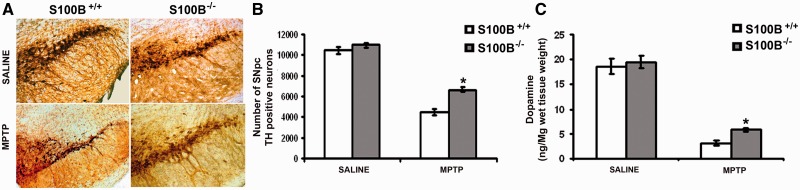
Using stereology, significantly more tyrosine hydroxylase-positive (54.4%) and Nissl-stained (89.7%) neurons survived in S100B−/− than in S100B+/+ mice after MPTP treatment (42.0% and 59.5%, respectively, P < 0.001; Fig. 4B and Supplementary Table 1). Correspondingly, the density of tyrosine hydroxylase-positive fibres in the striatum was decreased to 36% in S100B−/− MPTP-treated mice (compared with saline-treated mice), but to 14% in S100B+/+ MPTP-treated mice (Supplementary Table 1). Numbers of tyrosine hydroxylase-positive neurons and Nissl-stained cells were comparable between saline-treated S100B−/− and S100B+/+ mice (Fig. 4B and Supplementary Table 1).
Figure 4.
Genetic ablation of S100B rescues dopaminergic neurons from MPTP toxicity. Three weeks after saline treatment, numbers of tyrosine hydroxylase-positive neurons in the substantia nigra pars compacta are comparable between S100B−/− (knockout) and S100B+/+ (wild-type) mice when assessed with stereology (A, upper panels and B; Supplementary Table 1). However, S100B−/− mice show higher numbers of tyrosine hydroxylase-positive neurons in the substantia nigra pars compacta than do S100B+/+ mice, indicating a lowered vulnerability of the S100B−/− strain to MPTP (A, lower panels and B; Supplementary Table 1). Accordingly, striatal dopamine depletion as assessed with high-performance liquid chromatography 3 weeks after MPTP is less severe in S100B−/− mice than in S100B+/+ mice (C and Supplementary Table 2). Data are mean ± SEM for six mice per group. *P < 0.001 compared with MPTP-treated S100B+/+ mice (Newman–Keuls post hoc test).
To assess whether ablation of S100B influences striatal dopaminergic dysfunction after MPTP treatment, we determined levels of dopamine, 3,4-dihydroxyphenylacetic acid and homovanillic acid in the striata of S100B+/+ and S100B−/− mice after MPTP administration using high-performance liquid chromatography. Loss of striatal dopamine was significantly less pronounced in MPTP-treated S100B−/− mice when compared with MPTP-treated S100B+/+ mice (Fig. 4C and Supplementary Table 2).
No impairment of MPTP metabolism due to ablation of S100B
The main factor in MPTP-induced toxicity is its conversion to MPP+. Measurement of striatal MPP+ levels in S100B+/+ and S100B−/− mice, however, revealed comparable results (Supplementary Table 3).
MPTP toxicity is S100B mediated via RAGE and tumour necrosis factor-α in the mouse midbrain
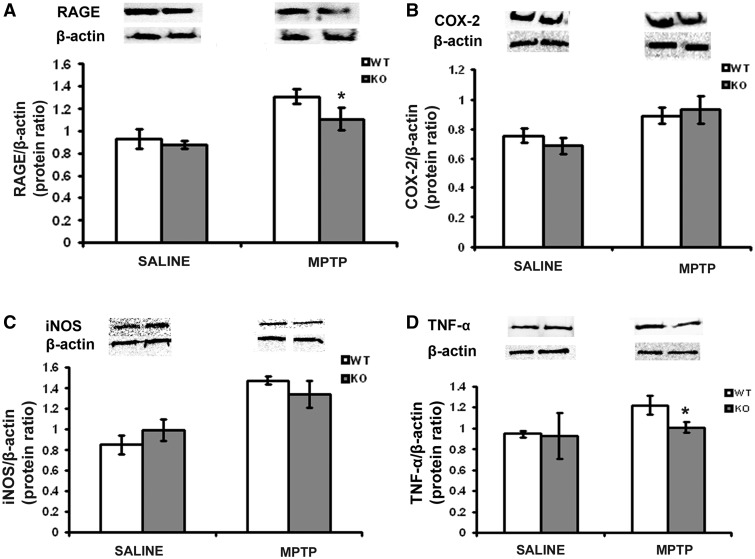
Assessment of the influence of S100B ablation on MPTP-induced effects revealed significantly lower RAGE protein levels in the midbrain of S100B−/− mice compared with S100B+/+ mice after MPTP administration (Fig. 5A). Comparable results were obtained for TNF-α (Fig. 5D), with TNF-α levels being lower in S100B−/− mice than in S100B+/+ mice after MPTP administration. COX-2 and inducible nitric oxide synthase protein levels were not significantly different between the mouse strains after MPTP administration (Fig. 5B and C).
Figure 5.
S100B-mediated MPTP toxicity via RAGE and TNF-α in the mouse midbrain area containing the substantia nigra pars compacta. In the immunoblot, ventral midbrain area containing the substantia nigra pars compacta MPTP-induced RAGE (A) and TNF-α protein levels (D) are lower in S100B−/− (knockout, KO) mice compared with S100B+/+ (wild-type, WT) mice. In contrast, protein levels of COX-2 (B) and inducible nitric oxide synthase (iNOS) (C) are comparable. Data are mean ± SEM for four to six mice per group. *P < 0.001 compared with MPTP-treated S100B+/+ mice (Newman–Keuls post hoc test).
Tumour necrosis factor-α is induced via RAGE in SH-SY5Y cells
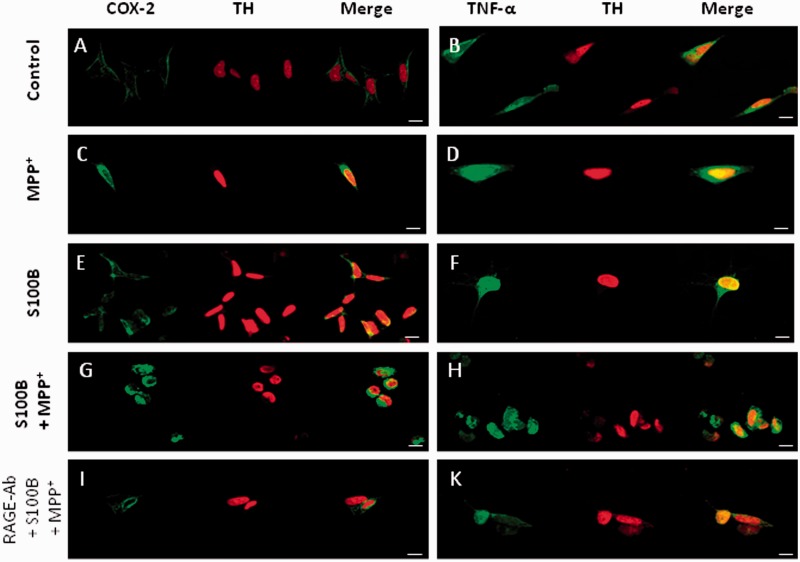
Treatment of SH-SY5Y cells with S100B and/or MPP+ led to increased staining intensity of COX-2 (Fig. 6C, E and G) and TNF-α (Fig. 6D, F and H) as visualized by confocal microscopy. Addition of RAGE neutralizing antibody (Fig. 6I and K) led to an attenuation of this intensity.
Figure 6.
TNF-α is induced via RAGE in SH-SY5Y cells. COX-2 (A) and TNF-α (B) immunocytochemistry in non-stimulated SH-SY5Y cells shows only faint staining, indicating low cytosolic levels of these proteins under resting conditions. Incubation with MPP+ leads to an intense COX-2 (C) and TNF-α (D) staining of the cytoplasm. Incubation with S100B leads to even more intense staining against COX-2 (E) and TNF-α (F). Co-incubation of S100B and a neutralizing RAGE antibody result in reduced COX-2 (I) and TNF-α (K) staining intensity (scale bars = 20 μm).
MPTP toxicity is S100B mediated via microgliosis
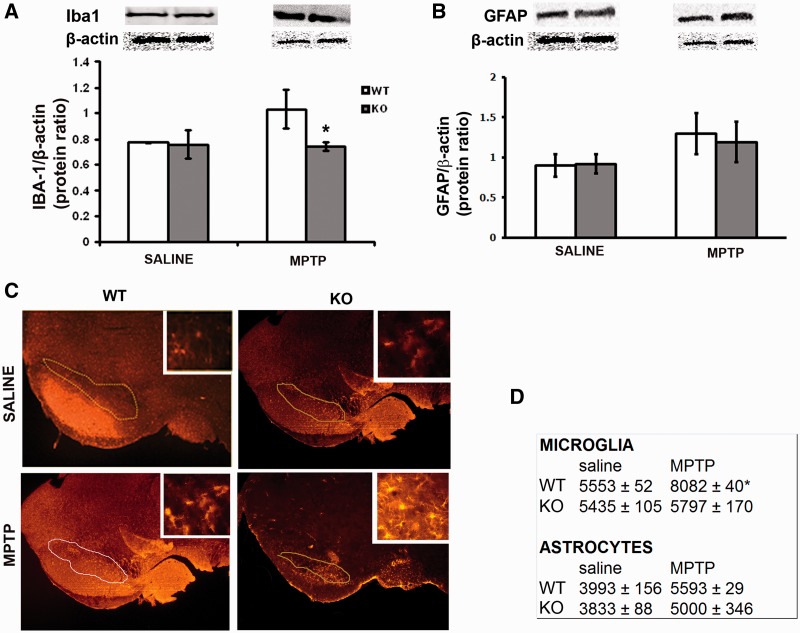
As S100B response is linked to inflammation, we assessed whether the protective effect of S100B ablation is associated with a reduction of MPTP-induced glial response. Mouse ventral midbrain area containing the substantia nigra pars compacta of S100B+/+ and S100B−/− mice after MPTP treatment was either prepared for western blotting or for immunostaining. They were stained for ionized calcium-binding adaptor molecule 1, a specific marker for microglial cells, and GFAP, a specific marker for astrocytes (Fig. 7C). When using western blot, ionized calcium-binding adaptor molecule 1 levels in the ventral midbrain containing the substantia nigra pars compacta were significantly higher in S100B+/+ than in S100B−/− mice (Fig. 7A). Similarly, stereological counting revealed a significantly higher number of ionized calcium-binding adaptor molecule 1-positive cells in the substantia nigra pars compacta of S100B+/+ mice than in the substantia nigra pars compacta of S100B−/− mice (Fig. 7D). Although astroglial cell count increased in both strains 3 weeks after MPTP treatment, GFAP protein levels and numbers of GFAP-positive cells did not differ between S100B+/+ and S100B−/− mice (Fig. 7B and D).
Figure 7.
Microglial and astrocytic markers in the ventral midbrain area containing the substantia nigra pars compacta after MPTP. In western blot, ionized calcium-binding adaptor molecule 1 (Iba1) levels in the ventral midbrain area containing the substantia nigra pars compacta of S100B+/+ [wild-type (WT)] mice are significantly increased 2 days after MPTP treatment (A and D), whereas they remain fairly unchanged in S100B−/− mice. Immunostaining reveals microgliosis in S100B+/+ mice, whereas there is no significant infiltration of microglia in S100B−/− [knockout (KO)] mice (C and D). Markers and counts of astrocytes show no differences between S100B+/+ and S100B−/− mice (D). Data are mean ± SEM for four to six mice per group. *P < 0.001 compared with MPTP-treated S100B+/+ mice (Newman–Keuls post hoc test).
Discussion
Elevated S100B level has been shown to be involved in neurotoxic pathways (Mrak et al., 1996; Rothermundt et al., 2003). Therefore, it is tempting to speculate that elevated S100B levels might be related to Parkinson’s disease-associated disease processes. This hypothesis is corroborated by recent results indicating that astrocytes—the main source of S100B—are fundamentally involved in the pathophysiology of Parkinson’s disease. Astrocytes can endocytose α-synuclein proteins secreted from neurons, can often have α-synuclein-positive inclusions in regions where Lewy bodies occur and, as a consequence of this process, can initiate inflammatory responses (Braak et al., 2007; Lee et al., 2011). Although there is no direct evidence for an interaction of amyloidogenic pathways and increased S100B expression in Parkinson’s disease, it is suggestive that such mechanisms lead to a vicious cycle with, hypothetically, increased intracellular nitric oxide and calcium burden, as well as apoptosis (Hu et al., 1997; Iuvone et al., 2007).
In this study, we detected increased S100B protein levels in the substantia nigra of patients with Parkinson’s disease, as well as in the ventral midbrain containing the substantia nigra pars compacta of MPTP-treated mice, a model for Parkinson’s disease. Elevated S100B messenger RNA levels were also seen in the same region, suggesting that increased expression of S100B may contribute to Parkinson’s disease pathogenesis. These changes occurred immediately after treatment with MPTP, suggesting that S100B upregulation could be the trigger for activation of other downstream molecules involved in the pathogenesis of Parkinson’s disease. Over time, protein levels returned to baseline, and messenger RNA levels even dropped below baseline. This indicates that these organisms are able to counteract the initial reaction simply by downregulation of the expression pathways. This can lead, despite increasing numbers of astrocytes at the lesion site, to relatively stable S100B levels that are close to baseline. In Parkinson’s disease, these regulatory mechanisms seem to be corrupted. This is corroborated by increased S100B protein levels in the substantia nigra and in the CSF of patients with Parkinson’s disease, compared with controls. How these regulation mechanisms are affected is a matter of further research.
The MPTP model leads to rapid and immediate cell death compared with cell death in human Parkinson’s disease, even when a relatively ‘subacute’ regimen of MPTP application is chosen (5 × 30 mg/kg intraperitoneally) (Tatton and Kish, 1997). Therefore, extrapolation of data from the MPTP model to human Parkinson’s disease requires particular caution. Nevertheless, this model is useful for indicating processes that might be involved in the pathogenesis and potential treatment strategies that, as in this study, need to be evaluated in the human disease condition (Langston et al., 1983; Langston and Irwin, 1986).
In the brain, trophic and toxic effects of extracellular S100B are mediated by RAGE (Hofmann et al., 1999). RAGE, a 45-kDa cell surface multi-ligand receptor, is found on neurons and microglial cells (Adami et al., 2004; Bianchi et al., 2011; Villarreal et al., 2011) and is involved in pro-inflammatory responses by activation of nuclear factor-κB (Yan et al., 1994; Villarreal et al., 2011), including the expression of interleukin 6 and TNF-α (Basta et al., 2002). In the current study, genetic ablation of S100B reduced levels not only of RAGE but also of TNF-α, as shown by western blotting. An increase in levels of the pro-inflammatory cytokine TNF-α is seen as a reaction to cell death as part of the inflammatory cascade. RAGE has been correlated to enhanced release of TNF-α and nuclear factor-κB and the activation of the Janus kinase-signal transducer and activator of transcription (STAT) pathway (Sriram et al., 2004). Furthermore, RAGE activation or the Janus kinase-2/STAT3 pathway, which can be activated through RAGE, is followed by an increased expression of COX-2 and inducible nitric oxide synthase (Neumann et al., 1999; Huang et al., 2001). These hypotheses for the action of RAGE are strengthened by our findings that both RAGE and S100B messenger RNA are increased in the MPTP mouse model of Parkinson’s disease. As we did not detect a relevant difference of COX-2 and inducible nitric oxide synthase protein levels after MPTP treatment in S100B−/− compared with S100B+/+ mice, we suggest that S100B-associated neurodegeneration is primarily mediated by the RAGE/TNF-α pathway. It is likely that COX-2 and inducible nitric oxide synthase are too far downstream in the S100B-mediated pathway, and thus, we were not able to detect any significant changes when using western blotting as a relatively crude quantification method. Our immunocytochemistry experiments (Fig. 6) make it indeed tempting to speculate that not only TNF-α but also COX-2 is, through the RAGE pathway, involved in central S100B action. COX-2 and inducible nitric oxide synthase are markers of neuroinflammation and oxidative stress, respectively. COX-2 activation has been shown to be involved in Parkinson’s disease neurodegeneration (Knott et al., 2000), and its activation leads to an increase of reactive oxygen species and to a release of pro-inflammatory cytokines such as TNF-α and interleukin 1 (Teismann et al., 2003). S100B has been shown to upregulate the expression of COX-2 in microglia via the Cdc42/Rac1–JNK and a Ras–Rac1–nuclear factor-κB pathway. This requires its binding to RAGE (Bianchi et al., 2007). Ablation of RAGE also led to a neuroprotective effect associated with reduced activation of nuclear factor-κB in the MPTP model of Parkinson’s disease (Teismann et al., 2012), indicating a causative influence of RAGE in this model.
We found a significant neuroprotective effect that, however, did not lead to a complete protection of nigral dopaminergic neurons. Two hypotheses of incomplete protection by S100B ablation can be considered. First, S100B ablation yielded only incomplete protection, as it did not lead to an attenuation of COX-2 and inducible nitric oxide synthase in vivo, both of which have been shown to be contributors to cell death in the MPTP model (Liberatore et al., 1999; Teismann et al., 2003). As these pathways still seem to be active, they may contribute to the cell degeneration, and thus, only a partial protection could be achieved. Second, it has been reported that low levels of S100B exert neuroprotective effects, thus promoting neuronal survival (Bianchi et al., 2007). Consequently, the beneficial and deleterious effects of S100B may have been ablated in a total knockout model. It would be interesting to see whether S100B knockdown leads to an enhanced cell survival.
The effect of S100B on Parkinson’s disease-related neurodegeneration may also be indirectly mediated. Our data indicate that genetic ablation of S100B reduces microglial response, strengthening the hypothesis that S100B activation is involved in the neuroinflammation seen in Parkinson’s disease. This could theoretically also be explained by a reduced requirement for microglia to remove cell debris after neuronal loss, which was less severe in S100B−/− mice (46% in S100B−/− mice versus 58% in S100B+/+ mice, see ‘Results’ section). However, we argue that the relatively small difference in neuronal loss between the strains cannot explain the relatively large difference in microgliosis observed in these strains (6.7% versus 45.5% increase of microglial cells after MPTP treatment; Fig. 7).
We detected higher CSF S100B levels in patients with Parkinson’s disease compared with controls, reaching an area under the curve of 0.76 in discriminating these two cohorts. This makes the protein a putative marker candidate that could be included in a Parkinson’s disease biomarker array. The facts that (i) S100B levels are about 20-fold higher in the CSF than in the serum and (ii) S100B exerts neurotoxic effects when increasing beyond a certain threshold (Hu et al., 1997; Li et al., 2000; Bianchi et al., 2007) underline the relevance of the findings from the human post-mortem and animal experiments presented here. However, it should be noted that a large proportion of patients with Parkinson’s disease had relatively low control-like CSF S100B levels and, vice versa, some controls had high CSF S100B levels (Fig. 2A). This may, at least partly, be explained by the fact that Parkinson’s disease, as a phenotype, consists of a (yet undefined) number of endophenotypes. Only some may develop clinical symptoms because of brain inflammation in general, and S100B dysregulation in particular.
Elevated CSF S100B levels have also been described in several short- and long-term brain pathologies, e.g. in Alzheimer’s disease (Petzold et al., 2003), frontotemporal lobar degeneration (Petzold et al., 2003), schizophrenia (Steiner et al., 2006), short-term brain injury (Kleindienst et al., 2010) and inflammatory diseases (e.g. Petzold et al., 2009). Interestingly, one study described elevated CSF S100B levels only in early stages of Alzheimer’s disease (but not in later stages). This led the authors to suggest that S100B plays a role in the initiation and/or facilitation of plaque formation (Peskind et al., 2001). Another study did not find significant differences of CSF S100B levels between patients with Alzheimer’s disease or dementia with Lewy bodies and controls (Mollenhauer et al., 2005).
If these CSF studies, the results by Mokuno et al. (1983) and our results are taken together, it seems plausible that patients with Parkinson’s disease tend to have higher CSF S100B levels compared with patients with amyloid β pathology, underscoring the relevance of S100B in this disease entity.
Serum S100B levels were not significantly different between patients with Parkinson’s disease and controls. This is in line with a recent publication showing comparable S100B levels between 40 patients with Parkinson’s disease and controls (Schaf et al., 2005). Therefore, serum S100B does not seem to be helpful as a diagnostic blood marker in Parkinson’s disease.
We found neither an association of single-nucleotide polymorphism rs3788266 with Parkinson’s disease occurrence nor with S100B levels. The polymorphism rs3788266 is, to our knowledge, the only known single-nucleotide polymorphism that clearly influences S100B levels and is associated with dopaminergic dysfunction (Liu et al., 2005; Roche et al., 2007). Our results argue against a crucial involvement of rs3788266 in the aetiology of Parkinson’s disease. However, the genetic results found in our assessed population must be interpreted with caution because of sample size and need confirmation in larger cohorts. Interestingly, in the model of MPTP where the neurodegenerative insult occurs rapidly and only once (in contrast to the neurodegenerative process underlying human Parkinson’s disease), both S100B and RAGE messenger RNA levels initially increased before falling below baseline while protein levels returned to baseline. This suggests that in predominantly healthy organisms such as the mice used here, there seems to be a form of counter regulation of the S100B–RAGE pathway to keep the system stable.
External factors (e.g. newly attracted astrocytes that further increase local S100B levels) may also influence maintenance of the underlying inflammatory reaction contributing to neurodegeneration. These latter mechanisms seem of particular interest for Parkinson’s disease pathophysiology in the light of the results presented here and the finding of Bierhaus et al. (2001), who showed that the RAGE-dependent activation of nuclear factor-κB may contrast with nuclear factor-κB activation mediated by pro-inflammatory cytokines by being a non–self-limiting process.
In conclusion, the picture emerging from our studies advocates a causative role of S100B in the pathogenesis of Parkinson’s disease. The results particularly indicate that binding of S100B to RAGE may lead to neurodegeneration and the activation of the inflammatory cascade, thus potentially contributing to the amplification of inflammation in Parkinson’s disease. The present study also suggests that S100B might be a viable target to generate new compounds aimed at slowing the progression of the disease.
Funding
This work was supported by Parkinson's Disease Foundation (IRGP 06 – 07), Royal Society (2006/R1) and Wellcome Trust (WT080782MF).
Supplementary material
Supplementary material is available at Brain online.
Acknowledgements
The authors are grateful to the staff of the Medical Research Facility for their help with the animal care. They also thank Professor Graeme Nixon for his assistance and help in the preparation of this manuscript.
Glossary
Abbreviations
- COX
cyclooxygenase
- MPP+
1-methyl-4-phenylpyridinium
- MPTP
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- RAGE
receptor for advanced glycation endproducts
- TNF
tumour necrosis factor
References
- Adami C, Bianchi R, Pula G, Donato R. S100B-stimulated NO production by BV-2 microglia is independent of RAGE transducing activity but dependent on RAGE extracellular domain. Biochim Biophys Acta. 2004;1742:169–77. doi: 10.1016/j.bbamcr.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Basta G, Lazzerini G, Massaro M, Simoncini T, Tanganelli P, Fu C, et al. Advanced glycation end products activate endothelium through signal-transduction receptor RAGE: a mechanism for amplification of inflammatory responses. Circulation. 2002;105:816–22. doi: 10.1161/hc0702.104183. [DOI] [PubMed] [Google Scholar]
- Bianchi R, Adami C, Giambanco I, Donato R. S100B binding to RAGE in microglia stimulates COX-2 expression. J Leukoc Biol. 2007;81:108–18. doi: 10.1189/jlb.0306198. [DOI] [PubMed] [Google Scholar]
- Bianchi R, Kastrisianaki E, Giambanco I, Donato R. S100B protein stimulates microglia migration via RAGE-dependent up-regulation of chemokine expression and release. J Biol Chem. 2011;286:7214–26. doi: 10.1074/jbc.M110.169342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierhaus A, Schiekofer S, Schwaninger M, Andrassy M, Humpert PM, Chen J, et al. Diabetes-associated sustained activation of the transcription factor nuclear factor-kappaB. Diabetes. 2001;50:2792–808. doi: 10.2337/diabetes.50.12.2792. [DOI] [PubMed] [Google Scholar]
- Braak H, Sastre M, Del Tredici K. Development of alpha-synuclein immunoreactive astrocytes in the forebrain parallels stages of intraneuronal pathology in sporadic Parkinson’s disease. Acta Neuropathol. 2007;114:231–41. doi: 10.1007/s00401-007-0244-3. [DOI] [PubMed] [Google Scholar]
- Businaro R, Leone S, Fabrizi C, Sorci G, Donato R, Lauro GM, et al. S100B protects LAN-5 neuroblastoma cells against Abeta amyloid-induced neurotoxicity via RAGE engagement at low doses but increases Abeta amyloid neurotoxicity at high doses. J Neurosci Res. 2006;83:897–906. doi: 10.1002/jnr.20785. [DOI] [PubMed] [Google Scholar]
- Donato R. Functional roles of S100 proteins, calcium-binding proteins of the EF-hand type. Biochim Biophys Acta. 1999;1450:191–231. doi: 10.1016/s0167-4889(99)00058-0. [DOI] [PubMed] [Google Scholar]
- Halliday GM, Stevens CH. Glia: initiators and progressors of pathology in Parkinson’s disease. Mov Disord. 2011;26:6–17. doi: 10.1002/mds.23455. [DOI] [PubMed] [Google Scholar]
- Hofmann MA, Drury S, Fu C, Qu W, Taguchi A, Lu Y, et al. RAGE mediates a novel proinflammatory axis: a central cell surface receptor for S100/calgranulin polypeptides. Cell. 1999;97:889–901. doi: 10.1016/s0092-8674(00)80801-6. [DOI] [PubMed] [Google Scholar]
- Hu J, Ferreira A, Van Eldik LJ. S100beta induces neuronal cell death through nitric oxide release from astrocytes. J Neurochem. 1997;69:2294–301. doi: 10.1046/j.1471-4159.1997.69062294.x. [DOI] [PubMed] [Google Scholar]
- Huang JS, Guh JY, Chen HC, Hung WC, Lai YH, Chuang LY. Role of receptor for advanced glycation end-product (RAGE) and the JAK/STAT-signaling pathway in AGE-induced collagen production in NRK-49F cells. J Cell Biochem. 2001;81:102–13. doi: 10.1002/1097-4644(20010401)81:1<102::aid-jcb1027>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Huttunen HJ, Kuja-Panula J, Sorci G, Agneletti AL, Donato R, Rauvala H. Coregulation of neurite outgrowth and cell survival by amphoterin and S100 proteins through receptor for advanced glycation end products (RAGE) activation. J Biol Chem. 2000;275:40096–105. doi: 10.1074/jbc.M006993200. [DOI] [PubMed] [Google Scholar]
- Iuvone T, Esposito G, De Filippis D, Bisogno T, Petrosino S, Scuderi C, et al. Cannabinoid CB1 receptor stimulation affords neuroprotection in MPTP-induced neurotoxicity by attenuating S100B up-regulation in vitro. J Mol Med. 2007;85:1379–92. doi: 10.1007/s00109-007-0233-y. [DOI] [PubMed] [Google Scholar]
- Kerkhoff C, Klempt M, Sorg C. Novel insights into structure and function of MRP8 (S100A8) and MRP14 (S100A9) Biochim Biophys Acta. 1998;1448:200–11. doi: 10.1016/s0167-4889(98)00144-x. [DOI] [PubMed] [Google Scholar]
- Kleindienst A, Meissner S, Eyupoglu IY, Parsch H, Schmidt C, Buchfelder M. Dynamics of S100B release into serum and cerebrospinal fluid following acute brain injury. Acta Neurochir Suppl. 2010;106:247–50. doi: 10.1007/978-3-211-98811-4_46. [DOI] [PubMed] [Google Scholar]
- Knott C, Stern G, Wilkin GP. Inflammatory regulators in Parkinson's disease: iNOS, lipocortin-1, and cyclooxygenases-1 and -2. Mol Cell Neurosci. 2000;16:724–39. doi: 10.1006/mcne.2000.0914. [DOI] [PubMed] [Google Scholar]
- Kogel D, Peters M, Konig HG, Hashemi SM, Bui NT, Arolt V, et al. S100B potently activates p65/c-Rel transcriptional complexes in hippocampal neurons: clinical implications for the role of S100B in excitotoxic brain injury. Neuroscience. 2004;127:913–20. doi: 10.1016/j.neuroscience.2004.06.013. [DOI] [PubMed] [Google Scholar]
- Langston JW, Ballard P, Tetrud JW, Irwin I. Chronic Parkinsonism in humans due to a product of meperidine-analog synthesis. Science. 1983;219:979–80. doi: 10.1126/science.6823561. [DOI] [PubMed] [Google Scholar]
- Langston JW, Irwin I. MPTP: current concepts and controversies. Clin Neuropharmacol. 1986;9:485–507. [PubMed] [Google Scholar]
- Lee HJ, Suk JE, Patrick C, Bae EJ, Cho JH, Rho S, et al. Direct transfer of alpha-synuclein from neuron to astroglia causes inflammatory responses in synucleinopathies. J Biol Chem. 2011;285:9262–72. doi: 10.1074/jbc.M109.081125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lees AJ, Hardy J, Revesz T. Parkinson’s disease. Lancet. 2009;373:2055–66. doi: 10.1016/S0140-6736(09)60492-X. [DOI] [PubMed] [Google Scholar]
- Li Y, Barger SW, Liu L, Mrak RE, Griffin WS. S100beta induction of the proinflammatory cytokine interleukin-6 in neurons. J Neurochem. 2000;74:143–50. doi: 10.1046/j.1471-4159.2000.0740143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberatore GT, Jackson-Lewis V, Vukosavic S, Mandir AS, Vila M, McAuliffe WG, et al. Inducible nitric oxide synthase stimulates dopaminergic neurodegeneration in the MPTP model of Parkinson disease. Nat Med. 1999;5:1403–9. doi: 10.1038/70978. [DOI] [PubMed] [Google Scholar]
- Liu J, Shi Y, Tang J, Guo T, Li X, Yang Y, et al. SNPs and haplotypes in the S100B gene reveal association with schizophrenia. Biochem Biophys Res Commun. 2005;328:335–41. doi: 10.1016/j.bbrc.2004.12.175. [DOI] [PubMed] [Google Scholar]
- Maetzler W, Schmid SP, Wurster I, Liepelt I, Gaenslen A, Gasser T, et al. Reduced but not oxidized cerebrospinal fluid glutathione levels are lowered in Lewy body diseases. Mov Disord. 2011a;26:176–81. doi: 10.1002/mds.23358. [DOI] [PubMed] [Google Scholar]
- Maetzler W, Stapf AK, Schulte C, Hauser AK, Lerche S, Wurster I, et al. Serum and cerebrospinal fluid uric acid levels in lewy body disorders: associations with disease occurrence and amyloid-beta pathway. J Alzheimers Dis. 2011b;27:119–26. doi: 10.3233/JAD-2011-110587. [DOI] [PubMed] [Google Scholar]
- Mokuno K, Kato K, Kawai K, Matsuoka Y, Yanagi T, Sobue I. Neuron-specific enolase and S-100 protein levels in cerebrospinal fluid of patients with various neurological diseases. J Neurol Sci. 1983;60:443–51. doi: 10.1016/0022-510x(83)90155-7. [DOI] [PubMed] [Google Scholar]
- Mollenhauer B, Cepek L, Bibl M, Wiltfang J, Schulz-Schaeffer WJ, Ciesielczyk B, et al. Tau protein, Abeta42 and S-100B protein in cerebrospinal fluid of patients with dementia with Lewy bodies. Dement Geriatr Cogn Disord. 2005;19:164–70. doi: 10.1159/000083178. [DOI] [PubMed] [Google Scholar]
- Mrak RE, Sheng JG, Griffin WS. Correlation of astrocytic S100 beta expression with dystrophic neurites in amyloid plaques of Alzheimer's disease. J Neuropathol Exp Neurol. 1996;55:273–9. doi: 10.1097/00005072-199603000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muramatsu Y, Kurosaki R, Watanabe H, Michimata M, Matsubara M, Imai Y, et al. Expression of S-100 protein is related to neuronal damage in MPTP-treated mice. Glia. 2003;42:307–13. doi: 10.1002/glia.10225. [DOI] [PubMed] [Google Scholar]
- Neumann A, Schinzel R, Palm D, Riederer P, Munch G. High molecular weight hyaluronic acid inhibits advanced glycation endproduct-induced NF-kappaB activation and cytokine expression. FEBS Lett. 1999;453:283–7. doi: 10.1016/s0014-5793(99)00731-0. [DOI] [PubMed] [Google Scholar]
- Nishiyama H, Knopfel T, Endo S, Itohara S. Glial protein S100B modulates long-term neuronal synaptic plasticity. Proc Natl Acad Sci USA. 2002;99:4037–42. doi: 10.1073/pnas.052020999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peskind ER, Griffin WS, Akama KT, Raskind MA, Van Eldik LJ. Cerebrospinal fluid S100B is elevated in the earlier stages of Alzheimer's disease. Neurochem Int. 2001;39:409–13. doi: 10.1016/s0197-0186(01)00048-1. [DOI] [PubMed] [Google Scholar]
- Petzold A, Brettschneider J, Jin K, Keir G, Murray NM, Hirsch NP, et al. CSF protein biomarkers for proximal axonal damage improve prognostic accuracy in the acute phase of Guillain-Barre syndrome. Muscle Nerve. 2009;40:42–9. doi: 10.1002/mus.21239. [DOI] [PubMed] [Google Scholar]
- Petzold A, Jenkins R, Watt HC, Green AJ, Thompson EJ, Keir G, et al. Cerebrospinal fluid S100B correlates with brain atrophy in Alzheimer's disease. Neurosci Lett. 2003;336:167–70. doi: 10.1016/s0304-3940(02)01257-0. [DOI] [PubMed] [Google Scholar]
- Roche S, Cassidy F, Zhao C, Badger J, Claffey E, Mooney L, et al. Candidate gene analysis of 21q22: support for S100B as a susceptibility gene for bipolar affective disorder with psychosis. Am J Med Genet B Neuropsychiatr Genet. 2007;144B:1094–6. doi: 10.1002/ajmg.b.30556. [DOI] [PubMed] [Google Scholar]
- Rothermundt M, Peters M, Prehn JH, Arolt V. S100B in brain damage and neurodegeneration. Microsc Res Tech. 2003;60:614–32. doi: 10.1002/jemt.10303. [DOI] [PubMed] [Google Scholar]
- Schaf DV, Tort AB, Fricke D, Schestatsky P, Portela LV, Souza DO, et al. S100B and NSE serum levels in patients with Parkinson’s disease. Parkinsonism Relat Disord. 2005;11:39–43. doi: 10.1016/j.parkreldis.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Sorci G, Bianchi R, Riuzzi F, Tubaro C, Arcuri C, Giambanco I, et al. S100B protein, a damage-associated molecular pattern protein in the brain and heart, and beyond. Cardiovasc Psychiatry Neurol. 2010;2010, pii:656481. doi: 10.1155/2010/656481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sriram K, Benkovic SA, Hebert MA, Miller DB, O’Callaghan JP. Induction of gp130-related cytokines and activation of JAK2/STAT3 pathway in astrocytes precedes up-regulation of glial fibrillary acidic protein in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of neurodegeneration: key signaling pathway for astrogliosis in vivo? J Biol Chem. 2004;279:19936–47. doi: 10.1074/jbc.M309304200. [DOI] [PubMed] [Google Scholar]
- Steiner J, Bielau H, Bernstein HG, Bogerts B, Wunderlich MT. Increased cerebrospinal fluid and serum levels of S100B in first-onset schizophrenia are not related to a degenerative release of glial fibrillar acidic protein, myelin basic protein and neurone-specific enolase from glia or neurones. J Neurol Neurosurg Psychiatry. 2006;77:1284–7. doi: 10.1136/jnnp.2006.093427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatton NA, Kish SJ. In situ detection of apoptotic nuclei in the substantia nigra compacta of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated mice using terminal deoxynucleotidyl transferase labelling and acridine orange staining. Neuroscience. 1997;77:1037–48. doi: 10.1016/s0306-4522(96)00545-3. [DOI] [PubMed] [Google Scholar]
- Teismann P, Sathe K, Bierhaus A, Leng L, Martin HL, Bucala R, et al. Receptor for advanced glycation endproducts (RAGE) deficiency protects against MPTP toxicity. Neurobiol Aging. 2012;33:2478–90. doi: 10.1016/j.neurobiolaging.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teismann P, Tieu K, Choi DK, Wu DC, Naini A, Hunot S, et al. Cyclooxygenase-2 is instrumental in Parkinson’s disease neurodegeneration. Proc Natl Acad Sci USA. 2003;100:5473–8. doi: 10.1073/pnas.0837397100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Eldik LJ, Zimmer DB. Secretion of S-100 from rat C6 glioma cells. Brain Res. 1987;436:367–70. doi: 10.1016/0006-8993(87)91681-7. [DOI] [PubMed] [Google Scholar]
- Villarreal A, Aviles Reyes RX, Angelo MF, Reines AG, Ramos AJ. S100B alters neuronal survival and dendrite extension via RAGE-mediated NF-kappaB signaling. J Neurochem. 2011;117:321–32. doi: 10.1111/j.1471-4159.2011.07207.x. [DOI] [PubMed] [Google Scholar]
- Wu DC, Jackson-Lewis V, Vila M, Tieu K, Teismann P, Vadseth C, et al. Blockade of microglial activation is neuroprotective in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson disease. J Neurosci. 2002;22:1763–71. doi: 10.1523/JNEUROSCI.22-05-01763.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan SD, Schmidt AM, Anderson GM, Zhang J, Brett J, Zou YS, et al. Enhanced cellular oxidant stress by the interaction of advanced glycation end products with their receptors/binding proteins. J Biol Chem. 1994;269:9889–97. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.