Abstract
Transplantation of neural stem cells provides a promising therapy for stroke. Its efficacy, however, might be limited because of massive grafted-cell death after transplantation, and its insufficient capability for tissue repair. Interleukin 6 is a pro-inflammatory cytokine involved in the pathogenesis of various neurological disorders. Paradoxically, interleukin 6 promotes a pro-survival signalling pathway through activation of signal transducer and activator of transcription 3. In this study, we investigated whether cellular reprogramming of neural stem cells with interleukin 6 facilitates the effectiveness of cell transplantation therapy in ischaemic stroke. Neural stem cells harvested from the subventricular zone of foetal mice were preconditioned with interleukin 6 in vitro and transplanted into mouse brains 6 h or 7 days after transient middle cerebral artery occlusion. Interleukin 6 preconditioning protected the grafted neural stem cells from ischaemic reperfusion injury through signal transducer and activator of transcription 3-mediated upregulation of manganese superoxide dismutase, a primary mitochondrial antioxidant enzyme. In addition, interleukin 6 preconditioning induced secretion of vascular endothelial growth factor from the neural stem cells through activation of signal transducer and activator of transcription 3, resulting in promotion of angiogenesis in the ischaemic brain. Furthermore, transplantation of interleukin 6-preconditioned neural stem cells significantly attenuated infarct size and improved neurological performance compared with non-preconditioned neural stem cells. This interleukin 6-induced amelioration of ischaemic insults was abolished by transfecting the neural stem cells with signal transducer and activator of transcription 3 small interfering RNA before transplantation. These results indicate that preconditioning with interleukin 6, which reprograms neural stem cells to tolerate oxidative stress after ischaemic reperfusion injury and to induce angiogenesis through activation of signal transducer and activator of transcription 3, is a simple and beneficial approach for enhancing the effectiveness of cell transplantation therapy in ischaemic stroke.
Keywords: cytokine preconditioning, oxidative stress, angiogenesis, ischaemic stroke, transplantation
Introduction
Stem cell-based approaches hold much promise as potential novel treatments for stroke (Bliss et al., 2010; Lindvall and Kokaia, 2010). A variety of clinical trials have been performed and others are currently underway (Banerjee et al., 2011). Experimental studies have shown that transplantation of neural stem cells (NSCs) can restore function after ischaemic stroke by replacing neurons or by trophic actions, including neuroprotection, promotion of angiogenesis, immunomodulation and axonal plasticity (Bliss et al., 2010). Despite promising results, transplantation of NSCs is confronted with the problem of poor grafted-cell survival (1–3%) because of a hostile host-brain environment (Hicks et al., 2009; Nakagomi et al., 2009), which might be caused by production of reactive oxygen species after ischaemic reperfusion injury and host inflammatory response mediators (Lo et al., 2003). Moreover, the capacity of naïve NSCs might be insufficient to induce adequate tissue repair against severe ischaemic insult. To enhance the effectiveness of cell transplant therapy, several remedial approaches have been suggested, such as ex vivo gene modification of stem cells for overexpression of pro-survival signalling molecules or paracrine factors of interest (Wei et al., 2005; Liu et al., 2006). However, although these methods exhibit a better transplantation outcome, a more beneficial, simpler and safer approach might be needed for future clinical application.
Interleukin 6 (IL-6) is a pro-inflammatory cytokine that is involved in the pathogenesis of various neurological disorders, including stroke (Suzuki et al., 2009). Previous clinical studies in patients with ischaemic stroke have demonstrated a positive correlation between serum IL-6 levels and infarct volume and long-term poor outcome (Beamer et al., 1995). This finding suggests that IL-6 induces an excessive inflammatory response, which may aggravate ischaemic cerebral damage. In contrast, accumulating paradoxical experimental data have revealed that IL-6 can promote a pro-survival signalling pathway through activation of signal transducer and activator of transcription 3 (STAT3) (Lecour and James, 2011). Phosphorylated STAT3 forms dimers that, when translocated to the nucleus, bind to the specific promoters of target genes and induce expression of genes involved in cytoprotection and angiogenesis (Hirano et al., 2000; Brivanlou and Darnell, 2002). Moreover, our recent studies have shown enhanced neuroprotection against ischaemic stroke by STAT3 activation and progression of ischaemic cerebral insult by STAT3 inhibition (Jung et al., 2009, 2011). These findings support our study rationale that IL-6 preconditioning may induce reprogramming of NSCs and accelerate amelioration of ischaemic stroke after transplantation. Therefore, the purpose of the present study was to determine whether preconditioning with IL-6 enhances the effectiveness of cell transplantation therapy in ischaemic stroke. We also sought to elucidate the underlying mechanisms of IL-6 preconditioning in NSCs.
Materials and methods
Details beyond the descriptions here are given in the online Supplementary material.
Animals
All animals were treated in accordance with Stanford University guidelines, and the animal protocols were approved by Stanford University’s Administrative Panel on Laboratory Animal Care. Homozygous green fluorescent protein (GFP) transgenic mice [C57BL/6-transgenic (UBC-GFP)30Scha/J; The Jackson Laboratory] were bred with heterozygous manganese-superoxide dismutase (SOD2) transgenic mice (C57BL/6 background, backcrossed with C57BL/6 for >10 generations) (Maier et al., 2006) to generate heterozygous GFP transgenic mice and heterozygous SOD2/GFP double transgenic mice. These animals were used for isolation of NSCs. We also used wild-type C57BL/6 mice (The Jackson Laboratory) for middle cerebral artery occlusion models.
Isolation and culturing of foetal neural stem cells
NSCs were harvested from the heterozygous GFP transgenic mice or heterozygous SOD2/GFP double transgenic mice (SOD2-NSCs) as described (Blurton-Jones et al., 2009), with some modification. In brief, bilateral subventricular zones from post-natal Day 1 mouse brains were dissected in Dulbecco’s PBS (14040-182; Invitrogen) and mechanically dissociated. The cells were collected and resuspended in Neurobasal™-A medium (10888-022; Invitrogen) containing B-27 supplement (12587-010; Invitrogen), l-glutamine (25030-081; Invitrogen), 20 ng/ml murine fibroblast growth factor-basic (450-33; PeproTech) and 10 ng/ml murine epidermal growth factor (315-09; PeproTech). Cells were grown on a 10-cm plastic dish precoated with poly-l-ornithine hydrobromide (P3655-100MG; Sigma-Aldrich) and laminin (L2020-1MG; Sigma-Aldrich) at 37°C and 5% CO2 as adherent monolayers. The medium was changed every 2 days, and cells were passaged once a week. Cells that had been passaged 4–10 times were used for the experiments.
The NSCs were treated with IL-6 (I9646; Sigma-Aldrich) before the in vitro experiments or transplantation. IL-6 was added to the cell culture medium (final concentration: 20 ng/ml) for 24 h, followed by washing three times with Dulbecco’s PBS to remove IL-6 from the NSCs before experiments.
Treatment of the cultures with oxygen–glucose deprivation
We used oxygen–glucose deprivation and reoxygenation, an in vitro model that best mimics in vivo cerebral ischaemia-reperfusion. NSCs were subjected to oxygen–glucose deprivation by replacing the medium with a buffered salt solution without glucose. The plates were placed in an anaerobic chamber (Plas Labs) at 37°C. After 8 h, the medium was replaced with the culture medium with glucose, and the plates were returned to a 5% CO2/95% air incubator for various reoxygenation periods. For the in vitro studies, the NSC culture dishes were allocated to the experimental groups using a coin toss method before treatment. An investigator blinded to the treatment performed the experimental interventions and assessment of the outcome. The sample size was four per group in all the in vitro studies.
In situ detection of superoxide anion production
Early production of superoxide anions was investigated with the use of hydroethidine as previously described (Murakami et al., 1998). Production of superoxide anions was shown by oxidized hydroethidine as diffuse signals and small particles in the cytosol. For the in vitro study, 5 μM hydroethidine solution (D23107; Invitrogen) was added to the cell culture medium. The cells were incubated for 5 min, followed by fixation with 4% paraformaldehyde in PBS for 15 min. For the in vivo study, hydroethidine solution [200 μl of 1 mg/ml in 1% dimethyl sulphoxide with saline (D11347; Invitrogen)] was administered intravenously immediately after transplantation of the NSCs (n = 4 per group). Animals were killed 1 h after administration, and tissue sections were prepared as described in the immunofluorescent staining section (Supplementary material). For fluorescent double staining of hydroethidine signals and GFP, sections were incubated with rabbit anti-GFP (1:100; G10362; Invitrogen), followed by Alexa Fluor® 488-conjugated donkey anti-rabbit IgG (A21206; Invitrogen). Slides were covered with VECTASHIELD® mounting medium with 4′,6 diamidino-2-phenylindole (DAPI) (H-1500; Vector Laboratories). The sections were observed with a fluorescence microscope (Axioplan 2; Carl Zeiss), and oxidized hydroethidine fluorescence was examined at an excitation of 510 nm and emission of >580 nm and was quantified with ImageJ software (version 1.42q; NIH).
Transfection of small interfering RNA
NSCs that were 60% confluent were transfected with 20 nM stealth STAT3 small interfering RNA (Invitrogen), SOD2 small interfering RNA (Invitrogen) or non-functioning negative-control small interfering RNA (12935-400; Invitrogen) using Lipofectamine® RNAiMAX (13778; Invitrogen) according to the manufacturer’s protocols. The target sequence of the mouse-specific STAT3 small interfering RNA mixture was as follows: AAACGUGAGCGACUCAAACUGCCCU. The target sequence of the mouse-specific SOD2-small interfering RNA mixture was as follows: UACUGAAGGUAGUAAGCGUGCUCCC. After 48 h of incubation, the NSCs were used for various experiments and transplantation.
Capillary-like tube formation assay
We used BD BioCoat™ Angiogenesis Systems: Endothelial Cell Tube Formation (354149; BD Biosciences Pharmingen) according to the manufacturer’s instructions. Briefly, bEnd.3 cells (CRL-2299; ATCC), a polyoma middle T-transformed mouse brain endothelial cell line, were cultured at 3.5 × 105 cells/ml for 14 h with the following: (i) regular NSC culture medium (Neurobasal™-A); (ii) conditioned medium from non-preconditioned NSCs transfected with control small interfering RNA; (iii) conditioned medium from IL-6 preconditioned NSCs transfected with control-small interfering RNA; (iv) conditioned medium from preconditioned NSCs transfected with STAT3 small interfering RNA; or (v) conditioned medium from preconditioned NSCs with a goat anti-mouse neutralizing antibody to vascular endothelial growth factor (VEGF) (10 μg/ml) (AF-493-NA; R&D Systems). After incubation with Calcein AM solution (8 μg/ml) (354216; BD Biosciences Pharmingen) for 30 min, images were acquired by confocal microscopy (LSM 510; Carl Zeiss). Total tube length was measured in three randomly selected microscopic fields using ImageJ software as described (Rikitake et al., 2002).
Focal cerebral ischaemia
Adult male C57BL/6 mice (26–30 g) were subjected to transient focal cerebral ischaemia by intraluminal middle cerebral artery blockade with a suture, as described previously (Kim et al., 2009), with some modifications. The mice were anaesthetized with 2.0% isoflurane in 30% oxygen and 70% nitrous oxide using a face mask. The rectal temperature was controlled at 37°C with a homeothermic blanket. Physiological parameters were monitored throughout the surgeries. After a midline skin incision, the right external carotid artery was exposed, and its branches were electrocoagulated. A 6-0 monofilament nylon suture coated with silicon rubber (6023PK5Re; Doccol Corp) was introduced into the right internal carotid artery through the external carotid artery stump. After 45 min of middle cerebral artery occlusion, blood flow was restored by withdrawal of the suture. The animals were maintained in an air-conditioned room at 20°C with ad libitum access to food and water before and after surgery.
Development of neurological impairment was assessed immediately after surgery, and mice that showed circling towards the paretic side were included in the study. Forty-one (of 253) mice died within 6 h after surgery before allocation to the experimental groups. No mice died after this period. Animals were randomized using a coin toss method 6 h after surgery before treatment. No mice were excluded from the study after the allocation to the experimental groups. All the experimental interventions and the assessment of the outcome were performed by an investigator blinded to treatment.
Intracerebral transplantation
The NSCs were transplanted 6 h or 7 days after the onset of stroke, using a 10-μl Hamilton syringe with a 33 G needle attached to a stereotaxic apparatus (David Kopf Instruments). The mice were given three 1.0-μl deposits of single cell suspension in Dulbecco’s PBS (1 × 105 cells per μl) along the anterior–posterior axis into the cortex at these coordinates: (i) anterior–posterior, +1.0; medial–lateral, +2.0; dorsal–ventral, −1.0; (ii) anterior–posterior, −0.5; medial–lateral, +2.5; dorsal–ventral, −1.0; (iii) anterior–posterior, −2.0; medial–lateral, +2.5; dorsal–ventral, −1.0. These targets approximated the penumbra area in the cortex. Deposits were delivered at 0.5 μl/min, and the needle was left in situ for 5 min post-injection before being slowly removed.
In situ labelling of DNA fragmentation in transplanted neural stem cells
Every eighth section (160 μm apart) containing the graft region (anterior–posterior, −0.5; medial–lateral, +2.5; dorsal–ventral, −1.0) was chosen for staining using the in situ cell death detection kit, TMR red (n = 4 per group). The sections were then incubated with rabbit anti-GFP (1:100; G10362; Invitrogen) and mouse anti-nestin (1:100; AB353; Millipore), followed by Alexa Fluor® 488-conjugated donkey anti-rabbit IgG (A21206; Invitrogen) and Alexa Fluor® 647-conjugated donkey anti-mouse IgG (A31571; Invitrogen). Slides were covered with VECTASHIELD® mounting medium with DAPI. Cells positive for terminal deoxynucleotidyl transferase-mediated uridine 5′-triphosphate-biotin nick end labelling (TUNEL), also stained with GFP, were counted using unbiased computational stereology (fractionator method using Stereo Investigator software, MicroBrightfield), as described (Kelly et al., 2004). We also counted the number of TUNEL-positive host brain cells at the coordinates anterior–posterior, −0.5; medial–lateral, +3.0; dorsal–ventral, −1.0.
Measurement of infarct size
The brain sections were stained with haematoxylin and eosin (n = 8 per group). We estimated the cortical/striatal infarct size as a percentage of the ipsilateral cortex/striatum using the following formula: [(area of contralateral cortex/striatum) − (area of remaining ipsilateral cortex/striatum)/(area of contralateral cortex/striatum) × 100]. The area of both sides of the cortex and striatum was measured on six serial coronal sections per brain (1 mm apart), and the area of the infarct was quantified for these six levels using Adobe Photoshop (Adobe Systems).
Behavioural analysis
A rotarod test and modified neurologic severity scores were evaluated by two individuals blinded to the mouse-treatment status on the day of the stroke surgery (baseline), and 1, 7, 14, 21 and 28 days after stroke (n = 8 per group). The modified neurologic severity scores are the result of a battery of motor and coordination tests assessing the severity of neurological deficits on a graded scale ranging from 0 to 14, where 0 represents normal function and 14 represents maximal deficits as described (Chen et al., 2005).
With the rotarod test, after 3 days of training before the stroke surgery, the mice were placed on the rotarod cylinder (ENV-577 M; Med Associates Inc.), and the time the animals remained on the rotarod was recorded. The speed was slowly increased from 4 to 40 rpm within a period of 5 min. The trial was ended if the animal fell off the rungs or gripped the device and spun around for two consecutive revolutions. The maximum duration on the device was recorded with three rotarod measurements on the day of the stroke surgery. Rotarod test data are presented as percentages of the maximal duration compared with the internal baseline control.
Statistical analysis
Behavioural data were assessed using repeated measures ANOVA. We used Scheffé’s post hoc analysis of the rotarod test and analysed the modified neurologic severity scores using the Steel–Dwass test. For other experimental data, comparisons among multiple groups were performed with one-way ANOVA, followed by Scheffé's post hoc analysis. Comparisons between two groups were achieved with Student’s unpaired t-test. We used Pearson’s correlation coefficient and Spearman’s rank correlation to assess the correlation between functional recovery and infarct size. Data are expressed as median for the modified neurologic severity scores and mean (SD) for the other experiments. Significance was accepted with P < 0.05.
Results
IL-6 preconditioning upregulated SOD2 and conferred cytoprotection on neural stem cells in vitro
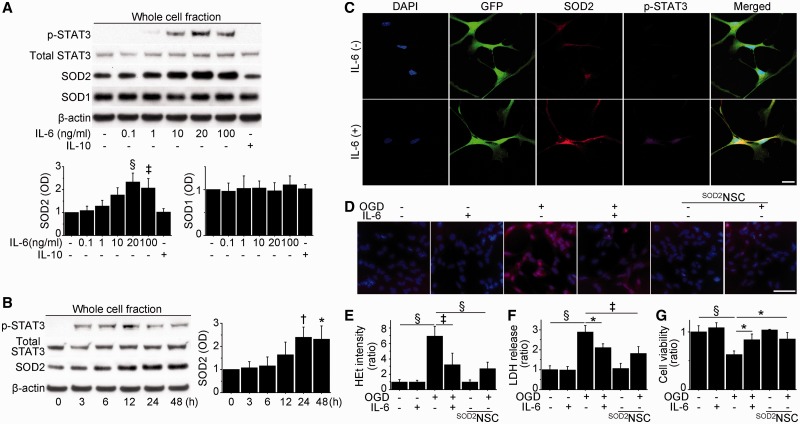
We used self-renewing and multipotent NSCs isolated from foetal GFP transgenic mice or SOD2/GFP double transgenic mice (Supplementary Fig. 1). Basal and post-ischaemic expression of SOD2, which is regulated by STAT3 in other cell types (Negoro et al., 2001; Terui et al., 2004), differed among cerebral endothelial cells, neurons, NSCs and SOD2-NSCs (Supplementary Fig. 2A). We first investigated the effects of IL-6 preconditioning on STAT3 activation and SOD2 expression in NSCs in vitro. The NSCs expressed IL-6Rα under normal conditions and after ischaemic injury (Supplementary Fig. 2B). Western blot analysis showed phosphorylation of STAT3 at Tyr705 by IL-6 preconditioning for 24 h, but not by IL-10 (Fig. 1A and Supplementary Fig. 2C). In addition, IL-6 preconditioning (20 and 100 ng/ml) significantly upregulated SOD2 expression, whereas copper/zinc-superoxide dismutase expression was unchanged. Moreover, IL-6 preconditioning resulted in a rapid increase in phosphorylated STAT3 from 3 h, and subsequent overexpression of SOD2 from 24 h that continued for up to 48 h (Fig. 1B). This IL-6-induced SOD2 upregulation was also maintained after ischaemic reperfusion injury (Supplementary Fig. 2D). Immunocytochemistry revealed a distribution of phosphorylated STAT3 predominantly in the nucleus and increased SOD2 signals in mitochondria after IL-6 preconditioning (Fig. 1C). Based on these findings, we performed 24 h of preconditioning with 20 ng/ml IL-6 for further experiments.
Figure 1.
Upregulation of SOD2 and reduced NSC death with IL-6 preconditioning in vitro. (A) Western blot analysis of NSCs pretreated with various concentrations of IL-6 or IL-10 (10 ng/ml) for 24 h. IL-6 preconditioning (20 and 100 ng/ml) significantly upregulated SOD2 expression in association with STAT3 activation. No change was observed in copper/zinc-superoxide dismutase (SOD1) expression. β-Actin was used as an internal control (n = 4). OD = optical density. (B) Western blot analysis of NSCs treated with IL-6 (20 ng/ml) and harvested at the indicated time points. IL-6 immediately phosphorylated STAT3 and induced SOD2 at later time points. β-Actin was used as an internal control (n = 4). (C) Fluorescent staining with DAPI (blue), GFP (green), SOD2 (red) and phosphorylated STAT3 (p-STAT3) (magenta) in NSCs with or without IL-6 preconditioning (20 ng/ml) for 24 h revealed the distribution of phosphorylated STAT3, predominantly in the nucleus, and increased SOD2 signals in mitochondria in preconditioned NSCs. Scale bar = 20 μm. (D and E) Fluorescent staining of NSCs with hydroethidine (red) and DAPI (blue). The increase in hydroethidine (HEt) signals after 8 h of oxygen–glucose deprivation (OGD), and 15 min of reoxygenation was reduced in preconditioned NSCs and SOD2-NSCs (n = 4). Scale bar = 50 μm. Lactate dehydrogenase (LDH); (F) and water soluble tetrazolium salts 1 (G) assays showed a significant reduction in NSC death and increased cell viability in preconditioned NSCs and SOD2-NSCs after 8 h of oxygen–glucose deprivation and 24 h of reoxygenation (n = 4). *P < 0.05, †P < 0.01, ‡P < 0.005, §P < 0.001.
NF-κB, which is also a downstream target of IL-6, is reported to regulate SOD2 expression in neurons (Mattson and Meffert, 2006). Western blot analysis revealed that IL-6 preconditioning increased expression of NF-κB in the NSCs, which was abolished by addition of 25 μM of BMS-345541 (an inhibitor of IκB kinase) (Supplementary Fig. 2E). However, IL-6-mediated SOD2 upregulation was not reduced by BMS-345541. These findings suggest that NF-κB does not play an important role in IL-6-induced SOD2 expression in NSCs.
We next asked whether IL-6 preconditioning could reduce NSC death after oxygen–glucose deprivation and reoxygenation. NSCs subjected to 8 h of oxygen–glucose deprivation and 15 min of reoxygenation exhibited a significant increase in hydroethidine signals in the cytosol, which represents production of superoxide anions (Fig. 1D and E). However, this signal increase was significantly reduced by IL-6 preconditioning. A lactate dehydrogenase assay showed that NSC death increased in a time-dependent manner with oxygen–glucose deprivation (Supplementary Fig. 3A). After 8 h of oxygen–glucose deprivation and 24 h of reoxygenation, the preconditioned NSCs had a significant reduction in death (28%) compared with the non-preconditioned NSCs (Fig. 1F). This cytoprotective effect was also supported by a water soluble tetrazolium salts 1 assay (Fig. 1G) and TUNEL staining (Supplementary Fig. 3B). Moreover, IL-6 preconditioning reduced death in NSCs subjected to the oxidative stimuli H2O2 and diethylenetriamine/nitric oxide (Supplementary Fig. 3C and D). We also examined whether overexpression of SOD2 on its own exerts cytoprotection using SOD2-NSCs. They showed less hydroethidine signalling and had better survival than non-preconditioned NSCs after oxygen–glucose deprivation and reoxygenation (Fig. 1E–G).
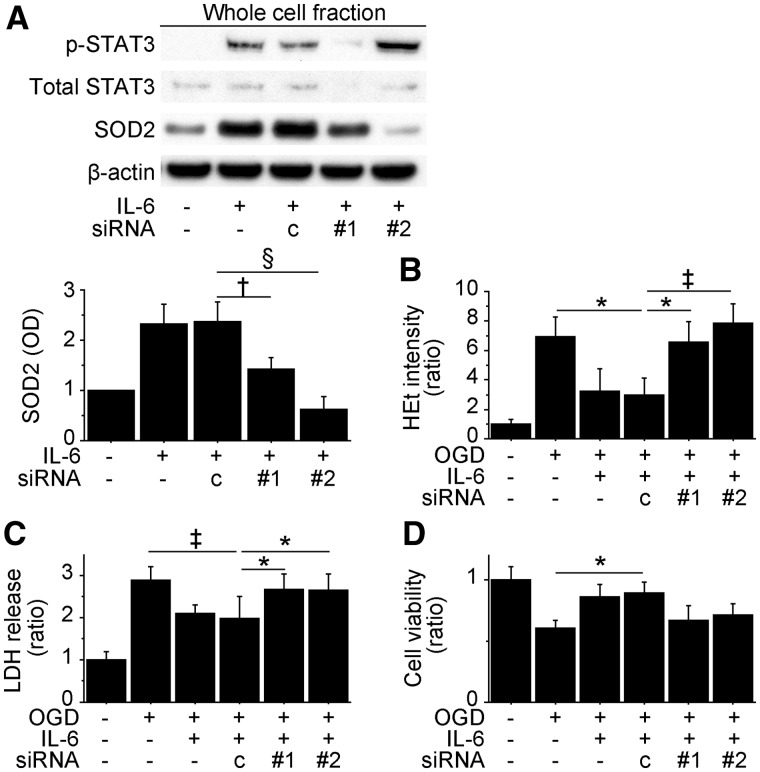
STAT3-mediated SOD2 expression was essential for IL-6-induced cytoprotection in vitro
To identify the roles of STAT3 and SOD2 in IL-6-induced cytoprotection, we pretreated NSCs with control-, STAT3- or SOD2 small interfering RNA before the experiments. STAT3 small interfering RNA strongly downregulated IL-6-induced expression of phosphorylated STAT3 and SOD2 (Fig. 2A). In addition, SOD2 expression was significantly inhibited by SOD2-small interfering RNA without changing phosphorylated STAT3 levels. IL-6-induced reduction in hydroethidine signals after oxygen–glucose deprivation and reoxygenation was reversed by transfecting the NSCs with either STAT3 or SOD2 small interfering RNA (Fig. 2B). Moreover, the lactate dehydrogenase and water soluble tetrazolium salts-1 assays demonstrated that STAT3- and SOD2-small interfering RNA abolished IL-6-induced cytoprotection after 8 h of oxygen–glucose deprivation and 24 h of reoxygenation (Fig. 2C and D). We also used the STAT3 inhibitor AG490 to show the primary role of STAT3 in IL-6-mediated cytoprotection. The lactate dehydrogenase and water soluble tetrazolium salts 1 assays revealed that AG490 (20 nM) significantly reduced IL-6-mediated cytoprotection after oxygen–glucose deprivation and reoxygenation (Supplementary Fig. 3E and F).
Figure 2.
Inhibition of STAT3 and SOD2 abolished IL-6-induced cytoprotection in vitro. Non-preconditioned NSCs and preconditioned NSCs were pretreated with control small interfering RNA (c), STAT3 small interfering RNA (#1) or SOD2 small interfering RNA (#2) before the experiments. (A) Western blot analysis demonstrated that SOD2 expression in the preconditioned NSCs was downregulated by STAT3 or SOD2 small interfering RNA transfection (n = 4). β-Actin was used as an internal control. (B) IL-6-induced reduction in hydroethidine (HEt) signals after 8 h of oxygen-glucose deprivation (OGD) and 15 min of reoxygenation was reversed by STAT3 or SOD2 small interfering RNA (n = 4). Death and viability of the NSCs analysed by lactate dehydrogenase (C) and water soluble tetrazolium salts 1 (D) assays after 8 h of OGD and 24 h of reoxygenation. STAT3 and SOD2 small interfering RNA abolished IL-6-induced cytoprotection (n = 4). *P < 0.05, †P < 0.01, ‡P < 0.005, §P < 0.001. LDH = lactate dehydrogenase.
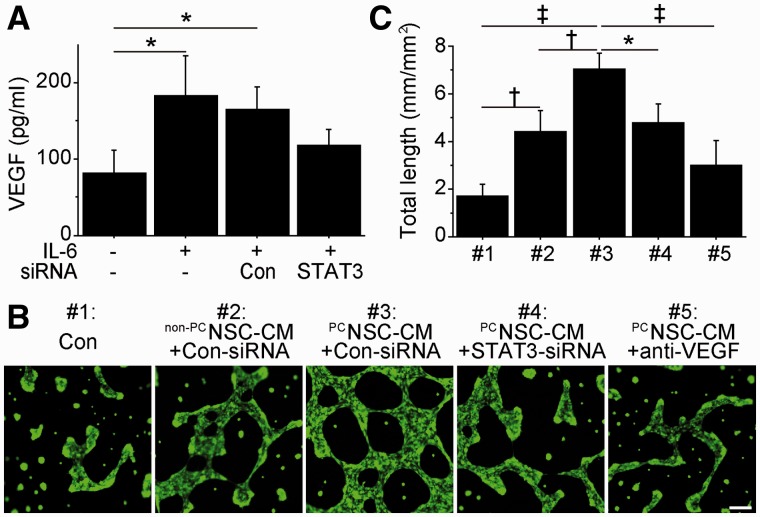
IL-6 preconditioning promoted vascular endothelial growth factor-induced capillary-like tube formation in vitro
Because STAT3 regulates gene expression of VEGF in other cell types (Wang et al., 2007), we tested whether IL-6 preconditioning could induce its expression in NSCs and could promote capillary-like tube formation in vitro. We measured VEGF levels in conditioned medium by sandwich ELISA, which showed that IL-6 preconditioning significantly increased VEGF levels (2.2-fold) under normal conditions. This IL-6-mediated VEGF expression was reversed by transfection with STAT3-small interfering RNA (Fig. 3A). In addition, IL-6 significantly induced VEGF expression even after oxygen–glucose deprivation and reoxygenation (Supplementary Fig. 3G). A capillary-like tube formation assay revealed that total tube length significantly increased when mouse cerebral endothelial cells were incubated with preconditioned NSCs-conditioned medium compared with those incubated with regular cell culture medium or non-preconditioned NSCs-conditioned medium (Fig. 3B and C). However, this increased tube formation was significantly inhibited by transfecting preconditioned NSCs with STAT3 small interfering RNA or by the addition of an anti-mouse VEGF neutralizing antibody. We also measured expression of secretoneurin and insulin-like growth factor 1, as STAT3 regulates expression of these two factors in other cell types. IL-6 preconditioning did not change the expression of either factor in NSCs (data not shown).
Figure 3.
IL-6-induced VEGF enhanced capillary-like tube formation in vitro. (A) In vitro ELISA of the conditioned medium from NSCs. IL-6 preconditioning increased VEGF levels, which were suppressed by STAT3-small interfering RNA transfection (n = 4). Con = control. (B and C) Capillary-like tube formation assay performed on mouse cerebral endothelial cells cultured with regular NSC culture medium (#1), conditioned medium (PC) from non-preconditioned (non-PC) NSCs transfected with control-small interfering RNA (#2), conditioned medium from preconditioned NSCs transfected with control-small interfering RNA (#3), conditioned medium from preconditioned NSCs transfected with STAT3-small interfering RNA (#4) or conditioned medium from preconditioned NSCs with a goat anti-mouse neutralizing antibody to VEGF (anti-VEGF) (#5). Preconditioned NSCs-conditioned medium with control small interfering RNA transfection resulted in the greatest increase in total tube length. This was abolished by STAT3 small interfering RNA transfection or by addition of an anti-VEGF neutralizing antibody (n = 4). Scale bar = 100 μm. *P < 0.05, †P < 0.01, ‡P < 0.001.
IL-6 preconditioning reduced grafted-cell death
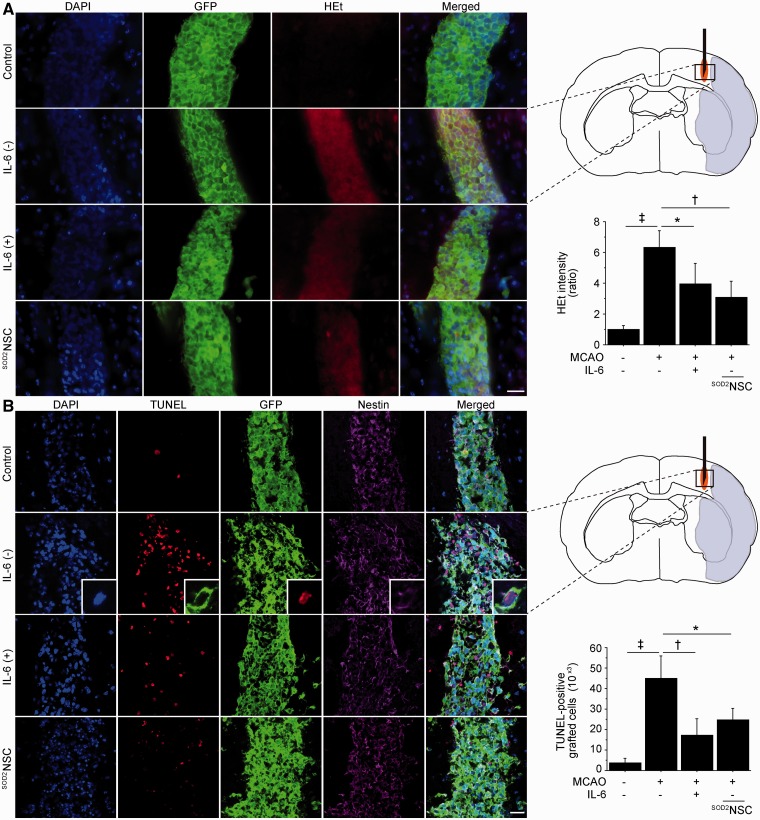
We then asked if IL-6 preconditioning could protect the grafted cells from ischaemic reperfusion injury in vivo. We transplanted non-preconditioned NSCs, preconditioned NSCs or SOD2-NSCs into the ischaemic penumbra 6 h after stroke (Supplementary Fig. 4A). When transplanted into the ischaemic brains, hydroethidine signals in the non-preconditioned NSCs increased remarkably 1 h after transplantation compared with those in the non-preconditioned NSCs in the intact brains (Fig. 4A). This signal increase was significantly reduced in the preconditioned NSCs and SOD2-NSCs. Next, we counted the number of TUNEL-positive cells, which were also stained with GFP, 2 days after stroke and transplantation. When transplanted into the peri-infarct cortex, the number of TUNEL-positive grafted cells increased ∼12 times as much as those in the intact brain (Fig. 4B). However, the number of TUNEL-positive grafted cells in the ischaemic brain was significantly reduced in the preconditioned NSC and SOD2-NSC groups by 62 and 45%, respectively. We also assessed the effects of the grafted cells on the host brain cells. The number of TUNEL-positive host brain cells beside the graft was significantly reduced in the preconditioned NSC group compared with the non-transplanted control and non-preconditioned NSC groups (Supplementary Fig. 4B). In addition, we measured the inflammatory response in the ischaemic brain 2 days after transplantation. Immunofluorescent staining of ED1 (an activated microglial marker) demonstrated a reduced number of ED1-positive cells in the preconditioned NSC group compared with the non-transplanted and non-preconditioned NSC groups (Supplementary Fig. 4C).
Figure 4.
Reduced grafted-cell death with IL-6 preconditioning in vivo. NSCs were transplanted into the brain 6 h after stroke. (A) Fluorescent staining with DAPI (blue), GFP (green) and hydroethidine (HEt, red) in brain sections 1 h after transplantation. Hydroethidine signals increased in the non-preconditioned NSCs under ischaemic reperfusion injury, but this signal increase was reduced in the preconditioned NSCs and SOD2-NSCs (n = 4). MCAO = middle cerebral artery occlusion. (B) Fluorescent staining with DAPI (blue), TUNEL (red), GFP (green) and nestin (magenta) 2 days after transplantation. IL-6 preconditioning and SOD2 overexpression significantly reduced the number of TUNEL-positive grafted cells in the ischaemic brain. The insets represent high magnification images showing the co-localization of TUNEL with a nestin- and GFP-positive grafted cell (n = 4). Scale bars = 20 μm. *P < 0.05, †P < 0.005, ‡P < 0.001.
IL-6 preconditioning increased survival of grafted cells
Twenty-eight days after stroke and transplantation, fluorescent staining with GFP revealed an extensive migration of the grafted cells towards the ischaemic lesion border in the non-preconditioned NSC and preconditioned NSC groups (Fig. 5A). No signs of tumour formation caused by the grafted NSCs were detected in any of the mice. Importantly, a significantly greater number of preconditioned NSCs survived than non-preconditioned NSCs in the ischaemic brains (Fig. 5B). We next analysed differentiation profiles and proliferation capacity of the NSCs. Fluorescent double staining of lineage-specific phenotype markers and GFP demonstrated that the grafted NSCs differentiated into neurons and astrocytes 28 days after stroke and transplantation (Fig. 5C). No GFP-positive cells were labelled with the oligodendrocyte precursor marker NG2. The percentage of neurons (10 versus 9%) and astrocytes (45 versus 42%) that differentiated from the grafted cells was similar between the non-preconditioned NSC and preconditioned NSC groups. Moreover, the percentage of Ki-67-positive grafted cells did not differ between the two groups 2 days after stroke and transplantation (Supplementary Fig. 5A and B).
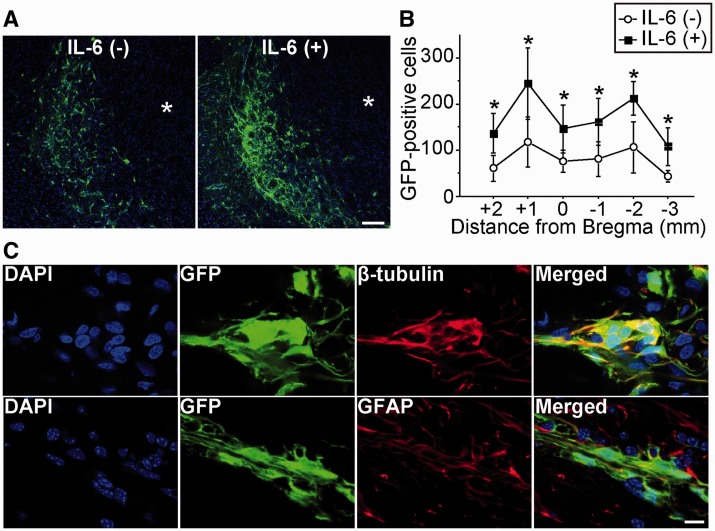
Figure 5.
Increased survival of grafted cells with IL-6 preconditioning in vivo. NSCs were transplanted into the brain 6 h after stroke. (A) Fluorescent staining with GFP (green) and DAPI (blue) revealed migration of the grafted cells towards the lesion border zone 28 days after stroke and transplantation. Asterisk indicates ischaemic lesion. Scale bar = 100 μm. (B) Quantification of the number of surviving grafted cells 28 days after stroke and transplantation. Specimens were picked up every 1 mm, and the number of GFP-positive cells was counted. IL-6 preconditioning significantly increased survival of the grafted cells (n = 4). (C) Fluorescent staining with GFP (green) and β-tubulin or glial fibrillary acidic protein (GFAP) (red) revealed that the grafted NSCs differentiated into neurons (β-tubulin+) and astrocytes (GFAP+) 28 days after stroke and transplantation. Nuclei were counterstained with DAPI. Scale bar = 10 μm. *P < 0.05.
Transplantation of preconditioned neural stem cells enhanced angiogenesis in vivo
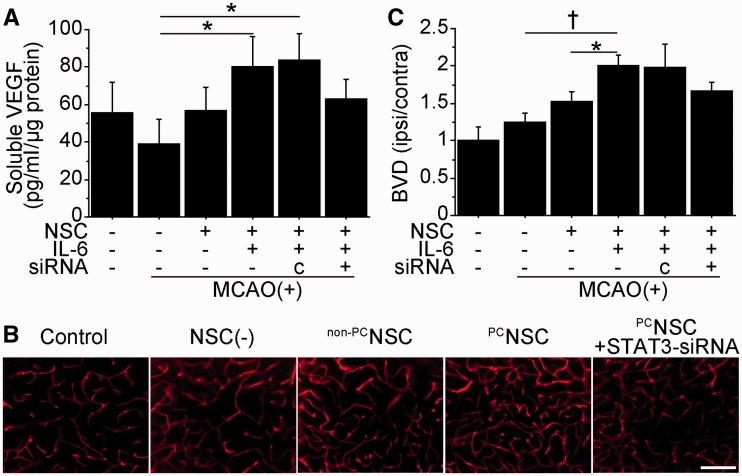
To examine whether transplantation of NSCs increases VEGF levels in the peri-infarct cortex, we performed a sandwich ELISA 2 days after transplantation. VEGF levels significantly increased in the preconditioned NSC group compared with the non-transplanted control group (2.0-fold) (Fig. 6A). However, this increased expression was blocked by transfecting the preconditioned NSCs with STAT3 small interfering RNA before transplantation. Immunofluorescent staining demonstrated increased expression of VEGF in the preconditioned NSC group (Supplementary Fig. 6A). Next, to investigate the effects of the NSCs on angiogenesis, the density of lectin-perfused vessels (blood vessel density) in the peri-infarct cortex was analysed 14 days after transplantation. The preconditioned NSC group showed a significantly increased blood vessel density compared with the non-transplanted control and non-preconditioned NSC groups (Fig. 6B and C). This blood vessel density increase in the preconditioned NSC group was reduced by 17% by transfection with STAT3 small interfering RNA. As early administration of VEGF aggravates cerebral oedema in ischaemic stroke (Zhang et al., 2000), we measured brain water content 2 days after transplantation. Water content in the ischaemic brains was similar among the groups (Supplementary Fig. 6B).
Figure 6.
Transplantation of preconditioned NSCs promoted angiogenesis in vivo. NSCs were transplanted into the brain 6 h after stroke. (A) In vivo ELISA of the cortex revealed a significant elevation of VEGF in the preconditioned NSC group 2 days after transplantation. Transfection with STAT3 small interfering RNA abolished this increase (n = 4). c = control small interfering RNA. Representative images of lectin-perfused vessels (B) and quantification of blood vessel density (BVD) (C) in the peri-infarct cortex 14 days after transplantation. Blood vessel density was significantly increased in the preconditioned NSC group, which was suppressed by STAT3 small interfering RNA transfection (n = 4). Scale bar = 100 μm. *P < 0.05, †P < 0.001.
Transplantation of preconditioned neural stem cells reduced infarct size and improved behavioural performance after ischaemic stroke
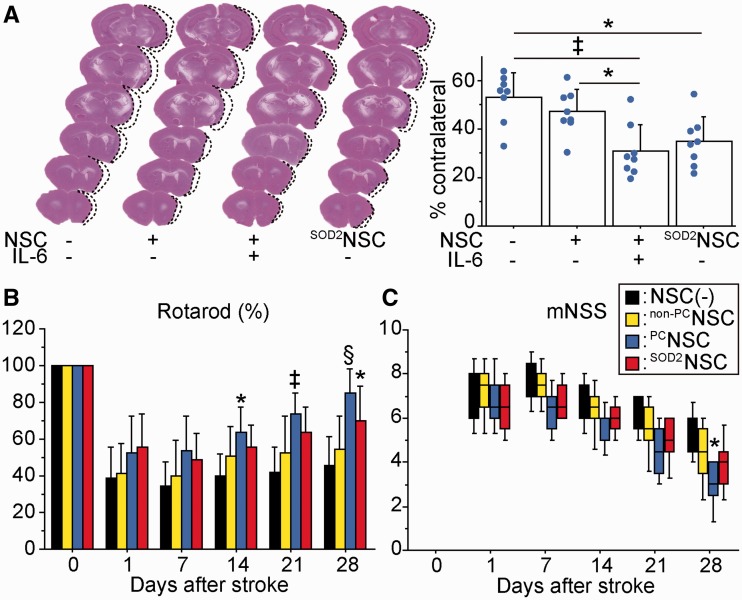
We next investigated whether transplantation of preconditioned NSCs could facilitate amelioration of ischaemic stroke. Twenty-eight days after stroke and transplantation, the cortical infarct significantly decreased by 42 and 34% in the preconditioned NSC group compared with the non-transplanted control and non-preconditioned NSC groups, respectively, as determined by haematoxylin and eosin staining (Fig. 7A). Reduction in infarct size was also observed in the SOD2-NSC group compared with the non-transplanted control group (35%). Infarct size in the striatum was similar among the four groups (63.5, 63.9, 61.3 and 61.8%). We next monitored neurological performance using the rotarod test and modified neurologic severity scores. The preconditioned NSC group showed a significant functional recovery from Days 14 through 28 using the rotarod test and on Day 28 using modified neurologic severity scores compared with the non-transplanted control group (Fig. 7B and C). In addition, the SOD2-NSC group showed enhanced functional recovery on Day 28 according to the rotarod test. Significant behavioural improvement was not observed in the non-preconditioned NSC group at any time points compared with the non-transplanted control group. We found a negative correlation between the rotarod test (Day 28) and the infarct size (r = −0.457, P < 0.01), and between the modified neurologic severity scores (Day 28) and the infarct size (P < 0.01).
Figure 7.
Effects of preconditioned NSCs on infarct size and behavioural performance. NSCs were transplanted into the brain 6 h after stroke. (A) Measurement of the infarct size by haematoxylin and eosin staining 28 days after stroke and transplantation. Cortical infarct size was significantly attenuated in the preconditioned NSC and SOD2-NSC groups compared with the non-transplanted control group (n = 4). Indices of behavioural performance using the rotarod test (B) and modified neurologic severity scores (mNSS) (C). Transplantation of preconditioned NSCs resulted in the greatest functional recovery 28 days after stroke and transplantation (n = 8). Black bars denote non-transplanted control group; yellow bars denote non-preconditioned NSC group; blue bars denote preconditioned NSC group; red bars denote SOD2-NSC group. The labels show P-value compared with the non-transplanted control group. *P < 0.05, ‡P < 0.005; §P < 0.001.
IL-6 preconditioning ameliorated ischaemic stroke through STAT3 activation
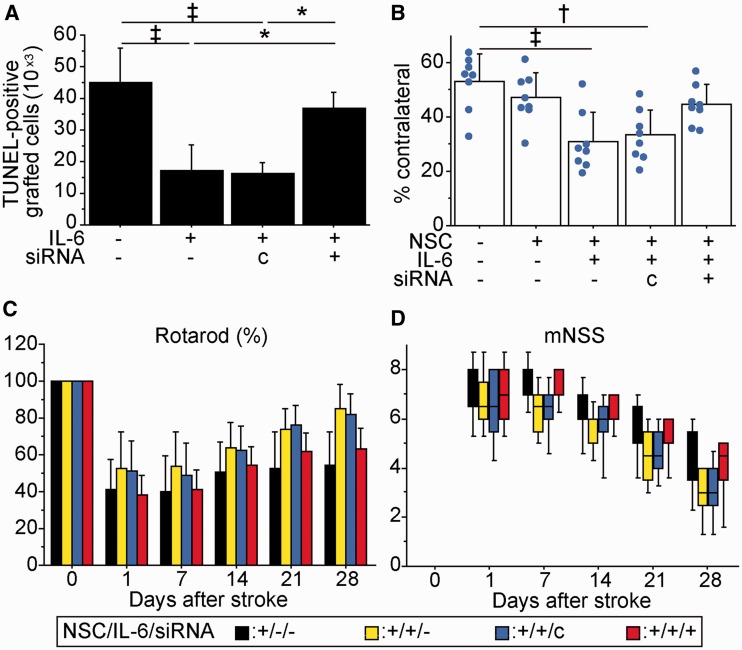
We transplanted preconditioned NSCs or non-preconditioned NSCs, pretreated with control- or STAT3-small interfering RNA, into the peri-infarct cortex 6 h after stroke. The decreased number of TUNEL-positive grafted cells with IL-6 preconditioning was reversed by transfection with STAT3 small interfering RNA (Fig. 8A). The preconditioned NSCs, also transfected with STAT3 small interfering RNA, had no attenuation of cortical infarct size (Fig. 8B). Furthermore, behavioural improvement offered by the preconditioned NSCs was suppressed by STAT3 small interfering RNA as assessed by the rotarod test and the modified neurologic severity scores (Fig. 8C and D).
Figure 8.
Inhibition of STAT3 abolished IL-6-induced amelioration of ischaemic stroke. Preconditioned NSCs or non-preconditioned NSCs, transfected with control or STAT3 small interfering RNA, were transplanted 6 h after stroke. (A) Stereological counting of TUNEL-positive grafted cells 2 days after stroke and transplantation. STAT3 small interfering RNA suppressed IL-6-induced cytoprotection (n = 4). (B) Cortical infarct size evaluated by haematoxylin and eosin staining 28 days after stroke and transplantation. The preconditioned NSCs, which also underwent STAT3 small interfering RNA pretreatment, had no attenuation of lesion size (n = 4). Behavioural performance analysed by the rotarod test (C) and mNSS (D). STAT3-small interfering RNA diminished the behavioural improvement observed in the preconditioned NSC group (n = 8). Black bars denote non-preconditioned NSC group; yellow bars denote preconditioned NSC group; blue bars denote preconditioned NSC group with control small interfering RNA transfection; red bars denote preconditioned NSC group with STAT3 small interfering RNA transfection. *P < 0.05, †P < 0.01, ‡P < 0.005.
Subacute delivery of preconditioned neural stem cells enhances functional recovery
To examine whether IL-6 preconditioning could also enhance the effectiveness of cell transplantation therapy at a later time point, we transplanted NSCs into the peri-infarct cortex 7 days after the onset of stroke. Two days after transplantation, IL-6 preconditioning significantly reduced the number of TUNEL-positive grafted cells (Supplementary Fig. 7A). Interestingly, subacute transplantation resulted in a reduced number of TUNEL-positive grafted cells compared with acute delivery. In accordance with this finding, hydroethidine signals in the host brain cells beside the graft were reduced at the subacute stage of stroke (Supplementary Fig. 7B). In addition, the preconditioned NSC group showed significantly increased blood vessel density compared with the non-transplanted control group 14 days after transplantation (Supplementary Fig. 7C). Haematoxylin and eosin staining revealed that cortical infarct size was similar among the non-transplanted control, non-preconditioned NSC and preconditioned NSC groups 28 days after stroke (53.5, 53.2 and 50.9%). Significant improvement in the rotarod test was observed in the preconditioned NSC group on Days 21 and 28 compared with the non-transplanted control group. This was diminished by transfecting the preconditioned NSCs with STAT3 small interfering RNA before transplantation (Supplementary Fig. 7D). Although statistical significance was not observed, the preconditioned NSC group showed behavioural improvement, as indicated by the modified neurologic severity scores, compared with the non-transplanted control group.
Discussion
We have shown in this study that preconditioning with IL-6 enhances the effectiveness of cell transplantation therapy in ischaemic stroke. The major findings are as follows: (i) IL-6 preconditioning protected NSCs from ischaemic reperfusion injury through STAT3-mediated SOD2 upregulation; (ii) preconditioned NSCs promoted angiogenesis through STAT3-induced VEGF expression; (iii) transplantation of preconditioned NSCs at the acute stage of stroke resulted in attenuation of infarct size; and (iv) transplantation of preconditioned NSCs at the acute and subacute stages of stroke accelerated improvement of behavioural performance. We believe that simultaneously conferring antioxidant and pro-angiogenic properties on grafted stem cells without the need for genetic modification is an effective and novel approach for the treatment of stroke. In addition, as IL-6 is not necessarily required to be carried by stem cells, our chemical preconditioning approach is simple and safe to follow from a clinical perspective.
Aside from its pro-inflammatory detrimental effects, IL-6 has been reported to promote activation of an intrinsic survival signalling pathway through STAT3 activation in neurons and cardiomyocytes (Jugdutt, 2010; Jung et al., 2011). In accordance with these reports, IL-6 preconditioning activated STAT3 in NSCs, leading to cytoprotection in the present study. In addition to STAT3 activation, IL-6 is reported to activate two other signalling pathways, phosphatidylinositol 3 kinase/Akt and Ras/extracellular signal-regulated kinase pathways, which might be involved in the protective effects against oxidative stress (Negoro et al., 2001). However, our loss-of-function experiments using AG490 and STAT3 small interfering RNA indicate that activation of STAT3 plays a primary role in the IL-6-induced antioxidant capacity and cytoprotection in NSCs, although we cannot exclude the possibility of involvement of other IL-6-mediated signalling pathways.
SOD2, a mitochondrial antioxidant enzyme for superoxide, is a primary cellular defence enzyme involved in protecting cells from oxidative stress (Chan, 1996). In our study, we found the vital link between IL-6 and SOD2 in NSCs as follows: first, IL-6 preconditioning induced SOD2 overexpression. Second, IL-6-induced SOD2 expression was blocked by STAT3 small interfering RNA transfection. These findings indicate that IL-6-mediated STAT3 activation induces SOD2 expression in NSCs. In line with our results, IL-6 is reported to upregulate SOD2 expression in hepatocytes, cortical neurons and the cerebral cortex through STAT3 activation (Terui et al., 2004; Jung et al., 2011). In contrast, NF-κB, which is also a downstream target of IL-6, is reported to regulate SOD2 expression in neurons (Mattson and Meffert, 2006). However, we found that inactivation of NF-κB by BMS-345541 did not influence IL-6-mediated SOD2 upregulation. Based on this finding, NF-κB is unlikely to regulate SOD2 expression in NSCs. Taken together, IL-6-induced activation of STAT3 plays a pivotal role in SOD2 expression in NSCs, although association of other IL-6-mediated pathways cannot be completely excluded. Further study is needed to clarify this important issue.
In the present study, we have found the relationship between SOD2 expression and cytoprotection in NSCs as follows: (i) IL-6 preconditioning, which induced SOD2 expression, reduced oxidative stress and conferred cytoprotection after ischaemic reperfusion injury; (ii) SOD2 overexpression using SOD2-NSCs resulted in cytoprotection; and (iii) STAT3 and SOD2 small interfering RNA, both of which significantly downregulated SOD2 expression, abolished IL-6-induced cytoprotection. Taken together, IL-6 preconditioning protects NSCs from oxidative stress by inducing SOD2. This finding agrees with our previous reports showing that SOD2-deficient mice had increased infarct size and apoptosis after cerebral ischaemia (Murakami et al., 1998; Fujimura et al., 1999), whereas overexpression of SOD2 provided neuroprotection (Maier et al., 2006). Our results indicate that SOD2 might be a potential therapeutic target for grafted stem cells.
Another interesting finding of this study is the induction of the pro-angiogenic VEGF with IL-6 preconditioning. Horie et al. (2011) have shown that NSCs ameliorate ischaemic stroke through secretion of VEGF. Because a reduction in VEGF expression was observed in NSCs transfected with STAT3 small interfering RNA, STAT3 contributes to its increased expression in preconditioned NSCs. This finding is compatible with other reports showing STAT3-induced VEGF expression in cardiomyocytes and mesenchymal stem cells (Funamoto et al., 2000; Wang et al., 2009). Stem cells genetically modified to overexpress VEGF enhance angiogenesis and improve functional recovery after stroke (Lee et al., 2007; Toyama et al., 2009). In-line with these studies, our in vitro study demonstrated promotion of capillary-like tube formation using the conditioned medium from preconditioned NSCs. Because VEGF is reported to regulate the proliferation of endothelial cells, increased expression of VEGF from the preconditioned NSCs might also enhance endothelial proliferation (Wang et al., 2008). More importantly, transplantation of preconditioned NSCs enhanced angiogenesis in the ischaemic brain, which was suppressed by STAT3 small interfering RNA transfection. Although early administration of VEGF aggravates cerebral oedema in ischaemic stroke (Zhang et al., 2000), transplantation of preconditioned NSCs did not induce cerebral oedema. This might be because slow and mild secretion of VEGF from the preconditioned NSCs did not contribute to cerebral oedema. Based on these findings, IL-6 preconditioning of stem cells can be an efficacious strategy to induce angiogenesis through STAT3 activation after ischaemic stroke.
A series of studies showed that STAT3 activation by IL-6 family cytokines promotes differentiation of NSCs into astrocytes or neurons (Bonni et al., 1997; Johansson et al., 2008). IL-6 is also reported to stimulate proliferation of NSCs through STAT3 activation (Kang and Kang, 2008). Contrarily, IL-6 preconditioning did not affect the differentiation profiles and proliferation capacity of NSCs in the present study. This discrepancy might be explained by the difference in incubation time and concentration of IL-6. In addition, IL-6 family cytokines might need the help of other cytokines, such as the bone morphogenetic protein family, to sufficiently induce differentiation (Nakashima et al., 1999). In the present study, a substantial number of the grafted NSCs with or without IL-6 preconditioning differentiated into astrocytes (45 and 42%, respectively) 28 days after transplantation, and some of them seemed to have formed glial scarring, which might hamper recovery from stroke. Nonetheless, NSCs have glial scar inhibitory effects through downregulation of genes promoting astrogliosis in the brain (Bacigaluppi et al., 2009). More importantly, because no signs of tumour formation were observed in the brains transplanted with the preconditioned NSCs 28 days post-grafting, we believe that our preconditioning approach securely exhibits its beneficial effects.
Because there is a clinical-grade bank of foetal-derived human NSCs, we expect that using foetal NSCs is a clinically realistic goal (Andres et al., 2011). Depending on the timing of transplantation, the mechanisms of action of the NSCs in the ischaemic brain might be different. In the present study, we transplanted NSCs at the acute stage of stroke (6 h after the onset of stroke) or at the subacute stage of stroke (7 days after stroke onset). If a treatment strategy focuses on neuroprotection, acute delivery will be critical (Bliss et al., 2010). If the cells act to enhance the endogenous repair process (e.g. angiogenesis and plasticity), subacute delivery would be appropriate, as these events are more prevalent in the first few weeks after stroke. Although clinical relevance may be limited, acute delivery of the preconditioned NSCs reduced infarct size and drastically improved behavioural performance compared with the non-transplanted control group. This might occur because increased survival of grafted cells with IL-6 preconditioning may lead to augmentation of the total amount of neuroprotective paracrine factors in the ischaemic brain, resulting in enhanced neuroprotection (Bliss et al., 2010). In addition, increased angiogenesis in the preconditioned NSC group would be involved in the prevention of cortical atrophy and accelerated improvement of neurological recovery (Chen et al., 2003; Fan et al., 2010; Horie et al., 2011). Furthermore, we cannot exclude the possibility that other mechanisms of stem cell action, such as immunomodulation and cell replacement, are enhanced by IL-6 preconditioning (Kelly et al., 2004; Bacigaluppi et al., 2009). In contrast, subacute delivery of the preconditioned NSCs also enhanced functional recovery compared with the non-transplanted control group, although functional improvement with subacute delivery was less than with acute delivery, and no changes were observed in infarct size. This functional recovery might be achieved not by neuroprotection but by enhancement of the endogenous repair mechanism, including angiogenesis. Taken together, IL-6 preconditioning beneficially effects stem cell transplantation at the acute and subacute stages of stroke.
In conclusion, we have demonstrated that IL-6 preconditioning reprograms NSCs to tolerate oxidative stress after ischaemic reperfusion injury and to induce angiogenesis through STAT3 activation, resulting in enhanced effectiveness of cell transplant therapy in ischaemic stroke. The beneficial effects of IL-6 preconditioning and the simplicity and easy adoptability make this approach highly appealing for future clinical applications.
Funding
National Institutes of Health [PO1 NS014543, RO1 NS025372 and RO1 NS038653] and James R. Doty Endowment.
Supplementary material
Supplementary material is available at Brain online.
Acknowledgements
The authors thank Liza Reola and Bernard Calagui for technical assistance, Cheryl Christensen for editorial assistance and Elizabeth Hoyte for assistance with the figures.
Glossary
Abbreviations
- DAPI
4′,6 diamidino-2-phenylindole
- GFP
green fluorescent protein
- NSC
neural stem cell
- STAT3
signal transducer and activator of transcription-3
- TUNEL
terminal deoxynucleotidyl transferase-mediated uridine 5′-triphosphate-biotin nick end labelling
- VEGF
vascular endothelial growth factor
References
- Andres RH, Horie N, Slikker W, Keren-Gill H, Zhan K, Sun G, et al. Human neural stem cells enhance structural plasticity and axonal transport in the ischaemic brain. Brain. 2011;134:1777–89. doi: 10.1093/brain/awr094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacigaluppi M, Pluchino S, Jametti LP, Kilic E, Kilic Ü, Salani G, et al. Delayed post-ischaemic neuroprotection following systemic neural stem cell transplantation involves multiple mechanisms. Brain. 2009;132:2239–51. doi: 10.1093/brain/awp174. [DOI] [PubMed] [Google Scholar]
- Banerjee S, Williamson D, Habib N, Gordon M, Chataway J. Human stem cell therapy in ischaemic stroke: a review. Age Ageing. 2011;40:7–13. doi: 10.1093/ageing/afq133. [DOI] [PubMed] [Google Scholar]
- Beamer NB, Coull BM, Clark WM, Hazel JS, Silberger JR. Interleukin-6 and interleukin-1 receptor antagonist in acute stroke. Ann Neurol. 1995;37:800–4. doi: 10.1002/ana.410370614. [DOI] [PubMed] [Google Scholar]
- Bliss TM, Andres RH, Steinberg GK. Optimizing the success of cell transplantation therapy for stroke. Neurobiol Dis. 2010;37:275–83. doi: 10.1016/j.nbd.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blurton-Jones M, Kitazawa M, Martinez-Coria H, Castello NA, Müller F-J, Loring JF, et al. Neural stem cells improve cognition via BDNF in a transgenic model of Alzheimer disease. Proc Natl Acad Sci USA. 2009;106:13594–9. doi: 10.1073/pnas.0901402106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonni A, Sun Y, Nadal-Vicens M, Bhatt A, Frank DA, Rozovsky I, et al. Regulation of gliogenesis in the central nervous system by the JAK-STAT signaling pathway. Science. 1997;278:477–83. doi: 10.1126/science.278.5337.477. [DOI] [PubMed] [Google Scholar]
- Brivanlou AH, Darnell JE., Jr Signal transduction and the control of gene expression. Science. 2002;295:813–8. doi: 10.1126/science.1066355. [DOI] [PubMed] [Google Scholar]
- Chan PH. Role of oxidants in ischemic brain damage. Stroke. 1996;27:1124–9. doi: 10.1161/01.str.27.6.1124. [DOI] [PubMed] [Google Scholar]
- Chen J, Zhang C, Jiang H, Li Y, Zhang L, Robin A, et al. Atorvastatin induction of VEGF and BDNF promotes brain plasticity after stroke in mice. J Cereb Blood Flow Metab. 2005;25:281–90. doi: 10.1038/sj.jcbfm.9600034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Zhang ZG, Li Y, Wang L, Xu YX, Gautam SC, et al. Intravenous administration of human bone marrow stromal cells induces angiogenesis in the ischemic boundary zone after stroke in rats. Circ Res. 2003;92:692–9. doi: 10.1161/01.RES.0000063425.51108.8D. [DOI] [PubMed] [Google Scholar]
- Fan Y, Shen F, Frenzel T, Zhu W, Ye J, Liu J, et al. Endothelial progenitor cell transplantation improves long-term stroke outcome in mice. Ann Neurol. 2010;67:488–97. doi: 10.1002/ana.21919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimura M, Morita-Fujimura Y, Kawase M, Copin J-C, Calagui B, Epstein CJ, et al. Manganese superoxide dismutase mediates the early release of mitochondrial cytochrome c and subsequent DNA fragmentation after permanent focal cerebral ischemia in mice. J Neurosci. 1999;19:3414–22. doi: 10.1523/JNEUROSCI.19-09-03414.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funamoto M, Fujio Y, Kunisada K, Negoro S, Tone E, Osugi T, et al. Signal transducer and activator of transcription 3 is required for glycoprotein 130-mediated induction of vascular endothelial growth factor in cardiac myocytes. J Biol Chem. 2000;275:10561–6. doi: 10.1074/jbc.275.14.10561. [DOI] [PubMed] [Google Scholar]
- Hicks AU, Lappalainen RS, Narkilahti S, Suuronen R, Corbett D, Sivenius J, et al. Transplantation of human embryonic stem cell-derived neural precursor cells and enriched environment after cortical stroke in rats: cell survival and functional recovery. Eur J Neurosci. 2009;29:562–74. doi: 10.1111/j.1460-9568.2008.06599.x. [DOI] [PubMed] [Google Scholar]
- Hirano T, Ishihara K, Hibi M. Roles of STAT3 in mediating the cell growth, differentiation and survival signals relayed through the IL-6 family of cytokine receptors. Oncogene. 2000;19:2548–56. doi: 10.1038/sj.onc.1203551. [DOI] [PubMed] [Google Scholar]
- Horie N, Pereira MP, Niizuma K, Sun G, Keren-Gill H, Encarnacion A, et al. Transplanted stem cell-secreted vascular endothelial growth factor effects poststroke recovery, inflammation, and vascular repair. Stem Cells. 2011;29:274–85. doi: 10.1002/stem.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson S, Price J, Modo M. Effect of inflammatory cytokines on major histocompatibility complex expression and differentiation of human neural stem/progenitor cells. Stem Cells. 2008;26:2444–54. doi: 10.1634/stemcells.2008-0116. [DOI] [PubMed] [Google Scholar]
- Jugdutt BI. Preventing adverse remodeling and rupture during healing after myocardial infarction in mice and humans. Circulation. 2010;122:103–5. doi: 10.1161/CIRCULATIONAHA.110.969410. [DOI] [PubMed] [Google Scholar]
- Jung JE, Kim GS, Chan PH. Neuroprotection by interleukin-6 is mediated by signal transducer and activator of transcription 3 and antioxidative signaling in ischemic stroke. Stroke. 2011;42:3574–9. doi: 10.1161/STROKEAHA.111.626648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung JE, Kim GS, Narasimhan P, Song YS, Chan PH. Regulation of Mn-superoxide dismutase activity and neuroprotection by STAT3 in mice after cerebral ischemia. J Neurosci. 2009;29:7003–14. doi: 10.1523/JNEUROSCI.1110-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang MK, Kang SK. Interleukin-6 induces proliferation in adult spinal cord-derived neural progenitors via the JAK2/STAT3 pathway with EGF-induced MAPK phosphorylation. Cell Prolif. 2008;41:377–92. doi: 10.1111/j.1365-2184.2008.00537.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly S, Bliss TM, Shah AK, Sun GH, Ma M, Foo WC, et al. Transplanted human fetal neural stem cells survive, migrate, and differentiate in ischemic rat cerebral cortex. Proc Natl Acad Sci USA. 2004;101:11839–44. doi: 10.1073/pnas.0404474101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim GS, Jung JE, Niizuma K, Chan PH. CK2 is a novel negative regulator of NADPH oxidase and a neuroprotectant in mice after cerebral ischemia. J Neurosci. 2009;29:14779–89. doi: 10.1523/JNEUROSCI.4161-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecour S, James RW. When are pro-inflammatory cytokines SAFE in heart failure? Eur Heart J. 2011;32:680–5. doi: 10.1093/eurheartj/ehq484. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Kim KS, Park IH, Kim SU. Human neural stem cells over-expressing VEGF provide neuroprotection, angiogenesis and functional recovery in mouse stroke model. PLoS One. 2007;2 doi: 10.1371/journal.pone.0000156. e156, doi:10.1371/journal.pone.0000156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindvall O, Kokaia Z. Stem cells in human neurodegenerative disorders—time for clinical translation? J Clin Invest. 2010;120:29–40. doi: 10.1172/JCI40543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Honmou O, Harada K, Nakamura K, Houkin K, Hamada H, et al. Neuroprotection by PIGF gene-modified human mesenchymal stem cells after cerebral ischaemia. Brain. 2006;129:2734–45. doi: 10.1093/brain/awl207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo EH, Dalkara T, Moskowitz MA. Mechanisms, challenges and opportunities in stroke. Nat Rev Neurosci. 2003;4:399–415. doi: 10.1038/nrn1106. [DOI] [PubMed] [Google Scholar]
- Maier CM, Hsieh L, Crandall T, Narasimhan P, Chan PH. Evaluating therapeutic targets for reperfusion-related brain hemorrhage. Ann Neurol. 2006;59:929–38. doi: 10.1002/ana.20850. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Meffert MK. Roles for NF-κB in nerve cell survival, plasticity, and disease. Cell Death Differ. 2006;13:852–60. doi: 10.1038/sj.cdd.4401837. [DOI] [PubMed] [Google Scholar]
- Murakami K, Kondo T, Kawase M, Li Y, Sato S, Chen SF, et al. Mitochondrial susceptibility to oxidative stress exacerbates cerebral infarction that follows permanent focal cerebral ischemia in mutant mice with manganese superoxide dismutase deficiency. J Neurosci. 1998;18:205–13. doi: 10.1523/JNEUROSCI.18-01-00205.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagomi N, Nakagomi T, Kubo S, Nakano-Doi A, Saino O, Takata M, et al. Endothelial cells support survival, proliferation, and neuronal differentiation of transplanted adult ischemia-induced neural stem/progenitor cells after cerebral infarction. Stem Cells. 2009;27:2185–95. doi: 10.1002/stem.161. [DOI] [PubMed] [Google Scholar]
- Nakashima K, Yanagisawa M, Arakawa H, Kimura N, Hisatsune T, Kawabata M, et al. Synergistic signaling in fetal brain by STAT3-Smad1 complex bridged by p300. Science. 1999;284:479–82. doi: 10.1126/science.284.5413.479. [DOI] [PubMed] [Google Scholar]
- Negoro S, Kunisada K, Fujio Y, Funamoto M, Darville MI, Eizirik DL, et al. Activation of signal transducer and activator of transcription 3 protects cardiomyocytes from hypoxia/reoxygenation-induced oxidative stress through the upregulation of manganese superoxide dismutase. Circulation. 2001;104:979–81. doi: 10.1161/hc3401.095947. [DOI] [PubMed] [Google Scholar]
- Rikitake Y, Hirata K, Kawashima S, Ozaki M, Takahashi T, Ogawa W, et al. Involvement of endothelial nitric oxide in sphingosine-1-phosphate–induced angiogenesis. Arterioscler Thromb Vasc Biol. 2002;22:108–14. doi: 10.1161/hq0102.101843. [DOI] [PubMed] [Google Scholar]
- Suzuki S, Tanaka K, Suzuki N. Ambivalent aspects of interleukin-6 in cerebral ischemia: inflammatory versus neurotrophic aspects. J Cereb Blood Flow Metab. 2009;29:464–79. doi: 10.1038/jcbfm.2008.141. [DOI] [PubMed] [Google Scholar]
- Terui K, Enosawa S, Haga S, Zhang HQ, Kuroda H, Kouchi K, et al. Stat3 confers resistance against hypoxia/reoxygenation-induced oxidative injury in hepatocytes through upregulation of Mn-SOD. J Hepatol. 2004;41:957–65. doi: 10.1016/j.jhep.2004.08.019. [DOI] [PubMed] [Google Scholar]
- Toyama K, Honmou O, Harada K, Suzuki J, Houkin K, Hamada H, et al. Therapeutic benefits of angiogenetic gene-modified human mesenchymal stem cells after cerebral ischemia. Exp Neurol. 2009;216:47–55. doi: 10.1016/j.expneurol.2008.11.010. [DOI] [PubMed] [Google Scholar]
- Wang M, Tan J, Coffey A, Fehrenbacher J, Weil BR, Meldrum DR. Signal transducer and activator of transcription 3–stimulated hypoxia inducible factor-1α mediates estrogen receptor-α–induced mesenchymal stem cell vascular endothelial growth factor production. J Thorac Cardiovasc Surg. 2009;138:163–71. doi: 10.1016/j.jtcvs.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Zhang W, Crisostomo P, Markel T, Meldrum KK, Fu XY, et al. STAT3 mediates bone marrow mesenchymal stem cell VEGF production. J Mol Cell Cardiol. 2007;42:1009–15. doi: 10.1016/j.yjmcc.2007.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Li X, Parra M, Verdin E, Bassel-Duby R, Olson EN. Control of endothelial cell proliferation and migration by VEGF signaling to histone deacetylase 7. Proc Natl Acad Sci USA. 2008;105:7738–43. doi: 10.1073/pnas.0802857105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei L, Cui L, Snider BJ, Rivkin M, Yu SS, Lee C-S, et al. Transplantation of embryonic stem cells overexpressing Bcl-2 promotes functional recovery after transient cerebral ischemia. Neurobiol Dis. 2005;19:183–93. doi: 10.1016/j.nbd.2004.12.016. [DOI] [PubMed] [Google Scholar]
- Zhang ZG, Zhang L, Jiang Q, Zhang R, Davies K, Powers C, et al. VEGF enhances angiogenesis and promotes blood-brain barrier leakage in the ischemic brain. J Clin Invest. 2000;106:829–38. doi: 10.1172/JCI9369. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.