Abstract
In this issue of Cancer Cell, Gruber et al. report that a significant proportion of children with acute megakaryoblastic leukemia acquire a translocation that confers enhanced BMP signaling and promotes self-renewal of hematopoietic progenitors. This study presents novel therapeutic targets that may lead to improved therapies for this aggressive leukemia.
Acute Megakaryoblastic Leukemia (AMKL) is a rare and deadly form of acute myeloid leukemia. There are three major subtypes of AMKL that differ from one another in their genetics and their prognosis: leukemia found in children with Down syndrome (DS), in children without DS, or in adults. Of these groups, the pathogenesis of DS-AMKL has the greatest clarity. Nearly 5% of children with DS-AMKL acquire GATA1 mutations in mid-gestation that lead to abnormal megakaryocyte development. It is believed that the combination of a GATA1 mutation and aberrant expression of ERG and DYRK1A, among other genes on chromosome 21, promotes a pre-leukemia named transient myeloproliferative disorder (TMD) (Malinge et al., 2012). Evolution of TMD to AMKL likely requires the acquisition of additional mutations in genes such as MPL and JAK2, which are associated with aberrant megakaryopoiesis in the myeloproliferative neoplasms (MPNs). It is also believed that GATA1 mutations confer hypersensitivity to treatment with cytosine arabinoside, which leads to a favorable outcome (Ge et al., 2004). In sharp contrast, adults with AMKL face a dismal prognosis with nearly all patients relapsing within one year of diagnosis (Tallman et al., 2000). Apart from sporadic mutations in JAK2 and MPL, little is known about the genetic basis of adult AMKL.
Although there have been many revelations about pediatric AMKL in children with DS, much less is known about the etiology of other pediatric cases. One exception is the presence of a recurring (1;22) translocation, which creates the OTT-MAL (or RBM15-MKL1) fusion unique to pediatric AMKL. This fusion leads to altered expression of Serum Response Factor (SRF) target genes and aberrant Notch pathway activation (Cheng et al., 2009; Mercher et al., 2009). Additionally, rare findings of JAK and MPL mutations have also been observed in this group.
To identify new mutations and chromosomal aberrations that define pediatric AMKL, Downing and colleagues took advantage of next-generation sequencing, a powerful tool that provides new insights into the genetics of cancer (Gruber et al., 2012). Paired-end sequencing of a discovery cohort consisting of 14 pediatric non-DS AMKL patients revealed structural variations that led to novel chimeric transcripts in 12 of the cases. Of note, half of the AMKL cases harbored a cryptic inversion on chromosome 16 inv(16)(p13.3q24.3) that led to fusion of CBFA2T3 and GLIS2. Two of these cases with the CBFA2T3-GLIS2 fusion also had a gain of chromosomal arm 21q, a common abnormality in AMKL. Remarkably, after screening a larger validation cohort and other leukemia samples, the fusion was detected in 27% of pediatric non-DS AMKL cases. The CBFA2T3-GLIS2 fusion was not observed in adult AMKL nor in any other form of myeloid leukemia. In addition, none of the samples with inv(16)(p13.3q24.3) had t(1;22), suggesting that these two recurring translocations are mutually exclusive. Surprisingly, 7 cases, including three cases with the novel fusion, also contained amplification of the Down syndrome critical region, implying that dysregulation of Hsa21 genes contributes to more than just the DS subtype of AMKL (Gruber et al., 2012).
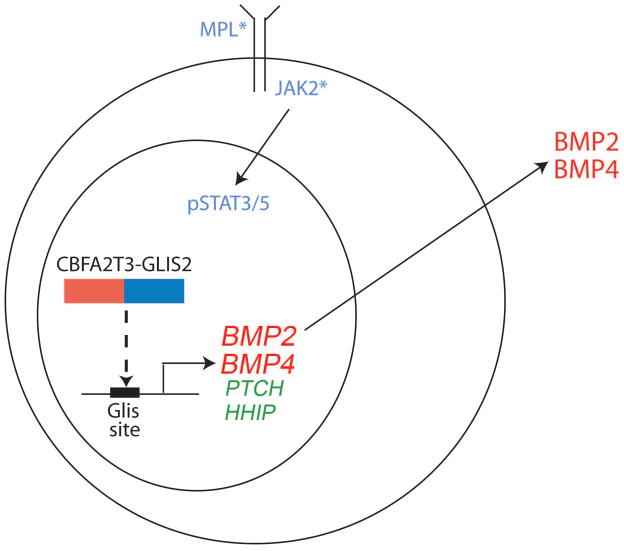
CBFA2T3 is a member of the ETO family of co-repressors that is expressed in hematopoietic cells and plays a role in regulating stem cell quiescence (Chyla et al., 2008). In contrast, the gene encoding GLIS2, which participates in the regulation of SHH signaling, is not expressed in hematopoietic cells. However, as a consequence of the fusion, the C-terminal zinc finger domain that binds the GLIS consensus sequence is fused to the N-terminal CBFA2T3 nervy domain that mediates protein-protein interactions and is expressed in blood cells. Gene expression studies comparing fusion-positive AMKLs against other forms of AML revealed that cases with the fusion showed altered expression of genes in the BMP, SHH, and WNT pathways. In particular, marked overexpression of BMP2 and BMP4 was observed (Figure). Of note, expression of CBFA2T3-GLIS2 or GLIS2 alone in Drosophila led to enhanced BMP signaling and the associated dpp gain-of-function phenotype, including shortened legs and wing blistering (Gruber et al., 2012).
Figure. Model of the activity of inv(16)(p13.3q24.3) gene fusion product in AMKL.
The CBFA2T3-GLIS2 fusion protein generated by inv(16)(p13.3q24.3) likely directly activates transcription of activators of BMP signaling BMP2 and BMP4 as well as inhibitors of SHH signaling PTCH and HHIP. BMP2/4 may act in an autocrine manner to foster growth of AMKL blasts or may alternatively signal in a paracrine manner to hematopoietic progenitors that, in turn, would promote the megakaryocytic lineage phenotype of the leukemia. In addition, mutations in MPL or JAK family members confer cytokine independence and likely cooperate with the fusion to promote AMKL.
The discovery of this novel recurring translocation raises a number of important questions. First, is the fusion a necessary and/or sufficient factor in leukemogenesis? Functional studies demonstrated that expression of the fusion protein, or GLIS2 alone, led to enhanced self-renewal of hematopoietic progenitors in vitro. This phenotype was blocked by dorsomorphin, a small molecule that interferes with BMP signaling to Smad effectors, suggesting that the increased expression of BMP downstream of the fusion is essential for the phenotype. Expression of the CBFA2T3-GLIS2 fusion transcript in mice, however, did not lead to leukemia, indicating that the fusion protein is not sufficient for leukemogenesis. It is likely that mutations, which confer cytokine independent growth, such as those in MPL or JAK family members, cooperate with the fusion to promote AMKL.
Second, is there a contribution by CBFA2T3 apart from driving expression of GLIS2 in hematopoietic cells? The finding that GLIS2 transduced hematopoietic progenitors failed to re-plate to the same extent as progenitors transduced with the fusion strongly suggests that there is an additional, as yet undefined, contribution by CBFA2T3. Alternatively, it is possible that loss of the N-terminal amino acids of GLIS2 in the fusion may alter GLIS2 function.
Third, what is the natural history of the disease? GATA1 mutations in DS-AMKL originate in the fetal liver and can be detected as early as 21 weeks of gestation (Taub et al., 2004). However, although GATA1 mutations in DS are suspected to cause TMD, not all babies with GATA1 mutations show an overt hematopoietic phenotype and the majority do not go on to develop AMKL. This finding is reminiscent of classical studies by Mel Greaves and colleagues who showed that chromosomal translocations can be detected at birth even in children who do not go on to develop leukemia (Greaves and Wiemel, 2003). The timing of the pediatric non-DS AMKL suggests that the fusion of CBFA2T3 and GLIS2 occurs in utero and within the fetal liver. Whether all instances of the fusion lead to leukemia remains to be determined, but the fact that expression of CBFA2T3-GLIS2 in mice does not confer leukemia suggests that additional events are required for full transformation. Similar to GATA1 mutations, the fusion is not detected in adult AMKL, implying perhaps that necessary cooperating events are not possible in bone marrow progenitors.
Fourth, how does the translocation specifically lead to pediatric AMKL? Previous studies have shown that increased BMP signaling induces the differentiation of CD34+ cells to megakaryocytes (Jeanpierre et al., 2008). This effect may be the result of upregulation of JAK/STAT signaling, as seen in MPNs and AMKL with activating mutations in MPL and JAK family members. However, the details of how activated BMP signaling, downstream of the CBFA2T3-GLIS2 fusion protein, imparts a megakaryocytic phenotype remains a mystery.
Finally, will BMP inhibitors prove to be effective new therapies for AMKL? Given the poor prognosis of AMKL and, in particular, cases with the CBFA2T3-GLIS2 fusion, development of novel therapies is essential. The observation that a BMP antagonist disrupts re-plating of hematopoietic progenitors suggests that inhibition of BMP signaling may provide therapeutic benefit. However, additional events that cooperate with the fusion to drive acute leukemia may circumvent anti-BMP therapy. Future pre-clinical and, if appropriate, clinical studies to determine the effectiveness of BMP inhibitors against human AMKL are needed.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Cheng EC, Luo Q, Bruscia EM, Renda MJ, Troy JA, Massaro SA, Tuck D, Schulz V, Mane SM, Berliner N, et al. Role for MKL1 in megakaryocytic maturation. Blood. 2009;113:2826–2834. doi: 10.1182/blood-2008-09-180596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chyla BJ, Moreno-Miralles I, Steapleton MA, Thompson MA, Bhaskara S, Engel M, Hiebert SW. Deletion of Mtg16, a target of t(16;21), alters hematopoietic progenitor cell proliferation and lineage allocation. Molecular & Cellular Biology. 2008;28:6234–6247. doi: 10.1128/MCB.00404-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge Y, Jensen TL, Stout ML, Flatley RM, Grohar PJ, Ravindranath Y, Matherly LH, Taub JW. The role of cytidine deaminase and GATA1 mutations in the increased cytosine arabinoside sensitivity of Down syndrome myeloblasts and leukemia cell lines. Cancer Res. 2004;64:728–735. doi: 10.1158/0008-5472.can-03-2456. [DOI] [PubMed] [Google Scholar]
- Greaves MF, Wiemel J. Origins of chromosome translocations in childhood leukaemia. Nat Rev Cancer. 2003;3:639–649. doi: 10.1038/nrc1164. [DOI] [PubMed] [Google Scholar]
- Gruber, et al. (this issue) [Google Scholar]
- Jeanpierre S, Nicolini FE, Kaniewski B, Dumontet C, Rimokh R, Puisieux A, Maguer-Satta V. BMP4 regulation of human megakaryocytic differentiation is involved in thrombopoietin signaling. Blood. 2008;112:3154–3163. doi: 10.1182/blood-2008-03-145326. [DOI] [PubMed] [Google Scholar]
- Malinge S, Bliss-Moreau M, Kirsammer G, Diebold L, Chlon T, Gurbuxani S, Crispino JD. Increased dosage of the murine chromosome 21 orthog Dyrk1a promotes megakaryoblastic leukemia in Down syndrome. Journal of Clinical Investigation. 2012;122:948–962. doi: 10.1172/JCI60455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercher T, Raffel GD, Moore SA, Cornejo MG, Baudry-Bluteau D, Cagnard N, Jesneck JL, Pikman Y, Cullen D, Williams IR, et al. The OTT-MAL fusion oncogene activates RBPJ-mediated transcription and induces acute megakaryoblastic leukemia in a knockin mouse model. J Clin Invest. 2009;119:852–864. doi: 10.1172/JCI35901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallman MS, Neuberg D, Bennett JM, Francois CJ, Paietta E, Wiernik PH, Dewald G, Cassileth PA, Oken MM, Rowe JM. Acute megakaryocytic leukemia: the Eastern Cooperative Oncology Group experience. Blood. 2000;96:2405–2411. [PubMed] [Google Scholar]
- Taub JW, Mundschau G, Ge Y, Poulik JM, Qureshi F, Jensen T, James SJ, Matherly LH, Wechsler J, Crispino JD. Prenatal origin of GATA1 mutations may be an initiating step in the development of megakaryocytic leukemia in Down syndrome. Blood. 2004;104:1588–1589. doi: 10.1182/blood-2004-04-1563. [DOI] [PubMed] [Google Scholar]