Abstract
Reaction time (RT) and event-related potential (ERP) measures were used to examine the relationships between psychopathic symptoms and three major attention networks (alerting, orienting, and executive attention) among a community sample of youth. Antisocial Process Screening Device (APSD; Frick and Hare 2001) total and subscale scores were negatively correlated with ERP measures of attentional alerting, indicating that youth with psychopathic symptoms had difficulty using warning cues to prepare for upcoming targets. APSD total scores were not related to performance on measures of orienting or executive attention, although weaker executive attention was found among youth with higher scores on the Impulsivity subscale. These findings support attention-based models of psychopathy and provide evidence of specific deficits in attentional alerting among youth with psychopathic traits. Deficiencies in attentional alerting may be related to noradrenergic functioning and may have cascading effects on higher order cognitive and affective processing.
Keywords: Attention, Psychopathy, Antisocial personality, ANT, Evoked potential
Introduction
Psychopathy is a complex personality disorder characterized by callous and manipulative treatment of others, lack of meaningful relationships, irresponsibility, persistent antisocial behavior, and lack of remorse or guilt. Although it is tempting to attribute psychopathy to maliciousness or willful misconduct, it is difficult to reconcile this view with the often self-defeating and capricious nature of psychopathic behavior (Cleckley 1982). Accordingly, modern theoretical and empirical work on psychopathy has focused on identifying intrinsic deficits that may account for the behavior of psychopathic individuals. Decades of empirical research have established that criminal offenders with psychopathy show subtle yet important abnormalities in emotion, cognition, language, and neuropsychological function when compared with nonpsychopathic offenders (see Blair 2005; Hiatt and Newman 2006). Interestingly, these deficits occur against a background of generally good executive function (Hare 1984; Hart et al. 1990; but see Blair et al. (2006)), and average or better intelligence (Johansson and Kerr 2005; Vitacco et al. 2005).
One of the most striking and well-replicated deficits of psychopathy is a difficulty using punishment-related cues, even when doing so would result in monetary gain or other tangible rewards. Although individuals with psychopathy generally react normally to direct punishment (e.g., Flor et al. 2002), they have difficulty inhibiting previously punished behaviors (e.g., Lykken 1957; Newman et al. 1990) and show weak physiological responses to both conditioned punishment cues (Flor et al. 2002; Hare 1982) and unpleasant images (Levenston et al. 2000; Patrick et al. 1993).
These punishment-related deficits have led to neurobiological models of psychopathy that emphasize amygdala dysfunction (Blair 2006). Several studies have reported functional (e.g., Glenn et al. 2009) and/or structural (e.g., Yang et al. 2009) abnormalities of the amygdala in psychopathic populations. However, abnormalities have also been found in a number of other brain structures (see Kiehl 2006; Weber et al. 2008). Moreover, although amygdala dysfunction appears to contribute to some aspects of psychopathy, it cannot account for all aspects of the disorder.
An important and fascinating aspect of psychopathy that cannot readily be accounted for by amygdala dysfunction is the context dependency of psychopathic individuals’ deficits. Psychopathic individuals’ ability to use punishment-related information, for example, is typically indistinguishable from that of controls when the importance of avoiding punishment is made explicit in the task instructions or is otherwise evident from the start of the task (e.g., Kiehl et al. 2000; Newman and Kosson 1986). Insensitivity to punishment cues is generally seen only when the punishment contingencies are not explicit or when punishment avoidance must compete with prepotent reward-seeking goals (Newman et al. 1997).
This context dependency has been interpreted as an attention-related failure to process potentially important information when it is peripheral to ongoing, goal-directed behavior (Newman 1998). Consistent with this interpretation, known as the Response Modulation Hypothesis (RMH), psychopathic individuals are insensitive to peripheral or contextual information even in the absence of punishment or other emotionally salient stimuli (see Hiatt and Newman 2006). Moreover, a recent study by Newman and colleagues (Newman et al. 2010) provided direct evidence that one of the hallmark punishment-related deficits of psychopathy, poor fear-potentiated startle (FPS), is critically dependent upon attention: psychopathic individuals showed deficient FPS when their attention was directed away from threat-related information, but showed normal FPS when the threat-related information was attended.
Attention thus may play a critical role in psychopathy, even potentially driving punishment-related deficits. However, it remains unclear which aspects of attention are affected. Attention is a multifaceted construct that is instantiated at multiple levels of processing and by multiple neurobiological systems. Although the attention-related deficit proposed by Newman’s RMH is conceptually well specified (Newman 1998), it is not readily linked to extant neurobiological or cognitive models of attention. Further, most of the evidence for attention dysfunction in psychopathy comes from performance on relatively complex tasks that involve a range of processes, such as suppressing dominant responses, switching between rule sets, and managing goals. With few exceptions (e.g., Harpur 1991), basic components of attention have not been directly examined.
Our study involved an initial examination of basic attention processes among youth with psychopathic features as assessed by the Antisocial Process Screening Device (APSD; Frick and Hare 2001). Recent studies have shown that many of the deficits seen in adult psychopathy are also present in youth with elevated APSD scores (e.g., Blair et al. 2001; Vitale et al. 2005). We assume that deficits in basic attention processes are present throughout development and may in fact be more visible in younger populations due to less developed compensatory strategies. Early identification of attention-related abnormalities may ultimately improve early treatment and prevention efforts, which is especially important given the difficulty of treating psychopathy in adulthood.
In this study, we examined the three attention processes described by Posner and colleagues as the alerting, orienting, and executive attention networks (Berger and Posner 2000). The alerting network is involved in achieving and maintaining an alert state and preparedness to react to stimuli and has been linked to the noradrenergic system (Coull et al. 2001) and the right parietal lobe (Corbetta and Shulman 2002). The orienting network is involved in allocating attention to specific locations in space and has been linked to the acetylcholine system (Phillips et al. 2000; Witte et al. 1997) and the superior and inferior parietal lobe (Corbetta et al. 2000; Coull et al. 2001; Rosen et al. 1999). The executive attention network is involved in target detection, error detection, and conflict resolution and has been linked to the ventral tegmental area dopaminergic system (Cools 2008; Granon et al. 2000), the anterior cingulate (Bush et al. 2000), and prefrontal cortex (Kadosh et al. 2008; Sandrini et al. 2008).
We used the Attention Network Task (ANT; Fan et al. 2002) to survey the integrity of these three attention networks. The ANT combines elements of traditional spatial cueing (Posner 1980) and flanker interference (Eriksen and Eriksen 1974) paradigms. Executive attention is assessed via flanker interference, and the alerting and orienting networks are estimated by the effects of preceding cue conditions on the speed of response to the target-flanker display.
The ANT provides fairly independent and reliable reaction-time (RT) estimates of the three attention networks (Fan et al. 2002). However, we were concerned about the sensitivity of RT measures for estimating the alerting and orienting networks. RT variability related to resolving flanker interference and subsequent motor response processes may be much larger than the cue effects, potentially obscuring variability related to the cue conditions. To improve the sensitivity of the alerting and orienting estimates, we used event-related potentials (ERPs) as well as RTs to estimate the three attention networks.
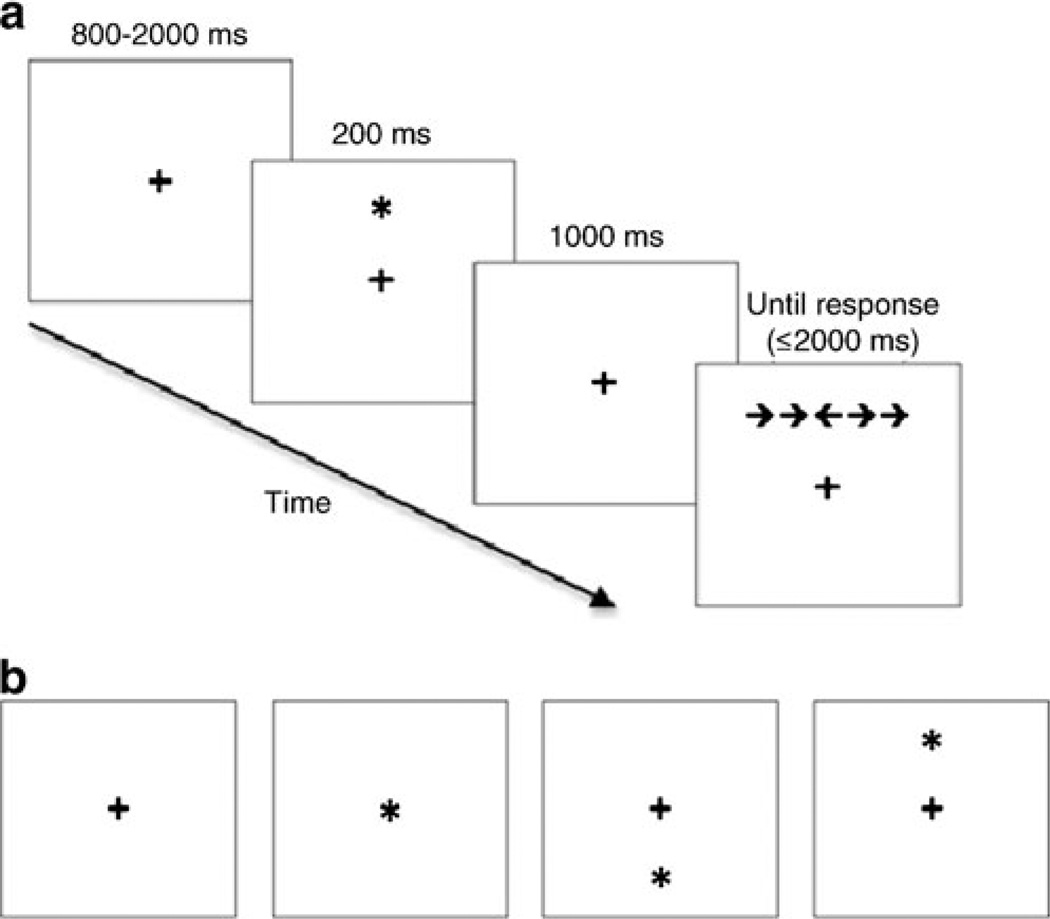
Alerting and orienting were assessed using the occipital P1 event-related potential (ERP) component. The P1 provides a relatively direct and sensitive index of where attention is directed in space, without contamination from response-related processes (see Hillyard and Anllo-Vento (1998)). The more a participant’s attention is directed toward an upcoming target, the larger the amplitude (voltage) of the P1 response to the target should be. Thus, the P1 response to the target should be smallest for the no cue condition, which provides neither temporal nor spatial information about the upcoming target, somewhat larger for the central cue condition, which provides temporal information about the upcoming target, and largest of all for the spatial cue condition, which allows participants to focus their attention directly on the location of the upcoming target (see Fig. 1).
Fig. 1.
a Schematic of ANT trial events, depicting a spatial cue and a left-arrow target with incongruent flankers; b possible cue conditions (no cue, central cue, lower spatial cue, upper spatial cue)
ERP indices of flanker interference are not as well established. Although fMRI studies have consistently shown that frontal executive areas, including the anterior cingulate cortex, are activated by conflict and interference (Ochsner et al. 2009; Wang et al. 2010), these findings are not readily translated to ERP measures. We chose to examine the N450, which has been associated with conflict processing on Stroop-like tasks (e.g., Larson et al. 2009; West et al. 2005), and the P3b, which is associated with cognitive effort and has shown flanker congruency effects in previous studies (Johnstone et al. 2009; Rueda et al. 2004; Wild-Wall et al. 2008). We expected incongruent flankers to produce a more negative N450 response and a more positive P3b response relative to congruent flankers.
Psychopathic individuals’ difficulty using information that is outside of their current attentional focus could be consistent with deficits in either alerting or orienting. Deficits in alerting would interfere with the detection of new or unexpected events by dampening perceptual sensitivity (i.e., stimuli must meet a higher threshold to capture attention), whereas deficits in orienting would make it difficult to redirect attention (and subsequent cognitive resources) to new information once it has been perceived. We therefore predicted that psychopathic symptoms would be associated with deficits in alerting and/or orienting.
We did not expect to find deficits in executive attention, given that existing studies have generally shown good executive function (EF) among psychopathic individuals, and there is a large body of work demonstrating normal (or better) conflict resolution on stroop and flanker paradigms (e.g., Hiatt et al. 2004; Zeier et al. 2009).
Finally, it is important to note that our hypotheses, like the findings reviewed throughout the introduction, are based on the measurement of psychopathy as a unitary construct (i.e., using total scores on rating scales). However, psychopathy can also be subdivided into two, three, or four correlated factors reflecting different clusters of symptoms (see Patrick 2006). There is increasing interest in examining the unique correlates of different factors, which may differ from those of the overarching construct. Of particular relevance to the current study, factor-based approaches have shown that while overall psychopathy scores are typically unrelated to EF, the antisocial/impulsive symptoms of psychopathy (Factor 2 in two-factor models) are often associated with lower EF, whereas the interpersonal/affective features (Factor 1 in two-factor models) are associated with average or better EF (e.g., Ross et al. 2007). To allow examination of differential effects, we report results for APSD subscales as well as the total score. However, we direct our hypotheses to APSD total scores.
Methods
Participants
Participants were 54 children and adolescents (22 male, 32 female) ages 9–14 years (M=11.76, SD=1.33) recruited from the Eugene, Oregon, community by means of flyers and newspaper advertisements. Youth at risk for psychopathology were oversampled by placing flyers at counseling and family service agencies. The sample was 86% Caucasian. A brief telephone screening was used to ensure that participants met the following inclusion criteria: normal or corrected-to-normal vision, functional use of both hands, English fluency, and no history of traumatic brain injury, epilepsy, or seizures. Participants were also required to be right-handed according to self-report on the Edinburgh Handedness Inventory (Laterality Quotient >40).
Measures
Antisocial Process Screening Device (APSD)
Parent report on the APSD (Frick and Hare 2001) was used to assess psychopathic characteristics. The APSD is a 20-item questionnaire designed to assess behaviors and attitudes in youth that are associated with adult psychopathy. Each item was rated on a 3-point scale (0=not at all true, 1=sometimes true, 2=definitely true), with higher scores indicating higher levels of psychopathic traits. The APSD produces age-normed T-scores for the overall total score and for three subscales (callous–unemotional (CU), narcissism (N), and impulsivity (I)). In our sample, the APSD total score demonstrated good internal consistency (Cronbach’s α=0.83). Subscale alphas ranged from 0.61 to 0.68.
Control Variables
To clarify whether any significant relationships between APSD total scores and attention network measures were specific to psychopathy per se, all significant zero-order correlations with APSD total scores were also examined after controlling for internalizing symptoms (INT), externalizing symptoms (EXT), attention-deficit/hyperactivity symptoms (ADHD), and executive functioning, as well as age and gender. INT, EXT, and ADHD scores were obtained from the Child Behavior Checklist (Achenbach and Rescorla 2001), and executive function scores were obtained from the Behavior Rating Inventory of Executive Functioning (Gioia et al. 2000).
Child Behavior Checklist (CBCL)
INT, EXT, and ADHD symptoms were assessed by parent report on the CBCL (Achenbach and Rescorla 2001). The CBCL is a 113-item questionnaire designed to assess behavioral and emotional problems in youth ages 6–18 years. Each item was rated on a 3-point scale indicating how often the parent observed each symptom in their child (0=not at all, 1=sometimes, 2=often). The CBCL produces age- and gender-normed T-scores indexing internalizing problems, externalizing problems, and total problems, as well as subscales such as attention problems, depression, and anxiety. ADHD symptoms were assessed using the DSM-oriented ADHD scale.
Behavior Rating Inventory of Executive Function (BRIEF)
General executive and self-regulatory skill was assessed by parent report on the BRIEF (Gioia et al. 2000). The 86-item BRIEF assesses behavior regulation (inhibition, shifting, emotional control) and metacognitive skills (initiation, planning, working memory, organization, self-monitoring). Each item was rated on a 3-point scale indicating how often the parent observed each behavior in their child (0=never, 1=sometimes, 2=often). The age-normed Global Executive Composite (GEC) T-score was used to control for general executive skill.
Attention Network Task
The ANT is a computerized task designed to assess alerting, orienting, and executive attention (see Fig. 1). Participants indicated the direction of a target arrow by pressing a left or right response key with their left or right index finger, respectively. Target arrows were flanked by two distractor arrows on either side, which could be either congruent (pointing in the same direction as the target, 50% probability) or incongruent (pointing in the opposite direction, 50% probability). The target and flankers appeared in either the upper or lower half of the screen (equiprobable) and remained visible until the participant responded or until 2 s elapsed.
Each target-flanker presentation was preceded by a 200-ms cue and a 1,000 ms cue-to-target interval. Cue conditions were equally divided between no cue (central fixation cross remained unchanged until the target appeared, 48 trials), central cue (asterisk in the center of the screen, providing temporal but not spatial information about the upcoming target, 48 trials), and spatial cue (asterisk occurs in target location, 100% valid, 48 trials) conditions. Each cue condition (including no cue) was preceded by a fixation cross of variable duration (800–2,000 ms).
RT and ERP measures of the three attention networks were obtained by comparing target responses under the following conditions: no cue versus central cue (alerting), central cue versus spatial cue (orienting), and congruent flankers versus incongruent flankers (executive attention).
Procedure
All procedures were carried out in one 2-hour laboratory testing session. After informed parent consent and child assent were obtained, the parent was shown to a waiting area to complete the questionnaires and the child was brought to the EEG laboratory. The ANT was one of several computerized tasks administered in counter balanced order during the testing session. Participants were instructed to keep their gaze focused on the central fixation point throughout the task and to respond as quickly and accurately as possible. Total time to complete all tasks was approximately 1 h.
EEG Data Processing and Analysis
Scalp electroencephalographic (EEG) data were acquired using a 256-channel HydroCel Geodesic Sensor Net (Electrical Geodesics Inc. [EGI], Eugene, OR). The data were analog filtered with a 0.01–100-Hz bandpass prior to being digitized at 250 Hz with a 16-bit A/D converter. The EEG was referenced to the vertex during acquisition.
A 60-Hz notch filter was applied prior to the analysis of the EEG data. EEG data were segmented off-line into 800-ms epochs spanning 200 ms before to 600 ms after the appearance of the target. Trials with incorrect behavioral responses were excluded from analyses. Individual epochs were screened for eye blinks, eye movements, bad channels, and other noncephalic artifacts. Uncontaminated trials were averaged to create the individual subject waveforms, which were baseline corrected over the 200-ms pretarget interval and transformed into an average reference representation. Individual-subject average waveforms were averaged together to derive the group grand-average wave-forms.
Based on previous research and the grand-average waveforms, the time windows used to extract the P1 and P3b components from the individual-subject waveforms were 100–180 ms and 300–380 ms post target, respectively. For these components, a positive peak was identified within the specified window for each individual subject, and an average was obtained for a 40-ms window centered on the peak. The N450 was measured as the average amplitude of the EEG between 350 ms and 500 ms post target. Separate averages were created for each participant for each of the two target conditions (incongruent or congruent flankers) and the three cue conditions (no cue, central cue, and spatial cue).
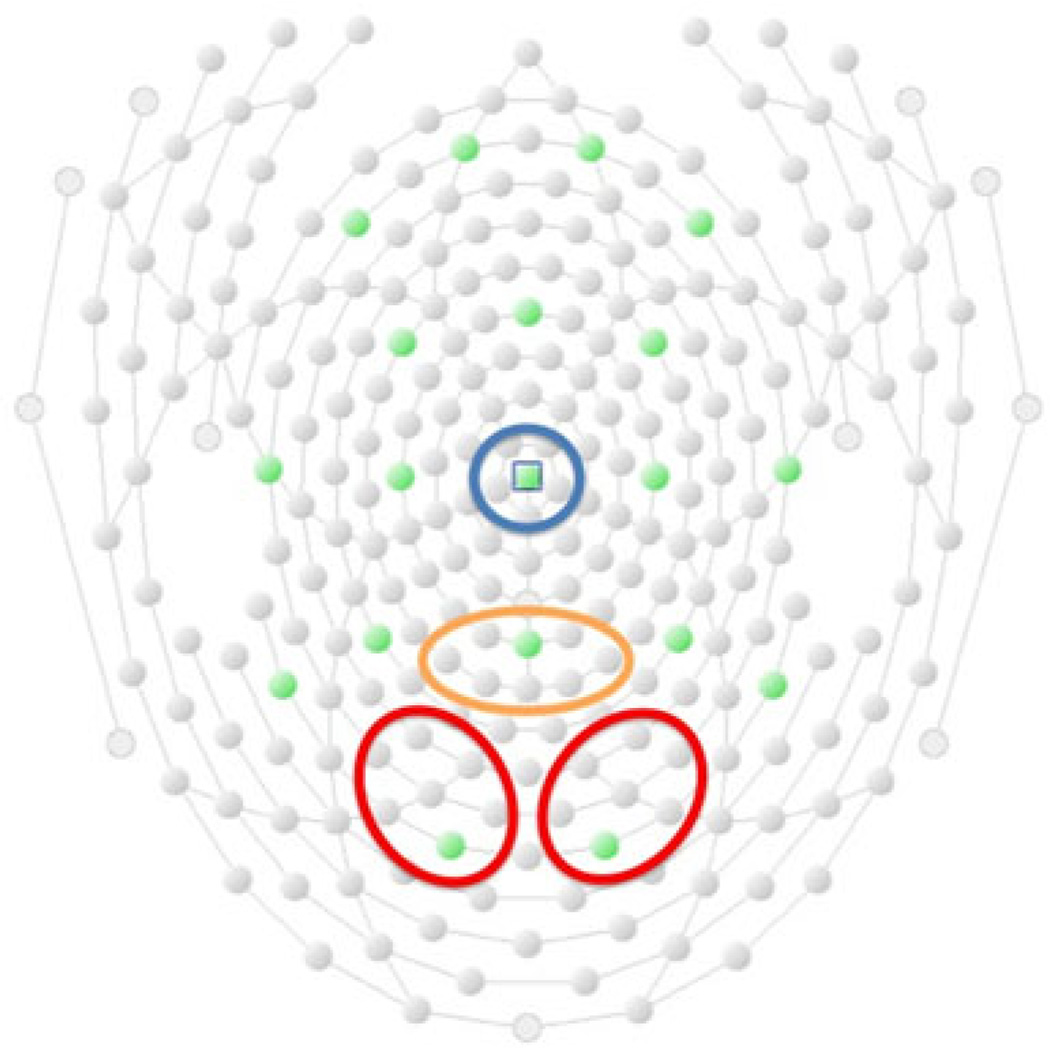
Electrodes used for quantification of the P1, P3b, and N450 components were selected based on previous studies and the locations of maximal component amplitude in the grand-average waveforms. The P1 component was measured using the average of 14 occipital-parietal sites (7 per hemisphere) that included O1 and O2 (Fig. 2, red; EGI sensor numbers: left 96, 97, 106, 107, 108, 115, 116; right 150, 151, 159, 160, 161, 169, 170), the P3b component was measured using the average of 8 parietal sites centered around Pz (Fig. 2, orange; EGI sensor numbers 100, 101, 129, 99, 110, 119, 128, 141), and the N450 was measured using the average of 6 central sites centered around Cz (Fig. 2, blue; EGI sensor numbers 9, 45, 81, 132, 186, VREF).
Fig. 2.
The occipital (red), parietal (orange), and medial frontal (blue) electrode groups used in statistical analyses
Results
Descriptive Statistics
Eleven participants (5 boys, 6 girls) were excluded from analyses because of EEG artifacts (eye blinks, eye movements, or bad channels) in greater than 33% of the target segments, resulting in a final sample of 43 participants (17 boys, 26 girls). APSD total and subscale scores for the final sample are presented in Table 1, along with selected scores from the CBCL and BRIEF. APSD scores did not differ by gender (all Fs<1.8, ps>0.19) and were not correlated with age (rs<0.16, ps>0.32).
Table 1.
Descriptive statistics for APSD, CBCL, and BRIEF Measures
| Mean t-score | SD | Range | ||
|---|---|---|---|---|
| APSD | Callous–unemotional | 48.81 | 9.23 | 37–70 |
| Narcissism | 48.77 | 9.46 | 39–72 | |
| Impulsivity | 49.93 | 7.64 | 35–69 | |
| Total | 48.35 | 8.62 | 35–70 | |
| CBCL | Internalizing | 51.33 | 11.06 | 33–72 |
| Externalizing | 47.67 | 8.70 | 34–64 | |
| ADHD | 55.40 | 6.26 | 50–72 | |
| BRIEF | Global executive composite | 53.93 | 9.23 | 36–76 |
APSD Antisocial Process Screening Device, CBCL Child Behavior Checklist, BRIEF Behavior Rating Inventory of Executive Function
Behavioral Results
Mean accuracy was 94.93% (range=81%–100%). APSD scores were not significantly correlated with mean RT (all rs<0.20, ps>0.20) or accuracy (all rs<0.24, ps>0.13).
RT difference scores were created to index alerting (central cue minus no cue), orienting (spatial cue minus central cue), and executive attention (incongruent flankers minus congruent flankers, hereafter referred to as conflict). The expected main effects were obtained for each of these measures: alerting t (42)=7.64, p<0.001; orienting t(42)=4.00, p<0.001; conflict t(42)=17.46, p<0.001, indicating that responses were faster following a central cue than no cue, faster following a spatial cue than a central cue, and faster for targets with congruent versus incongruent flankers. Means and standard deviations are presented in Table 2.
Table 2.
Mean reaction times by Cue and Flanker conditions
| Mean RT | SD | ||
|---|---|---|---|
| Condition | No cue | 773.53 | 147.64 |
| Central cue | 736.06 | 141.60 | |
| Spatial cue | 702.74 | 136.01 | |
| Incongruent | 808.85 | 145.26 | |
| Congruent | 671.92 | 135.58 | |
| Difference score | Alerting | 37.47 | 32.19 |
| Orienting | 33.32 | 54.66 | |
| Conflict | 136.93 | 51.44 |
APSD Total Score
Individual differences were examined by means of bivariate correlations between each of the three attention measures and the APSD total score. To ensure that the attention measures were not confounded by overall RT differences, the difference scores were corrected for overall RT by dividing each difference score by the sum of the operands; for example, alerting=(no cue RT−central cue RT) / (no cue RT+central cue RT). APSD total score was not correlated with RT measures of alerting (r=−0.09, p=0.55) or orienting (r=−0.05, p=0.73). There was a trend-level positive correlation between APSD total score and conflict (r=0.24, p=0.12), but it did not survive after controlling for externalizing symptoms (partial r=0.16, p=0.32), ADHD symptoms (partial r=0.13, p=0.41), or GEC (partial r=0.05, p=0.78).
Subscale Analyses
Higher scores on the Impulsivity subscale were associated with increased flanker interference (r=0.38, p<0.05). There were no other significant correlations between APSD subscales and any of the RT measures (all rs<0.22, ps>0.15)1.
ERP Results
Preliminary Analyses
Descriptive Statistics
After exclusion of EEG segments that were contaminated by noncephalic artifacts, each target condition (congruent, incongruent) had an average of 60 trials, M (SD)=60.0 (7.65), range=35–72, and each cue condition (no, center, spatial) had an average of 40 trials, M (SD)=40.3 (5.41), range=26–48. The number of good segments was correlated with age (r=0.31, p<0.05), but not with APSD total scores (r=0.15, p=0.36).
One participant was excluded from further analysis because of ERP difference scores that were greater than three standard deviations from the mean, resulting in a total of 42 participants for the ERP analyses. The exclusion of this participant had no meaningful effects on the previously reported RT results.
Main Effects
ERP amplitude difference scores were computed to create measures of alerting (central cue minus no cue), orienting (spatial cue minus central cue), and conflict (incongruent flankers minus congruent flankers). Alerting and orienting effects were examined for P1, and conflict effects were examined for the P3b and N450 components.
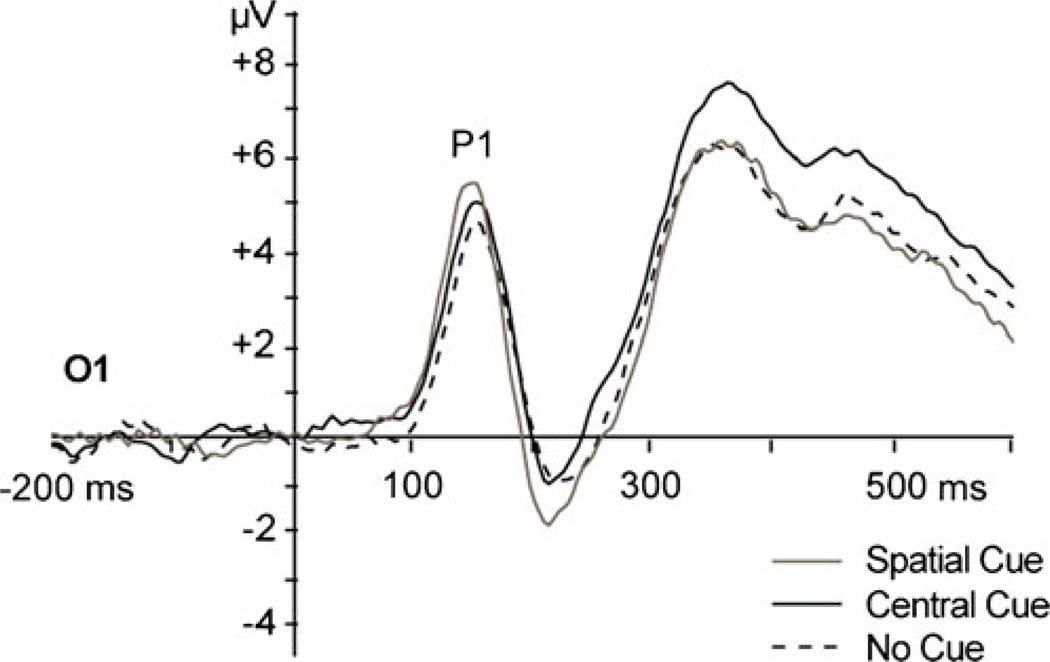
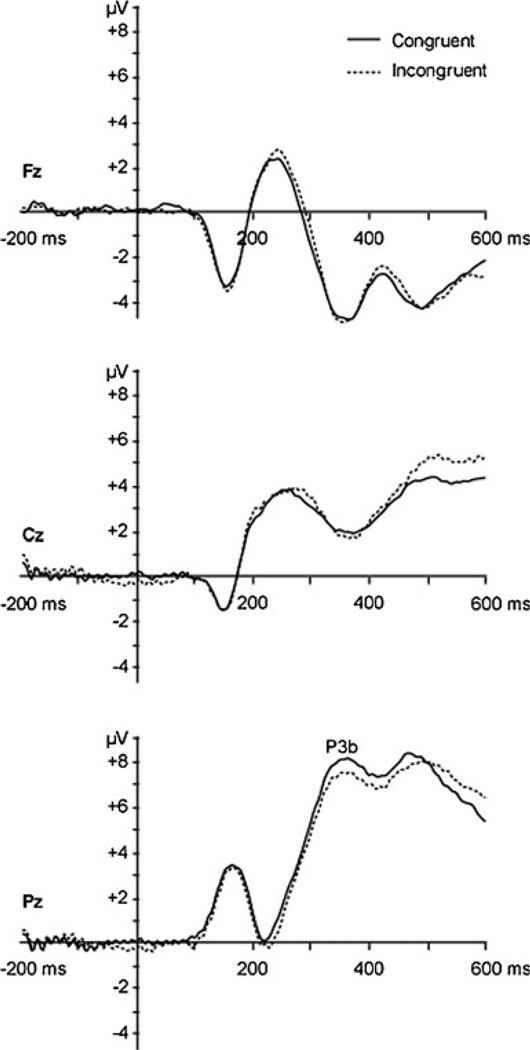
Before examining individual differences, we conducted a series of one-sample t-tests to identify which components showed significant main effects of attention. The occipital P1 component showed significant effects for both alerting, M (SD)=1.54 (2.07) µV; t(41)=4.82, p<0.001, and orienting, M (SD)=1.04 (1.60) µV; t(41)=4.22, p<0.001, indicating that, as expected, the target-evoked P1 was larger for targets preceded by central cues versus no cues, and for targets preceded by spatial cues versus central cues. A significant P3b conflict effect was found over parietal sites, M (SD)=−0.96 (1.97) µV; t(41)=−3.17, p<0.01. Contrary to expectations, the P3b was larger for targets with congruent versus incongruent flankers. The N450 conflict effect was not significant (t(41)=1.57, p=0.13). Grand-average waveforms for each cue condition are shown for electrode site O1 in Fig. 3. Grand-average waveforms for the flanker conditions are shown for sites Fz, Cz, and Pz in Fig. 4.
Fig. 3.
Target-locked grand-average waveforms for cue conditions at occipital site O1. Target onset occurred at time zero
Fig. 4.
Target locked grand-average waveforms for incongruent and congruent flankers at Fz, Cz, and Pz. Target onset occurred at time zero
Individual Differences: ERP Amplitude
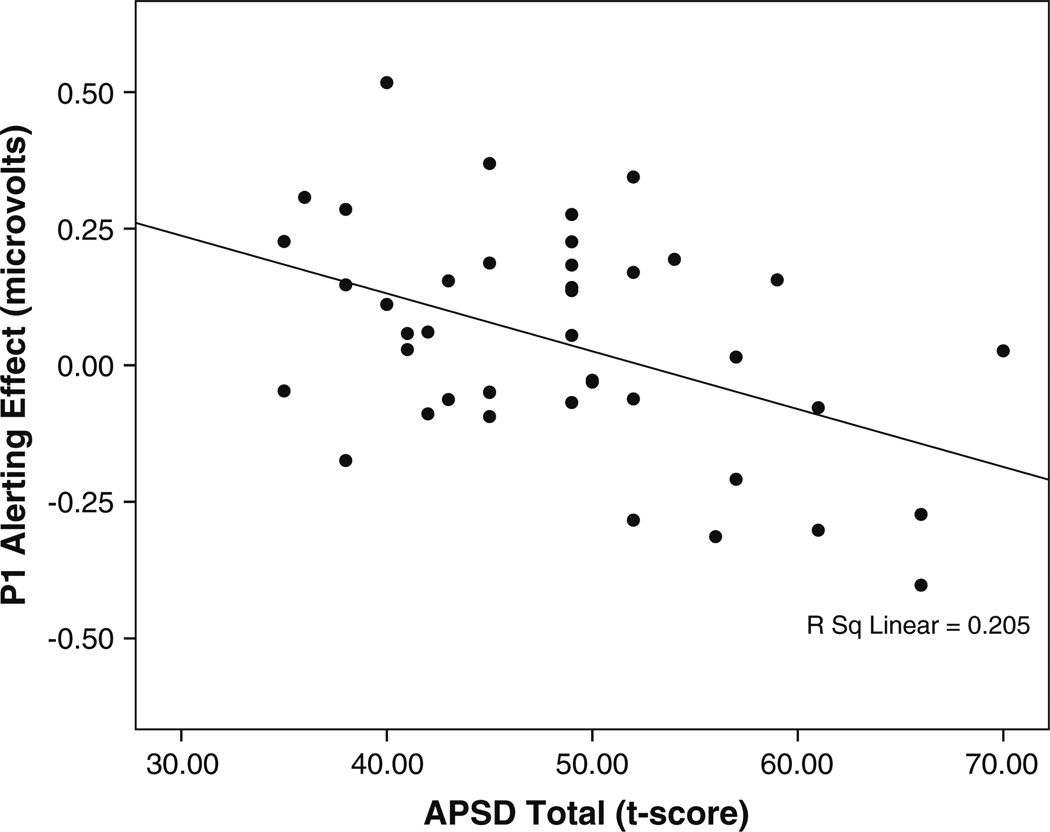
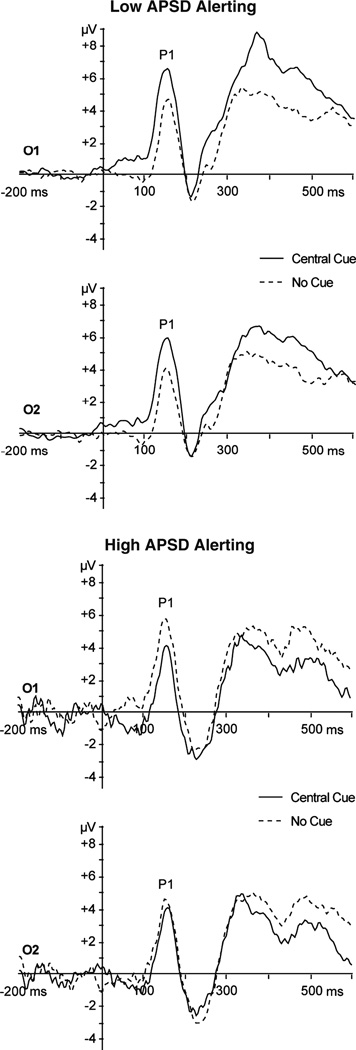
Individual differences were examined for components that showed significant main effects of attention (alerting P1, orienting P1, and conflict P3b). Difference scores were corrected for mean amplitude. Bivariate correlations between the APSD total score and the ERP attention measures revealed a significant negative correlation with alerting (r=−0.45, p<0.01), indicating weaker alerting among youth with higher APSD scores (see Fig. 5). This relationship remained significant after controlling for age, gender, INT, EXT, ADHD, and GEC. Average alerting waveforms for participants with high (top 20%; n=7) and low (bottom 20%; n=7) APSD total scores are presented in Fig. 6. APSD total score was not correlated with ERP indices of orienting (r=0.23, p=0.15) or conflict (r=0.01, p=0.95).
Fig. 5.
Correlation between APSD Total score and the P1 alerting effect
Fig. 6.
Target-locked grand average alerting waveforms for participants in the highest and lowest APSD quintiles. Waveforms are shown for occipital sites O1 and O2. Target onset occurred at time zero
Subscale Analyses
The relationship between APSD and P1 alerting was comparable and significant across all APSD subscales (r=−0.38, −0.39, and −0.33 for the CU, N, and I subscales, respectively; all ps<0.05). There were no other significant correlations with any of the ERP measures (all rs<0.22, ps>0.15).
Breaking Down the Difference Score
To better understand the processes underlying the reduced P1 alerting effects among youth with higher APSD scores, we examined correlations between APSD total score and P1 amplitude for each of the three cue conditions (no cue, central cue, spatial cue). APSD was negatively correlated with the P1 response to targets preceded by the central cue (r=−0.33, p<0.05), but not with the no cue or spatial cue conditions (rs=−0.04 and −0.15, respectively; ps>0.33). In multiple linear regression with the three cue conditions entered simultaneously, only the central cue condition was significantly related to APSD, t(41)=−3.29, p<0.01. These results suggest that youth with psychopathic characteristics had particular difficulty using the central cue to prepare for the upcoming target.
Discussion
We examined the relationships between three attention networks (alerting, orienting, and executive attention) and psychopathic symptoms in a community sample of youth and found an association between psychopathic symptoms and reduced attentional alerting. For youth with higher APSD scores, attention to the target, as assessed by the early sensory response over visual cortex (P1), was not improved by the prior presentation of a central, temporal cue. This relationship between psychopathy and alerting persisted even after controlling for global executive skills, ADHD symptoms, and symptoms of general psychopathology (internalizing and externalizing). APSD total scores were not associated with either behavioral or ERP indices of attentional orienting and executive attention, although there was evidence of weak executive attention among youth with higher scores on the Impulsivity subscale.
Despite the strong association between psychopathy and the P1 alerting response, there was no relationship between psychopathy and the RT measure of alerting. That is, the reduction in attention indicated by the P1 did not translate to slower behavioral responses to the target. This finding is consistent with our assumption that ERP measures are more sensitive than RT measures of alerting and orienting using the ANT (see also MacLeod et al. (2010)). The behavioral response is well downstream of the initial ERP response to the target (i.e., 700 versus 150 ms) and is affected not only by attention to the target, but also by the processes involved in discriminating the target from the distractors, resolving flanker interference, and planning and executing the motor response. RT differences in alerting might be more evident on simple target-detection tasks that strongly emphasize response speed.
Although psychopathy, when assessed as a unitary construct, is generally not associated with executive skill deficits, we did find a trend-level positive correlation between APSD total scores and the reaction-time measure of executive attention (flanker interference). However, this result was not unique to psychopathy, as evidenced by the lack of correlation after controlling for externalizing, ADHD, or general executive skill. Subscale analyses indicated that this result was driven by the Impulsivity subscale. This pattern of findings is consistent with a host of previous studies linking general antisociality and disinhibition to poor executive functioning (see Morgan and Lilienfeld 2000). It is also consistent with studies showing that the antisocial/impulsive symptoms of psychopathy (Factor 2 in two-factor models) are associated with weaknesses in executive functioning (e.g., Sellbom and Verona 2007), whereas the core personality features (Factor 1 in two-factor models), like the syndrome as a whole, are associated with normal or better executive skills (e.g., Ishikawa et al. 2001).
The lack of group differences on either the N450 or P3b indices of executive attention is complicated by our failure to find the expected ERP main effects for flanker interference. Flanker congruency did not significantly affect the amplitude of the N450 component, and P3b amplitude was unexpectedly larger for congruent than for incongruent flankers. We had predicted larger P3b amplitudes for incongruent flankers based on previous studies with children and adolescents (Johnstone et al. 2009; Rueda et al. 2004). However, other studies have reported smaller P3b amplitudes for incongruent flankers (e.g., Doucet and Stelmack 2000), and our result is consistent with a recent study using the ANT in healthy adults (Neuhaus et al. 2010). The reasons for the contradictory results across studies are unclear. Although our lack of group differences in ERP measures of executive attention is consistent with prior evidence of good executive functioning among psychopathic individuals, further study may be warranted using tasks that produce more robust ERP main effects for executive attention.
What might cause the reduced attentional alerting among youth with psychopathic symptoms? One possibility is that their attention becomes “stuck” on the central, temporal cue. In contrast to the no cue and spatial cue conditions, the central cue condition presents a cue in a nontarget location. Upon appearance of the target, participants must move their attention from the central cue to the target.
There is some evidence that psychopathic individuals have difficulty shifting their attention. However, this evidence generally involves shifting between competing tasks under highly demanding conditions (e.g., Kosson and Newman 1986). Our study involved only one “task” (i.e., respond to the central arrow) and is therefore unlikely to tap into these task-shifting weaknesses. In addition, two previous studies that used paradigms with cued shifts of spatial attention found normal or better attention shifts among psychopathic individuals (Harpur 1991; Howland et al. 1993).
Another possible explanation is that youth with psychopathic symptoms had more difficulty estimating the 1-second delay interval between the cue and the target. Interestingly, a recent study by Luman et al. (2008) found persistent underestimation of a 1-second interval among youth with ADHD, most of whom also had conduct disorder (CD) and/or oppositional defiant disorder (ODD). However, there were few correlations between CD/ODD symptoms and time estimation. Further, Howland et al. (1993) used a spatial cueing paradigm with a 1-second cue–target interval and found no RT differences between psychopathic and nonpsychopathic offenders.
Alternatively, the reduced P1 alerting effect among youth with psychopathic symptoms may arise from deficits in the neurobiological systems that are involved in attentional alerting. Attentional alerting has been linked to a norepinephrine (NE)-driven network involving the locus coeruleus (LC) and the right frontal and right lateral parietal cortex (Coull et al. 2001; Witte and Marrocco 1997). Although psychopathy has not, to our knowledge, previously been linked with the attentional alerting system, there have been independent suggestions of both right hemisphere (Hiatt et al. 2002; Hiatt and Newman 2006) and NE (Blair 2003) involvement in the disorder.
Interestingly, many of the functions that have been associated with the neural underpinnings of the alerting network, such as conditioned arousal (Sara 1985), attention to unexpected and potentially aversive stimuli (Brenner and Mirmiran 1988), responsiveness to changing reward contingencies (Bouret and Sara 2004; Dalley et al. 2001), avoidance of commission errors (Chamberlain et al. 2006), and the detection of sad facial expressions (Harmer et al. 2001) are similar to functions that are disrupted in psychopathy (Blair and Mitchell 2009; Hiatt and Newman 2006).
More broadly, alerting network dysfunction could explain, at least in part, the attention dysfunction postulated by Newman’s RMH (Newman 1998). According to the RMH, most symptoms of psychopathy can be understood in terms of an attention-related failure to process potentially important information when it is peripheral to ongoing, goal-directed behavior (Hiatt and Newman 2006; Newman and Lorenz 2003). Dysfunction in the LC-NE system may contribute to this failure, as the LC plays an important role in “the ability to rapidly redirect attention and change the course of action in response to a novel stimulus of potential behavioral significance” (Benarroch (2009), p. 1701).
Thus, it is at least plausible that alerting network dysfunction could account for many of the deficits that are characteristic of psychopathy. If our preliminary evidence of alerting system dysfunction can be replicated and confirmed by future studies, it would suggest the possible utility of interventions that target alerting network function, via either behavioral (Posner 2008) or pharmacological (e.g., noradrenaline reuptake inhibitors) means.
Interpretation of our findings should be tempered by the limitations of our study. First, our sample of adolescents was fairly normative and had relatively mild levels of psychopathic symptoms. Although the relationship between APSD scores and attentional alerting was robust, it is difficult to argue that this relationship is specific to psychopathy as defined by Cleckley (1982) and as assessed in adult criminals by the Psychopathy Checklist–Revised (PCL–R; Hare 2003). However, it is reassuring that the relationship was unaffected by controlling for executive functioning and symptoms of ADHD, INT, and EXT. In addition, previous studies have demonstrated many parallels between APSD and PCL–R results (e.g., Blair et al. 2001; Blair et al. 2005; Vitale et al. 2005).
A second limitation is that our ERP measures were based on a relatively small number of trials, particularly for the cue-related effects. Because the P1 component is relatively small in amplitude, it is generally recommended that hundreds of trials be administered to reliably separate the P1 from ongoing background noise (e.g., Luck 2005). The smaller number of trials in our study may affect the stability of the P1 components, and our finding requires replication. It is likely that the relatively small number of trials would also make it more difficult to detect individual differences, and in this respect it is notable that we found individual differences in P1 but not in the larger P3b component.
In conclusion, we found that youth with higher levels of psychopathic symptoms showed weaker attentional alerting, as assessed by the P1 ERP response. Attentional orienting and executive attention were not related to APSD total scores. These findings support attention-based models of psychopathy and provide evidence of specific deficits in attentional alerting among youth with psychopathic traits. Deficiencies in attentional alerting may be the result of abnormal noradrenergic functioning, which may have cascading effects on higher order cognitive and affective processes.
Acknowledgments
This project was supported by National Institutes of Health grants MH82127 to KHR and MH20012 and DA018760 to TJD.
Footnotes
At the suggestion of an anonymous reviewer, we also conducted multiple regression analyses with simultaneous entry of the three APSD subscales in order to examine regression coefficients for the unique variance of each APSD subscale with each of the ANT measures (RT and ERP measures of AL, OR, and FL). Results were consistent with the bivariate correlations reported above.
Contributor Information
Kristina Hiatt Racer, Email: kdhiatt@uoregon.edu, Child and Family Center, University of Oregon, 195 W. 12th Ave., Eugene, OR 97401, USA.
Tara Torassa Gilbert, Electrical Geodesics Inc., Eugene, OR, USA.
Phan Luu, Department of Psychology, University of Oregon, Eugene, OR, USA; Electrical Geodesics Inc., Eugene, OR, USA.
Joshua Felver-Gant, Department of School Psychology, University of Oregon, Eugene, OR, USA.
Yalchin Abdullaev, Department of Psychology, University of Oregon, Eugene, OR, USA; Pioneer Pacific College, Nursing Program, Springfield, OR, USA.
Thomas J. Dishion, Child and Family Center, University of Oregon, 195 W. 12th Ave., Eugene, OR 97401, USA Department of Psychology, University of Oregon, Eugene, OR, USA; Department of School Psychology, University of Oregon, Eugene, OR, USA.
References
- Achenbach TM, Rescorla LA. Manual for ASEBA school-age forms & profiles. Burlington: University of Vermont, Research Center for Children, Youth, & Families; 2001. [Google Scholar]
- Benarroch EE. The locus ceruleus norepinephrine system: functional organization and potential clinical significance. Neurology. 2009;73:1699–1704. doi: 10.1212/WNL.0b013e3181c2937c. [DOI] [PubMed] [Google Scholar]
- Berger A, Posner MI. Pathologies of brain attentional networks. Neuroscience and Biobehavioral Reviews. 2000;24:3–5. doi: 10.1016/s0149-7634(99)00046-9. [DOI] [PubMed] [Google Scholar]
- Blair RJR. Neurobiological basis of psychopathy. The British Journal of Psychiatry. 2003;182:5–7. doi: 10.1192/bjp.182.1.5. [DOI] [PubMed] [Google Scholar]
- Blair RJR. Applying a cognitive neuroscience perspective to the disorder of psychopathy. Development and Psychopathology. 2005;17:865–891. doi: 10.1017/S0954579405050418. [DOI] [PubMed] [Google Scholar]
- Blair RJR. The emergence of psychopathy: Implications for the neuropsychological approach to developmental disorders. Cognition. 2006;101(2):414–442. doi: 10.1016/j.cognition.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Blair RJR, Mitchell DGV. Psychopathy, attention, and emotion. Psychological Medicine. 2009;39:543–555. doi: 10.1017/S0033291708003991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair RJR, Colledge E, Murray L, Mitchell DGV. A selective impairment in the processing of sad and fearful expressions in children with psychopathic tendencies. Journal of Abnormal Child Psychology. 2001;29(6):491–498. doi: 10.1023/a:1012225108281. [DOI] [PubMed] [Google Scholar]
- Blair RJR, Budhani S, Colledge E, Scott S. Deafness to fear in boys with psychopathic tendencies. Journal of Child Psychology and Psychiatry. 2005;46:327–336. doi: 10.1111/j.1469-7610.2004.00356.x. [DOI] [PubMed] [Google Scholar]
- Blair KS, Newman C, Mitchell DGV, Richell RA, Leonard A, Morton J. Differentiating among prefrontal substrates in psychopathy: neuropsychological test findings. Neuropsychology. 2006;20:153–165. doi: 10.1037/0894-4105.20.2.153. [DOI] [PubMed] [Google Scholar]
- Bouret S, Sara SJ. Reward expectation, orientation of attention and locus coeruleus-medial frontal cortex interplay during learning. The European Journal of Neuroscience. 2004;20:791–802. doi: 10.1111/j.1460-9568.2004.03526.x. [DOI] [PubMed] [Google Scholar]
- Brenner E, Mirmiran M. Functions of the noradrenergic innervation of the rat brain: coping with the unexpected. Brain Dysfunction. 1988;1:57–70. [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends in Cognitive Sciences. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Chamberlain SR, Müller U, Blackwell AD, Robbins TW, Sahakian BJ. Neurochemical modulation of response inhibition and probabilistic learning in humans. Science. 2006;311:861–863. doi: 10.1126/science.1121218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleckley H. The mask of sanity. St. Louis: Mosby; 1982. [Google Scholar]
- Cools R. Role of dopamine in the motivational and cognitive control of behavior. The Neuroscientist. 2008;14:381–395. doi: 10.1177/1073858408317009. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews. Neuroscience. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Kincade J, Ollinger JM, McAvoy MP, Shulman GL. Voluntary orienting is dissociated from target detection in human posterior parietal cortex. Nature Neuroscience. 2000;3:292–297. doi: 10.1038/73009. [DOI] [PubMed] [Google Scholar]
- Coull JT, Nobre AC, Frith CD. The noradrenergic a2 agonist clonidine modulated behavioural and neuroanatomical correlates of human attentional orienting and alerting. Cerebral Cortex. 2001;11:73–84. doi: 10.1093/cercor/11.1.73. [DOI] [PubMed] [Google Scholar]
- Dalley JW, McGaughy J, O’Connell MT, Cardinal RN, Levita L, Robbins TW. Distinct changes in cortical acetylcholine and noradrenaline efflux during contingent and noncontingent performance of a visual attentional task. The Journal of Neuroscience. 2001;21:4908–4914. doi: 10.1523/JNEUROSCI.21-13-04908.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doucet C, Stelmack RM. An event-related potential analysis of extraversion and individual differences in cognitive processing speed and response execution. Journal of Personality and Social Psychology. 2000;78:956–964. doi: 10.1037//0022-3514.78.5.956. [DOI] [PubMed] [Google Scholar]
- Eriksen BA, Eriksen CW. Effects of noise letters upon the identification of a target letter in a nonsearch task. Perception & Psychophysics. 1974;16:143–149. [Google Scholar]
- Fan J, McCandliss BD, Sommer T, Raz A, Posner MI. Testing the efficiency and independence of attentional networks. Journal of Cognitive Neuroscience. 2002;14:340–347. doi: 10.1162/089892902317361886. [DOI] [PubMed] [Google Scholar]
- Flor H, Birbaumer N, Hermann C, Ziegler S, Patrick CJ. Aversive Pavlovian conditioning in psychopaths: peripheral and central correlates. Psychophysiology. 2002;39(4):505–518. doi: 10.1017.S0048577202394046. [DOI] [PubMed] [Google Scholar]
- Frick PJ, Hare RD. Antisocial process screening device. Toronto: Multi-Health Systems; 2001. [Google Scholar]
- Gioia GA, Isquith PK, Guy SC, Kenworthy L. Behavior rating inventory of executive function. Child Neuropsychology. 2000;6(3):235–238. doi: 10.1076/chin.6.3.235.3152. [DOI] [PubMed] [Google Scholar]
- Glenn AL, Raine A, Schug RA. The neural correlates of moral decision-making in psychopathy. Molecular Psychiatry. 2009;14(1):5–6. doi: 10.1038/mp.2008.104. [DOI] [PubMed] [Google Scholar]
- Granon S, Passetti F, Thomas KL, Dalley JW, Everitt BJ, Robbins TW. Enhanced and impaired attentional performance after infusion of the D1 dopaminergic receptor agents into rat prefrontal cortex. The Journal of Neuroscience. 2000;20:1208–1215. doi: 10.1523/JNEUROSCI.20-03-01208.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare RD. Psychopathy and physiological activity during anticipation of an aversive stimulus in a distraction paradigm. Psychophysiology. 1982;19(3):266–271. doi: 10.1111/j.1469-8986.1982.tb02559.x. [DOI] [PubMed] [Google Scholar]
- Hare RD. Performance of psychopaths on cognitive tasks related to frontal lobe function. Journal of Abnormal Psychology. 1984;93:133–140. doi: 10.1037//0021-843x.93.2.133. [DOI] [PubMed] [Google Scholar]
- Hare RD. The Hare psychopathy checklist—revised. 2nd ed. Toronto: Multi-Health Systems; 2003. [Google Scholar]
- Harmer CJ, Perrett DI, Cowen PJ, Goodwin GM. Administration of beta-adrenoceptor blocker propranolol impairs the processing of facial expressions of sadness. Psychopharmacology. 2001;154:383–389. doi: 10.1007/s002130000654. [DOI] [PubMed] [Google Scholar]
- Harpur TJ. Visual attention in psychopathic criminals. Unpublished doctoral dissertation. Vancouver, British Columbia, Canada: University of British Columbia; 1991. [Google Scholar]
- Hart SD, Forth AE, Hare RD. Performance of criminal psychopaths on selected neuropsychological tests. Journal of Abnormal Psychology. 1990;99:374–379. doi: 10.1037//0021-843x.99.4.374. [DOI] [PubMed] [Google Scholar]
- Hiatt KD, Newman JP. Understanding psychopathy: The cognitive side. In: Patrick CJ, editor. Handbook of psychopathy. New York: Guilford; 2006. pp. 334–352. [Google Scholar]
- Hiatt KD, Lorenz AR, Newman JP. Assessment of emotion and language processing in psychopathic offenders: Results from a dichotic listening task. Personality and Individual Differences. 2002;32:1255–1268. [Google Scholar]
- Hiatt KD, Schmitt WA, Newman JP. Stroop tasks reveal abnormal selective attention among psychopathic offenders. Neuropsychology. 2004;18:50–59. doi: 10.1037/0894-4105.18.1.50. [DOI] [PubMed] [Google Scholar]
- Hillyard SA, Anllo-Vento L. Event-related brain potentials in the study of visual selective attention. Proceedings of the National Academy of Sciences. 1998;95:781–787. doi: 10.1073/pnas.95.3.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howland EW, Kosson DS, Patterson CM, Newman JP. Altering a dominant response: Performance of psychopaths and low socialization college students on a cued reaction time task. Journal of Abnormal Psychology. 1993;102:379–387. doi: 10.1037//0021-843x.102.3.379. [DOI] [PubMed] [Google Scholar]
- Ishikawa SS, Raine A, Lencz T, Bihrle S, Lacasse L. Autonomic stress reactivity and executive functions in successful and unsuccessful criminal psychopaths from the community. Journal of Abnormal Psychology. 2001;110(3):423–432. doi: 10.1037//0021-843x.110.3.423. [DOI] [PubMed] [Google Scholar]
- Johansson P, Kerr M. Psychopathy and intelligence: a second look. Journal of Personality Disorders. 2005;19(4):357–369. doi: 10.1521/pedi.2005.19.4.357. [DOI] [PubMed] [Google Scholar]
- Johnstone SJ, Barry RJ, Markovska V, Dimoska A, Clarke AR. Response inhibition and interference control in children with AD/HD: a visual ERP investigation. International Journal of Psychophysiology. 2009;72:145–153. doi: 10.1016/j.ijpsycho.2008.11.007. [DOI] [PubMed] [Google Scholar]
- Kadosh RC, Kadosh KC, Henik A, Linden DEJ. Processing conflicting information: facilitation, interference, and functional connectivity. Neuropsychologia. 2008;46:2872–2879. doi: 10.1016/j.neuropsychologia.2008.05.025. [DOI] [PubMed] [Google Scholar]
- Kiehl KA. A cognitive neuroscience perspective on psychopathy: evidence for paralimbic system dysfunction. Psychiatry Research. 2006;142(2–3):107–128. doi: 10.1016/j.psychres.2005.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiehl KA, Smith AM, Hare RD, Liddle PF. An event-related potential investigation of response inhibition in schizophrenia and psychopathy. Biological Psychiatry. 2000;48(3):210–221. doi: 10.1016/s0006-3223(00)00834-9. [DOI] [PubMed] [Google Scholar]
- Kosson DS, Newman JP. Psychopathy and the allocation of attentional capacity in a divided-attention situation. Journal of Abnormal Psychology. 1986;95:257–263. [PubMed] [Google Scholar]
- Larson MJ, Kaufman DAS, Perlstein WM. Conflict adaptation and cognitive control adjustments following traumatic brain injury. Journal of the International Neuropsychological Society. 2009;15:927–937. doi: 10.1017/S1355617709990701. [DOI] [PubMed] [Google Scholar]
- Levenston GK, Patrick CJ, Bradley MM, Lang PJ. The psychopath as observer: emotion and attention in picture processing. Journal of Abnormal Psychology. 2000;109(3):373–385. [PubMed] [Google Scholar]
- Luck SJ. An introduction to the event-related potential technique. Cambridge: MIT; 2005. [Google Scholar]
- Luman M, Oosterlaan J, Sergeant JA. Modulation of response timing in ADHD, effects of reinforcement valence and magnitude. Journal of Abnormal Child Psychology. 2008;36:445–456. doi: 10.1007/s10802-007-9190-8. [DOI] [PubMed] [Google Scholar]
- Lykken DT. A study of anxiety in the sociopathic personality. Journal of Abnormal and Social Psychology. 1957;55(1):6–10. doi: 10.1037/h0047232. [DOI] [PubMed] [Google Scholar]
- MacLeod JW, Lawrence MA, McConnell MM, Eskes GA, Klein RM, Shore DI. Appraising the ANT: psychometric and theoretical considerations of the Attention Network Test. Neuropsychology. 2010;24(5):637–651. doi: 10.1037/a0019803. [DOI] [PubMed] [Google Scholar]
- Morgan AB, Lilienfeld SO. A meta-analytic review of the relation between antisocial behavior and neuropsychological measures of executive function. Clinical Psychology Review. 2000;20(1):113–136. doi: 10.1016/s0272-7358(98)00096-8. [DOI] [PubMed] [Google Scholar]
- Neuhaus AH, Urbanek C, Opgen-Rhein C, Hahn E, Ta TMT, Koehler S, et al. Event-related potentials associated with Attention Network Test. International Journal of Psychophysiology. 2010;76(2):72–79. doi: 10.1016/j.ijpsycho.2010.02.005. [DOI] [PubMed] [Google Scholar]
- Newman JP. Psychopathic behavior: An information processing perspective. In: Cooke DJ, Hare RD, Forth A, editors. Psychopathy: Theory, research and implications for society. Dordrecht: Kluwer; 1998. pp. 81–104. [Google Scholar]
- Newman JP, Kosson DS. Passive avoidance learning in psychopathic and nonpsychopathic offenders. Journal of Abnormal Psychology. 1986;95:252–256. [PubMed] [Google Scholar]
- Newman JP, Lorenz AR. Response modulation and emotion processing: Implications for psychopathy and other dysregulatory psychopathology. In: Davidson RJ, Scherer K, Goldsmith HH, editors. Handbook of affective sciences. New York: Oxford University Press; 2003. pp. 1043–1067. [Google Scholar]
- Newman JP, Patterson CM, Howland EW, Nichols SL. Passive avoidance in psychopaths: the effects of reward. Personality and Individual Differences. 1990;11:1101–1114. [Google Scholar]
- Newman JP, Schmitt WA, Voss WD. The impact of motivationally neutral cues on psychopathic individuals: assessing the generality of the response modulation hypothesis. Journal of Abnormal Psychology. 1997;106:563–575. doi: 10.1037//0021-843x.106.4.563. [DOI] [PubMed] [Google Scholar]
- Newman JP, Curtin JJ, Bertsch JD, Baskin-Sommers AR. Attention moderates the fearlessness of psychopathic offenders. Biological Psychiatry. 2010;67(1):66–70. doi: 10.1016/j.biopsych.2009.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Hughes B, Robertson ER, Cooper JC, Gabriel JDE. Neural systems supporting the control of affective and cognitive conflicts. Journal of Cognitive Neuroscience. 2009;21(9):1841–1854. doi: 10.1162/jocn.2009.21129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick CJ. Back to the future: Cleckley as a guide to the next generation of psychopathy research. In: Patrick CJ, editor. Handbook of Psychopathy. 2006. pp. 605–617. [Google Scholar]
- Patrick CJ, Bradley MM, Lang PJ. Emotion in the criminal psychopath: Startle reflex modulation. Journal of Abnormal Psychology. 1993;102(1):82–92. doi: 10.1037//0021-843x.102.1.82. [DOI] [PubMed] [Google Scholar]
- Phillips JM, McAlonan K, Robb WG, Brown VJ. Cholingeric neurotransmission influences covert orientation of visuospatial attention in the rat. Psychopharmacology. 2000;150:112–116. doi: 10.1007/s002130000437. [DOI] [PubMed] [Google Scholar]
- Posner MI. Orienting of attention. The Quarterly Journal of Experimental Psychology. 1980;41A:19–45. doi: 10.1080/00335558008248231. [DOI] [PubMed] [Google Scholar]
- Posner MI. Measuring alertness. In: Plaff DW, Kieffer BL, editors. Molecular and biophysical mechanisms of arousal, alertness, and attention, Annals of the New York Academy of Sciences. Malden: Blackwell; 2008. pp. 193–199. [DOI] [PubMed] [Google Scholar]
- Rosen AC, Rao SM, Caffarra P, Scaglioni A, Bobholz JA, Woodley SJ, et al. Neural basis of endogenous and exogenous spatial orienting: a functional MRI study. Journal of Cognitive Neuroscience. 1999;11:135–152. doi: 10.1162/089892999563283. [DOI] [PubMed] [Google Scholar]
- Ross SR, Benning SD, Adams Z. Symptoms of executive dysfunction are endemic to secondary psychopathy: an examination in criminal offenders and noninstitutionalized young adults. Journal of Personality Disorders. 2007;21:384–399. doi: 10.1521/pedi.2007.21.4.384. [DOI] [PubMed] [Google Scholar]
- Rueda MR, Posner MI, Rothbart MK, Davis-Stober CP. Development of the time course for processing conflict: an event-related potentials study with 4 year olds and adults. BMC Neuroscience. 2004;5(39) doi: 10.1186/1471-2202-5-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandrini M, Rossini PM, Miniussi C. Lateralized contribution of prefrontal cortex in controlling task-irrelevant information during verbal and spatial working memory tasks: RTMS evidence. Neuropsychologia. 2008;46:2056–2063. doi: 10.1016/j.neuropsychologia.2008.02.003. [DOI] [PubMed] [Google Scholar]
- Sara SJ. The locus coeruleus and cognitive function: attempts to relate noradrenergic enhancement of signal/noise in the brain to behavior. Physiological Psychology. 1985;13:151–162. [Google Scholar]
- Sellbom M, Verona E. Neuropsychological correlates of psychopathic traits in a non-incarcerated sample. Journal of Research in Personality. 2007;41:276–294. [Google Scholar]
- Vitacco MJ, Neumann CS, Jackson RL. Testing a four-factor model of psychopathy and its association with ethnicity, gender, intelligence, and violence. Journal of Consulting and Clinical Psychology. 2005;73:466–476. doi: 10.1037/0022-006X.73.3.466. [DOI] [PubMed] [Google Scholar]
- Vitale JE, Newman JP, Bates JE, Goodnight J, Dodge KA, Pettit GS. Deficient behavioral inhibition and anomalous selective attention in a community sample of adolescents with psychopathic traits and low-anxiety traits. Journal of Abnormal Child Psychology. 2005;33:461–470. doi: 10.1007/s10802-005-5727-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Liu X, Guise KG, Knight RT, Ghajar J, Fan J. Effective connectivity of the fronto-parietal network during attentional control. Journal of Cognitive Neuroscience. 2010;22(3):543–553. doi: 10.1162/jocn.2009.21210. [DOI] [PubMed] [Google Scholar]
- Weber S, Habel U, Amunts K, Schneider F. Structural brain abnormalities in psychopaths: A review. Behavioral Sciences & the Law. 2008;26(1):7–28. doi: 10.1002/bsl.802. [DOI] [PubMed] [Google Scholar]
- West R, Jakubek K, Wymbs N, Perry M, Moore K. Neural correlates of conflict processing. Experimental Brain Research. 2005;167(1):38–48. doi: 10.1007/s00221-005-2366-y. [DOI] [PubMed] [Google Scholar]
- Wild-Wall N, Falkenstein M, Hohnsbein J. Flanker interference in young and older participants as reflected in event-related potentials. Brain Research. 2008;1211:72–84. doi: 10.1016/j.brainres.2008.03.025. [DOI] [PubMed] [Google Scholar]
- Witte EA, Marrocco RT. Alteration of brain noradrenergic activity in rhesus monkeys affects the alerting component of covert orienting. Psychopharmacology. 1997;132:315–323. doi: 10.1007/s002130050351. [DOI] [PubMed] [Google Scholar]
- Witte EA, Davidson MC, Marrocco RT. Effects of altering brain cholinergic activity on covert orienting of attention: comparison of monkey and human performance. Psychopharmacology. 1997;132:324–334. doi: 10.1007/s002130050352. [DOI] [PubMed] [Google Scholar]
- Yang Y, Raine A, Narr KL, Colletti P, Toga AW. Localization of deformations within the amygdala in individuals with psychopathy. Archives of General Psychiatry. 2009;66(9):986–994. doi: 10.1001/archgenpsychiatry.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeier JD, Maxwell JS, Newman JP. Attention moderates the processing of inhibitory information in primary psychopathy. Journal of Abnormal Psychology. 2009;118:554–563. doi: 10.1037/a0016480. [DOI] [PMC free article] [PubMed] [Google Scholar]