Abstract
Obesity is associated with a chronic low inflammatory state characterized by elevated levels of chemokines. Monocyte chemoattractant protein-1 (MCP-1) is a member of the cysteine-cysteine (CC) chemokine family and is increased in obesity. The purpose of this study was to identify loci regulating serum MCP-1 in obese Hispanic children from the Viva La Familia Study. A genome-wide association (GWA) analysis was performed in 815 children, ages 4-19 years, using genotypes assayed with the Illumina HumanOmni1-Quad v1.0 BeadChips. All analyses were performed in SOLAR using a linear regression-based test under an additive model of allelic effect, while accounting for the relatedness of family members via a kinship variance component. The strongest association for MCP-1 levels was found with a non-synonymous single nucleotide polymorphism (SNP), rs12075 resulting in an amino acid substitution (Asp42Gly) in the Duffy antigen receptor for chemokines (DARC) gene product (minor allele frequency = 43.6%, p = 1.3 × 10−21) on chromosome 1. Four other DARC SNPs were also significantly associated with MCP-1 levels(p < 10−16-10−6). The Asp42Gly variant was associated with higher levels of MCP-1 and accounted for approximately 10% of its variability. In addition, MCP-1 levels were significantly associated with SNPs in chemokine receptor 3 (CCR3) and caspase recruitment domain family, member 9 (CARD9). In summary, the association of the DARC Asp42Gly variant with MCP-1 levels replicates previous GWA results substantiating a potential role for DARC in the regulation of pro-inflammatory cytokines.
Keywords: Obesity, inflammation, polymorphism, effect size, variance, kinship
INTRODUCTION
Obesity is a major contributor to and precedes several metabolic disorders such as insulin resistance, dyslipidemia, hyperglycemia and hypertension [1]. Obesity is characterized as a chronic low inflammatory state [2]. Chemokines and proinflammatory cytokines and hormones released by adipose tissue underlie the chronic inflammatory profile associated with obesity [3]. Chemokines and their receptors play a crucial role in directing the movement of leukocytes throughout the body contributing to adaptive immune response and the pathogenesis of a variety of diseases including atherosclerosis, diabetes and obesity [1, 4]. The largest family of chemokines consists of cysteine-cysteine (CC) chemokines that attract mononuclear cells to sites of chronic inflammation. Monocyte chemoattractant protein 1 (MCP-1) or chemokine CC ligand 2 (CCL2) is the most thoroughly characterized CC chemokine and is central to the inflammatory process [5, 6]. MCP-1 is produced mainly in endothelial and smooth cells. MCP-1 is involved in the recruitment of leukocytes to sites of inflammation, promotion of angiogenesis and maturation of immune cells. Its levels are elevated in a broad spectrum of chronic inflammatory diseases including obesity, insulin resistance, sepsis, cancer, autoimmune diseases and atherosclerosis. Other CC chemokines include macrophage inflammatory protein (MIP)-1 (CCL3), MIP-1 (CCL4), and RANTES (CCL5). A second family of chemokines consists of CXC chemokines such as interleukin-8 (CXCL8) which attracts polymorphonuclear leukocytes to sites of acute inflammation. The third family is the CX3C family; fractalkine (CX3CL1) is the only member.
Chemokines activate surface receptors that are seven-transmembrane domain G-protein coupled receptors which in turn activate signaling cascades that result in the rearrangement, change of shape, and cell movement of actin. The chemokine receptor cysteine-cysteine (CC) receptor 2 (CCR2) is known to influence inflammation associated with obesity [7]. Expression of CCR2 in adipose tissue was observed to be increased in obese individuals and associated with systemic inflammation [8]. However, CC2R is not the only receptor for MCP-1; another receptor, Duffy antigen receptor for chemokines (DARC), mediates the interactions of MCP-1 with erythrocytes and endothelial cells [9]. In circulation, MCP-1 is bound to erythrocyte DARC that acts as a chemokine receptor/reservoir of proinflammatory cytokines. DARC regulates free chemokine activity and presumably releases chemokines to local environments when needed.
The Viva La Familia Study was designed to investigate genetic and environmental factors affecting obesity and its comorbidities in Hispanic children. Previously, we reported that genetic factors associated with obesity also were associated with increased fasting serum levels of MCP-1 [10]. We conducted a genome-wide scan to identify chromosomal or quantitative trait loci (QTL) affecting the variation in cytokines in these children. Significant heritability was observed for serum MCP-1 (h2 = 0.62) [10]. The univariate genome-wide scan showed suggestive evidence for QTLs influencing the variation in MCP-1 levels on chromosomes 11 (logarithm of odds (LOD) score = 2.2) and 1 (LOD = 2.0) [10]. To further pursue these findings, we conducted a genome-wide association study (GWAS) and here report our results for MCP-1 levels in Hispanic children of the Viva La Familia Study.
MATERIAL AND METHODS
Study design and participants
The Viva La Familia Study represents a family-based cohort highly enriched for obesity. Families were selected based on an obese proband between the ages of 4 and 19 years. Accordingly, 51% of the children were classified as obese (> 95th BMI percentile) with BMI z-scores ranging from 2.3 to 4.5 [11]. The majority of the parents were either overweight (34%) or obese (57%). Subjects (n=934) were from 319 Hispanic families enrolled in the Viva La Familia Study in 2000-2004. Anthropometric and body composition measurements were performed on parents and children. Fasting blood samples were drawn for biochemistry profiling of the children, and genotyping of children and parents. Subjects and study procedures are described in detail in a previous publication ([11]. All children and their parents gave written informed consent or assent. The protocol was approved by the Institutional Review Board for Human Subject Research for Baylor College of Medicine and Affiliated Hospitals and the Texas Biomedical Research Institute.
Phenotyping
A blood sample was drawn in the morning after a 12-h fast. Serum samples were obtained from whole blood after clotting. Fasting serum concentration of MCP-1 was measured by enzyme-linked immunosorbent assay (ELISA) (interassay CV 5%) (R & D Systems, Minneapolis, MN).
Genome-wide association analysis (GWA analysis) in Hispanic children
A GWA analysis for 815 children was conducted using single nucleotide polymorphism (SNP) assays included on the Illumina HumanOmni1-Quad v1.0 BeadChips (Illumina, San Diego, CA). Genotype calls were obtained after scanning on the Illumina BeadStation 500GX and analysis using the GenomeStudio software. Our genotyping error rate (based on duplicates) was 2 per 100,000 genotypes. The average call rate per individual sample was 97%. Specific SNPs were removed from analysis if they had call rates <95% (about 4,000 SNPs) or deviated from Hardy-Weinberg equilibrium at a 5% FDR (12 SNPs). SNP genotypes were checked for Mendelian consistency using the program SimWalk2 [12]. The evidence for population stratification was assessed and estimates of the allele frequencies and their standard errors were obtained using SOLAR [13].
Measured genotype analysis
GWA analyses were performed using the SOLAR program [13]. Each SNP genotype was converted in SOLAR to a covariate measure equal to 0, 1, or 2 copies of the minor allele (or, for missing genotypes, the weighted covariate based on imputation). These covariates were included in the variance-components mixed models for measured genotype analyses [14] versus null models that incorporated the random effect of kinship and fixed effects such as age, sex, their interaction and higher order terms. For the initial GWA screen, we tested each SNP covariate independently as a 1 degree of freedom likelihood ratio test. A derived alpha value, from simulations using the family data, of 1 × 10−7 or less was considered significant.
RESULTS
The mean serum MCP-1 levels were 317± 117 and 309 ± 117 pg/ml for boys and girls, respectively. The strongest association for MCP-1 levels was found with a non-synonymous single nucleotide polymorphism (SNP), rs12075 (Asp42Gly) in the Duffy antigen receptor for chemokines (DARC) gene (minor allele frequency = 43.6%, p = 1.3 × 10−21). MCP-1 levels also were associated with five other SNPs in DARC (p < 10−16-10−6) (Table 1). The minor alleles of all DARC SNPs were significantly associated with higher levels of MCP-1. SNP rs12075 (Asp42Gly) accounted for approximately 10% of the variability in MCP-1 levels. Together, these six SNPs accounted for 34.1% of the variability in MCP-1 levels.
Table 1.
DARC SNPs genotype specific means of MCP-1 levels
| SNP | Minor allele/major allele |
Minor allele frequency |
Mean effect size (%)* |
p-value | GG** (pg/mL) |
AG** (pg/mL) |
AA** (pg/mL) |
|---|---|---|---|---|---|---|---|
| rs12075 | A /G | 0.43 | 10.2 | 1.21 × 10−21 | 271.69 (98.4) | 317.30 (105.1) | 386.83 (141.5) |
| rs2427837 | A /G | 0.12 | 3.2 | 7.0 × 10−08 | 304.20 (110.9) | 350.15 (128.1) | 411.23 (168.8) |
| rs3027012 | A /G | 0.17 | 3.6 | 2.9 × 10−07 | 301.41 (115.8) | 344.59 (115.8) | 389.63 (118.1) |
| rs3027016 | A/G | 0.11 | 2.8 | 2.4 × 10−06 | 306.58 (113.3) | 341.01 (122.9) | 428.37 (152.5) |
| rs863002 | A/G | 0.24 | 6.0 | 4.2 × 10−13 | 292.15 (107.5) | 343.18 (121.9) | 386.10 (129.9) |
| rs863006 | A /G | 0.42 | 8.3 | 1.1 × 10−16 | 274.34 (99.1) | 321.32 (108.9) | 378.75 141.6) |
Mean effect size- Proportion of variance contributed by the SNP
Mean (SD)
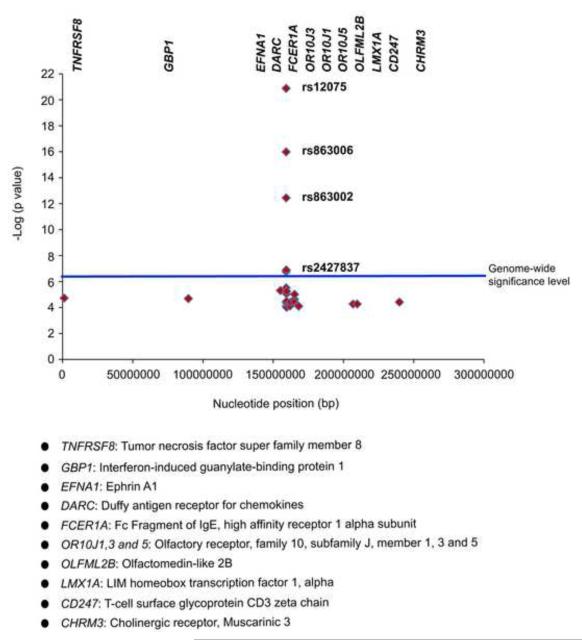
Suggestive evidence of associations of MCP-1 levels with SNPs in other regions of chromosome 1 were found in ephrin A1(EFNA1), Guanylate-binding protein-1 (GBP1), Fc Fragment of IgE, high affinity receptor 1 alpha subunit (FCER1A), olfactory receptor, family 10, subfamily J, member 1, 3 and 5 (OR10J1, OR10J3, OR10J5), olfactomedin-like 2B (OLFML2B), Fc receptor-like protein B (FCRLB), LIM homeobox transcription factor 1, alpha (LMX1A) and cholinergic receptor, Muscarinic 3 (CHRM3), with p-values ranging between 4. 9 × 10−6 and 7.0 × 10−5 (Figure 1).
Figure1.
Associations of serum MCP-1 levels with DARC SNPs on chromosome 1. TNFRSF8: Tumor necrosis factor super family member 8; GBP1: Interferon-induced guanylate-binding protein 1; EFNA1: Ephrin A1; DARC: Duffy antigen receptor for chemokines; FCER1A: Fc Fragment of IgE, high affinity receptor 1 alpha subunit; OR10J1,3 and 5: olfactory receptor, family 10, subfamily J, member 1, 3 and 5; OLFML2B: olfactomedin-like 2B; LMX1A: LIM homeobox transcription factor 1, alpha; CD247: T-cell surface glycoprotein CD3 zeta chain; CHRM3: cholinergic receptor, Muscarinic 3
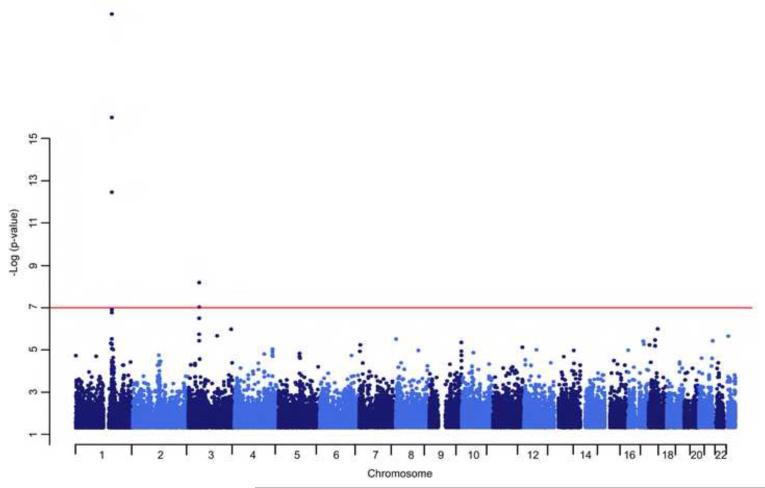
The GWAS (Figure 2) revealed other highly significant associations for MCP-1 levels: snp2- 1167588 on chromosome 2 (p = 6.5 × 10−8); rs7652290, rs7645716 and rs12636651 in chemokine receptor 3 (CCR3) (p = 3.3 × 10−7, 9.6 × 10−8 and 6.6 × 10−9, respectively) on chromosome 3; rs34971035 in caspase recruitment domain family, member 9 (CARD9) (p = 4.6 × 10−8) on chromosome 9; and snp10-4307576 (p = 4.6 × 10−8) on chromosome 10. On chromosome 11, there were no significant associations detected, despite our original suggestive linkage signal in this region.
Figure2.
Genome-wide association analysis of MCP-1
To estimate whether the SNPs associated with MCP-1 levels account for the variation in the phenotypes that generated the original linkage signal on chromosome 1, we conducted a linkage analysis conditional on the missense polymorphism (rs12075). This analysis is based on the premise that if the SNP explains the linkage completely, then the LOD score will be reduced to zero. Our original linkage signal of LOD equal to 2.0 was reduced to 0.9 indicating that this SNP in DARC accounts partially, but not completely, for the variation in serum MCP-1 in Hispanic children.
DISCUSSION
The major finding of this study is the strong association of serum MCP-1 with SNPs in the DARC gene in Hispanic children of the Viva La Familia Study. Given that DARC is a major receptor for CC chemokines, this association strongly suggests a major role of DARC in the regulation of the circulating levels of the CC chemokine, MCP-1. Our study is the first to report an association of serum MCP-1with DARC in children of Hispanic origin and replicates the findings of a GWAS conducted in Caucasian adults [15].
Schnabel et al [15] reported the results of a GWAS of serum MCP-1 and showed a strong association with DARC SNPs. This study, conducted in Caucasian adults from the Framingham Heart Study, found the SNP rs12075 to have the strongest association with MCP-1 levels, accounting for approximately 20% of the residual variability in MCP-1 levels. This particular SNP (Asp42Gly) has been shown to discriminate between 2 blood group antigens, (F by (aspartate) and F ab (glycine) [16]. They also found a significant association of DARC with levels of RANTES. They replicated their findings in the MONICA/KORA and ARIC Studies. In the current study, we replicated this association in Hispanic children and observed the strongest association of MCP-1 levels with rs12075 which accounted for approximately 10% of the variation in serum MCP-1 levels. In the Framingham Heart Study and MONICA/KORA studies, the minor allele (G) of rs12075 had a frequency equal to 45.6%. Based on the rs12075 genotypes, MCP-1 concentrations were 389 pg/mL for AA, 318 pg/mL for AG, and 259 pg/mL for GG. These are similar to our findings; in both studies the G allele is associated with lower levels of MCP-1. However, allele frequencies differ between our studies. In our study the minor allele is (A) [15]. Our study found that rs12075 explains approximately 10% (34.1% for five SNPs) of the variation in serum MCP-1 which is much less than that reported by Schnabel et al. but much higher than those reported by recent GWAS [17]. In a family-based study of more than 4000 individuals from Sardinia aged 14-102 years, a GWAS of inflammatory markers also found significant association between DARC SNPs and MCP-1 levels [18].Since our original QTL encompassed the DARC gene, we also conducted a conditional linkage analysis with rs12075. Importantly, we also found that rs12075 accounts for more than 50% of the linkage signal indicating a strong influence on the variation in serum MCP-1. Interestingly, the same SNPs from DARC were not associated with plasma MCP-1 [15]. We could not confirm this result due to the unavailability of plasma MCP-1 measurements in our study.
Suggestive evidence of association was also found for MCP-1 levels on chromosome 1 with SNPs in EFNA1, GBP1, FCER1A, OR10J1, OR10J3, OR10J5, OLFML2B, FCRLB, LMX1A and CHRM3. This was a replication of a GWAS conducted in Framingham Heart study where SNPs in FCER1A OR10J1 and OR10J3 genes were significantly associated with serum levels of MCP-1 [17]. In our study, these SNPs explained about 4-7% of the variability in MCP-1 levels. The FCER1A gene codes for a Fc, fragment for IgE which is known to have a positive association with MCP-1, particularly in mast cells in both humans and rodents [19,20].
The expression of MCP-1 is increased in obesity and plays a role recruiting macrophages into the adipose tissue of obese individuals [21]. In a study conducted in mice, it was found that short-term high fat diet increases the expression of MCP-1 RNA in adipose tissue and hypertrophied adipocytes [21], confirming findings reported earlier by Kanda et al. [1]. They not only found an increased expression of MCP-1 in adipose tissue in mice fed a high-fat diet, but also found that the increased MCP-1 contributes to insulin resistance and hepatic steatosis in obese mice [1]. Increased MCP-1 levels have also been reported in human obesity. In a study conducted in obese women, increased MCP-1 levels were associated with fat mass as well as visceral adipose tissue [22]. Similarly, a study in Mexican American children found elevated levels of MCP-1 in obese/overweight when compared to healthy weight children [23]. In another study in type 2 diabetic individuals, MCP-1 levels were increased and significantly correlated with diabetes duration and coronary artery disease phenotypes [24]. The same was observed in children with type 1 diabetes [25]. Thus, MCP-1 plays an important role in obesity-associated inflammation. In the Hispanic children of our study, we previously reported significant correlations of cytokines, including MCP-1, with obesity-related traits [10].
In the current GWAS, MCP-1 levels were primarily associated with SNPs in genes that play a role in chemokine/cytokine regulation and inflammation. Besides DARC, MCP-1 levels were associated with SNPs in CCR3 and CARD9. The CCR3 encodes an eotaxin receptor which mediates circulating levels of RANTES, MCP-2, MCP-3 and MCP-4, besides eotaxin. Huber et al. [8] found increased expression of CCR3 in subcutaneous and visceral adipose tissue in obese patients as compared to lean controls. Likewise, expression of CCR3 was greater in the adipose tissue of high-fat diet-induced obese mice as compared to lean controls [26]. Studies by Schnabel et al [15] and Naitza et al.[18] found suggestive association of serum MCP-1 with CCR2 which is also a MCP-1 receptor and is in the same region of chromosome 3 as CCR3. The CARD9 gene encodes proteins that induce nuclear factor kappa-B (NFKB) activity through IKK. Hsu et al [27] showed that CARD9 was required for the activation of proinflammatory cytokines through its activation of p38 and JNK kinases and thus important for an innate immune response to intracellular pathogens.
The DARC gene is located on 1q22-q23 and codes for a receptor for MCP-1, interleukin 8 (IL8) and other inflammatory chemokines [28]. DARC binds CXC and CC chemokines with high affinity and therefore plays an important role in the homeostasis of chemokines [29]. It helps transport chemokines from the site of inflammation to the blood stream through the endothelial barrier [28]. It has also been shown to regulate leukocyte recruitment during inflammation [30]. Previously, DARC has been shown to mediate infection of HIV-1 from red blood cells to target cells, thus affecting HIV susceptibility [31], to protect against malaria [32], to mediate infection in acute lung injury [29], and to attenuate angiogenesis [33]. A study by Liu et al. [32] showed a link between DARC expression and renal disease. They localized DARC RNA in renal venules and found it to be upregulated in inflammation in children [34]. Circulating levels of another inflammatory marker C-reactive protein (CRP) were associated with DARC SNPs [18] indicating a multifaceted role for DARC in inflammation.
To summarize, strong associations between SNPs in DARC gene, particularly rs12075, and serum MCP-1 concentration identify a potential candidate gene that can be further explored for its role in inflammatory pathways. In addition, this replication of European GWAS of MCP-1 in our study of Hispanic children confirms the importance of DARC in the regulation of MCP-1. Given the role of MCP-1 in inflammation and as a potential link between obesity and its metabolic complications such as type 2 diabetes and atherosclerosis, further analyses of DARC SNPs may lead to a better understanding of pathogenesis of inflammation in related diseases
Highlights.
Obesity is associated with a chronic low inflammatory state
Genetic factors associated with obesity are also associated with serum monocyte chemoattractant protein-1 (MCP-1) levels
A GWAS in obese Hispanic children showed strong association between DARC gene and serum MCP-1 levels.
Serum MCP-1 concentration was also significantly associated with CCR3 and CARD9 genes.
The association of the DARC with serum MCP-1 levels replicates previous GWAS results.
ACKNOWLEDGEMENTS
We thank all the families who participated in the VIVA LA FAMILIA Study. The authors wish to acknowledge the technical assistance of Grace-Ellen Meixner, Margie Britten and Maria del Pilar Villegas. This work was supported by the National Institutes of Health (NIH) [DK080457], and the USDA/ARS [Cooperative Agreement 6250-51000-053]. Work performed at the Texas Biomedical Research Institute in San Antonio, Texas was conducted in facilities constructed with support from the Research Facilities Improvement Program of the National Center for Research Resources, NIH [C06 RR013556, C06 RR017515]. The contents of this publication do not necessarily reflect the views or policies of the USDA, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S.
Abbreviations
- GWA
Genome-wide association
- SNP
Single nucleotide polymorphism
- DARC
Duffy antigen receptor for chemokines
- CARD9
caspase recruitment domain family, member 9
- MCP-1
Monocyte chemoattractant protein-1
- TNFRSF8
Tumor necrosis factor super family member 8
- GBP1
Interferon-induced guanylate-binding protein 1
- EFNA1
Ephrin A1
- FCER1A
Fc Fragment of IgE, high affinity receptor 1 alpha subunit
- OR10J1,3 and 5
olfactory receptor, family 10, subfamily J, member 1, 3 and 5
- OLFML2B
olfactomedin-like 2B
- LMX1A
LIM homeobox transcription factor 1, alpha
- CD247
T-cell surface glycoprotein CD3 zeta chain
- CHRM3
cholinergic receptor, Muscarinic 3
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Kanda H, Tateya S, Tamori Y, Kotani K, Hiasa K, Kitazawa R, Kitazawa S, Miyachi H, Maeda S, Egashira K, Kasuga M. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest. 2006;116:1494–1505. doi: 10.1172/JCI26498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Maffei M, Funicello M, Vottari T, Gamucci O, Costa M, Lisi S, Viegi A, Ciampi O, Bardi G, Vitti P, Pinchera A, Santini F. The obesity and inflammatory marker haptoglobin attracts monocytes via interaction with chemokine (C-C motif) receptor 2 (CCR2) BMC Biol. 2009;7:87. doi: 10.1186/1741-7007-7-87. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Hotamisligil GS. Role of endoplasmic reticulum stress and c-Jun NH2-terminal kinase pathways in inflammation and origin of obesity and diabetes. Diabetes. 2005;54(Suppl.2):S73–S78. doi: 10.2337/diabetes.54.suppl_2.s73. [DOI] [PubMed] [Google Scholar]
- [4].Murdoch C, Finn A. Chemokine receptors and their role in inflammation and infectious diseases. Blood. 2000;95:3032–3043. [PubMed] [Google Scholar]
- [5].Xu L, Ashkenzi A, Chaudhari A. Duffy antigen/receptor for chemokines (DARC) attenuates angiogenesis by causing senescence in endothelial cells. Angiogenesis. 2007;10:307–318. doi: 10.1007/s10456-007-9084-y. [DOI] [PubMed] [Google Scholar]
- [6].Dahlman I, Kaaman M, Olsson T, Tan GD, Beckerton AS, Wahlen K, Andersson J, Nordstrom EA, Blomqvist L, Sjogren A, Forsgran M, Attersand A, Arner P. A unique role of monocyte chemoattractant protein 1 among chemokines in adipose tissue of obese subjects. J Clin Endocrinol Metab. 2005;90:5834–5840. doi: 10.1210/jc.2005-0369. [DOI] [PubMed] [Google Scholar]
- [7].Weisberg SP, Hunter D, Huber R, Lemieux J, Slaymaker S, Vaddi K, Charo I, Leibel RL, Ferrante AW., Jr. CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J Clin Invest. 2006;116:115–124. doi: 10.1172/JCI24335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Huber J, Kiefer FW, Zeyda M, Ludvik B, Silberhumer GR, Prager G, Zlabinger GJ, Stulnig TM. CC chemokine and CC chemokine receptor profiles in visceral and subcutaneous adipose tissue are altered in human obesity. J Clin Endocrinol Metab. 2008;93:3215–3221. doi: 10.1210/jc.2007-2630. [DOI] [PubMed] [Google Scholar]
- [9].Rull A, Camps J, Alonso-Villaverde C, Joven J. Insulin resistance, inflammation and obesity: Role of monocyte chemoattractant protein-1 (or CCL2) in the regulation of metabolism. Mediators Inflamm. 2010:pii326580. doi: 10.1155/2010/326580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Cai G, Cole SA, Butte NF, Smith CW, Mehta NR, Voruganti VS, Proffitt JM, Comuzzie AG. A genetic contribution to circulating cytokines and obesity in children. Cytokine. 2008;44:242–247. doi: 10.1016/j.cyto.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Butte NF, Cai G, Cole SA, Comuzzie AG. Viva la Familia Study: genetic and environmental contributions to childhood obesity and its comorbidities in the Hispanic population. Am J Clin Nutr. 2006;84:646–654. doi: 10.1093/ajcn/84.3.646. [DOI] [PubMed] [Google Scholar]
- [12].Sobel E, Lange K. Descent graphs in pedigree analysis: applications to haplotyping, location scores, and marker sharing statistics. Am J Hum Genet. 1996;58:1323–1337. [PMC free article] [PubMed] [Google Scholar]
- [13].Almasy L, Blangero J. Multipoint quantitative-trait linkage analysis in general pedigrees. Am J Hum Genet. 1998;62:1198–1121. doi: 10.1086/301844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Boerwinkle E, Chakraborty R, Sing CF. The use of measured genotype information in the analysis of quantitative phenotypes in man. I. Models and analytical methods. Ann Hum Genet. 1986;50:181–194. doi: 10.1111/j.1469-1809.1986.tb01037.x. [DOI] [PubMed] [Google Scholar]
- [15].Schnabel RB, Baumert J, Barbalic M, Dupuis J, Ellinor PT, Durda P, Dehghan A, Bis JC, Illig T, Morrison AC, Jenny NS, Keaney JF, Jr, Gieger C, Tilley C, Yamamoto JF, Khuseyinova N, Heiss G, Doyle M, Blankenberg S, Herder C, Walston JD, Zhu Y, Vasan RS, Klopp N, Boerwinkle E, Larson MG, Psaty BM, Peters A, Ballantyne CM, Witteman JC, Hoogeveen RC, Benjamin EJ, Koenig W, Tracy RP. Duffy antigen receptor for chemokines (Darc) polymorphism regulates circulating concentrations of monocyte chemoattractant protein-1 and other inflammatory mediators. Blood. 2010;115:5289–5299. doi: 10.1182/blood-2009-05-221382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Chanock SJ. The DARC side of GWAS. Blood. 2010;115:5285–5286. doi: 10.1182/blood-2010-02-263699. [DOI] [PubMed] [Google Scholar]
- [17].Benjamin EJ, Dupuis J, Larson MG, Lunetta KL, Booth SL, Govindaraju DR, Kathiresan S, Keaney JF, Jr., Keyes MJ, Lin J-P, Meigs JB, Robins SJ, Rong J, Schnabel R, Vita JA, Wang TJ, Wilson PWF, Wolf PA, Vasan RS. Genome-wide association with select biomarker traits in the Framingham Heart Study. BMC Medical Genetics. 2007;8(Suppl1):S11. doi: 10.1186/1471-2350-8-S1-S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Naitza S, Porcu E, Steri M, Taub DD, Mulas A, Xiao X, Strait J, Dei M, Lai S, Busonero F, Maschio A, Usala G, Zoledziewska M, Sidore C, Zara I, Pitzalis M, Loi A, Virdis F, Piras R, Deidda F, Whalen MB, Crisponi L, Concas A, Podda C, Uzzau S, Scheet P, Longo DL, Lakatta E, Abecasis GR, Cao A, Sclessinger D, Uda M, Sanna S, Cucca F. A genome-wide association scan on the levels of markers of inflammation in Sardinians reveals associations that underpin its complex regulation. PLoS Genetics. 2012;8:e1002480. doi: 10.1371/journal.pgen.1002480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Gosset P, Tillie-Leblond I, Oudin S, Parmentier O, Wallaert B, Joseph M, Tonnel AB. Production of chemokines and proinflammatory amd anti-inflammatory cytokines by human alveolar macrophages activated by IgE receptors. J Allergy Clin Immunol. 1999;103:289–297. doi: 10.1016/s0091-6749(99)70504-x. [DOI] [PubMed] [Google Scholar]
- [20].Eglite S, Morin JM, Metzger H. Synthesis and secretion of monocyte chemotactic protein-1 stimulated by the high affinity receptor for IgE. J Immunol. 2003;170:2680–2687. doi: 10.4049/jimmunol.170.5.2680. [DOI] [PubMed] [Google Scholar]
- [21].Ito A, Suganami T, Miyamoto Y, Yoshimasa Y, Takeya M, Kamei Y, Ogawa Y. Role of MAPK Phosphatase-1 in the induction of monocyte cehmoattractant protein-1 during the course of adipocyte hypertrophy. J Biol Chem. 2007;282:25445–25452. doi: 10.1074/jbc.M701549200. [DOI] [PubMed] [Google Scholar]
- [22].Malavazos AE, Cereda E, Morricone L, Coman C, Corsi MM, Ambrosi B. Monocyte chemoattractant protein 1: a possible link between visceral adipose tissue-associated inflammation and subclinical echocardiographic abnormalities in uncomplicated obesity. Eur J Endocrinol. 2005;153(6):871–877. doi: 10.1530/eje.1.02033. [DOI] [PubMed] [Google Scholar]
- [23].Breslin WL, Johnston CA, Strohacker K, Carpenter KC, Davidson TR, Moreno JP, Foreyt JP, McFarlin BK. Obese Mexican American children have elevated MCP-1, TNF-α, Monocyte concentration and dyslipidemia. Pediatrics. 2012;129:e1180–1186. doi: 10.1542/peds.2011-2477. [DOI] [PubMed] [Google Scholar]
- [24].Harsimran K, Singh AA, Guruvinder S, Sharda S, Vasudha S. Plasma monocyte chemoattractant protein-1 as risk marker in type 2 diabetes mellitus and coronary artery disease in North Indians. Diab Vasc Dis Res. 2009;6:288–290. doi: 10.1177/1479164109346364. [DOI] [PubMed] [Google Scholar]
- [25].Zineh I, Beitelshees AL, Silverstein JH, Haller MJ. Serum monocyte chemoattractant protein-1 concentrations associate with diabetes status but not arterial stiffness in children with type 1 diabetes. Diabetes care. 2009;32:564–467. doi: 10.2337/dc08-1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Zeyda M, Gollinger K, Kriehuber E, Kiefer FW, Neuhofer A, Stulnig TM. Newly identified adipose tissue macrophage populations in obesity with distinct chemokine and chemokine receptor expression. Int J Obes. 2010;34:1684–1694. doi: 10.1038/ijo.2010.103. [DOI] [PubMed] [Google Scholar]
- [27].Hsu Y-MS, Zhang Y, You Y, Wang D, Li H, Duramand O, Qin X-F, Dong C, Lin X. The adaptor protein CARD9 is required for innate immune responses to intracellular pathogens. Nature Immun. 2007;8:198–205. doi: 10.1038/ni1426. [DOI] [PubMed] [Google Scholar]
- [28].Pruenster M, Rot A. Throwing light on DARC. Biochemical Society Transactions. 2006;34(part 6) doi: 10.1042/BST0341005. [DOI] [PubMed] [Google Scholar]
- [29].Zarbock A, Bishop J, Muller H, Schmolke M, Buschmann K, Van Aken H, Singbartl Chemokine homeostasis vs. chemokine presentation during severe acute lung injury: the other side of the Duffy antigen receptor for chemokines. Am J Physiol Lung Cell Mol Physiol. 2010;298:L462–L471. doi: 10.1152/ajplung.00224.2009. [DOI] [PubMed] [Google Scholar]
- [30].Dawson T, Lentsch AB, Wang Z, Cowhig JE, Rot A, Maeda N, Peiper SC. Exaggerated response to endotoxin in mice lacking the Duffy antigen/receptor for chemokines (DARC) Blood. 2000;96:1681–1684. [PubMed] [Google Scholar]
- [31].He W, Neil S, Kulkarni H, Wright E, Agan BK, Marconi VC, Dolan MJ, Weiss RA, Ahuja SK. Duffy antigen receptor for chemokines mediates trans –infection of HIV-1 from red blood cells to target cells and affects HIV-AIDS susceptibility. Cell Host and Microbe. 2008;4:52–62. doi: 10.1016/j.chom.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Pogo AO, Chaudhari A. The Duffy protein: a malarial and chemokine receptor. Semin Hematol. 2000;37:122–129. doi: 10.1016/s0037-1963(00)90037-4. [DOI] [PubMed] [Google Scholar]
- [33].Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Liu XH, Hadley TJ, Xu L, Peiper SC, Ray PE. Up-regulation of duffy antigen receptor expression in children with renal disease. Kidney International. 1999;55:1491–1500. doi: 10.1046/j.1523-1755.1999.00385.x. [DOI] [PubMed] [Google Scholar]