Summary
Nongastrointestinal-type mucinous borderline tumors have been described as displaying endocervical and serous differentiation and hence have been termed “endocervical-type” mucinous borderline tumors, “mixed epithelial papillary cystadenoma of borderline malignancy of mullerian type” or “atypical proliferative seromucinous tumors”. A striking feature of these tumors is their frequent association with endometriosis, which has been reported in a third to a half of cases. This is an unusual finding as pure endocervical and serous tumors are not usually associated with endometriosis. ARID1A is a recently identified tumor suppressor, which frequently loses its expression and is mutated in endometrium-related carcinomas including ovarian clear cell, ovarian endometrioid and uterine endometrioid carcinomas. Although ARID1A mutations and expression have been studied in gynecological cancer, the expression pattern of ARID1A has not been investigated in ovarian atypical proliferative (borderline) tumors. In this study, we analyzed ARID1A expression in serous, gastrointestinal-type and endocervical-type (seromucinous) mucinous, and endometrioid atypical proliferative (borderline) tumors using immunohistochemistry and performed mutational analysis in selected cases. We observed loss of ARID1A staining in 8 (33%) of 24 seromucinous tumors. In contrast, ARID1A staining was retained in all the other 32 tumors except in one endometrioid tumor (p<0.01). Mutational analysis was performed on two representative seromucinous tumors, which showed complete loss of ARID1A. Both tumors harbored somatic inactivating ARID1A mutations. Previous studies have reported loss of expression and/or mutation of ARID1A in 30–57% of endometrioid and clear cell carcinomas but only rarely in serous tumors. The findings in this study, showing a significantly higher frequency of loss of ARID1A expression in endocervical-type (seromucinous) tumors, presumably due to mutation, compared to the other histologic types suggest that they are molecularly related to endometrioid and clear cell tumors.
Keywords: ARID1A, ovarian borderline tumor
Introduction
In 1988, Rutgers and Scully reported two different types of mucinous borderline tumors that were distinct from the more typical gastrointestinal-type mucinous tumor. One of these was designated “Müllerian mucinous papillary cystadenoma of borderline malignancy” and the other “mixed-epithelial papillary cystadenoma of borderline malignancy of Müllerian type” (1, 2). The former was characterized by a population of cells that were for the most part of endocervical-type whereas the latter were composed of endocervical type mucinous cells as well as a mixture of serous, endometrioid and indifferent cells with abundant eosinophilic cytoplasm. In our experience these nongastrointestinal type mucinous tumors almost always contain a mixture of multiple cell types. Moreover, their behavior and clinical features, including their frequent association with endometriosis, suggests, that they are closely related variants. Unlike their gastrointestinal type counterpart, which is typically glandular, the endocervical-type tumors are papillary and contain ciliated cells. Because of these features, under low magnification they closely resemble serous borderline tumors, which led us in 2002 to resurrect the term “seromucinous” to describe this group (3). In this report, we have used the various terms interchangeably. Since then we have been struck by their similarity to endometrial-type tumors. Specifically, the endometrial surface epithelium is ciliated and endocervical-type mucinous differentiation is quite common in endometrial proliferative lesions. These features along with the frequent association with endometriosis suggest that the endocervical-type mucinous tumors are closely related to endometrioid tumors.
We and others have recently reported somatic sequence mutations in a tumor suppressor gene termed ARID1A in 46–57% of ovarian clear cell carcinomas (4, 5), 40% of uterine endometrioid carcinomas (6) and 30% of ovarian endometrioid carcinomas (5) but rare (≤ 10%) in other types of carcinomas. Importantly, a close correlation between the mutational status and the expression pattern of ARID1A was found (5, 6). Loss of ARID1A immunoreactivity was found in 42–59% ovarian clear cell carcinomas, 21% ovarian endometrioid carcinomas and 26–34% uterine endometrioid carcinomas (5, 6). These findings prompted us to hypothesize that seromucinous tumors might display a similar molecular profile to endometrioid and clear cell tumors. Accordingly, we undertook an immunohistochemical analysis of a group of atypical proliferative (borderline) seromucinous tumors and compared them to serous, gastrointestinal-type mucinous, and endometrioid atypical proliferative tumors in an attempt to confirm our hypothesis.
Materials and Methods
Tissue Material
Paraffin embedded tissue sections of a total of 57 ovarian atypical proliferative (borderline) tumors were obtained from the Department of Pathology of the Johns Hopkins Hospital over the past 10 years. Some of them were the consultation cases to one of the authors (RJK and IMS). The use of the archival materials was approved by the internal review board of both institutions. For mutational analysis, genomic DNA isolated from case 314 and case 620 was used. Both samples consisted of fresh tumor cells that were isolated by incubating tumor fragments with 0.5% trypsin and EDTA at 37°C for 20 min with agitation. The tumor cells on the surface of papillae were carefully scraped off and the epithelial cells were cultured overnight. Red and white blood cells were removed after several washes before the attached epithelial cells were harvested for DNA purification using the Qiagne Blood DNA kit.
Immunohistochemistry
Immunohistochemical analysis was performed on tissue sections from ovarian atypical proliferative (borderline) tumors (13 serous, 8 endometrioid, 12 gastrointestinal type mucinous and 24 seromucinous tumors. A polyclonal rabbit anti-ARID1A antibody (Sigma-Aldrich HPA005456) was used for immunohistochemistry; the specificity of the antibody was validated in a previous report (6). Antigen retrieval was performed by placing sections in citrate buffer (pH 6.0), which were then placed in an autoclave at 120 °C for 10 minutes. The sections were incubated with the rabbit antibody at a dilution of 1:200 overnight at 4 °C. A positive reaction was detected using the EnVision+System (Dako, Carpinteria, CA). Tumor stromal cells served as positive internal controls. Only nuclear staining was scored. In this study, we defined “complete loss” as negative ARID1A staining in ≥ 90% of tumor cells. A previous study demonstrated that loss of nuclear expression correlated with mutation of the gene. Hence, absence of nuclear staining (diffuse or focal) was considered positive for gene mutation.
Mutation analysis
Two tumor samples from SMBT-1 and SMBT-2 were analyzed for somatic ARID1A mutations in all exons (form exon 1 to exon 20). Normal tissues from the matched cases were also sequenced. Tumor cells were enriched by affinity purification from fresh tissues using Dynal Epithelial enriched beads. The method of PCR and nucleotide sequences of PCR primers were previously reported (7). Sanger’s sequencing was performed on the purified PCR products. Sequence variations were detected using Mutation Surveyor DNA Variant Analysis software (SoftGenetics, Stage College, PA).
Results
The ARID1A immunostaining pattern in all subtypes of ovarian atypical proliferative (borderline) tumors is summarized in Table 1. Atypical proliferative seromucinous tumors (APSMTs) demonstrated the most frequent loss of ARID1A immunoreactivity as this occurred in 8 (33%) of 24 cases. Among these 8 tumors, 7 showed complete loss of ARID1A staining and one demonstrated clonal loss with focal tumor areas devoid of ARID1A immunoreactivity. In contrast, the serous and gastrointestinal mucinous tumors did not show any loss of ARID1A staining; one endometrioid tumor exhibited complete loss. Thus, the frequency of loss of ARID1A expression in APSMT was higher than other types of atypical proliferative tumors (p< 0.01, Fisher’s exact test, two-tailed). For all ARID1A negative staining tumors, stromal cells were intensely positive for ARID1A, which served as an internal positive control (Fig. 1). Morphologically, APSMTs are papillary and nearly always contain a mixture of cells including endocervical-type cells, ciliated cells, rounded and hobnail cells with abundant eosinophilic cytoplasm and sometimes squamous cells. Although it has been reported that loss of ARID1A expression in tumors correlates very well with inactivating somatic mutation of ARID1A, we selected two representative APSMTs (SMBT-1 and SMBT-2), which showed complete loss of ARID1A staining for mutational analysis to confirm that finding. All the exons (exon 1- exon 20) of the gene from both tumor and normal tissue were sequenced. We found one-base pair deletion at 3216 (3216delA) in SMBT-1 and a 7-base pair deletion at 2165 (2165_2171delACCAGAT) in SMBT-2. Based on chromatograms, we did not detect a second (wild-type) peak, indicating that the somatic sequence mutations were accompanied by either an interstitial deletion or a chromosomal arm loss in the other allele. The deletion mutations resulted in a frameshift and introduction of a stop codon, which results in a truncating protein. Since APSMTs had the most frequent ARID1A loss, we further correlated ARID1A expression with clinicopathological features. Seven (41%) of 17 APSMTs with documented assessment of endometriosis in pathology reports contained concurrent endometriosis (no evidence of atypical endometriosis) and two tumors were associated with adenomyosis. None of the other tumors were associated with endometriosis (Table 2). As shown in Table 2, there was no significant correlation between ARID1A immunostaining pattern, the patients’ age and the presence of endometriosis. The small number of cases precluded a definitive conclusion. ARID1A immunoreactivity was also determined in three representative ARID1A-negative APSMTs of which the adjacent endometriosis (endometriotic cyst) epithelium was available for staining. We found that in all three cases, ARID1A immunoreactivity was also lost in the epithelium of endometriotic cysts (Fig. 2).
Table 1.
ARID1A immunoreactivity in ovarian atypical proliferative (borderline) tumors.
| Serous | Endometrioid | Gastrointestinal type mucinous |
Seromucinous | |
|---|---|---|---|---|
| Cases with loss of ARID1A immunoreactivity | 0 | 1 | 0 | 8 |
| Total case number | 13 | 8 | 12 | 24 |
| % of cases with loss of expression | 0 | 13 | 0 | 33 |
Fig. 1.
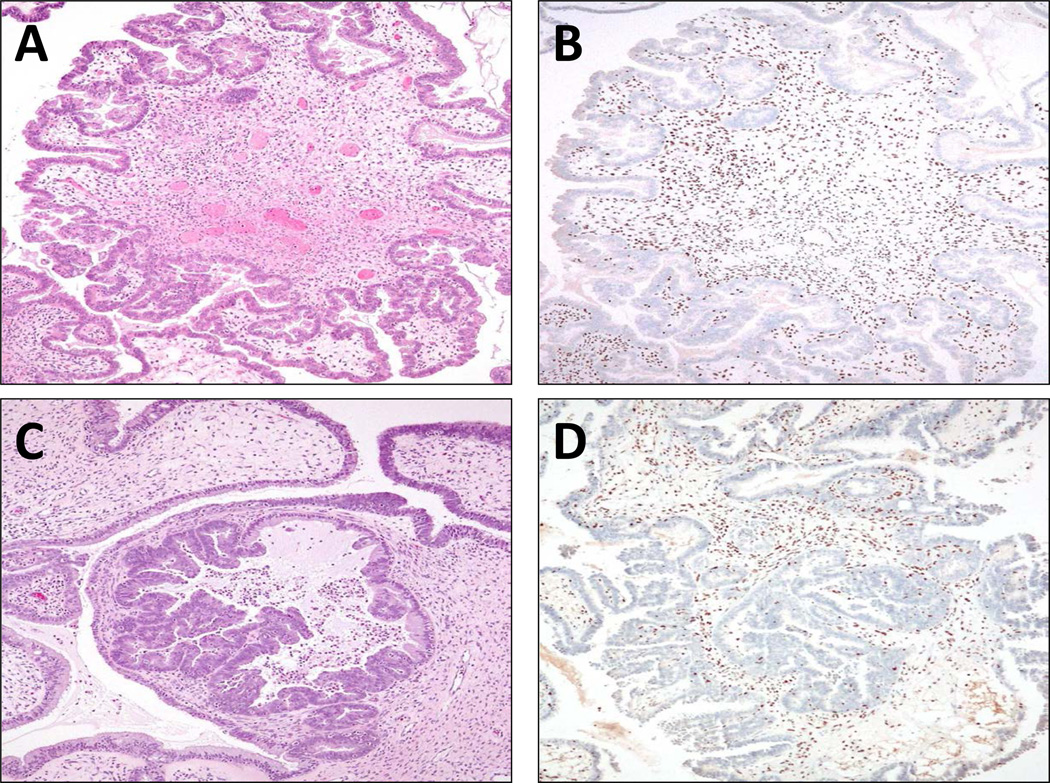
ARID1A immunoreactivity in two representative atypical proliferative (borderline) seromucinous tumors. Both tumors show a characteristic morphological feature of a seromucinous tumor containing mixed serous and mucinous epithelium, forming complex papillary fronds. Tumor cells are negative for ARID1A staining but the tumor stromal cells are positive for ARID1A expression. Mutational analysis showed somatic ARID1A mutations in both tumors. A and B: SMBT-1; C and D: SMBT-2.
Table 2.
Clinicopathological features and ARID1A expression in ovarian seromucinous borderline tumors
| Case | age | Endometriosis or adenomyosis | Associated lesion | ARID1A |
|---|---|---|---|---|
| SMBT-1 | 67 | no | no | lost |
| SMBT-2 | 50 | no | complex atypical hyperplasia | lost |
| SMBT-3 | 39 | no | no | lost |
| SMBT-4 | 45 | no | no | positive |
| SMBT-5 | 66 | no | no | positive |
| SMBT-6 | 52 | endometriosis | EMC + CCC | positive |
| SMBT-7 | 59 | no | no | positive |
| SMBT-8 | 51 | no | mature teraroma | positive |
| SMBT-9 | 44 | adenomyosis | no | positive |
| SMBT-10 | 37 | endometriosis | no | positive |
| SMBT-11 | 46 | endometriosis + mucinous and endometrioid borderline tumor | no | positive |
| SMBT-12 | 85 | NA | NA | positive |
| SMBT-13 | 33 | no | no | positive |
| SMBT-14 | 46 | endometriosis + adenomyosis | no | lost |
| SMBT-15 | 49 | NA | no | positive |
| SMBT-16 | 39 | NA | no | clonal lost |
| SMBT-17 | 53 | NA | NA | lost |
| SMBT-18 | 66 | no | no | positive |
| SMBT-19 | 62 | NA | NA | positive |
| SMBT-20 | 39 | endometriosis | no | positive |
| SMBT-21 | NA | NA | NA | positive |
| SMBT-22 | 44 | endometriosis | uterine endometrioid CA | lost |
| SMBT-23 | 51 | endometriosis | no | lost |
| SMBT-24 | 56 | NA | no | positive |
Fig. 2.
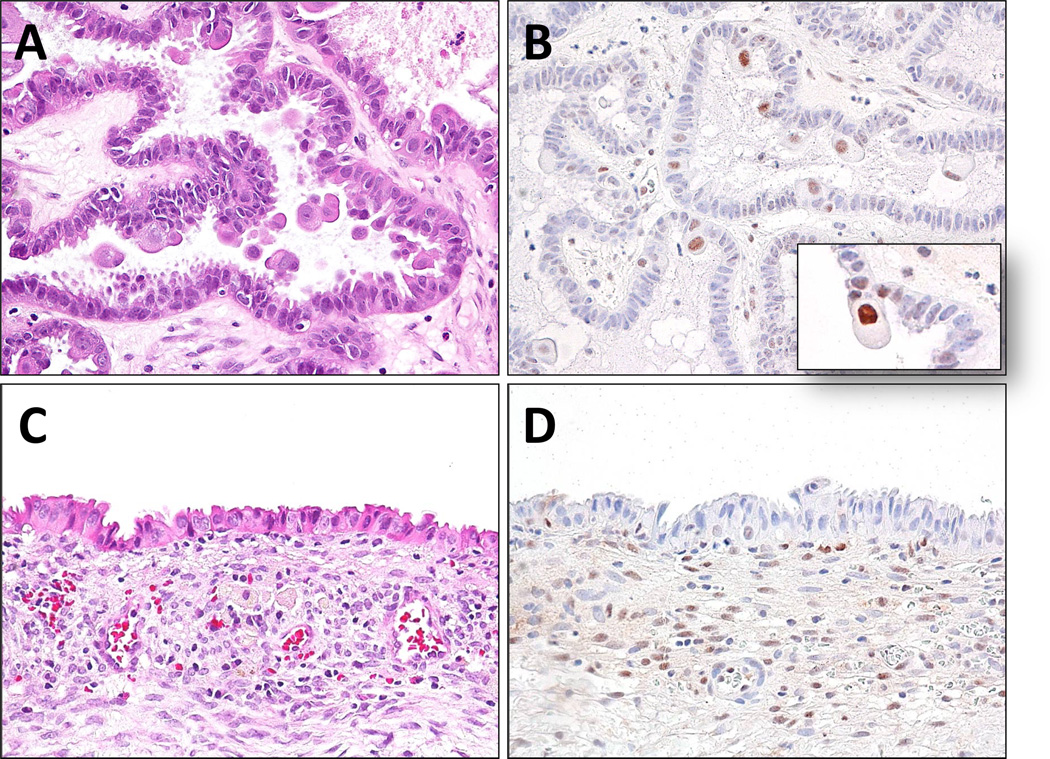
ARID1A expression in SMBT-23 which contains both atypical proliferative (borderline) seromucinous tumor (A, B) and associated endometriotic cyst (C, D). ARID1A staining was not detected in the epithelial cells of the endometriotic cyst (stromal cells serving as the positive control) and in the majority of tumors cells from the seromucinous tumor. There are occasional ARID1A positive tumor cells in the seromucinous tumor component and those positive cells contain abundant eosinophilic cytoplasm and appear extruded from the epithelial layer (inset).
Discussion
Chromatin remodeling is essential for almost all nuclear activities including DNA replication, transcription, DNA methylation and DNA repair (8–16). Thus, it is not surprising that aberration in chromatin remodeling activity, as a result of gene mutations or amplification involving various chromatin remodeling factors, is one of the mechanisms in the pathogenesis of human cancer (17). For example, somatic inactivating mutations have been detected in several SWI/SNF chromatin remodeling genes including PBRM1 BAF180) (18) in renal cell carcinoma, BRG1 (SMARCA4) in lung carcinoma (19, 20) and ARID2 in hepatocellular carcinoma (21). Amplification of Rsf-1, a gene participating in ISWI chromatin remodeling, has been demonstrated to promote chromosomal instability, propel tumor progression, and contribute to disease aggressiveness in ovarian and oral cancer (22–26). ARID1A BAF250A) is a chromatin remodeling factor which promotes the formation of the SWI/SNF chromatin remodeling complexes containing BRG1 or BRM. ARID1A has been proposed as a candidate tumor suppressor gene based on the finding of frequent inactivating mutations of ARID1A in a variety of gynecological malignancies. In fact, a recent study has demonstrated that restoring wild-type ARID1A expression in ovarian cancer cells that harbor ARID1A mutations is sufficient to suppress cell proliferation and tumor growth in mice, whereas silencing of ARID1A expression in non-transformed epithelial cells enhances cellular proliferation and tumorigenicity (27).
In this report, we found that atypical proliferative seromucinous tumors (APSMTs) frequently lost ARID1A immunoreactivity compared to the other tumor types. Except for loss of expression of ARID1A in one of 8 atypical proliferative (borderline) endometrioid tumors, all the other tumors in this study failed to show loss of ARID1A expression. This frequency of loss of ARID1A immunoreactivity in the atypical proliferative (borderline) endometrioid tumors (8%) is substantially lower than the 21% previously reported in ovarian endometrioid carcinoma (5). This is most likely related to the limited number of cases analyzed.
We demonstrated somatic mutations of ARID1A in two representative APSMTs with complete loss of ARID1A expression, confirming the utility of immunohistochemistry as a surrogate for mutational analysis. Because ARID1A mutations and loss of expression are, in general, restricted to endometrium-related lesions, either uterine endometrioid carcinoma, ovarian clear cell carcinoma, ovarian endometrioid carcinoma, or atypical endometriosis (5–7, 28, 29), the above data provide molecular evidence that APSMT is closely related to endometrial-type proliferative lesions. Moreover, of the 24 APSMTs, approximately one third of cases in which clinical data were available were associated with endometriosis. Interestingly, one of these endometriosis associated APSMTs also contained a clear cell and endometrioid carcinoma. In contrast, none of the other tumors in the study were associated with endometriosis.
In this study, we observed “clonal loss” of ARID1A staining in one of the APSMTs, which was characterized by relatively large groups of ARID1A negative tumor cells in a background of ARID1A positive tumor cells. In a previous study of uterine endometrioid carcinomas, we found a similar clonal loss of ARID1A immunoreactivity associated with ARID1A mutation (27). The nature and significance of clonal loss of ARID1A waits further study.
In summary, we analyzed different types of atypical proliferative (borderline) tumors for their expression of ARID1A, a newly identified tumor suppressor gene, and found that, as compared to other types of atypical proliferative (borderline) tumors, APMSTs frequently lost ARID1A expression. Moreover, in two of these cases, ARID1A inactivating mutations were found. These findings along with the frequent association with endometriosis and infrequent expression of WT-1 (30) strongly suggest a closer relationship of these tumors to endometrioid and clear cell tumors than to mucinous and serous tumors.
Acknowledgements
This study was supported by NIH/NCI grants, CA165807 and CA129080.
Footnotes
Conflict of interest statement
The authors declare that there are no conflicts of interest.
References
- 1.Rutgers JL, Scully RE. Ovarian mullerian mucinous papillary cystadenomas of borderline malignancy. A clinicopathologic analysis. Cancer. 1988;61:340–348. doi: 10.1002/1097-0142(19880115)61:2<340::aid-cncr2820610225>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 2.Rutgers JL, Scully RE. Ovarian mixed-epithelial papillary cystadenomas of borderline malignancy of mullerian type. A clinicopathologic analysis. Cancer. 1988;61:546–554. doi: 10.1002/1097-0142(19880201)61:3<546::aid-cncr2820610321>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 3.Shappell HW, Riopel MA, Smith Sehdev AE, Ronnett BM, Kurman RJ. Diagnostic criteria and behavior of ovarian seromucinous (endocervical-type mucinous and mixed cell-type) tumors: atypical proliferative (borderline) tumors, intraepithelial, microinvasive, and invasive carcinomas. Am J Surg Pathol. 2002;26:1529–1541. doi: 10.1097/00000478-200212000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Jones JM, Cui XS, Medina D, Donehower LA. Heterozygosity of p21WAF1/CIP1 enhances tumor cell proliferation and cyclin D1-associated kinase activity in a murine mammary cancer model. Cell Growth Differ. 1999;10:213–222. [PubMed] [Google Scholar]
- 5.Wiegand KC, Shah SP, Al-Agha OM, et al. ARID1A mutations in endometriosis-associated ovarian carcinomas. N Engl J Med. 2010;363:1532–1543. doi: 10.1056/NEJMoa1008433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guan B, Mao TL, Panuganti PK, et al. Mutation and loss of expression of ARID1A in uterine low-grade endometrioid carcinoma. Am J Surg Pathol. 2011;35:625–632. doi: 10.1097/PAS.0b013e318212782a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jones S, Wang TL, Shih IM, et al. Frequent mutations of chromatin remodeling gene ARID1A in ovarian clear cell carcinoma. Science. 2010;330:228–231. doi: 10.1126/science.1196333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu H, Mulholland N, Fu H, Zhao K. Cooperative activity of BRG1 and Z-DNA formation in chromatin remodeling. Mol Cell Biol. 2006;26:2550–2559. doi: 10.1128/MCB.26.7.2550-2559.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nie Z, Xue Y, Yang D, et al. A specificity and targeting subunit of a human SWI/SNF family-related chromatin-remodeling complex. Mol Cell Biol. 2000;20:8879–8888. doi: 10.1128/mcb.20.23.8879-8888.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang W, Cote J, Xue Y, et al. Purification and biochemical heterogeneity of the mammalian SWI-SNF complex. EMBO J. 1996;15:5370–5382. [PMC free article] [PubMed] [Google Scholar]
- 11.Van Rechem C, Boulay G, Leprince D. HIC1 interacts with a specific subunit of SWI/SNF complexes, ARID1A/BAF250A. Biochem Biophys Res Commun. 2009;385:586–590. doi: 10.1016/j.bbrc.2009.05.115. [DOI] [PubMed] [Google Scholar]
- 12.Ho L, Crabtree GR. Chromatin remodelling during development. Nature. 2010;463:474–484. doi: 10.1038/nature08911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reisman D, Glaros S, Thompson EA. The SWI/SNF complex and cancer. Oncogene. 2009;28:1653–1668. doi: 10.1038/onc.2009.4. [DOI] [PubMed] [Google Scholar]
- 14.Gao X, Tate P, Hu P, Tjian R, Skarnes WC, Wang Z. ES cell pluripotency and germ-layer formation require the SWI/SNF chromatin remodeling component BAF250a. Proc Natl Acad Sci U S A. 2008;105:6656–6661. doi: 10.1073/pnas.0801802105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krosl J, Mamo A, Chagraoui J, et al. A mutant allele of the Swi/Snf member BAF250a determines the pool size of fetal liver hemopoietic stem cell populations. Blood. 2010;116:1678–1684. doi: 10.1182/blood-2010-03-273862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alessio N, Squillaro T, Cipollaro M, Bagella L, Giordano A, Galderisi U. The BRG1 ATPase of chromatin remodeling complexes is involved in modulation of mesenchymal stem cell senescence through RB-P53 pathways. Oncogene. 2010;29:5452–5463. doi: 10.1038/onc.2010.285. [DOI] [PubMed] [Google Scholar]
- 17.Gui Y, Guo G, Huang Y, et al. Frequent mutations of chromatin remodeling genes in transitional cell carcinoma of the bladder. Nat Genet. 2011 doi: 10.1038/ng.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Varela I, Tarpey P, Raine K, et al. Exome sequencing identifies frequent mutation of the SWI/SNF complex gene PBRM1 in renal carcinoma. Nature. 2011;469:539–542. doi: 10.1038/nature09639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Medina PP, Romero OA, Kohno T, et al. Frequent BRG1/SMARCA4-inactivating mutations in human lung cancer cell lines. Hum Mutat. 2008;29:617–622. doi: 10.1002/humu.20730. [DOI] [PubMed] [Google Scholar]
- 20.Rodriguez-Nieto S, Canada A, Pros E, et al. Massive parallel DNA pyrosequencing analysis of the tumor suppressor BRG1/SMARCA4 in lung primary tumors. Hum Mutat. 2010 doi: 10.1002/humu.21415. [DOI] [PubMed] [Google Scholar]
- 21.Li M, Zhao H, Zhang X, et al. Inactivating mutations of the chromatin remodeling gene ARID2 in hepatocellular carcinoma. Nat Genet. 2011 doi: 10.1038/ng.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shih le M, Sheu JJ, Santillan A, et al. Amplification of a chromatin remodeling gene, Rsf-1/HBXAP, in ovarian carcinoma. Proc Natl Acad Sci U S A. 2005;102:14004–14009. doi: 10.1073/pnas.0504195102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Choi JH, Sheu JJ, Guan B, et al. Functional analysis of 11q13.5 amplicon identifies Rsf-1 (HBXAP) as a gene involved in paclitaxel resistance in ovarian cancer. Cancer Res. 2009;69:1407–1415. doi: 10.1158/0008-5472.CAN-08-3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sheu JJ, Choi JH, Yildiz I, et al. The Roles of Human Sucrose Nonfermenting Protein 2 Homologue in the Tumor-Promoting Functions of Rsf-1. Cancer Res. 2008;68:4050–4057. doi: 10.1158/0008-5472.CAN-07-3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sheu JJ, Guan B, Choi JH, et al. Rsf-1, a chromatin remodeling protein, induces DNA damage and promotes genomic instability. J Biol Chem. 2010;285:38260–38269. doi: 10.1074/jbc.M110.138735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fang FM, Li CF, Huang HY, et al. Overexpression of a chromatin remodeling factor, Rsf-1/HBXAP, correlates with aggressive oral squanous cell carcinoma. Am J Pathol. 2011 doi: 10.1016/j.ajpath.2011.01.043. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guan B, Wang TL, Shih IM. ARID1A, a factor that promotes formation of SWI/SNF-mediated chromatin remodeling, is a tumor suppressor in gynecologic cancers. Cancer Res. 2011 doi: 10.1158/0008-5472.CAN-11-1562. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wiegand KC, Lee AF, Al-Agha OM, et al. Loss of BAF250a (ARID1A) is frequent in high-grade endometrial carcinomas. J Pathol. 2011;224:328–333. doi: 10.1002/path.2911. [DOI] [PubMed] [Google Scholar]
- 29.Maeda D, Mao T-L, Fukayama M, et al. Clinicopathological significance of loss of ARID1A Immunoreactivity in ovarian clear cell carcinoma. Int J Mol Sci. 2010;11:5120–5128. doi: 10.3390/ijms11125120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vang R, Gown AM, Barry TS, Wheeler DT, Ronnett BM. Ovarian atypical proliferative (borderline) mucinous tumors: gastrointestinal and seromucinous (endocervical-like) types are immunophenotypically distinctive. Int J Gynecol Pathol. 2006;25:83–89. doi: 10.1097/01.pgp.0000177125.31046.fd. [DOI] [PubMed] [Google Scholar]