Abstract
Older adults exhibit higher morbidity and mortality from infectious diseases compared to the general population. The introduction and rapid spread of West Nile virus (WNV) throughout the continental United States since 1999 has highlighted the challenge of protecting older adults against emerging pathogens: to this day there is no therapy or vaccine approved for human use against West Nile encephalitis (WNE). Here we describe characterization of T and B cell responses in old mice following vaccination with RepliVAX WN, a novel WNE vaccine based on single-cycle flavivirus (SCFV) particles. In adult mice, RepliVAX WN induced a robust and long-lasting CD4+ and CD8+ T cell and antibody (B cell) responses against natural WNV epitopes, similar to those elicited by primary WNV infection. Primary and memory T and B cell responses in old mice against RepliVAX WN vaccination were significantly lower than those seen in younger mice, similar to the response of old mice to infection with WNV. Surprisingly, both the quality and the quantity of the recall antibody (Ab) and T cell responses in vaccinated old mice were improved to equal or exceeded those in adult animals. Moreover, these responses together (but not individually) were sufficient to protect both old and adult mice from severe WNV disease upon challenge. Therefore, at least two cycles of in vivo restimulation are needed for selection and expansion of protective lymphocytes in older populations and that live, single-cycle virus vaccines that stimulate both cellular and humoral immunity can protect older individuals against severe viral disease.
Introduction
West Nile virus (WNV) was introduced into the United States during the summer of 1999. By 2005 the virus had spread throughout the continental US, progressing from an isolated outbreak to a nationwide epidemic and documenting the potential for the rapid spread of an emerging infectious disease. Age and immunocompromised status were found to be the strongest predictors of vulnerability to this infection (1–3). Developing safe and effective means to protect these susceptible groups against WNV and other emerging or bioterror-introduced infections has become a major priority.
Despite this urgent need to protect vulnerable populations, there is currently no approved human vaccine or therapeutic to protect against WNV disease. WNV is a member of the Flaviviridae family, within the Flavivirus genus, which contains approximately 40 viruses capable of causing human disease. To date, vaccines are available for only three flavivirus diseases: yellow fever (YF), Japanese encephalitis (JE) and tick-borne encephalitis virus (TBE). The TBE and JE vaccines approved for use in developed countries are inactivated virus vaccines, while the YF vaccine is a live-attenuated virus vaccine. As is often the case with inactivated vaccines, the TBE and JE vaccines have limited potency and require multiple vaccinations and repeated boosters to be effective at preventing disease (2, 4), but also are considered safe, as they have low incidence of potentially life threatening side effects. By contrast, the live attenuated YF vaccine only requires one immunization to provide protection against disease (5), but does have the potential to cause disease in the immunosuppressed (6) or those over 50 and is contraindicated for these populations(7).
The incidence of WNV infection is fairly uniform with age (8) and in most immunocompetent humans the infection is asymptomatic (9, 10). However severe WNV disease, which includes the involvement of the central nervous system (manifested as meningitis and encephalitis) disproportionately afflicts older adults with a lethality of 10% and a mean age at death of 78 years (3, 8). Persons between 50–59 years of age have a 10-times higher incidence of severe disease and persons aged 80 years or greater have a 43-times higher incidence of severe disease compared to adults between 20–40 years of age (3, 11). Moreover there is an increase in morbidity in older adults, with many patients suffering symptoms from WNE that can last for several years following infection (12). The overall mortality in the first year post-infection of older individuals is also significantly increased compared to age-matched controls (3) further demonstrating the impact of the infection on this vulnerable population.
Vulnerability of older adults to infection is believed to be caused by a decline of the immune defense due to the aging of the immune system, also called immunosenescence (13, 14). Both cell-autonomous and population-based disorders have been described with advanced age (rev. in (13–17), and while these seem to most severely affect the adaptive immune system, it is less clear which ones are most important and whether and to what extent they can be manipulated to correct immunosenescence and improve immune protection in the aged. The most effective tool against infectious diseases remains vaccination. However, the same defects that impair immune responses against infection also prevent effective responses to vaccines (18). In older individuals, it is not clear whether this results from the lack of an adequate reserve of lymphocytes to respond to vaccination/infection (numerical/quantitative problem), or whether their lymphocytes (or other cells of the immune system) are inherently unable to respond by mounting a protective response (qualitative problem). This issue is critical because the answer would dictate practical approaches to immune intervention – rejuvenation in the case of the former issue, vaccination/immunostimulation in the case of the latter. To begin to answer this question, we tested the ability of a rationally engineered vaccine to induce primary, memory and recall responses in adult and old mice against WNV. We observed greatly depressed primary (defined as the response at the peak of acute phase, 7–8 days post-infection) and similarly low memory (defined as responsiveness to Ag 45 days post-vaccination) responses to vaccination in old animals. By contrast, recall responses in vaccinated old animals, which involved secondary expansion of Ag-specific cells, were high in magnitude; protective Ab responses were much improved during the recall response; and T cell responses equaled or surpassed those in adults in both magnitude and function. Protection required the presence of both Ag-specific T and B cells. This suggests that secondary stimulation is crucial to expand and differentiate sufficient numbers of responding lymphocytes in old animals and that some of the important defects in immunosenescence may be correctable by single or perhaps repeated vaccination.
Materials and Methods
Mice
Old (18–22 month) and adult (3 months old) C57BL/6 (B6) mice were purchased from the National Institute of Aging breeding colony (Harlan Sprague-Dawley, Indianapolis, IN) and the Jackson Laboratory (Bar Harbor, ME). C57BL/6 μMT−/− and C57BL/6 Rag1−/− mice were purchased from the Jackson Laboratory (Bar Harbor, ME), and bred at the Oregon Health & Science University (OHSU) West Campus or the University of Arizona vivaria. All animals were housed and bred under specific pathogen-free conditions and experiments conducted under the Institutional Animal Care and Use Committee, and the Institutional Biosafety Committee approvals in accordance with all applicable federal, state, and local regulations. All West Nile virus experiments were completed within a United States Department of Agriculture (USDA) inspected biosafety level 3 facility.
Virus, peptides and cell lines
The RepliVAX WN used in all experiments was isolated by limiting dilution purification from a mixed population produced by serial passaging of RepliVAX WN in WNV-C expressing cells that had been as previously described (19). Sequence analysis of the truncated C, prM, and N-terminus 30% of E of this isolate confirmed a single amino acid change (serine to cysteine) at the signalase cleavage site preceding the mature prM protein that enhanced growth in vitro (19). The infectious unit (IU) titer of the RepliVAX WN was determined by immunohistochemical staining of Vero cells infected with serial dilutions of RepliVAX WN, as previously described (20). West Nile virus strain 385–99, a kind gift from Robert Tesh, MD (University of Texas Medical Branch), was used for all challenge experiments, and its titer (expressed as PFU) was determined by plaque assay on Vero cells using standard methods.
E 641–655, NS3 1616–1630, NS3 2066–2080, and NS4b 2488–2496 peptides (20, 21) were purchased from 21st Century Biochemicals, diluted in 10% H2O, 90% DMSO and stored at −80° C. Vero (WHO) cells were grown in Dulbecco’s modified Eagle’s medium (DMEM) and EL-4s in RPMI supplemented with antibiotics and 5% fetal calf serum. EL-4 cells were infected with WNV using a multiplicity of infection (MOI) of 10 for 30 hours prior to use for 51Cr assays. All cells were periodically monitored to ensure that they were mycoplasma negative, and were cultured under aseptic conditions.
Flow cytofluorometric (FCM) analysis and Intracellular cytokine staining (ICCS)
Cytokine-producing T cells were detected using the Foxp3 Staining Buffer Set (eBioscience, San Diego, CA) as recommended by the manufacturer. Briefly, single-cell splenocyte suspensions obtained from experimental animals were incubated with 1μM peptide in the presence of 5μg/ml brefeldin A (Sigma Aldrich, St. Louis, MO) for 6 h at 37 °C. After six hours the cells were washed and incubated overnight in the presence of a saturating dose of surface antibodies against CD8, CD3, CD4, CD11a, CD127, CD44 and CD62L (BD-PharMingen, San Diego, CA; eBioscience; Biolegend, San Diego, CA). After washing, the cells were fixed, permeabilized and intracellular antibodies against IFN-γ, NFα (Ebioscience), and GzB (Invitrogen, Carlsbad, CA) were added for 30 minutes. The samples were then washed and analyzed using an LSR II or Fortessa cytometers (Becton Dickinson Immunocytometry Systems, Franklin Lakes, NJ) and Flow-Jo software (Treestar, Ashland, OR).
Vaccination/Infection, challenge and cytotoxic T lymphocyte assay
Mice were infected by intraperitoneal (i.p.) injection with 0.16–50 PFU of WNV virus per mouse, and/or vaccinated by i.p. injection with 1×102–1×106 IU of RepliVAX WN per mouse, as denoted in the figure legends. Mice were challenged 45–60 days post-vaccination with 1000 – 2000 pfu WNV as denoted in figure legends. Seven or eight days after infection or challenge, lymphocytes were isolated and used for direct flow cytometry (FCM) analysis, direct ex-vivo restimulation for cytokine production, and direct ex vivo 51Cr assay using indicated target cells. Percent specific lysis was calculated as [(E − S)/(M − S)]×100, where E represents the counts per minute released from targets incubated with lymphocytes, S represents the counts per minute released from target cells incubated with no lymphocytes and M represents the counts per minute released from cells after lysis with 1% Nonidet P40 (USB, Cleveland, OH).
ELISA
Enzyme-linked immunosorbent assays (ELISAs) used to determine serum Ab titers against WNV E and NS1 proteins have been previously described (19). Briefly, immulon 2HB microtiter plates (Thermo Labsystems, Franklin, MA) were coated overnight at 4°C with soluble forms of WNV E or NS1 proteins produced from replicon bearing cell lines under serum-free cultivation conditions (19). Wells were blocked with ELISA blocking buffer (phosphate buffered saline containing 5% normal horse serum and 0.05% Tween-20) after which sera samples diluted 1:100 in ELISA blocking buffer were incubated in the antigen-coated wells. After thorough washing, a goat anti-mouse IgG HRP (horseradish peroxidase) -conjugated Ab (KPL, Gaithersburg, MD) was incubated in the wells, the wells were thoroughly washed, and bound enzyme was detected with a soluble HRP substrate (TMB, Sigma, St. Louis, MO). HRP reaction was stopped with HCl, the optical density of the reaction products quantitated spectrophotometrically at 450nm, and values corrected for background activity detected from wells that received ELISA blocking buffer containing no animal sera samples. Neutralization assay was performed as previously described (19). Briefly, serial dilutions of pooled sera were incubated with 100pfu of WNV overnight at 4°C. Each serum dilution containing virus was then used to infect confluent Vero cells for 2 hours. Cells were overlaid with agarose and 2 days later agarose containing neutral red (0.2%) was added. Plaques were counted on the fifth and sixth day after infection. Results are expressed as the serum dilution that produced a 90% reduction of plaques.
Passive serum transfer experiments
Serum was collected from mice at different times following vaccination, infection, and challenge as indicated in each figure. At each time point, mice were euthanized and 600uL of blood was collected via cardiac puncture into 200uL of PBS. The blood was allowed to clot and serum was collected. Transfers were performed on a one donor mouse to one recipient mouse basis and serum was administered in a single i.p. injection. Recipient mice were challenged the next day with 1,000–2,000pfu of WNV either subcutaneously (s.c.) or i.p. as indicated.
WNV specific T cell analysis and plaque assay in brain
Mice were vaccinated by i.p. injection with 1×105 IU of RepliVAX WN per mouse. 45 days following vaccination, mice were challenged with 2000pfu WNV by i.p. injection. Eight days after challenge, the mice were sacrificed and perfused with 1X PBS. Brain was collected and disassociated through 0.22uM cell strainers. 200uL was saved for plaque assay. Lymphocytes were isolated over a percoll gradient and used for direct flow cytometry (FCM) analysis, Viral titer was determined by plaque assay by serially diluting sample onto Vero cells. After co-culture of the virus with the cells for 2 hours, cells were overlaid with agarose and 2 days later agarose containing neutral red (0.2%) was added. Plaques were counted on the fifth and sixth day after infection to determine viral load.
Statistical analyses
Fisher’s exact test and log rank test was used to analyze results from survival experiments. Statistical significance observed between groups was analyzed using unpaired Student’s t test. Virus titers are represented as the geometric mean titer (GMT) and statistical significance was calculated using the Mann Whitney test. All calculations were performed using Prism statistical software (GraphPad, San Diego, CA).
RESULTS
RepliVAX WN as a vaccination tool
WNV contains a single-strand positive-sense ~11 kb RNA genome that is translated into a single polyprotein which is then co-and post-translationally cleaved into three structural [capsid (C), pre/membrane (prM) and envelope (E)] and seven non-structural (NS1, NS2a, NS2b, NS3, NS4a, NS4b, and NS5) proteins. These ten multifunctional proteins play a role in invasion, entry, viral replication, assembly, and modulation of host cell functions, including the immune response (rev. in (22, 23)). The WNV structural proteins, C, M (produced by cleavage of prM in the exocytic pathway) and E, package a single copy of the RNA genome in a 50 nm particle. Many of the antigenic determinants that induce the immune response (particularly neutralizing antibodies) against WNV are found within E and are the focus for the development of several potential vaccines (24).
The ability to rationally design vaccines to prevent diseases caused by viruses has benefited from recent development of reverse genetics systems and an increased understanding of the correlates of protection. One vaccine platform that takes advantage of these developments is a single-cycle flavivirus (SCFV) known as RepliVAX WN (19, 25). RepliVAX WN consists of the same surface components as WNV but its genome contains a large deletion in the gene encoding the essential C protein. High titers of RepliVAX WN can be produced in packaging cells expressing functional WNV C encoded by a Venezuelan equine encephalitis virus replicon (19, 25). RepliVAX WN binds and enters cells in a manner indistinguishable from WNV, and the RepliVAX WN genome is efficiently translated and replicated to produce all of the WNV proteins except C (25), which prevents these infected cells from producing progeny virus. However, the presence of all other virion proteins components affords generation and secretion of highly immunogenic sub-viral particles (SVPs) believed to be the basis for RepliVAX-induced humoral immunity (25, 26). These particles are smaller but antigenically indistinguishable from WNV particles and are therefore expected to stimulate immune response similar to the one produced by WNV infection. RepliVAX WN has been shown to be safe and efficacious in preventing WNV-induced disease in mice (25)(Widman, 2008) and hamsters (19, 27), and demonstrated safety, potency and efficacy in a non-human primate model of WNV infection(28). To date, however, neither the relative contribution of the cellular immune response to RepliVAX WN-mediated protection nor the ability of this vaccine to induce protective responses in old mice has been examined.
Characterization of CD8+ and CD4+ T cell responses to RepliVAX WN in adult B6 mice
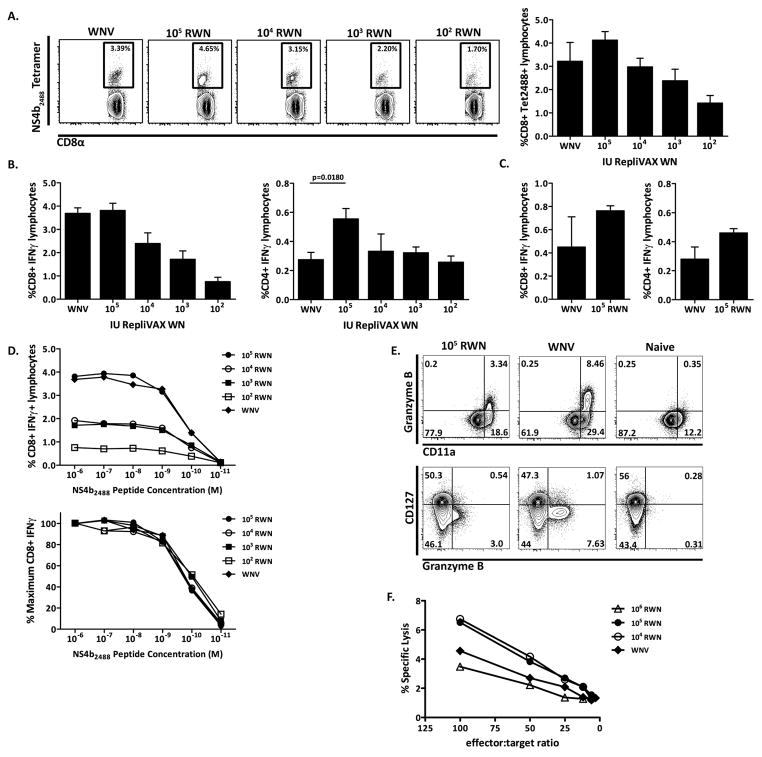
We first sought to determine whether vaccination with RepliVAX WN induces T cell responses. SCFVs such as RepliVAX WN have the ability to generate large quantities of viral proteins within an infected cell (Widman and Mason, unpublished data), providing plenty of material for viral antigen processing and presentation. Moreover, infection of mice using SCFVs stimulates strong innate immune responses (29)(Widman and Mason, unpublished data), which should increase antigen processing and presentation. Our recent work showed that the main WNV CD8 and CD4 epitopes in B6 mice reside within the NS4b, E and NS3 segments of the virus (20, 21), and thus RepliVAX WN encodes all the proteins critical to T cell stimulation. To examine cellular responses, we immunized mice with decreasing doses of RepliVAX WN (1×106–1×102 IU) by the i.p. route and compared the induction of T cell responses. We measured the percentage of antigen-specific CD8+ T cells responding to the immunodominant NS4b WNV epitope in B6 mice using the NS4b2488:Db tetramer, (20, 30). 1×105 IU of RepliVAX WN induced a T cell response comparable to the one induced by 50 pfu WNV delivered by the i.p. route, in terms of percentage and number of antigen-specific CD8+ T cells detected at 7 days post-immunization (Fig. 1A and data not shown). Moreover, the percentage of NS4b2488- specific CD8+ T cells elicited in response to RepliVAX WN was dose-dependent. Similar results were obtained when we evaluated the percentage of responding CD8+ and CD4+ T cells 7 days post-immunization by measuring IFNγ production using an intracellular cytokine staining assay (ICCS) (Fig. 1B). Immunization with 1×105 IU of RepliVAX WN generated similar percentages of NS4b2488-specific CD8 and E and NS3-specific CD4+ T cells compared to WNV infection, and decreasing doses of RepliVAX WN generated decreasing percentages of antigen-specific CD8+ and CD4+ T cells. In fact, with CD4+ T cells, larger percentages and numbers of CD4+ T cells were induced by 1×105 IU RepliVAX WN than by WNV infection (Fig. 1B, right panel and not shown). RepliVAX WN vaccination also generated T cells that responded to several other viral determinants including all other previously identified CD8+ and CD4+ T cell epitopes (data not shown) ((20, 21). Taken together, these results illustrate the ability of RepliVAX WN vaccination to generate robust responses to a broad array of natural T cell epitopes.
Figure 1. Magnitude and quality of antigen-specific T cells in response to RepliVAX WN vaccination and WNV infection.
A. CD8+ NS4b2488 tetramer+ T cells 7 days post-infection from mice given decreasing doses of RepliVAX WN. Left panel – Example of CD8+ NS4b2488 tetramer+ T cell staining 7 days post-infection, completed by FCM and gated on CD8. Results from a representative mouse at each dose from one out of two similar experiments are shown. Right panel – Quantification of CD8+ NS4b2488 tetramer+ staining showing decreasing doses of RepliVAX WN administered to mice. CD8 tetramer staining was collected by FCM and gated on CD8. N=4 mice per dose, x+/− SEM. One of two experiments. B. Left panel – Quantification of CD8+ T cells from day 7 post-infection by IFNγ ICCS, showing decreasing doses of RepliVAX WN administered to mice. CD8+ T cells were stimulated with 1×10−6M NS4b2488 peptide for 6 hours in the presence of BFA and analyzed by ICCS. Results depict x+/− SEM from n=4 mice per dose. Right panel – Quantification of CD4+ T cells from day 7 post-infection by IFNγ ICCS, showing decreasing doses of RepliVAX WN administered to mice. CD4+ T cells were stimulated with 1×10−6M E641 and NS31616+2066 peptide for 6 hours in the presence of BFA and analyzed by ICCS. Results depict x+/− SEM from n=4 mice per dose, from one of two similar experiments. C. Left panel – Quantification of CD8+ T cells from day 45 post-infection by IFNγ ICCS, comparing 105 IU RepliVAX WN to 50pfu of WNV administered to mice. CD8+ T cell ICCS was performed as above. n=4 mice per dose, x+/− SEM. Right panel – Quantification of CD4+ T cells from day 45 post-infection by IFNγ ICCS, comparing 105 IU RepliVAX WN to 50pfu of WNV administered to mice. CD4+ T cell ICCS was performed as above. Results depict x+/− SEM from n=4 mice per dose, from one representative example of two experiments. D. Top panel – Quantification by ICCS of day 7 CD8+ IFNγ+ T cells stimulated with decreasing doses of NS4b2488 peptide. CD8+ T cells stimulated with 1×10−6 – 1×10−11M NS4b2488 peptide for 6 hours in BFA and analyzed by ICCS. Results depict x+/− SEM from n=4 mice per dose of RepliVAX WN. Bottom panel – CD8+ T cell avidity curve with results expressed as a percentage of maximum CD8+ T cell IFNγ production in the presence of saturating peptide concentrations of NS4b2488 (1×10−6M). Results depict x+/− SEM from n=4 mice per dose of RepliVAX WN. One of two similar experiments is shown. E. Representative example of direct ex-vivo immunophenotyping of day 7 splenocytes, from RepliVAX WN-vaccinated, WNV-infected or naïve mice. Dot plots are gated on CD8. Staining was completed as described in materials and methods. Results shown are from one mouse of four per experiment and from one representative experiment of two. F. Day 7 CD8+ T cell response assayed by direct ex-vivo 6-hour 51Cr-release assay with NS4b peptide coated EL-4 T cells and control cells. n=4 mice in the WNV infected group, 5 mice in all other groups. One representative example of two experiments.
We next evaluated the ability of RepliVAX WN to induce formation of antigen-specific immunological memory. To this end, we used ICCS to determine the percentage of CD8+ and CD4+ T cells that would recognize viral determinants and produce IFNγ when stimulated with peptide antigen 45 days post-immunization (Fig. 1C). Antigen-specific memory CD8+ and CD4+ T cells induced by vaccination were present at similar frequencies compared to those induced by WNV infection 45 days post-exposure.
To determine the functional quality (also called functional avidity) of RepliVAX WN-induced responses, we next compared the ability of CD8+ T cells generated by RepliVAX WN immunization or WNV infection to respond to decreasing concentrations of peptide (Fig. 1D). For comparison, we expressed the results as a percentage of maximum CD8+ T cell IFNγ production obtained in the presence of saturating concentrations of NS4b2488 (10−6M). Results obtained indicate that in adult mice RepliVAX WN vaccination generates CD8+ T cells of comparable functional avidity to those generated within the context of a primary WNV infection (Fig. 1D, bottom panel). This was observed across a wide range of RepliVAX WN doses.
We next evaluated the differentiation phenotype of the responding CD8+ T cell population by FCM. We found that both WNV infection and RepliVAX WN vaccination generated GzB +, CD11ahi CD127low CD8+ T cells (Fig. 1E), a phenotype that corresponds to fully mature effector CD8+ T cells (31, 32). To test functional relevance of the robust direct ex-vivo GzB expression, we evaluated the cytotoxic potential of RepliVAX WN-generated CD8+ T lymphocytes 7 days post-infection using a direct ex vivo 51Cr release assay. Again, RepliVAX WN vaccination was largely indistinguishable from WNV infection in that it generated CD8 T cells that were capable of recognizing and lysing cognate peptide-coated EL-4 target cells with similar efficiency to those generated by whole virus infection (Fig. 1F).
Mechanism of protection by RepliVAX WN
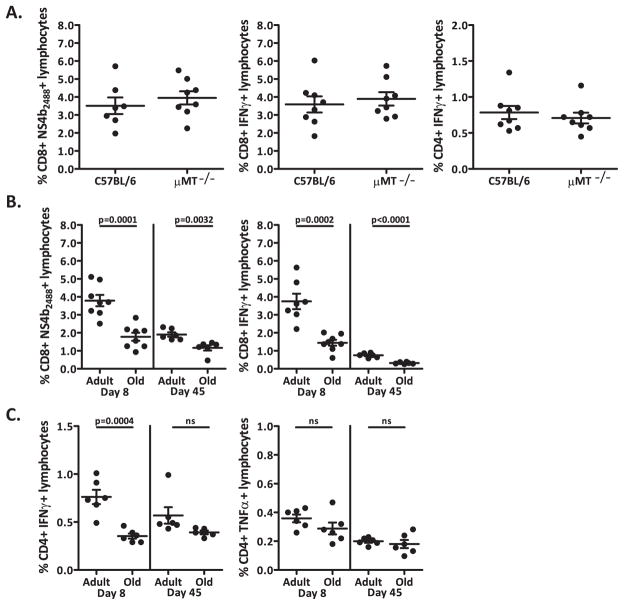
We next examined whether the T cell response alone is sufficient to provide protection against lethal WNV challenge 45 days after RepliVAX WN vaccination. To that effect, we immunized B cell-deficient mice (μMT−/−) and age-matched B6 mice i.p. with 1×105 IU RepliVAX WN. Using ICCS we evaluated the CD8+ and CD4+ T cell IFNγ response 8 days post-immunization (Fig. 2A). RepliVAX WN elicited equivalent percentages of CD8+ T cells specific for the NS4b2488 epitope in both μMT−/− mice and B6 controls; and the same was true for antigen-specific CD4+ T cells produced by RepliVAX WN vaccination in both strains of mice. These data show that RepliVAX WN vaccination can generate a robust CD8+ and CD4+ T cell effector response in B cell-deficient mice early after vaccination and that this response is equivalent, both in magnitude and function, to that of wild-type adult B6 mice.
Figure 2. Antigen-specific T cell response to RepliVAX WN and WNV.
A. Left panel – CD8+ NS4b2488 tetramer+ T cells 8 days post-vaccination from uMT−/− and B6 mice inoculated with 105 IU of RepliVAX WN. Middle panel – Quantification of day 8 CD8+ T cells by IFNγ ICCS from μ MT−/− and B6 mice inoculated with 105 IU of RepliVAX WN. ICCS of CD8+ T cells stimulated with 1×10−6M NS4b2488 peptide for 6 hours in BFA. Results depict x+/− SEM from n=8 mice per group. Right panel – Quantification by IFNγ ICCS of day 8 CD4+ T cells from μMT−/− and B6 mice inoculated with 105 IU of RepliVAX WN. ICCS of CD4+ T cells stimulated with 1×10−6M E641 and NS31616+2066 peptide for 6 hours in BFA. Results depict x+/− SEM from n=8 mice per group, from one of three similar experiments. B. Left panel – CD8+ NS4b2488 tetramer+ T cells 8 and 45 days post-vaccination from old and adult B6 mice inoculated with 105 IU of RepliVAX WN. Right panel – Quantification by IFNγ ICCS of days 8 and 45 of CD8+ T cells from old and adult mice immunized with 1×105 IU RepliVAX WN. ICCS of CD8+ T cells stimulated with 1×10−6M NS4b2488 peptide for 6 hours in BFA. Results depict x+/− SEM from n=8 mice per group. C. Quantification by IFNγ (Left panel) and TNFα (Right panel) ICCS of days 8 and 45 of CD4+ T cells from old and adult mice immunized with 1×105 IU RepliVAX WN. ICCS of CD4+ T cells stimulated with 1×10−6M E641 and NS31616+2066 peptide for 6 hours in BFA. Results depict x+/− SEM from n=8 mice per group, from one of two similar experiments.
Immune Responses to RepliVAX WN in old mice
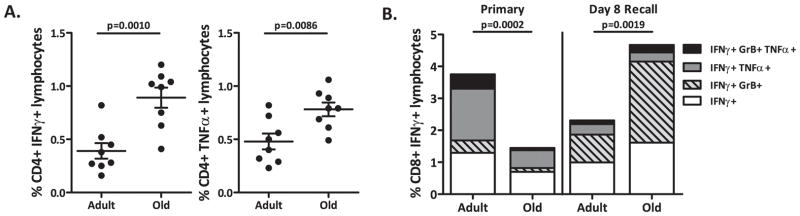
We have previously shown that old mice are more susceptible to severe WNV disease than adult mice due to severe defects in primary T cell effector maturation (33). These experiments demonstrated the existence of both qualitative (incompletely “armed” effector T cells) and quantitative (insufficient numbers of Ag-specific T cells in the CNS) defects: transfer experiments showed that adult naïve CD4+ or CD8+ T cells could singlehandedly protect RAG−/− mice from low-dose WNV challenge, whereas old CD4+ or CD8+ T cells had no protective effect (33). The role of antibodies was not addressed in these studies and it remained unclear which of the T cell defects were primarily responsible for the observed phenotype and whether they could be corrected by immunostimulation. We therefore initiated experiments to test whether stimulation with RepliVAX WN could generate an antigen-specific response in aged mice, and whether such a response would be protective. We first used FCM to evaluate the phenotype of CD8+ T cells 8 days after i.p. vaccination with 1×105 IU of RepliVAX WN (Fig. 2B). RepliVAX WN vaccination generated a measurable but poor primary T cell response (measured at 8 days post vaccination) in old mice compared to adults, as measured by the percentage of NS4b2488 tetramer-positive CD8+ T cells (Fig. 2B, left panel) or IFNγ antigen-specific CD8+ and CD4+ T cells [Fig. 2B right panel and Fig. 2C left panel, respectively] with a significant decrease in the percentage of IFNγ+-CD8+ T cells (p=0.0002) and IFNγ+-CD4+ T cells (p=0.0004) in old mice compared to the adult mice after a 6-hour peptide stimulation. This is reminiscent of profound age-related defects in primary T cell responses seen in other models of infectious diseases (34), including those seen during primary infection with WNV (33). Interestingly, these age-related defects persisted in the CD8+ memory population generated by vaccination as measured by both NS4b2488 tetramer staining (Figure 2B, left panel) or peptide restimulation (Fig. 2B, right panel) but were much less pronounced (and not significantly different from the adults) in the CD4+ memory population (Fig. 2C, left panel) TNFα+ CD4+ T cells do not show an age-related defect in either the primary or memory response (Fig. 3, right panel). These results may indicate that CD8+ T cell defects are more pronounced than those in CD4+ cells; however, additional comparisons will be needed to reach firm conclusions.
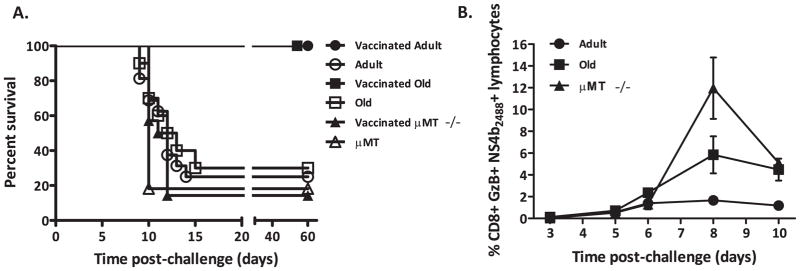
Figure 3. Animal survival and CD8 T cell response after lethal WNV challenge.
A. Survival of RepliVAX WN vaccinated and unvaccinated μMT−/, adult C57BL/6 and old C57BL/6 mice following i.p. challenge with 2000pfu of WNV 385–99. μMT−/− mice were not protected by RepliVAX WN vaccination. C57BL/6 vaccinated mice survived at a significantly higher rate (p<0.0001) than μMT−/− vaccinated animals. RepliVAX WN vaccinated old and adult mice were protected from lethal WNV challenge. Vaccinated mice survived at a significantly higher rate (adult p<0.0001, old p=0.0007) than unvaccinated mice. B. Quantification of antigen specific CD8+ T cells in the blood after challenge with WNV. Measurement on days 3, 5, 6, 8, and 10 post-infection by CD8+ NS4b2488 tetramer+ GzB+ FCS. At day 8, uMT−/− mice responded significantly more than old B6 (P<0.01) and old mice responded significantly more than adult B6 (P<0.001). Results depict x+/− SEM from n=8 mice per group, from one of two similar experiments.
Protective effects of RepliVAX WN in vulnerable mouse populations
All of these results prompted us to examine whether the responses generated by RepliVAX may be sufficient to protect old or B cell deficient animals. We found that RepliVAX WN vaccination significantly protected adult and, surprisingly, old mice from a lethal challenge with WNV (adult vaccinated vs. control p<0.0001, old vaccinated vs. control p=0.0007, Fig. 3A) whereas unvaccinated mice succumbed to WNV infection at this dose regardless of age. By contrast, μMT−/− mice were not protected by vaccination and no improvement in their survival was detected (Fig. 3A). We examined T cell responses in these mice after vaccination and challenge, and discovered that vaccinated μMT−/− mice had a robust recall T cell response, which was increased as compared to vaccinated adult B6 mice (p<0.001) and was somewhat higher, but not significantly different, from that in old B6 mice (Fig. 3B). These experiments indicate that RepliVAX WN vaccination generates a weak memory T cell response in old and μMT−/− mice, which upon further stimulation with viral challenge is nonetheless able to generate robust effector immunity. That immunity, however, was not sufficient to protect against lethal WNV infection in the absence of the Ab response, implying that humoral immunity is necessary for protection in this model.
The role of humoral immunity in vaccinated old mice
Contribution of Ab to RepliVAX WN mediated immunity has been previously demonstrated in outbred Swiss Webster mice, where RepliVAX WN induced strong Ab responses against the WNV E and NS1 proteins (19), and strong Ab responses against E protein are known to mediate protection against WNV infection (see (35, 36) and references therein). However, the extent to which Ab can protect old mice from lethal WNV infection has not been evaluated. To that end, we sought to determine the contribution of Ab to the protection of vaccinated old mice from lethal WNV challenge.
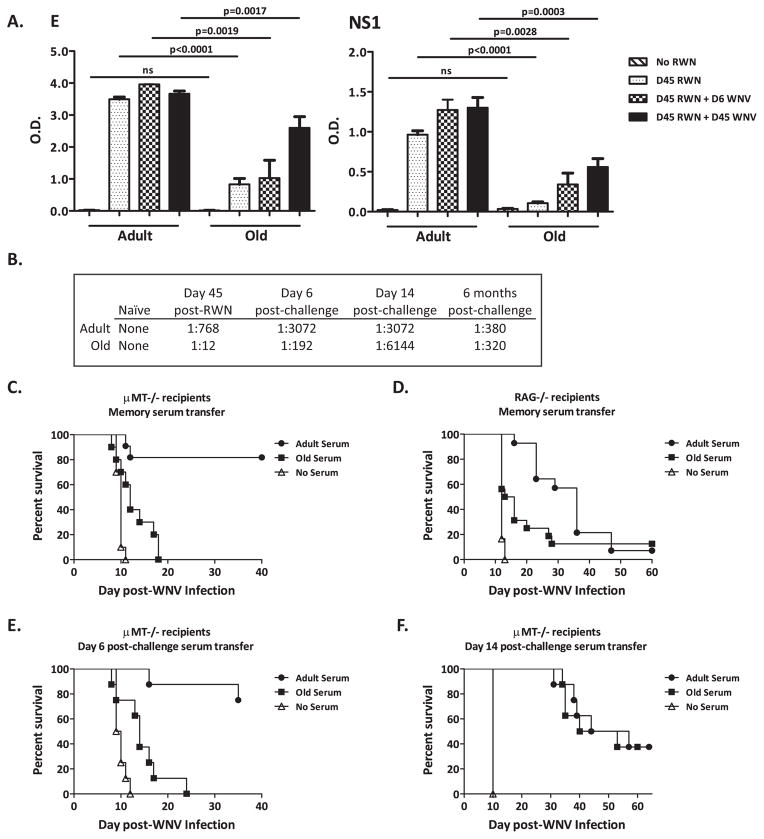
We first assessed IgG responses against the WNV E and NS1 proteins by ELISA during memory (45 days post-vaccination) and recall (6 and 45 days post-challenge) phases following vaccination in old and adult mice. Vaccination with 1×105 IU of RepliVAX WN induced Ab responses against E and NS1 in both adult and old mice (Fig. 4) demonstrating the ability of RepliVAX WN to induce Ab response against WNV antigens known to play important roles in protective immunity against flavivirus diseases (26, 37–39). However, old mice exhibited poor Ab response as compared to adult mice (adult vaccinated vs. old vaccinated p<0.0001 for both E and NS1) 45 days post-vaccination (Fig. 4A). Both old and adult mice show an increase in the amount of total E and NS1 specific Ab following WNV challenge. Nonetheless, while improved, total levels in the old mice remained significantly lower compared to adults, even 45 days post-challenge (Fig. 4A). Functionally, the neutralizing Ab response in old mice also exhibited a reduced titer in the memory (day 45 post-vaccination) and early recall (day 6 post–challenge; Fig. 4B) phases when compared to adults, indicating that not only is there less Ab, but that the Ab in old mice is of suboptimal quality. However, by day 14 post-challenge the neutralizing Ab response became as robust (e.g. of equal quality) in old as in adult mice and the neutralization titers remained of the same magnitude 6 months post-challenge (Fig. 4B). This suggested that during a second round of affinity maturation, Ab generated in old mice improves in quality and is therefore contributing more significantly to protection.
Figure 4. Characterization of the Ab contribution to successful vaccination.
A. Ab titers against WNV E (Left panel) and NS1 (Right panel) from C57BL/6 mice immunized with 105 IU of RepliVAX WN and challenged with WNV as determined by ELISA on day 45 post-vaccination, and day 6 and 45 post-challenge. Results depict x+/− SEM from n=4–20 mice per group from one of two similar experiments and statistical differences are represented in the figure. B. Neutralizing antibody titers of pooled serum from old and adult animals. Serum was collected45 days post-vaccination and 6 days, 14 days, and 6 months post-challenge and assayed for neutralizing capacity. n=8 mice per group. C. Serum transfer from vaccinated old and adult B6 mice to μMT−/−. μMT−/− mice that received serum from adult vaccinated mice survived WNV challenge (2000 pfu WNV, i.p.) better than those that received serum from old vaccinated mice (p<0.0001). Compiled data from two experiments, n=10–20 mice per group. D. Serum transfer from vaccinated old and adult B6 mice to RAG−/−. RAG−/− mice that received serum from adult vaccinated mice survived WNV infection (1000 pfu WNV, s.c.) longer than those that received serum from old vaccinated mice (p=0.0289) but had no difference in overall survival percentages. n=8 mice per group. E. Serum transfer from vaccinated and challenged old and adult B6 mice to μMT−/− from day 6 post-challenge. μMT−/− mice that received serum from adult vaccinated mice survived WNV infection (2000 pfu WNV, i.p.) better than those that received serum from old vaccinated mice (p=0.0002). n=8 mice per group. F. Serum transfer from vaccinated and challenged old and adult B6 mice to μMT−/− from day 14 post-challenge. μMT−/− mice that received serum from adult vaccinated mice survived WNV infection (2000 pfu WNV, i.p.) equally as well as those that received serum from old vaccinated mice (p=ns). n=8 mice per group.)
Given that vaccinated old mice survive challenge, we sought to determine the biological potency of Ab response. To that effect, we passively transferred serum from old and adult mice sacrificed at day 45 post-vaccination into μMT−/− mice and challenged them with WNV (Fig. 4C). μMT−/− mice receiving serum from adult vaccinated mice survived challenge while μMT−/− mice receiving serum from old vaccinated mice did not (adult vaccinated vs. old vaccinated p=0.0002). This result suggests that even in the context of an adult primary T cell response contributed by the μMT−/− mice, Ab from old mice is insufficient for protection. To further determine if Ab from the adult vaccinated mice was sufficient for protection, we repeated this experiment with RAG−/− mice as recipients (Fig. 4D) and observed that even serum from adult vaccinated mice could not confer protection albeit both old and, to a greater extent, adult serum extended survival in recipient mice. To test whether Ab generated even further into the recall response was protective we repeated these experiments with serum harvested on day 6 post-challenge (Fig. 4E) and day 14 post-challenge (Fig. 4F) and determined that serum from old mice does become more protective as the Ab recall response matures. This is demonstrated by better protection of the μMT−/− mice with serum from day 14 post-challenge as compared to the protection conferred by serum from day 6 post-challenge. It is likely that this result reflects both an increase in quantity and quality of the Ab Importantly, Ab still did not confer complete protection to the μMT−/− mice with an overall survival of 37.5% for μMT−/− mice receiving either old or adult serum (Fig. 4F), likely due to the finite Ab half-life.
Recall stimulation of primed old T cells restores their function to adult levels
We next sought to examine whether restimulation of primed old T cells may have improved their quantitative (numbers of effector T cells) and qualitative (polyfunctionality) features important for immune defense. To test whether vaccination may have primed for such an expansion, we challenged vaccinated old and adult mice with WNV. Although primary vaccination of old mice did not generate a robust primary or memory T cell response, vaccinated old C57BL/6 mice challenged with 2,000 pfu of WNV exhibited a robust CD4+ and CD8+ T cell response that equaled or surpassed the response measured in adult vaccinated animals (Fig. 5A and B, respectively). Of interest, whereas old mice showed an improved recall response, adult mice showed no such improvement over the levels seen in primary response. We explain this lack of additional expansion by the presence of a strong preexisting Ab response in the adults, which rapidly limits secondary infection in primed adult mice, leading to diminished T cell activation during challenge.
Figure 5. Recall responses to WNV in vaccinated adult and old B6 mice.
A. Quantification by IFNγ and TNFα ICCS of CD4+ T cells from old and adult mice on day8 post-WNV challenge. Cells stimulated with 1×10−6M E641 and NS31616+2066 peptide for 6 hours in BFA. Results depict x+/− SEM from n=8 mice per group, from one of three similar experiments. B. Quantification by IFNγ ICCS of CD8+ T cells from old and adult mice on day8 post-WNV challenge. T cells stimulated with 1×10−6M NS4b2488 peptide for 6 hours in BFA. IFNγ response broken down into percentages also expressing TNFα and/or GzB. Results depict x+/− SEM from n=8 mice per group, from one of three similar experiments.
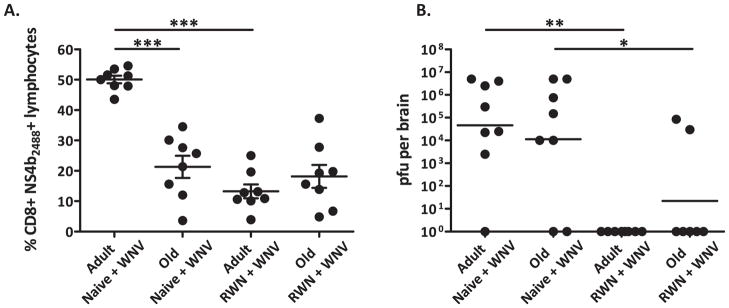
During recall, old CD8+ T cells also closely resembled adult T cells in polyfunctional profiles, with the majority expressing both IFNγ and GzB, an effector molecule associated with increased target lysis (Fig. 5B). CD4+ T cells also show improved function during recall as demonstrated by expanded IFNγ and TNFα expressing populations in old mice (Fig. 5A). To further demonstrate the potency of the CD8+ T cell response we evaluated T cells trafficking to the brain following challenge and viral titers in the brain. Unlike in our prior study (33), in this experiment on day 8 we did not see the significant difference between viral loads in old and adult brains, which is likely due to the fact that sometimes these changes do not become manifest until later (day 10 and beyond). However, we did find that vaccinated animals had fewer WNV specific T cells in the brain on day 8 post-challenge as compared non-vaccinated mice (Fig. 6A), particularly in the adult mice, and that viral titers were lower in the brains of vaccinated mice regardless of age (Fig. 6B). We did observe that in two vaccinated old mice there were positive viral titers in the brain and expect that this is due to timing and that they just have not yet cleared the virus. Most importantly, the strongly improved T-cell responses upon challenge in old mice correlated with protection from infection (Fig. 3A). While not formally demonstrated, these results suggest that vaccination may afford better control of WNV neuroinvasion.
Figure 6. RepliVAX WN vaccination prevents WNV encephalitis.
A. CD8+ NS4b2488 tetramer+ T cells isolated from the brains of vaccinated (RWN+WNV) and non-vaccinated (Naïve + WNV) adult and old mice on day 8 post-WNV challenge. Results depict x+/− SEM from n=8 mice per group, from one of two experiments. B. WNV virus titers in the brains of vaccinated (RWN+WNV) and non-vaccinated (Naïve + WNV) adult and old mice 8 days post-WNV challenge. Results depict GMT from n=8 mice per group, from one of two experiments.
These experiments allow us to make two important conclusions. First, secondary stimulation (restimulation) is critical for antiviral protection in old mice, regardless of whether the protection is ultimately provided by Ab or both T cells and Ab (an issue that will be investigated outside of the scope of this manuscript). Second, and more importantly, vaccination with a live-attenuated single-cycle vaccine was able to expand a small population of cells from aged mice that, upon secondary stimulation and further expansion, were able to provide protective immunity to old animals.
Discussion
WNV is widespread throughout the world, and is known to cause severe disease in immunocompromised and older adult populations. Vaccination of the later group presents unique challenges, as the immune systems of the older adults respond to infection differently than in younger adults. While many laboratories have designed WNV vaccine candidates that have been shown to be successful in protecting immunocompetent mice from disease (40–45), the exact immune mechanisms necessary and sufficient to provide protection from WNV-induced disease after vaccination have not been delineated for these candidates. Furthermore, little is known about the effectiveness of these vaccines in high-risk populations, the most likely target for a WNV vaccine. For adult animals, it is know that humoral immunity can provide protection during the establishment of disease (46), while cellular immunity is critical in clearing WNV infection since mice with defects in cytotoxic T lymphocyte (CTL) responses exhibit reduced ability to clear WNV infections (47, 48), and transfers of WNV-specific adult CD8+ (20, 30) or CD4+ T cells (21, 49) protect against lethal WNV. Our results indicate that in adult mice, RepliVAX WN vaccination can generate robust T and B (Ab) cell responses, quantitatively similar to those seen during WNV infection, and that these T and B cells survive to become memory cells. These data also indicate that RepliVAX WN vaccination allows the processing and presentation of both structural and non-structural protein antigens by both major histocompatibility complex-encoded (MHC) class I and II molecules, unlike an inactivated or particulate vaccine formulations, which cannot express non-structural proteins.
Our prior published (33) and current submitted work (Smithey, M. et al., submitted) identified discrete defects in old T cell differentiation and expansion in response to viral and bacterial infection, respectively. Moreover, it is well established that old age corresponds to a numerical decline in the naïve T cell pool (rev. in (14)), suggesting that fewer naïve T cells may be available to respond to microbial challenge in old organisms. Therefore, a fundamental question is whether an old immune system can be stimulated so as to expand sufficient numbers of fully differentiated, protective T cells and Ab-producing cells to achieve protection against infection. To answer that question, we employed RepliVAX WN, with a goal to test (i) whether RepliVAX WN vaccination was capable of inducing an immune response in old mice; (ii) whether this response was sufficient to afford protection from a lethal WNV challenge; and (iii) if protective, what was the mechanism of protection.
We found that RepliVAX WN vaccination was capable of generating detectable primary and memory T cell responses in old mice, which were significantly lower than those in adult counterparts (Fig. 2). B cell (Ab) responses generated in these mice were similarly much lower, as measured by total and neutralizing titers (Fig. 4). We therefore did not expect to find that vaccinated old mice survive high-dose i.p. challenge with WNV, whereas we expected full protection in their adult counterparts that exhibited much more robust primary and memory responses. In seeking the mechanism of this protection, we showed that secondary stimulation with the infectious virus was absolutely critical to the increased quality of both T and B cell responses in old mice. The role of humoral immunity in RepliVAX WN vaccination was demonstrated in μMT−/− mice, which mounted strong T cell responses but could not fend off lethal infection in the absence of Ab. Adult-derived memory Ab (collected at day 45 post-vaccination) conferred protection to these animals, whereas memory Ab from old mice did not, further stressing that memory generated after primary response in old mice remains suboptimal and insufficiently protective. The protective role of T cells in RepliVAX WN vaccination was demonstrated in μMT−/− mice, which could not be protected indefinitely by the transfer of serum (Ab) alone. Moreover, that experiment also revealed a qualitative difference between the adult and old serum generated in the memory phase: while memory serum from old animals marginally extended lifespan, adult memory serum provided more pronounced survival extension.
Secondary stimulation of previously vaccinated old mice with lethal WNV not only revealed strong protective immunity, but also led to improved quantity and quality of old T cell and Ab responses. Thus, CD8+ cells in old mice equaled or surpassed their adult counterparts in numbers and polyfunctional phenotype after this secondary stimulation (Fig. 5), and old Ab from serum collected on day 14 post-secondary stimulation was able to provide the same extent of protection as adult serum to μMT−/− mice in a challenge experiment (Fig 4F). Moreover, the Ab response of old animals exhibited strong neutralizing activity, equal to or superior to that seen in adult counterparts, which persisted for at least 6 months (Fig. 4B).
Finally, we examined the brain infiltrate and found that in the course of lethal challenge, there were fewer T cells and less or no virus in the brains of vaccinated old and adult mice, suggesting improved control of neuroinvasion as a consequence of vaccination. We did not formally test whether secondary stimulation can generate either Ab or T cells which can singlehandedly confer protection against WNV, a feat that cannot be accomplished in the course of primary response even in adult mice. Experiments to answer that question, as well as those addressing the exact extent of additional proliferation and differentiation required to improve immune function are currently in progress. Nonetheless, our results support the idea that a third generation of rationally attenuated vaccines, such as RepliVAX WN, administered with multiple rounds of boosting, may hold keys to improvement of functional immunity in the older population.
Acknowledgments
Supported by the USPHS awards N01 50027 (J.N-Z.) and T32 AI007472 (J.B.) from the National Institute of Allergy and Infectious Diseases, National Institutes of Health. Portions of this work were also supported by a grant to P.W.M from NIAID through the Western Regional Center of Excellence for Biodefense and Emerging Infectious Disease Research (NIH grant U54 AI057156) and the Sealy Center for Vaccine Development. D.G.W. received support from a James D. McLaughlin fellowship for a portion of this work.
We wish to thank members of the Nikolich and Mason laboratories for helpful discussion and support throughout this work and the NIAID Tetramer facility for the expert production of tetramers, and the Arizona Cancer Center/Arizona Research Laboratories -Division of Biotechnology for providing the instrumentation via the Flow Cytometry Core Facility.
Abbreviations
- FCM
Flow cytometry
- WNE
West Nile virus encephalitis
- WNV
West Nile virus
- WNV
capsid protein- C
- WNV
envelope protein- E
References
- 1.Asnis DS, Conetta R, Teixeira AA, Waldman G, Sampson BA. The West Nile Virus outbreak of 1999 in New York: the Flushing Hospital experience. Clin Infect Dis. 2000;30:413–418. doi: 10.1086/313737. [DOI] [PubMed] [Google Scholar]
- 2.Hayes EB, Sejvar JJ, Zaki SR, Lanciotti RS, Bode AV, Campbell GL. Virology, pathology, and clinical manifestations of West Nile virus disease. Emerg Infect Dis. 2005;11:1174–1179. doi: 10.3201/eid1108.050289b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murray K, Baraniuk S, Resnick M, Arafat R, Kilborn C, Cain K, Shallenberger R, York TL, Martinez D, Hellums JS, Hellums D, Malkoff M, Elgawley N, McNeely W, Khuwaja SA, Tesh RB. Risk factors for encephalitis and death from West Nile virus infection. Epidemiol Infect. 2006;134:1325–1332. doi: 10.1017/S0950268806006339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferguson M, Kurane I, Wimalaratne O, Shin J, Wood D. WHO informal consultation on the scientific basis of specifications for production and control of inactivated Japanese encephalitis vaccines for human use, Geneva, Switzerland, 1–2 June 2006. Vaccine. 2007;25:5233–5243. doi: 10.1016/j.vaccine.2007.05.034. [DOI] [PubMed] [Google Scholar]
- 5.Barnett ED. Yellow fever: epidemiology and prevention. Clin Infect Dis. 2007;44:850–856. doi: 10.1086/511869. [DOI] [PubMed] [Google Scholar]
- 6.McMahon AW, Eidex RB, Marfin AA, Russell M, Sejvar JJ, Markoff L, Hayes EB, Chen RT, Ball R, Braun MM, Cetron M. Neurologic disease associated with 17D-204 yellow fever vaccination: a report of 15 cases. Vaccine. 2007;25:1727–1734. doi: 10.1016/j.vaccine.2006.11.027. [DOI] [PubMed] [Google Scholar]
- 7.Monath TP, Lee CK, Julander JG, Brown A, Beasley DW, Watts DM, Hayman E, Guertin P, Makowiecki J, Crowell J, Levesque P, Bowick GC, Morin M, Fowler E, Trent DW. Inactivated yellow fever 17D vaccine: development and nonclinical safety, immunogenicity and protective activity. Vaccine. 28:3827–3840. doi: 10.1016/j.vaccine.2010.03.023. [DOI] [PubMed] [Google Scholar]
- 8.Mostashari F, Bunning ML, Kitsutani PT, Singer DA, Nash D, Cooper MJ, Katz N, Liljebjelke KA, Biggerstaff BJ, Fine AD, Layton MC, Mullin SM, Johnson AJ, Martin DA, Hayes EB, Campbell GL. Epidemic West Nile encephalitis, New York, 1999: results of a household- based seroepidemiological survey. Lancet. 2001;358:261–264. doi: 10.1016/S0140-6736(01)05480-0. [DOI] [PubMed] [Google Scholar]
- 9.Petersen LR, Marfin AA. West Nile virus: a primer for the clinician. Ann Intern Med. 2002;137:173–179. doi: 10.7326/0003-4819-137-3-200208060-00009. [DOI] [PubMed] [Google Scholar]
- 10.Weiss D, Carr D, Kellachan J, Tan C, Phillips M, Bresnitz E, Layton M. Clinical findings of West Nile virus infection in hospitalized patients, New York and New Jersey, 2000. Emerg Infect Dis. 2001;7:654–658. doi: 10.3201/eid0704.010409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nash D, Mostashari F, Fine A, Miller J, O’Leary D, Murray K, Huang A, Rosenberg A, Greenberg A, Sherman M, Wong S, Layton M. The outbreak of West Nile virus infection in the New York City area in 1999. N Engl J Med. 2001;344:1807–1814. doi: 10.1056/NEJM200106143442401. [DOI] [PubMed] [Google Scholar]
- 12.Sejvar JJ. The long-term outcomes of human West Nile virus infection. Clin Infect Dis. 2007;44:1617–1624. doi: 10.1086/518281. [DOI] [PubMed] [Google Scholar]
- 13.Cambier J. Immunosenescence: a problem of lymphopoiesis, homeostasis, microenvironment, and signaling. Immunol Rev. 2005;205:5–6. doi: 10.1111/j.0105-2896.2005.00276.x. [DOI] [PubMed] [Google Scholar]
- 14.Nikolich-Zugich J. Ageing and life-long maintenance of T-cell subsets in the face of latent persistent infections. Nat Rev Immunol. 2008;8:512–522. doi: 10.1038/nri2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Akbar AN, Fletcher JM. Memory T cell homeostasis and senescence during aging. Curr Opin Immunol. 2005;17:480–485. doi: 10.1016/j.coi.2005.07.019. [DOI] [PubMed] [Google Scholar]
- 16.Pawelec G, Akbar A, Caruso C, Effros R, Grubeck-Loebenstein B, Wikby A. Is immunosenescence infectious? Trends Immunol. 2004;25:406–410. doi: 10.1016/j.it.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 17.Miller RA. The aging immune system: primer and prospectus. Science. 1996;273:70–74. doi: 10.1126/science.273.5271.70. [DOI] [PubMed] [Google Scholar]
- 18.McElhaney JE. Influenza: a preventable lethal disease. J Gerontol A Biol Sci Med Sci. 2002;57:M627–628. doi: 10.1093/gerona/57.10.m627. [DOI] [PubMed] [Google Scholar]
- 19.Widman DG, Ishikawa T, Fayzulin R, Bourne N, Mason PW. Construction and characterization of a second-generation pseudoinfectious West Nile virus vaccine propagated using a new cultivation system. Vaccine. 2008;26:2762–2771. doi: 10.1016/j.vaccine.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 20.Brien JD, Uhrlaub JL, Nikolich-Zugich J. Protective capacity and epitope specificity of CD8(+) T cells responding to lethal West Nile virus infection. Eur J Immunol. 2007;37:1855–1863. doi: 10.1002/eji.200737196. [DOI] [PubMed] [Google Scholar]
- 21.Brien JD, Uhrlaub JL, Nikolich-Zugich J. West nile virus-specific CD4 T cells exhibit direct antiviral cytokine secretion and cytotoxicity and are sufficient for antiviral protection. J Immunol. 2008;181:8568–8575. doi: 10.4049/jimmunol.181.12.8568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brinton MA. The molecular biology of West Nile Virus: a new invader of the western hemisphere. Annu Rev Microbiol. 2002;56:371–402. doi: 10.1146/annurev.micro.56.012302.160654. [DOI] [PubMed] [Google Scholar]
- 23.Campbell GL, Marfin AA, Lanciotti RS, Gubler DJ. West Nile virus. Lancet Infect Dis. 2002;2:519–529. doi: 10.1016/s1473-3099(02)00368-7. [DOI] [PubMed] [Google Scholar]
- 24.Dauphin G, Zientara S. West Nile virus: recent trends in diagnosis and vaccine development. Vaccine. 2007;25:5563–5576. doi: 10.1016/j.vaccine.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 25.Fayzulin R, Scholle F, Petrakova O, Frolov I, Mason PW. Evaluation of replicative capacity and genetic stability of West Nile virus replicons using highly efficient packaging cell lines. Virology. 2006;351:196–209. doi: 10.1016/j.virol.2006.02.036. [DOI] [PubMed] [Google Scholar]
- 26.Mason P, Pincus S, Fournier M, Mason T, Shope R, Paoletti E. Japanese Encephalitis Virus-Vaccinia Recombinants Produce Particulate Forms of the Structural Membrane Proteins and Induce High Levels of Protein against Lethal JEV Infection. Virology. 1991;180:294–305. doi: 10.1016/0042-6822(91)90034-9. [DOI] [PubMed] [Google Scholar]
- 27.Widman DG, Ishikawa T, Winkelmann ER, Infante E, Bourne N, Mason PW. RepliVAX WN, a single-cycle flavivirus vaccine to prevent West Nile disease, elicits durable protective immunity in hamsters. Vaccine. 2009;27:5550–5553. doi: 10.1016/j.vaccine.2009.07.016. [DOI] [PubMed] [Google Scholar]
- 28.Widman DG, Ishikawa T, Giavedoni LD, Hodara VL, Garza Mde L, Montalbo JA, Travassos Da Rosa AP, Tesh RB, Patterson JL, Carrion R, Jr, Bourne N, Mason PW. Evaluation of RepliVAX WN, a single-cycle flavivirus vaccine, in a non-human primate model of West Nile virus infection. Am J Trop Med Hyg. 82:1160–1167. doi: 10.4269/ajtmh.2010.09-0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bourne N, Scholle F, Silva MC, Rossi SL, Dewsbury N, Judy B, De Aguiar JB, Leon MA, Estes DM, Fayzulin R, Mason PW. Early production of type I interferon during West Nile virus infection: role for lymphoid tissues in IRF3-independent interferon production. J Virol. 2007;81:9100–9108. doi: 10.1128/JVI.00316-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Purtha WE, Myers N, Mitaksov V, Sitati E, Connolly J, Fremont DH, Hansen TH, Diamond MS. Antigen-specific cytotoxic T lymphocytes protect against lethal West Nile virusencephalitis. Eur J Immunol. 2007;37:1845–1854. doi: 10.1002/eji.200737192. [DOI] [PubMed] [Google Scholar]
- 31.Marsland BJ, Nembrini C, Schmitz N, Abel B, Krautwald S, Bachmann MF, Kopf M. Innate signals compensate for the absence of PKC-{theta} during in vivo CD8(+) T cell effector and memory responses. Proc Natl Acad Sci U S A. 2005;102:14374–14379. doi: 10.1073/pnas.0506250102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaech SM, Tan JT, Wherry EJ, Konieczny BT, Surh CD, Ahmed R. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. 2003;4:1191–1198. doi: 10.1038/ni1009. [DOI] [PubMed] [Google Scholar]
- 33.Brien JD, Uhrlaub JL, Hirsch A, Wiley CA, Nikolich-Zugich J. Key role of T cell defects in age-related vulnerability to West Nile virus. J Exp Med. 2009;206:2735–2745. doi: 10.1084/jem.20090222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Messaoudi I, Lemaoult J, Guevara-Patino JA, Metzner BM, Nikolich-Zugich J. Age-related CD8 T cell clonal expansions constrict CD8 T cell repertoire and have the potential to impair immune defense. J Exp Med. 2004;200:1347–1358. doi: 10.1084/jem.20040437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oliphant T, Nybakken GE, Engle M, Xu Q, Nelson CA, Sukupolvi-Petty S, Marri A, Lachmi BE, Olshevsky U, Fremont DH, Pierson TC, Diamond MS. Antibody recognition and neutralization determinants on domains I and II of West Nile Virus envelope protein. J Virol. 2006;80:12149–12159. doi: 10.1128/JVI.01732-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oliphant T, Diamond MS. The molecular basis of antibody-mediated neutralization of West Nile virus. Expert Opin Biol Ther. 2007;7:885–892. doi: 10.1517/14712598.7.6.885. [DOI] [PubMed] [Google Scholar]
- 37.Schlesinger JJ, Brandriss MW, Walsh EE. Protection against 17D yellow fever encephalitis in mice by passive transfer of monoclonal antibodies to the nonstructural glycoprotein gp48 and by active immunization with gp48. J Immunol. 1985;135:2805–2809. [PubMed] [Google Scholar]
- 38.Pincus S, Mason PW, Konishi E, Fonseca BA, Shope RE, Rice CM, Paoletti E. Recombinant vaccinia virus producing the prM and E proteins of yellow fever virus protects mice from lethal yellow fever encephalitis. Virology. 1992;187:290–297. doi: 10.1016/0042-6822(92)90317-i. [DOI] [PubMed] [Google Scholar]
- 39.Chung KM, Nybakken GE, Thompson BS, Engle MJ, Marri A, Fremont DH, Diamond MS. Antibodies against West Nile Virus nonstructural protein NS1 prevent lethal infection through Fc gamma receptor-dependent and -independent mechanisms. J Virol. 2006;80:1340–1351. doi: 10.1128/JVI.80.3.1340-1351.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang T, Anderson JF, Magnarelli LA, Bushmich S, Koski RA, Fikrig E. West Nile virus envelope protein: role in diagnosis and immunity. 2001. p. 325. [PubMed] [Google Scholar]
- 41.Davis B, Chang G, Cropp B, Roehrig J, Martin D, Mitchell C, Bowen R, Bunning M. West Nile virus recombinant DNA vaccine protects mouse and horse from virus challenge and expresses in vitro a noninfectious recombinant antigen that can be used in enzyme-linked immunoabsorbent assays. J Virol. 2001;75:4040. doi: 10.1128/JVI.75.9.4040-4047.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang J, Kim J, Hwang D, Choo A, Dang K, Maguire H, Kudchodkar S, Ramanathan M, Weiner D. Induction of potent Th1-type immune responses from a novel DNA vaccine for West Nile virus New York isolate (WNV-NY1999) 2001;809 doi: 10.1086/323395. [DOI] [PubMed] [Google Scholar]
- 43.Chin JF, Chu JJ, Ng ML. The envelope glycoprotein domain III of dengue virus serotypes 1 and 2 inhibit virus entry. Microbes Infect. 2007;9:1–6. doi: 10.1016/j.micinf.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 44.Tesh RB, Arroyo J, Travassos Da Rosa AP, Guzman H, Xiao SY, Monath TP. Efficacy of killed virus vaccine, live attenuated chimeric virus vaccine, and passive immunization for prevention of West Nile virus encephalitis in hamster model. Emerg Infect Dis. 2002;8:1392–1397. doi: 10.3201/eid0812.020229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Arroyo J, Miller C, Catalan J, Myers GA, Ratterree MS, Trent DW, Monath TP. ChimeriVax-West Nile virus live-attenuated vaccine: preclinical evaluation of safety, immunogenicity, and efficacy. J Virol. 2004;78:12497–12507. doi: 10.1128/JVI.78.22.12497-12507.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Diamond M, Shrestha B, Marri A, Mahan D, Engle M. B cells and antibody play critical roles in the immediate defense of disseminated infection by West Nile encephalitis virus. J Virol. 2003:2578–2586. doi: 10.1128/JVI.77.4.2578-2586.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang T, Scully E, Yin Z, Kim JH, Wang S, Yan J, Mamula M, Anderson JF, Craft J, Fikrig E. IFN-gamma-producing gamma delta T cells help control murine West Nile virus infection. J Immunol. 2003;171:2524–2531. doi: 10.4049/jimmunol.171.5.2524. [DOI] [PubMed] [Google Scholar]
- 48.Shrestha B, Diamond MS. The role of CD8+ T cells in the control of West Nile virus infection. J Virol. 2004;78:8312–8321. doi: 10.1128/JVI.78.15.8312-8321.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sitati E, Diamond MS. CD4+ T Cell responses are required for clearance of West Nile Virus from the central nervous system. J Virol. 2006;80:12060–12069. doi: 10.1128/JVI.01650-06. [DOI] [PMC free article] [PubMed] [Google Scholar]