Abstract
Background
Several lines of evidence support a pathophysiological role of immunity in atherosclerosis. Tyrosine nitrated proteins, a footprint of oxygen and nitrogen derived oxidants generated by cells of the immune system, are enriched in atheromatous lesions and in circulation of coronary artery disease (CAD) subjects. However, the consequences of possible immune reactions triggered by the presence of nitrated proteins in subjects with clinically documented atherosclerosis have not been explored.
Methods and Results
Specific immunoglobulins that recognize 3-nitrotyrosine epitopes were identified in human lesions, as well as in circulation of CAD subjects. The levels of circulating immunoglobulins against 3-nitrotyrosine epitopes were quantified in CAD patients (n=374) and subjects without CAD (non CAD controls, n=313). A ten-fold increase in the mean level of circulating immunoglobulins against protein-bound 3-nitrotyrosine was documented in the CAD subjects (3.75 ± 1.8 μg antibody Eq/mL plasma vs. 0.36 ± 0.8 μg antibody Eq/mL plasma), and was strongly associated with angiographic evidence of significant CAD.
Conclusions
The results of this cross sectional study suggest that post-translational modification of proteins via nitration within atherosclerotic plaque-laden arteries and in circulation serve as neoepitopes for elaboration of immunoglobulins, thereby providing an association between oxidant production and the activation of the immune system in CAD.
Keywords: atherosclerosis, immune system, antibodies, antigens, nitric oxide, free radicals
INTRODUCTION
Chronic inflammation, oxidative processes and the activation of the immune system are implicated in the pathogenesis of atherosclerosis.1-3 The detection and quantification of tyrosine nitrated proteins in human and animal models of atherosclerosis has provided one of the mechanistic links between chronic inflammation and oxidative processes in coronary artery disease (CAD). Tyrosine nitration is a covalent post-translational modification of proteins that arises from the reaction of protein tyrosine residues with nitric oxide-derived oxidants.4-6 Using mass-spectrometry-based proteomic approaches, site-specific nitration of proteins with pathobiological relevance to CAD have been revealed 7, 8, while quantification of 3-nitrotyrosine in plasma using stable isotope dilution liquid chromatography tandem mass spectrometry (LC-MS/MS) demonstrated its association with CAD.9 Despite the potential utility of 3-nitrotyrosine as a biomarker, at the individual protein level, it remains unclear whether protein nitration is responsible for alterations in cellular function that imparts an increased risk for disease development or unfavorable outcomes. Emerging data implicate tyrosine nitration as an activator of immune responses, including the elaboration of immunoglobulins, in inflammatory diseases such as acute lung injury, osteoarthritis, rheumatoid arthritis, and systemic lupus erythematosus.10-12 Moreover, a possible role of immune modulation by protein nitration can be evoked in other prevalent vascular diseases such as angiotensin II-induced hypertension in which oxidants, T cells and infiltrating monocytes contribute to the disease pathogenesis.13, 14 In this study, we provide clinical data demonstrating that circulating immunoglobulins that recognize protein-bound 3-nitrotyrosine are increased in CAD subjects and are associated with enhanced risk of angiographic evidence of significant CAD.
MATERIALS AND METHODS
Subjects
Stable subjects undergoing elective diagnostic cardiac evaluation with coronary angiography were prospectively recruited to participate. All blood draws were from fasting subjects, venous sampling into EDTA (lavender top) vacutainers prior to heparin administration from sequential consenting subjects at the time of cardiac catheterization. Blood was immediately placed either on ice or within a refrigerator, and subsequently centrifuged for 15 minutes at 10,000 x g at 4°C. Plasma was maintained between 0-4°C and aliquots were frozen at −80 °C within 2 hours of blood draw. Subjects who experienced acute coronary syndrome within 30 days of sample collection or had significant end-organ dysfunction were excluded. All subjects included in the present studies were confirmed to be troponin I (Abbott Architect assay) negative. Angiographic evidence of significant CAD was defined by the presence of at least 50% stenosis in one or more major coronary vessel as judged by at least two cardiologists blinded to patient identification and history. The demographic data for the subjects are reported in the Table 1. Subjects with no history of CAD, significant angiographic (using ≥50% stenosis as cutoff) evidence of cardiovascular disease, peripheral artery disease, or other significant end organ dysfunction were considered “non-CAD subjects”. The reasons for cardiac catheterization within the study cohort (subjects can have one or more reasons) included: history of positive or indeterminate stress test (46%), evaluation for possible ischemic causes of symptoms (69%), preoperative evaluation (14%), and history of cardiomyopathy (3%). All study participants gave informed consent, and the study was approved by the Cleveland Clinic Institutional Review Board.
Table 1.
Baseline Subject Characteristics by CAD status
| All subjects (n=687) |
Non-CAD (n=313) |
With CAD (n=374) |
P | |
|---|---|---|---|---|
| Demographics and Cardiovascular Risk Factors | ||||
| Age (years) | 68±10 | 61±8 | 70±9 | <0.001 |
| Male (%) | 67 | 37 | 81 | <0.001 |
| Diabetes mellitus (%) | 37 | 17 | 46 | <0.001 |
| Hypertension (%) | 73 | 56 | 80 | <0.001 |
| Smokers (Former/Current, %) |
67 | 52 | 73 | <0.001 |
| Prior myocardial infarction (%) |
32 | 0 | 48 | <0.001 |
|
| ||||
|
Laboratory Data
| ||||
| LDL cholesterol (mg/dL) | 96(79-119) | 110(90-130) | 91(76-112) | <0.001 |
| HDL cholesterol (mg/dL) | 35(28-41) | 39(34-50) | 32(27-39) | <0.001 |
| Triglycerides (mg/dL) | 115(86-159) | 104(79-143) | 119(90-169) | 0.006 |
| Total leukocyte count (WBCP/hpf) |
6.12(5.117.37) | 5.87(4.61-6.95) | 6.24(5.21-7.57) | 0.003 |
| Uric acid (mg/dL) | 6.3(5.1-7.3) | 5.6(4.7-6.7) | 6.6(5.5-7.6) | 0.003 |
| hsCRP (mg/L) | 2.53(1.076.66) | 1.94(0.85-5.29) | 2.83(1.24-7.08) | 0.004 |
| Myeloperoxidase (pg/mL) | 114(74-246) | 104(70-167) | 124(80-286) | 0.001 |
| Creatinine clearance (ml/min/1.73m2) |
88(66-110) | 97(83-122) | 79(62-106) | <0.001 |
|
| ||||
|
Baseline Medications
| ||||
| Aspirin | 74 | 60 | 81 | <0.001 |
| Beta-adrenergic blockers | 58 | 47 | 62 | 0.002 |
| Angiotensin converting enzyme inhibitors |
53 | 34 | 61 | <0.001 |
| Statin therapy | 54 | 26 | 67 | <0.001 |
Values expressed in mean ± standard deviation or median (interquartile range). Abbreviations: LDL = low-density lipoprotein; HDL = high-density lipoprotein; hsCRP = high-sensitivity C-reactive protein.
Materials
Free 3-nitrotyrosine and the synthetic octapeptide containing two 3-nitrotyrosine residues (CGnitroYGGGnitroYG) (CPC Scientific, San Jose, CA) were conjugated to horseradish peroxidase (HRP) following a previously published one step procedure.10 Affinity purified polyclonal anti-nitrotyrosine antibodies were generated and characterized extensively 15, 16, 8 and used as reference immunoglobulin. Synthetic peptides comprising amino acids 213-219 of human apolipoprotein A-I sequence (213LAEYHAK219) with either tyrosine in position 216 or 3-nitrotyrosine (213LAEnitroYHAK219) were used for ligand competition. Human fibrinogen (America Diagnostica, Stamford, CT), fibronectin and ceruloplasmin were nitrated by addition of 3 consecutive bolus additions of peroxynitrite (final concentration 100 μM) in 0.1 M phosphate buffer containing 100 μM DTPA and 25 mM bicarbonate, pH 7.2. These proteins were chemically nitrated and used to screen for the circulating immunoglobulin isotype.
Immunonohistochemistry
To probe for tyrosine nitrated proteins sections from human carotid atheromatous lesions were fixed in cold methanol-acetone solution for 20 min at −20 °C. Staining for tyrosine-nitrated proteins was performed using rabbit polyclonal affinity purified anti-nitrotyrosine antibodies (7 μg/mL) raised against the synthetic peptide described previously.15,16 A goat anti-rabbit secondary antibody conjugated to HRP (Biorad, Hercules, CA) was used for signal detection. As control for the specificity of the antibody binding, the anti-3-nitrotyrosine antibody was pre-incubated with 250 μM 3-nitrotyrosine octapeptide or with 250 μM of the corresponding tyrosine octapeptide for 1 hour at room temperature. Other control experiments included; pre-incubation with 2.5 mM free 3-nitrotyrosine, reduction of tissue nitrotyrosine to aminotyrosine using 0.5 M dithionite in 0.1 N sodium hydroxide for 20 min at room temperature, as well as omission of the primary antibody. Oil red-O, trichrome staining and 3,3′-diaminobenzidine (DAB)-chromogenic detection of immunoglobulins was performed in human lesions fixed in 4% paraformaldehyde as described previously.8, 15,16
Affinity purification of immunoglobulins
Proteins that bind to 3-nitrotyrosine were affinity isolated using an Amino Link Plus Immobilization Kit (Pierce, Rockford, IL), as described previously with minor modifications.8 Briefly, 10 mg of plasma proteins (combining plasma from three CAD patients) were diluted in binding buffer (50 mM potassium phosphate, 400 mM NaCl, pH 7.5), applied to the column, and incubated for 1 hour, at room temperature. Unbound protein fractions were eluted by centrifugation with binding buffer. Bound proteins were eluted with 0.1 M citrate, pH 2.5, and neutralized with 1 M Tris, pH 9. Bound fractions were concentrated and buffer exchanged using Millipore 10 kDa centrifugal filter units (Millipore Corp., Billerica, MA). The protein fractions were separated on 10% SDS-PAGE and transferred toImmobilon-P PVDF membranes (Millipore Corp., Bedford, MA), at 100V and 4° C. After blocking with 3% BSA in TBS-T, the membranes were incubated with either goat anti-human IgG-IRDye 800 CW (Rockland Immunochemicals, Inc. Boyertown, PA) or mouse anti-human IgM (1:2500, Sigma, St. Louis, MO) plus goat anti-mouse IgG conjugated to Alexa Fluor 680 (1:5000, Invitrogen, Eugene, OR) in blocking buffer. Images were obtained with an Odyssey near infrared imaging system (LI-COR, Inc. Lincoln, NE) at 700 and 800 nm.
Human arterial tissue was obtained from the National Disease Resource Institute (Philadelphia, PA) or the Cleveland Clinic. Tissues (approximately 1 gram) were cleaned of connective tissue and blood, snap frozen in liquid nitrogen, and pulverized in the frozen state and stored at −80 °C until use. Frozen tissue powder was thawed in 5 ml extraction buffer containing protease inhibitor cocktail (Sigma, St Louis MO), and further macerated with a Tissue-Tek homogenizer operated on full power for 10 seconds. This procedure was repeated 4 times per tissue sample. Tissue extracts were centrifuged at 700 x g for 5 min and 21,000 x g twice for 10 min to remove particulate material. Supernatants were combined and loaded to appropriate columns for affinity enrichment.
Isotype determination
ELISA methodologies were developed to determine the isotype and the sub-isotype of the circulating immunoglobulin that recognize protein-bound 3-nitrotyrosine. First, to differentiate between IgG and IgM, 100 μl of 10 μg/ml of the synthetic 3-nitrotyrosine octapeptide described above was diluted in 50 mM bicarbonate buffer, pH 9 and added in the wells of 96 well plates (MAXI-SORP, Nunc, Roskilde, Denmark). After overnight incubation at 4° C the plates were washed and incubated in the presence of 100 μl/well 3% BSA in TBS-T (working buffer), for 2 hr at room temperature. Human plasma from CAD patients (5 mg of plasma protein/mL) were added to each well and incubated for 2 hr at room temperature. Anti-human IgG and anti-human IgM HRP-conjugated antibodies (Sigma, Saint Louis, MO) diluted to 1:5,000 in working buffer were added to appropriate series of wells. Peroxidase activity bound to each well was determined with 3,3′,5,5′ tetramethylbenzidine (TMB, Pierce Biotechnology, Inc., Rockford, IL) substrate.
To determine the IgG subclass of the antibody, microplates were coated with 100 μL of 5 μg/mL solution of nitrated and native ceruloplasmin, fibronectin, and fibrinogen. The plates were incubated overnight at 4° C. After blocking, 100 μl of human plasma (diluted 1:40) alone or in the presence of 10 μM synthetic 3-nitrotyrosine octapeptide (specificity control) in blocking buffer were added to each well and incubated for 1hr, followed by the addition of 100 μl/well of 1:2,000 dilutions of monoclonal HRP-conjugated anti-human IgG 1, 2, 3, and 4 (Sigma, Saint Louis, MO). Absorbance was measured at 450 nm on a micro-plate reader (Spectra-Max 250, Molecular Devices Corp., Sunnyvale, CA). The background absorbance obtained with unmodified proteins was subtracted from the absorbance of the samples that reacted with the nitrated protein antigens.
Ligand competition ELISA
A competition ELISA described previously was implemented to determine the levels and specificity of circulating immunoglobulins that recognize 3-nitrotyrosine in human plasma.10 Briefly, each well of a 96-well microtiter plate (MAXI-SORP, Nunc. Roskilde, Denmark) was coated with 50 μl of serial dilutions of human plasma (0.02-10 mg of protein/ml) or a single dilution (as indicated in the appropriate figure legend), in 50 mM sodium bicarbonate buffer, pH 9. After blocking, 75 nM horseradish peroxidase (HRP)-conjugated 3-nitrotyrosine or a HRP-conjugated synthetic octapeptide containing two 3-nitrotyrosine residues (CGnitroYGGGnitroYG) were added in 0.1% BSA in TRIS buffered saline with 0.05% tween-20 (TBS-T), and incubated for 1 hr. Peroxidase activity bound to each well was determined using the H2O2-peroxidase mediated oxidation of TMB measuring absorbance at 450 nm. To test the specificity of detection the absorbance at 450 nm was competed in each sample by inclusion of excess free, unlabeled, 3-nitrotyrosine (2.5 mM). In some experiments the CGnitroYGGGnitroY-G octapeptide was used as competitor.
A polyclonal rabbit anti-nitrotyrosine IgG antibody (0.08-40 μg of protein/ml) characterized in detail previously15, 16 was run each day along with plasma samples. The antibody titer in each sample was calculated using the absorbance at 450 nm of the samples (Abs450nm), and the average of the parameters of the dose-response curve obtained with the polyclonal anti-nitrotyrosine antibody using the following equation:
where 200 represents the sample dilution and the average values for the parameters A, B, C, and D were 0.07, 2.21, 0.04, and 1.57, respectively, denoting minimal absorbance, slope at the linear part of the curve, the dose at the central point, and the maximal absorbance, respectively. Antibody titers in human plasma are therefore expressed as μg equivalents of the polyclonal antibody per mL of plasma (μg Ab Eq/mL plasma).
Isolation of nitrated proteins for mass spectrometry
Proteins bearing the 3-nitrotyrosine epitope were affinity captured from human CAD plasma by using the polyclonal anti-3-nitrotyrosine antibody conjugated to a recombinant protein A column (Pierce, Rockford, IL). Pooled plasma from six CAD patients (14 mg total protein) were loaded into the column in 0.1 M Hepes, pH 7.4 (binding buffer), and incubated overnight at 4° C. The column was washed successively with 10 mL of the following solutions: binding buffer, 0.5 M NaCl, and binding buffer again. Bound fractions were eluted with 10 mL of 0.1 M glycine, pH 2.5, containing 0.15 M NaCl. After concentration and buffer exchange using Centriprep YM-10 filters (Millipore Corp., Billerica, MA), proteins were separated on 10% SDS-PAGE, and stained with colloidal blue (Invitrogen, Carlsbad, CA). An identical gel was transferred to PVDF membrane for immunodetection of protein 3-nitrotyrosine using the polyclonal anti-nitrotyrosine antibody.
Gel-LC-MS/MS analysis
Colloidal blue stained gels were cut into 11 × 2mm slices and in-gel digested with trypsin as previously described.8 Tryptic peptide digests were analyzed by a LTQ linear IT mass spectrometer (Thermo Electron, San Jose, CA) coupled to an Eksigent 2D LC system (Eksigent Technologies, Livermore, CA) and autosampler. Buffers A and B were 0.1 % formic acid/1 % methanol and 80% acetonitrile/0.1% formic acid/1 % methanol, respectively. Peptides were loaded isocratically onto a C18 trap column (75 μm i.d. x 25 mm; New Objective Proteopep 2) at a flow rate of 1 μL per minute in 2% B. Peptides were then eluted onto a C18 analytical column (75 μm i.d. x 150 mm; New Objective Proteopep 2). A linear gradient was then initiated at a flow rate of 300 nL per minute for 90 min from 3-40% B. The mass spectrometer was set to repetitively scan m/z from 375 to 1600 followed by data-dependent MS/MS scans on the five most abundant ions with dynamic exclusion enabled.
Generation and evaluation of SEQUEST peptide assignments
DTA files were generated from the MS/MS spectra extracted from RAW data files (intensity threshold of 1000; minimum ion count of 50) and processed by the ZSA and Correction algorithms of the SEQUEST Browser program. DTA files were submitted to Sorcerer-SEQUEST (ver. 3.11, rev 11; Sagen Research, San Jose, CA) using the following parameters: Database searching was performed against a Uniprot database containing Homo sapiens sequences from Swiss-Prot plus common contaminants, which were then reversed and appended to the forward sequences (91,522 entries). The database was indexed with the following parameters: mass range of 600 - 3500, tryptic cleavages with a maximum of 1 missed cleavage and static modifications of cysteine by carboxyamidomethylation (+57 amu). The DTA files were searched with a 2.0 amu peptide mass tolerance, 1.0 amu fragment ion mass tolerance, and variable modification of methionine (+16 amu). Potential sequence-to-spectrum peptide assignments generated by Sorcerer-SEQUEST were loaded into Scaffold (version 2.2; Proteome Software, Portland, OR) to validate protein identifications and perform manual inspection of MS/MS spectra containing 3-nitrotyrosine. Protein identifications were accepted at a threshold of ≥ 99 % protein confidence with ≥ 2 unique peptides at ≥ 80 % confidence. From these proteins, manual inspection of 3-nitrotyrosine-containing MS/MS spectra were performed using the following criteria: (1) assignment of the majority of fragment ion abundance, (2) 3-nitrotyrosine (+45 amu) modification supported by either y- or b- ions series (≥ 5 consecutive fragments), and (3) correctly assigned charge state and diagnostic markers, such as N-terminal proline, C-terminus aliphatic amino acids, and loss of H2O/ammonia consistent with amino acid sequence.
Statistical Analysis
The Student’s t-test or Wilcoxon-Rank sum test for continuous variables and chi-square test for categorical variables were used to examine the difference between the groups (CAD vs. non-CAD). The relationship between plasma immunoglobulin levels and risk for having CAD was determined by calculating Odds ratio (OR) and 95% confidence intervals (95%CI) using multiple logistic regression treating immunoglobulin levels against protein-bound nitrotyrosine as a continuous variable. Models adjusted for traditional risk factors (age, gender, systolic blood pressure, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, smoking), or traditional risk factors plus history of myocardial infarction (MI), and medications (aspirin, ACE, statin, beta-blockers). Cubic spline curves with 95% confidence intervals were constructed to illustrate the relationship between anti-nitrotyrosine immunoglobulin levels and odds ratio of angiographic evidence of significant CAD. All analyses were performed using R 2.13.1 (Vienna, Austria) and p-values <0.05 were considered statistically significant.
RESULTS
Nitrated proteins in human atherosclerotic lesions and plasma
The presence of nitrated proteins in atherosclerotic lesions and/or plasma of CAD patients could serve as neoepitopes, triggering an immune response and the elaboration of immunoglobulins. Therefore, we first sought to document the presence of nitrated proteins by immunohistochemistry in human lesions and by mass spectrometry in lesion extracts as well as plasma of subjects with CAD. A typical atherosclerotic lesion in human carotid artery stained with Oil red O is depicted in Figures 1 A and B. The immunofluorescence images after staining for nitrated protein epitopes is depicted in Figures 1C and D indicated the presence of nitrated proteins within the lesion, consistent with previous studies in human atherosclerotic lesions.6 The specificity of the anti-nitrotyrosine antibodies used for staining was confirmed by competition experiments with excess of the nitrated octapeptide (CGnitroYGGGnitroYG). Moreover, staining with anti-human IgG also revealed the presence of immunoglobulins in the lesions (Fig. 1G). Nitrated proteins extracted from atherosclerotic lesions and plasma were affinity enriched using the previously characterized polyclonal anti-nitrotyrosine antibody.15, 16. As a control, lesion extracts were incubated with a non-specific rabbit immunoglobulin under the same conditions (representative western blots confirming selective enrichment are depicted in supplementary figure 1). The bound fractions were separated by 1D SDS-PAGE, and analyzed by liquid chromatography tandem mass spectrometry (LC-MS/MS). Only proteins that were identified in the bound fraction of anti-nitrotyrosine-enriched samples are reported (Supplementary Table 1). Within the lesion, several proteins that have been previously reported to be modified by tyrosine nitration such as apolipoprotein AI (apoA-I), beta chain of fibrinogen, α1-antitrypsin and complement were identified.7, 17-19 A similar analysis in human plasma revealed for the first time the presence of specific peptide sequences that contained the nitrated tyrosine residue. Several of these sequences belong to immunoglobulins (supplementary table 2). Interestingly one specific tyrosine nitrated peptide [(K)SGTASVVCLLNNFnitroYPR(E)] from human immunoglobulin was also identified in the plasma of atherosclerosis rodent models; specifically, the low-density lipoprotein receptor (LDLr) and apobec-1 null double-knockout mice, as well as the LDLr/apobec-1 knockout mice lacking apoA-I (LA–apoA-I-/-).8 Collectively the data indicate that nitrated proteins are present in human atherosclerotic lesions as well as plasma of subjects with CAD and could serve as antigenic neoepitopes to trigger the activation of the immune system.
Figure 1. Immunohistochemical detection of nitrated proteins in human atherosclerotic lesions.
Panel A and B: Oil red O staining of the atherosclerotic lesion in human carotid. (A) Low magnification (1.25x) and (B) high magnification (20x). Panels C-E: Serial cross sections were incubated with polyclonal anti-3-nitrotyrosine antibody (C). The immunoreactive nitrated proteins were visualized in the lesions by green fluorescence after incubation with secondary anti-rabbit/Alexa Fluor 488 antibody. Immunoreactivity was reduced by competition of the primary anti-3-nitrotyrosine antibody binding with the 250 μM 3-nitrotyrosine-containing octapeptide (D) but not by 250 μM tyrosine-containing octapeptide (E). Nuclei are stained blue with DAPI. The results are representative of staining 2 different human lesions. Panel F and G: Trichrome staining of the carotid lesion (F) and immunostaining with anti-human IgG antibody revealed the presence of IgG immunoglobulins within and around the lesion (G). Immunoreactivity (brown color) was developed with DAB. Bars indicate 100 μm.
Affinity chromatography identified immunoglobulins that recognize 3-nitrotyrosine and nitrated proteins
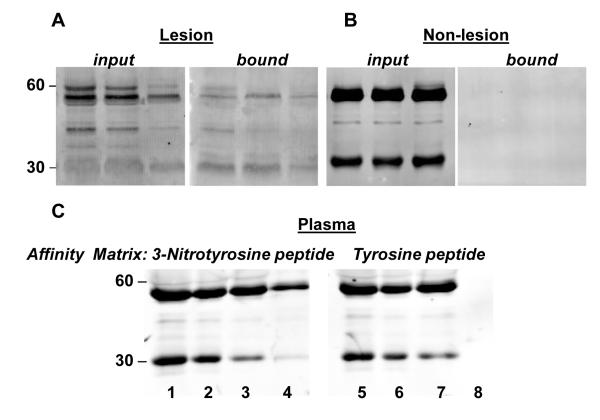
Protein extracts of human aorta atherosclerotic lesions were allowed to bind to affinity columns conjugated with 3-nitrotyrosine. After binding and exhaustive washing, the bound fractions were eluted, separated by SDS-PAGE and analyzed by Western blot using anti-human IgG antibodies (Fig. 2A). The presence of immunoglobulins that bound to 3-nitroyrosine was documented in the lesion tissue extract. As controls, tissue from the same aorta free of lesion was used and analyzed in parallel. Equivalent loading of extracts derived from aorta segments from the same tissue, but without visible atherosclerotic lesions, did not show immunoreactive bands for IgG in the bound fraction eluted from the 3-nitrotyrosine affinity column (Fig. 2A).
Figure 2. Affinity detection and isotyping of anti-nitrotyrosine immunoglobulins from aortic lesions and CAD plasma.
Panel A: Representative western blots from three different atherosclerotic lesion extracts (input) and bound fractions eluted from a 3-nitrotyrosine affinity column using anti-human IgG antibodies. Panel B: Representative western blots from the same vessels as in Panel A using the equivalent protein extracts from the same tissue without visible lesion. Immunoreactivity is observed only in the fractions originating from lesion areas. Panel C: Affinity captured plasma proteins using either immobilized 3-nitrotyrosine containing octapeptide (Lanes 1-4) or tyrosine containing octapeptide (Lanes 5-8) probed with polyclonal anti-human IgG. Lane 1 and 5, un-fractionated input plasma; lane 2 and 6, unbound fraction; lane 3 and 7, wash fractions; lane 4 and 8, bound fractions eluted with 0.1 M citrate buffer, pH 2.5. Data are representative of six independent experiments with similar results.
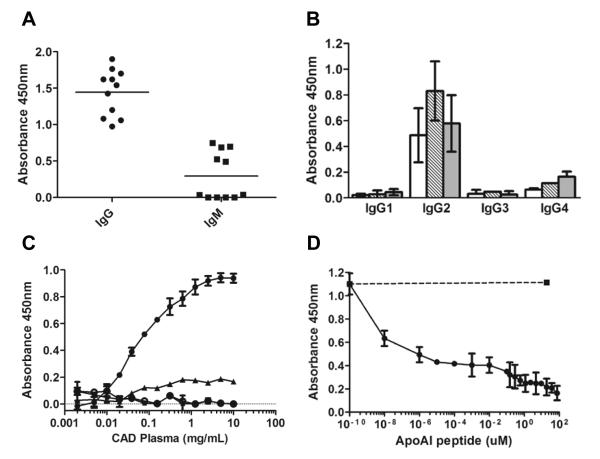
Pooled plasma from CAD subjects was allowed to bind to affinity columns that were made by conjugating either the nitrated tyrosine octapeptide (CGnitroYGGGnitroYG) or the equivalent tyrosine peptide (CGYGGGYG). The data in Figure 2B indicate the presence of IgG in the human plasma of CAD subjects that specifically bind the nitrated tyrosine peptide but not the tyrosine peptide. Plasma proteins recovered in the bound fraction from the nitrated tyrosine octapeptide affinity column also showed immunoreactivity toward human IgM antibodies. By employing an ELISA in which the 3-nitrotyrosine octapeptide was used to bind the immunoglobulins and the respective anti-human immunoglobulin to develop, we quantified the relative levels of IgG and IgM in human CAD subjects plasma. Plasma immunoglobulins that recognize the nitrotyrosine modification were predominantly IgG, since the signal was nearly 5-fold higher for IgG as compared to IgM (1.4 ± 0.3 and 0.3 ± 0.3 average absorbance respectively, Fig. 3a). Therefore, subsequent studies focused on quantifying the levels of circulating IgG immunoglobulin isoform in subjects. The IgG present in human plasma of CAD subjects was further characterized by isotyping using an ELISA in which potential endogenous 3-nitrotyrosine containing proteins: nitrated ceruloplasmin, nitrated fibronectin, and nitrated fibrinogen were used as antigens. The predominant isotype that recognized preferentially the nitrated proteins was IgG2 (Fig 3B).
Figure 3. Screening for circulating immunoglobulins that recognize 3-nitrotyrosine.
Panel A: IgG and IgM levels in human plasma were measured by ELISA in ten randomly selected CAD plasma subjects, using the 3-nitrotyrosine octapeptide to bind the immunoglobulins and the respective anti-human immunoglobulin for detection. Panel B: IgG subclass was determined by ELISA in three independent CAD plasma samples, using nitrated ceruloplasmin (white bars), nitrated fibronectin (dashed bars) and nitrated fibrinogen (grey bars) as capturing antigen, and HRP-conjugated mouse anti-human IgG 1, IgG2, IgG 3, and IgG 4 for detection. Panel C: Typical antigen-antibody binding curves from 3 randomly selected CAD subjects each performed in triplicate, using as ligand the 3-nitrotyrosine-octapeptide conjugated to HRP (●). No binding was observed using the same tyrosine-containing octapeptide conjugated to HRP (○). The binding of the 3-nitrotyrosine-octapeptide conjugated to HRP was eliminated with 75 μM unlabeled 3-nitrotyrosine-octapeptide (▴) or by 250 μM 3-nitrotyrosine (∎). Panel D: The binding of the 3-nitrotyrosine-octapeptide conjugated to HRP to 3 different plasma samples, each performed in triplicate, was also eliminated with increasing concentrations of apolipoprotein A-I peptide 213-219 (213L-A-E-nitroY-H-A-K219) which contained 3-nitrotyrosine in position 216 (●) but not by the same peptide that contained tyrosine in position 216 (∎). Data reports mean ± standard deviation.
ELISA development to quantify plasma levels of anti-nitrotyrosine immunoglobulins in non-CAD and CAD subjects
We next sought to determine whether plasma immunoglobulins that recognize the neoepitope 3-nitrotyrosine are more prevalent within subjects with CAD. For these studies, we modified a competition ELISA described previously for use in human plasma and mouse models of atherosclerosis.10, 8 Typical antigen-antibody binding curves using the nitrotyrosine-containing octapeptide conjugated to HRP are shown in Figure 3, panel C. No binding was observed using the same tyrosine-containing octapeptide. The specificity of binding was confirmed by competing the binding of the 3-nitrotyrosine peptide-conjugated HRP with excess free 3-nitrotyrosine (2.5 mM), as well as un-conjugated nitrotyrosine peptide (75 μM). In all subsequent analyses the difference between the absorbance in the absence versus presence of excess 3-nitrotyrosine (2.5 mM), considered as background, was used to calculate the titer of circulating anti-3-nitrotyrosine immunoglobulins. Furthermore, the binding of immunoglobulins to the HRP-conjugated nitrotyrosine peptide was competed by a peptide derived from apoA-I (213LAEnitroYHAK219) containing 3-nitrotyrosine in place of tyrosine in position 216. Maximal inhibition was obtained at 1 μM 213LAEnitroYHAK219 (Fig. 3, panel D). At the same concentration, the unmodified apoA-I peptide, 213LAEYHAK219, was unable to compete (Fig. 3, panel D) confirming the specificity of the immunoglobulins towards protein tyrosine nitrated epitopes.
Plasma levels of antibodies that recognize protein nitrotyrosine are increased in subjects with CAD
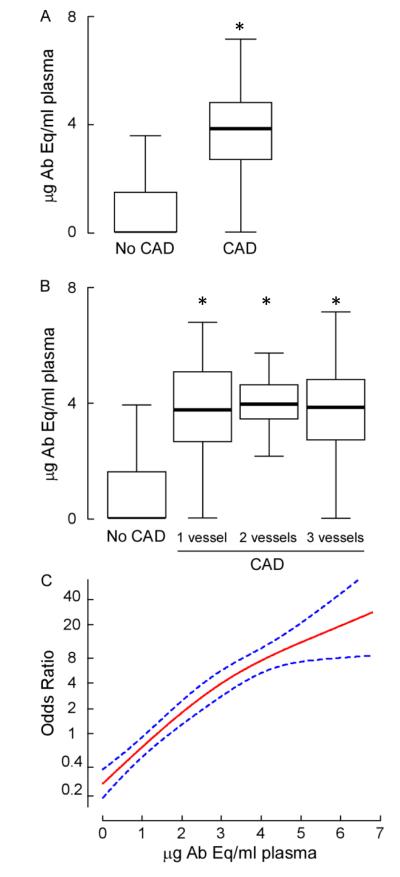
Plasma levels of anti-3-nitrotyrosine immunoglobulins were quantified in subjects with and without CAD as documented during elective cardiac evaluations, as described under Methods. Subjects with clinically documented CAD had greater than ten times higher levels of anti-3-nitrotyrosine immunoglobulins (3.75 ± 1.8 μg Ab Eq/mL plasma) as compared to non-CAD individuals (0.36 ± 0.8 μg Eq/mL plasma; p<0.001 for comparison with CAD). Interestingly, subjects with angiographic evidence of significantly-obstructed (≥50% stenosis) CAD showed increased plasma anti-3-nitrotyrosine immunoglobulin titers compared to non-CAD subjects, regardless of the number of coronary arteries affected by CAD (Fig. 4, panel B). A striking dose-dependent positive association was observed between plasma levels of anti-nitrotyrosine IgG and angiographic evidence of significant CAD; in fact, because the non-CAD cohort had very low levels of anti-3-nitrotyrosine immunoglobulins (non-detectable in 76 % of the samples), a remarkably large odds ratio was observed for anti-3-nitrotyrosine IgG titer versus the presence of significant angiographic evidence of CAD (Fig. 4, panel C). Multilogistic regression analyses indicated that the robust association of immunoglobulin levels against protein 3-nitrotyrosine with cardiovascular risk remains significant even following adjustments for multiple traditional risk factors, including age, gender, systolic blood pressure, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, smoking, history of MI and medications (aspirin, angiotensin converting enzyme inhibitors, statin, beta-blockers) (Table 2).
Figure 4. Anti-nitrotyrosine circulating immunoglobulins in CAD.
Panel A: Plasma levels of anti-3-nitrotyrosine circulating immunoglobulins were quantified in clinically documented CAD subjects vs. subjects without significant angiographic evidence of CAD. Antibody equivalents were calculated from standard curves performed with a polyclonal anti-nitrotyrosine antibody. Panel A and B: Box-whisker plots of immunoglobulin levels encompass the 25th and 75th percentiles and lines within boxes represent median values. Bars represent the 2.5th and 97.5th percentiles. Statistically significant differences in immunoglobulin content were found between CAD and non-CAD (p<0.0001, Kruskal-Wallis test). Panel C: Odds ratio and 95% confidence interval (dotted lines) for the relationship between plasma levels of anti-nitrotyrosine immunoglobulins and angiographic evidence of significant CAD were calculated as described under Methods.
Table 2.
Relationship between immunoglobulin against 3-nitrotyrosine and CAD prevalence.
| μg Ab Eq/mL Plasma | ||
|---|---|---|
| CAD | Unadjusted OR | 12.45 (9.14-16.97)* |
| Adjusted OR (1) | 12.43 (7.57-20.42)* | |
| Adjusted OR (2) | 14.18 (8.02-25.06)* |
Results of fitting one univariate and two multivariable logistic regression models treating analyte (immunoglobulin against 3-nitrotyrosine) as a continuous variable. Shown are adjusted odds ratio and 95%CI for prevalent CAD per standard deviation change in log-transformed analyte measured.
Model 1: adjusted for traditional risk factors include age, gender, systolic blood pressure, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, smoking and diabetes.
Model 2: adjusted for traditional risk factors include age, gender, systolic blood pressure, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, smoking, diabetes, history of MI, aspirin, ACE, statin, beta-blockers.
P<0.001.
DISCUSSION
Clinical evidence and data from animal models have indentified various forms of oxidized lipoproteins and heat shock proteins as immunogens that induce adaptive immune responses, including antibody elaboration, that modulate atherosclerotic lesion formation in experimental models of atherosclerosis.20-29 Most studies have predominantly focused on covalent adducts formed by addition of electrophilic lipid peroxidation products to proteins and their relationship to the development and progression of atherosclerosis.20-26 Moreover, the levels of circulating IgM that recognized oxidized forms of low density lipoprotein with covalently adducted lipid oxidation products have been reported to correlate with severity of atherosclerosis in mice models and in some studies with the progression of atherosclerosis in coronary or carotid vascular beds, or with risk for myocardial infarction and death in humans.25 Collectively these data highlight the critical importance of understanding the immune system responses in CAD and raise the possibility of novel therapeutic treatments for atherosclerosis.30, 31 The data also underscores the need for identifying antigens and immune responses that are mechanistically linked to the underlying pathophysiology of cardiovascular diseases.
Protein tyrosine nitration is considered to be a post-translational modification reflective of inflammatory-mediated oxidative processes involving nitric oxide-derived oxidants. Although the ability of nitrated proteins to induce the generation of specific antibodies in experimental animals has long been known15, 16, 32-36, the contribution of this post-translational protein modification as a trigger of immune reactions in pathology has just recently been explored.10-12 In this study we demonstrated for the first time the association of CAD, the most common and life threatening cardiovascular condition, with increased plasma levels of immunoglobulins reactive against proteins modified by nitration. The binding of plasma immunoglobulins to target 3-nitrotyrosine-containing “bait” (HRP conjugated to 3-nitrotyrosine or 3-nitrotyrosine containing octapeptide) served as a competition-based ELISA to quantify autoantibody titers for 3-nitrotyrosine recognizing immunoglobulins within subject’s plasma. The specificity of the methodology was confirmed by numerous approaches, including demonstration that the 3-nitrotyrosine binding activity in plasma was specifically outcompeted by free 3-nitrotyrosine, as well as by 3-nitrotyrosine-modified peptides and proteins, ruling out nonspecific binding. In particular, a nitrated peptide reflecting an endogenous nitrated tyrosine residue identified in apoA-I recovered from human atherosclerotic plaque7, but not the native tyrosine containing peptide, was able to out-compete the binding of the 3-nitrotyrosine-coupled HRP “bait” to immunoglobulins. Moreover, the circulating immunoglobulins were able to recognize nitrated fibrinogen and other nitrated plasma proteins but not the native counterparts (Fig. 3B). These data support a possible specific antigenic role of those endogenous nitrated proteins as the neo-epitope that triggers anti-nitrotyrosine immunoglobulins. It is remarkable that both of these proteins (apoA1 and fibrinogen) have been previously reported to be selectively nitrated in plasma from CAD patients relative to healthy controls, and that nitration of these particular proteins may have a potential functional role in cardiovascular events. In the case of fibrinogen, nitration increases the rate as well as the extent of fibrin cloth formation17, 18 potentially leading to enhanced thrombotic risk and adverse cardiovascular events. On the other hand, 3-nitrotyrosine modified apoA-I was found in serum and human atherosclerotic lesions. Further, exposure of apoA-1 to myeloperoxidase, an enzymatic source of nitric oxide-derived oxidants, creates a dysfunctional form of HDL with diminished ABCA1-dependent cholesterol efflux capacity and reduced lecithin cholesterol acyl transferase activity.7, 37 The molecular mechanisms for the production of immunoglobulins with specific and selective epitope recognition for 3-nitrotyrosine have been explored in mouse models.35, 36 Termination of self-tolerance and escaping of negative selection after active immunization with nitrated peptides was documented.35,38 Moreover, monoclonal antibodies that recognize only nitrated α-synuclein (including stereospecific clones that recognized one of the four nitrated tyrosine residues in α-synuclein) but not the unmodified protein or other tyrosine-nitrated proteins32, as well as antibodies recognizing only nitrated Mn Superoxide dismutase33, and only nitrated tau34 have been reported. In one model of mouse neurodegeneration the specific immune response to nitrated α-synuclein produced a vigorous immunoinflammatory response that led to the degeneration of dopamine-producing neurons.39
The pathophysiological relevance of circulating immunoglobulins targeting protein-3-nitrotyrosine remains to be determined. Indeed, despite the strikingly elevated levels of anti-3-nitrotyrosine antibodies in CAD subjects, it is possible that the antibody response within atherosclerotic plaque may promote a protective role, with immunoglobulins targeting proteins bearing potentially threatening modifications for destruction. Further, the relatively high risk cohort of subjects examined (those undergoing elective diagnostic cardiac evaluations) makes the results of the present study of unclear relevance when translated to a community based screen. It also remains to be determined whether elevated levels of 3-nitrotyrosine targeted antibodies are increased in subjects prior to clinically overt development of atherosclerotic CAD, and whether increased levels may serve as a sentinel of increased risk of major adverse cardiac events or not. Further studies are needed to both identify the role; if any, of 3-nitrotyrosine immunoglobulins in CAD pathophysiology, as well as whether anti-3-nitrotyrosine immunoglobulin titer carries prognostic value and is modulated by CAD targeting therapies.
Supplementary Material
Supplementary figure 1 Affinity enrichment of nitrated proteins from human atherosclerotic lesions. Proteins extracted from atherosclerotic human lesions (0.84 mg of protein) were incubated with Dyna-beads/protein-A cross-linked to a polyclonal anti-nitrotyrosine antibody in lysis buffer (150 mM NaCl, 1.5 mM MgCl2, 2 mM Hepes, pH 7.4, 10% glycerol, 1% Triton X-100, 1 mM PMSF, 1/100 Sigma protease inhibitor without metal chelators). Enrichment using the same beads cross-linked to a non-specific IgG were used as control. Bound fractions from the beads were eluted using 0.1 M citrate buffer, pH 2.5, and the fractions were separated in 10% SDS-PAGE, transferred to PVDF and probed with a polyclonal anti-nitrotyrosine antibody. (A) Colloidal blue stain and (B) western blot developed with an anti- nitrotyrosine polyclonal antibody. Lanes: 1) Input un-fractionated lesion protein extract; 2) Unbound fraction from the anti- nitrotyrosine antibody beads. 3) Bound fraction from the anti-nitrotyrosine antibody beads; 4) Bound fraction from the non-specific IgG beads. Representative data from 3 different human lesions.
Supplementary figure 2 Affinity enrichment for nitrated proteins from human CAD plasma. Pooled plasma CAD subjects was applied to a agarose column that a polyclonal anti-nitrotyrosine antibody was ligated. After binding and extensive washing the bound proteins were eluted with 0.1 M glycine, pH 2.5, containing 0.15 M NaCl and separated in 10% SDS-PAGE. (A) Colloidal blue stain and (B) western blot developed with an anti-nitrotyrosine polyclonal antibody. Lanes 1, and 4, un-fractionated plasma (input); Lanes 2, and 5, last wash before elution; Lanes 3, and 6, eluted bound proteins.
Supplementary figure 3 Typical binding curve and specificity of the reference anti-nitrotyrosine antibody. Polyclonal antibody 609 was generated using the nitrated tyrosine octapeptide (CGnitroYGGGnitroYG) as antigen as described in detail previously (15). The binding of the antibody to nitrated proteins (◆) was competed by the inclusion of 250 μM 3-nitrotyrosine (◻) or 10 μM nitrated tyrosine octapeptide (⌂). Data reports mean ± standard deviation.
Supplementary Table 1. Proteins that bound to anti-nitrotyrosine antibodies in human atherosclerotic lesions.
Supplementary Table 2. Nitrated proteins and the corresponding modified peptides in human CAD plasma.
Clinical evidence and data from animal models have identified a pathophysiological role of immunity in atherosclerosis. In this study we have identified and quantified specific immunoglobulins that recognize nitrated tyrosine epitopes in human atherosclerotic lesions as well as in circulation of subjects with clinically documented coronary artery disease (CAD). Protein tyrosine nitration is a post-translational modification reflective of inflammatory-mediated oxidative processes involving nitric oxide-derived oxidants that is known to elicit the elaboration of immunoglobulins in animal models. A highly significant ten-fold increase in the mean level of circulating immunoglobulins against protein 3-nitrotyrosine was documented in the CAD subjects. The levels of these specific immunoglobulins were positively associated with increased odds ratio for the presence of angiographic evidence of significant CAD. Further studies are needed to explore the pathophysiological relevance of circulating immunoglobulins against nitrated proteins in CAD pathophysiology. Additional studies are also required to test whether anti-3-nitrotyrosine immunoglobulin titer carries prognostic value and is modulated by CAD targeting therapies.
Acknowledgements
We thank Dr. Ian Blair (University of Pennsylvania) and Dr. David W. Speicher (Wistar Institute) for the use of mass spectrometers and discussions; Dr. Steven H. Seeholzer and the Protein Core at the Children’s Hospital of Philadelphia Research Institute for assistance with mass spectrometry data analysis.
Funding Sources The work was supported by grants from the National Institutes of Health HL54926, HL103918 and ES013508 NIEHS Center of Excellence in Environmental Toxicology to HI, P01HL098055 and P01HL076491 to SLH, and R01HL103931 and 1P20HL113452 to WHWT. HI is the Gisela and Dennis Alter Research Professor of Pediatric Neonatology at the Children’s Hospital of Philadelphia Research Institute. SLH is also partially supported by a gift from the Leonard Krieger Fund.
Footnotes
Disclosures Dr. Tang reports having received research grant support from Abbott Laboratories, and served as consultants for Medtronic Inc. and St. Jude Medical. Dr. Hazen reports being named as co-inventor on pending and issued patents held by the Cleveland Clinic relating to cardiovascular diagnostics, having been paid as a consultant by the following companies: Cleveland Heart Lab, Inc., Esperion, Liposciences Inc., Merck & Co., Inc., and Pfizer Inc., having received research funds from Abbott, Cleveland Heart Lab, Esperion and Liposciences, Inc., and having the right to receive royalty payments for inventions or discoveries related to cardiovascular diagnostics from Abbott Laboratories, Cleveland Heart Lab, Inc., Frantz Biomarkers, Liposciences, Inc., and Siemens.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473:317–25. doi: 10.1038/nature10146. [DOI] [PubMed] [Google Scholar]
- 2.Hansson GK, Libby P. The immune response in atherosclerosis: a double-edged sword. Nat Rev Immunol. 2006;6:508–519. doi: 10.1038/nri1882. [DOI] [PubMed] [Google Scholar]
- 3.Binder CJ, Chang M-K, Shaw PX, Miller YI, Hartvigsen K, Dewan A, Witztum JL. Innate and acquired immunity in atherogenesis. Nature Med. 2002;8:1218–1226. doi: 10.1038/nm1102-1218. [DOI] [PubMed] [Google Scholar]
- 4.Ischiropoulos H, Zhu L, Chen J, Tsai J-HM, Martin JC, Smith CD, Beckman JS. Peroxynitrite-mediated tyrosine nitration catalyzed by superoxide dismutase. Arch. Biochem. Biophys. 1992;298:431–437. doi: 10.1016/0003-9861(92)90431-u. [DOI] [PubMed] [Google Scholar]
- 5.Hazen SL, Zhang R, Shen Z, Wu W, Podrez EA, MacPherson JC, Schmitt D, Mitra SN, Mukhopadhyay C, Chen Y, Cohen PA, Hoff HF, Abu-Soud HM. Formation of nitric oxide-derived oxidants by myeloperoxidase in monocytes: pathways for monocyte-mediated protein nitration and lipid peroxidation In vivo. Circ Res. 1999;85:950–9588. doi: 10.1161/01.res.85.10.950. [DOI] [PubMed] [Google Scholar]
- 6.Beckman JS, Ye YZ, Anderson PG, Chen J, Accavitti MA, Tarpey MM, White CR. Extensive nitration of protein tyrosines in human atherosclerosis detected by immunohistochemistry. Biol Chem Hoppe Seyler. 1994;375:81–88. doi: 10.1515/bchm3.1994.375.2.81. [DOI] [PubMed] [Google Scholar]
- 7.Zheng L, Nukuna B, Brennan ML, Sun M, Goormastic M, Settle M, Schmitt D, Fu X, Thomson L, Fox PL, Ischiropoulos H, Smith JD, Kinter M, Hazen SL. Apolipoprotein A-I is a selective target for myeloperoxidase-catalyzed oxidation and functional impairment in subjects with cardiovascular disease. J. Clin. Invest. 2004;114:529–541. doi: 10.1172/JCI21109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parastatidis I, Thomson L, Fries DM, Moore RE, Tohyama J, Fu X, Hazen SL, Heijnen HF, Dennehy MK, Liebler DC, Rader DJ, Ischiropoulos H. Increased protein nitration burden in the atherosclerotic lesions and plasma of apolipoprotein A-I deficient mice. Circ. Res. 2007;101:368–76. doi: 10.1161/CIRCRESAHA.107.157537. [DOI] [PubMed] [Google Scholar]
- 9.Shishehbor MH, Aviles RJ, Brennan ML, Fu X, Goormastic M, Pearce GL, Gokce N, Keaney JF, Jr, Penn MS, Sprecher DL, Vita JA, Hazen SL. Association of nitrotyrosine levels with cardiovascular disease and modulation by statin therapy. JAMA. 2003;289:1675–1680. doi: 10.1001/jama.289.13.1675. [DOI] [PubMed] [Google Scholar]
- 10.Thomson LM, Christie J, Vadseth C, Lanken PN, Fu X, Hazen SL, Ischiropoulos H. Identification of immunoglobulins that recognize 3-nitrotyrosine in patients with acute lung injury following major trauma. Am. J. Respir. Cell Mol. Biol. 2007;36:152–157. doi: 10.1165/rcmb.2006-0288SM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khan F, Siddiqui AA. Prevalence of anti-3-nitrotyrosine antibodies in the joint synovial fluid of patients with rheumatoid arthritis, osteoarthritis and systemic lupus erythematosus. Clin Chim Acta. 2006;370:100–107. doi: 10.1016/j.cca.2006.01.020. [DOI] [PubMed] [Google Scholar]
- 12.Khan F, Siddiqui AA, Ali R. Measurement and significance of 3-nitrotyrosine in systemic lupus erythematosus. Scand J Immunol. 2006;64:507–514. doi: 10.1111/j.1365-3083.2006.01794.x. [DOI] [PubMed] [Google Scholar]
- 13.Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C, Harrison DG. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med. 2007;204:2449–2460. doi: 10.1084/jem.20070657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wenzel P, Knorr M, Kossmann S, Stratmann J, Hausding M, Schuhmacher S, Karbach SH, Schwenk M, Yogev N, Schulz E, Oelze M, Grabbe S, Jonuleit H, Becker C, Daiber A, Waisman A, Münzel T. Lysozyme M-positive monocytes mediate angiotensin II-induced arterial hypertension and vascular dysfunction. Circulation. 2011;124:1370–1381. doi: 10.1161/CIRCULATIONAHA.111.034470. [DOI] [PubMed] [Google Scholar]
- 15.Fries DM, Paxinou E, Themistocleous M, Swanberg E, Griendling KK, Salvemini D, Slot JW, Heijnen HF, Hazen SL, Ischiropoulos H. Expression of inducible nitric-oxide synthase and intracellular protein tyrosine nitration in vascular smooth muscle cells: role of reactive oxygen species. J. Biol. Chem. 2003;278:22901–22907. doi: 10.1074/jbc.M210806200. [DOI] [PubMed] [Google Scholar]
- 16.Heijnen HF, van Donselaar E, Slot JW, Fries DM, Blachard-Fillion B, Hodara R, Lightfoot R, Polydoro M, Spielberg D, Thomson L, Regan EA, Crapo J, Ischiropoulos H. Subcellular localization of tyrosine-nitrated proteins is dictated by reactive oxygen species generating enzymes and by proximity to nitric oxide synthase. Free Rad. Biol. Med. 2006;40:1903–1913. doi: 10.1016/j.freeradbiomed.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 17.Vadseth C, Souza JM, Thomson L, Seagraves A, Nagaswami C, Scheiner T, Torbet J, Vilaire G, Bennett JS, Murciano JC, Muzykantov V, Penn MS, Hazen SL, Weisel JW, Ischiropoulos H. Pro-thrombotic state induced by post translational modification of fibrinogen by reactive nitrogen species. J. Biol. Chem. 2004;279:8820–8826. doi: 10.1074/jbc.M306101200. [DOI] [PubMed] [Google Scholar]
- 18.Parastatidis I, Thomson L, Burke A, Chernysh I, Nagaswami C, Visser J, Stamer S, Liebler DC, Koliakos G, Heijnen HF, Fitzgerald GA, Weisel JW, Ischiropoulos H. Fibrinogen β-chain tyrosine nitration is a prothrombotic risk factor. J. Biol. Chem. 2008;283:33846–33853. doi: 10.1074/jbc.M805522200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gole MD, Souza JM, Choi I, Hertkorn C, Malcolm S, Foust RF, 3rd, Finkel B, Lanken PN, Ischiropoulos H. Plasma proteins modified by tyrosine nitration in acute respiratory distress syndrome. Am. J. Physiol. Lung Mol. Cell. Physiol. 2000;278:L961–967. doi: 10.1152/ajplung.2000.278.5.L961. [DOI] [PubMed] [Google Scholar]
- 20.Salonen JT, Ylä-Herttuala S, Yamamoto R, Butler S, Korpela H, Salonen R, Nyyssönen K, Palinski W, Witztum JL. Autoantibody against oxidized LDL and progression of carotid atherosclerosis. Lancet. 1992;339:883–887. doi: 10.1016/0140-6736(92)90926-t. [DOI] [PubMed] [Google Scholar]
- 21.Shaw PX, Horkko S, Tsimikas S, Chang MK, Palinski W, Silverman GJ, Chen PP, Witztum JL. Human-derived anti-oxidized LDL autoantibody blocks uptake of oxidized LDL by macrophages and localizes to atherosclerotic lesions in vivo. Arterioscler Thromb Vasc Biol. 2001;21:1333–1339. doi: 10.1161/hq0801.093587. [DOI] [PubMed] [Google Scholar]
- 22.Zhu J, Quyyumi AA, Rott D, Csako G, Wu H, Halcox J, Epstein SE. Antibodies to human heat-shock protein 60 are associated with the presence and severity of coronary artery disease: evidence for an autoimmune component of atherogenesis. Circulation. 2001;103:1071–1075. doi: 10.1161/01.cir.103.8.1071. [DOI] [PubMed] [Google Scholar]
- 23.Hulthe J, Wiklund O, Hurt-Camejo E, Bondjers G. Antibodies to oxidized LDL in relation to carotid atherosclerosis, cell adhesion molecules, and phospholipase A(2) Arterioscler Thromb Vasc Biol. 2001;21:269–274. doi: 10.1161/01.atv.21.2.269. [DOI] [PubMed] [Google Scholar]
- 24.Fredrikson GN, Hedblad B, Berglund G, Alm R, Ares M, Cercek B, Chyu KY, Shah PK, Nilsson J. Identification of immune responses against aldehyde-modified peptide sequences in apoB associated with cardiovascular disease. Arterioscler Thromb Vasc Biol. 2003;23:872–878. doi: 10.1161/01.ATV.0000067935.02679.B0. [DOI] [PubMed] [Google Scholar]
- 25.Karvonen J, Päivansalo M, Kesäniemi YA, Hörkkö S. Immunoglobulin M type of autoantibodies to oxidized low-density lipoprotein has an inverse relation to carotid artery atherosclerosis. Circulation. 2003;108:2107–2112. doi: 10.1161/01.CIR.0000092891.55157.A7. [DOI] [PubMed] [Google Scholar]
- 26.Chou MY, Fogelstrand L, Hartvigsen K, Hansen LF, Woelkers D, Shaw PX, Choi J, Perkmann T, Bäckhed F, Miller YI, Hörkkö S, Corr M, Witztum JL, Binder CJ. Oxidation-specific epitopes are dominant targets of innate natural antibodies in mice and humans. J Clin Invest. 2009;119:1335–1349. doi: 10.1172/JCI36800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.George J, Afek A, Gilburd B, Blank M, Levy Y, Aron-Maor A, Levkovit H, Shaish A, Goldberg I, Kopolovic J, Harats D, Shoenfeld Y. Induction of early atherosclerosis in LDL-receptor-deficient mice immunized with beta2-glycoprotein I. Circulation. 1998;98:1108–1115. doi: 10.1161/01.cir.98.11.1108. [DOI] [PubMed] [Google Scholar]
- 28.Schiopu A, Bengtsson J, Söderberg I, Janciauskiene S, Lindgren S, Ares MP, Shah PK, Carlsson R, Nilsson J, Fredrikson GN. Recombinant human antibodies against aldehyde-modified apolipoprotein B-100 peptide sequences inhibit atherosclerosis. Circulation. 2004;110:2047–2052. doi: 10.1161/01.CIR.0000143162.56057.B5. [DOI] [PubMed] [Google Scholar]
- 29.Foteinos G, Afzal AR, Mandal k, Jahangiri M, Xu Q. Anti-heat shock protein 60 autoantibodies induce atherosclerosis in apolipoprotein E-deficient mice via endothelial damage. Circulation. 2005;112:1206–1213. doi: 10.1161/CIRCULATIONAHA.105.547414. [DOI] [PubMed] [Google Scholar]
- 30.Nilsson J, Hansson GK. Autoimmunity in atherosclerosis: a protective response losing control? J. Intern. Med. 2008;263:464–478. doi: 10.1111/j.1365-2796.2008.01945.x. [DOI] [PubMed] [Google Scholar]
- 31.Nilsson J, Kovanen PT. Will autoantibodies help to determine severity and progression of atherosclerosis? Curr Opin Lipidol. 2004;15:499–503. doi: 10.1097/00041433-200410000-00002. [DOI] [PubMed] [Google Scholar]
- 32.Giasson BI, Duda JE, Murray IV, Chen Q, Souza JM, Hurtig HI, Ischiropoulos H, Trojanowski JQ, Lee VM. Oxidative damage linked to neurodegeneration by selective alpha-synuclein nitration in synucleinopathy lesions. Science. 2000;290:985–989. doi: 10.1126/science.290.5493.985. [DOI] [PubMed] [Google Scholar]
- 33.Xu S, Ying J, Jiang B, Guo W, Adachi T, Sharov V, Lazar H, Menzoian J, Knyushko TV, Bigelow D, Schöneich C, Cohen RA. Detection of sequence-specific tyrosine nitration of manganese SOD and SERCA in cardiovascular disease and aging. Am. J. Physiol. Heart Circ. Physiol. 2006;290:2220–2227. doi: 10.1152/ajpheart.01293.2005. [DOI] [PubMed] [Google Scholar]
- 34.Reyes JF, Fu Y, Vana L, Kanaan NM, Binder LI. Tyrosine nitration within the proline-rich region of Tau in Alzheimer’s disease. Am J Pathol. 2011;178:2275–85. doi: 10.1016/j.ajpath.2011.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Birnboim HC, Lemay AM, Lam DK, Goldstein R, Webb JR. MHC class II-restricted peptides containing the inflammation-associated marker 3-nitrotyrosine evade central tolerance and elicit a robust cell-mediated immune response. J Immunol. 2003;171:528–532. doi: 10.4049/jimmunol.171.2.528. [DOI] [PubMed] [Google Scholar]
- 36.Herzog J, Maekawa Y, Cirrito TP, Illian BS, Unanue ER. Activated antigen-presenting cells select and present chemically modified peptides recognized by unique CD4 T cells. Proc Natl Acad Sci USA. 2005;102:7928–7933. doi: 10.1073/pnas.0502255102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zheng L, Settle M, Brubaker G, Schmitt D, Hazen SL, Smith JD, Kinter M. Localization of nitration and chlorination sites on apolipoprotein A-I catalyzed by myeloperoxidase in human atheroma and associated oxidative impairment in ABCA1-dependent cholesterol efflux from macrophages. J Biol Chem. 2005;280:38–47. doi: 10.1074/jbc.M407019200. [DOI] [PubMed] [Google Scholar]
- 38.Nagaraj S, Gupta K, Pisarev V, Kinarsky L, Sherman S, Kang L, Herber DL, Schneck J, Gabrilovich DI. Altered recognition of antigen is a mechanism of CD8+ Tcell tolerance in cancer. Nat Med. 2007;13:828–835. doi: 10.1038/nm1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Benner EJ, Banerjee R, Reynolds AD, Sherman S, Pisarev VM, Tsiperson C, Nemachek P, Ciborowski S, Przedborsk S, Mosley RL, Gendelman HE. Specific immune response to nitrated a-synuclein produced a vigorous immuno-inflammatory response in mice that led to the degeneration of dopamine-producing neurons. PLoS ONE. 2008;3:e1376. doi: 10.1371/journal.pone.0001376. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figure 1 Affinity enrichment of nitrated proteins from human atherosclerotic lesions. Proteins extracted from atherosclerotic human lesions (0.84 mg of protein) were incubated with Dyna-beads/protein-A cross-linked to a polyclonal anti-nitrotyrosine antibody in lysis buffer (150 mM NaCl, 1.5 mM MgCl2, 2 mM Hepes, pH 7.4, 10% glycerol, 1% Triton X-100, 1 mM PMSF, 1/100 Sigma protease inhibitor without metal chelators). Enrichment using the same beads cross-linked to a non-specific IgG were used as control. Bound fractions from the beads were eluted using 0.1 M citrate buffer, pH 2.5, and the fractions were separated in 10% SDS-PAGE, transferred to PVDF and probed with a polyclonal anti-nitrotyrosine antibody. (A) Colloidal blue stain and (B) western blot developed with an anti- nitrotyrosine polyclonal antibody. Lanes: 1) Input un-fractionated lesion protein extract; 2) Unbound fraction from the anti- nitrotyrosine antibody beads. 3) Bound fraction from the anti-nitrotyrosine antibody beads; 4) Bound fraction from the non-specific IgG beads. Representative data from 3 different human lesions.
Supplementary figure 2 Affinity enrichment for nitrated proteins from human CAD plasma. Pooled plasma CAD subjects was applied to a agarose column that a polyclonal anti-nitrotyrosine antibody was ligated. After binding and extensive washing the bound proteins were eluted with 0.1 M glycine, pH 2.5, containing 0.15 M NaCl and separated in 10% SDS-PAGE. (A) Colloidal blue stain and (B) western blot developed with an anti-nitrotyrosine polyclonal antibody. Lanes 1, and 4, un-fractionated plasma (input); Lanes 2, and 5, last wash before elution; Lanes 3, and 6, eluted bound proteins.
Supplementary figure 3 Typical binding curve and specificity of the reference anti-nitrotyrosine antibody. Polyclonal antibody 609 was generated using the nitrated tyrosine octapeptide (CGnitroYGGGnitroYG) as antigen as described in detail previously (15). The binding of the antibody to nitrated proteins (◆) was competed by the inclusion of 250 μM 3-nitrotyrosine (◻) or 10 μM nitrated tyrosine octapeptide (⌂). Data reports mean ± standard deviation.
Supplementary Table 1. Proteins that bound to anti-nitrotyrosine antibodies in human atherosclerotic lesions.
Supplementary Table 2. Nitrated proteins and the corresponding modified peptides in human CAD plasma.