Abstract
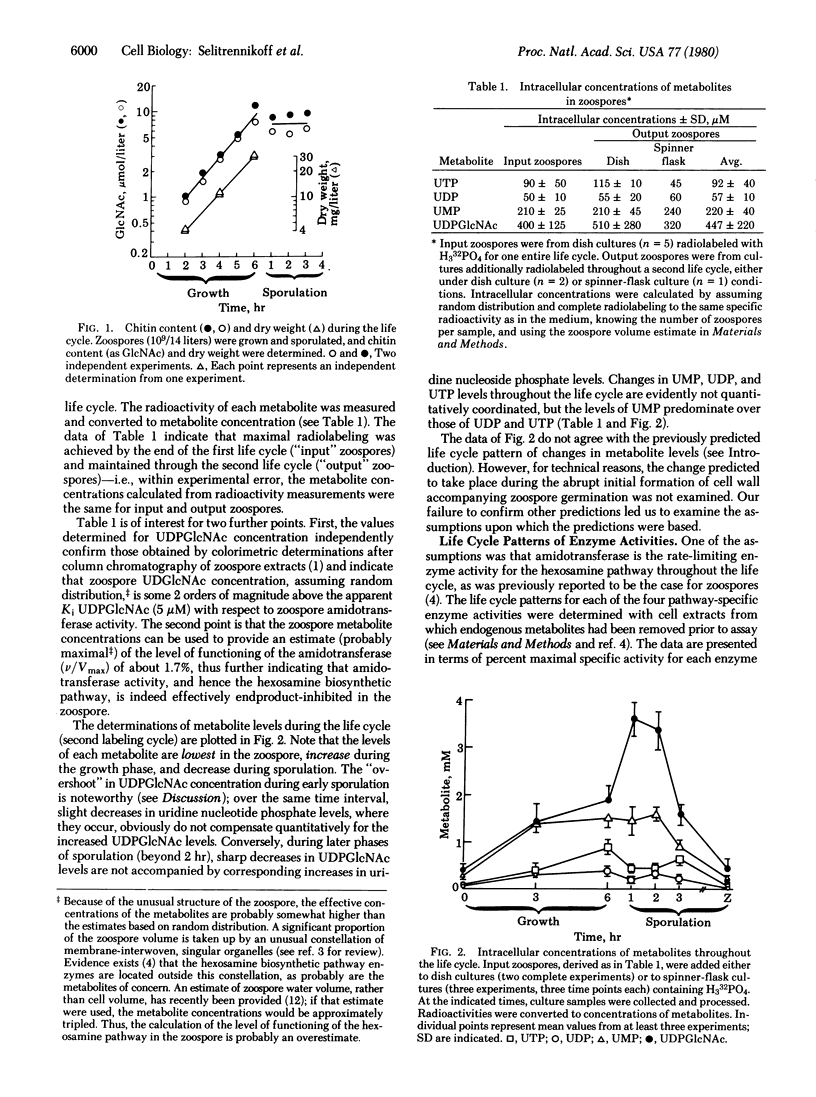
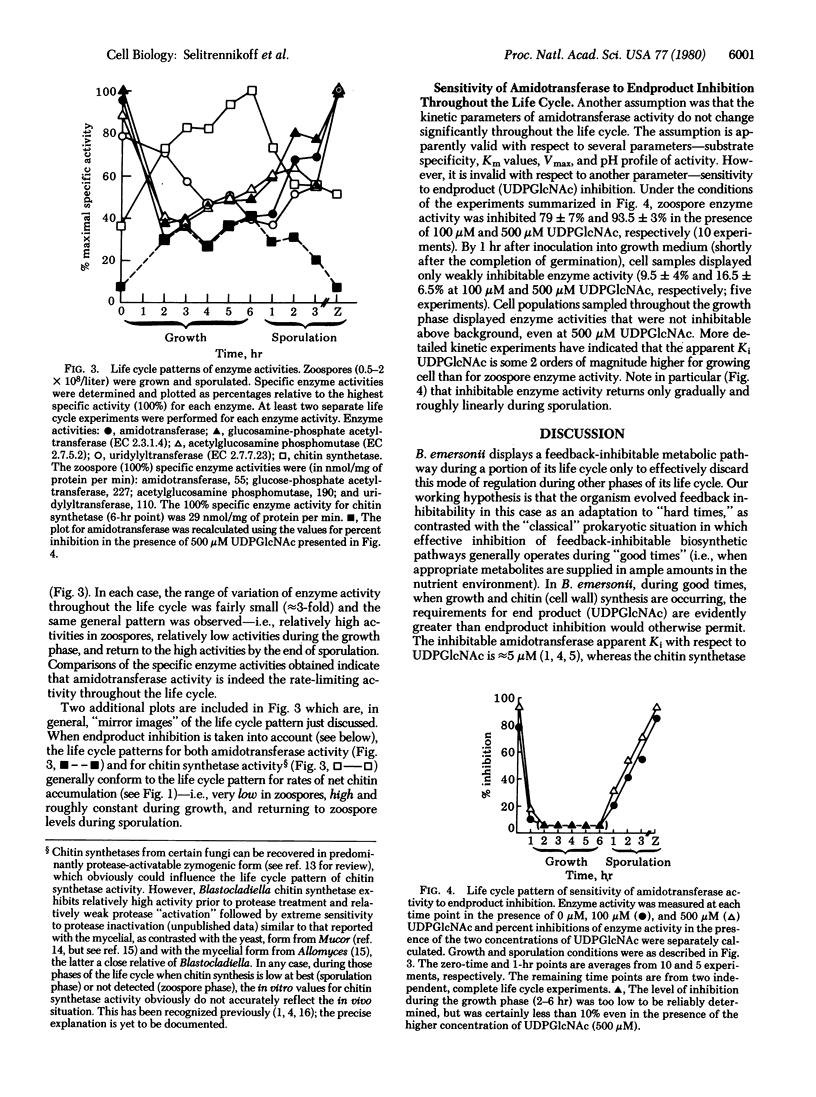
Chitin, a homopolymer of N-acetylglucosamine (GlcNAc), is the major macromolecular constituent of Blastocladiella emersonii cell walls. Zoospores do not possess a wall nor do they contain sufficient total hexosamine to account for the chitin content of the wall abruptly formed during germination. UDPGlcNAc, both the endproduct of hexosamine biosynthesis and the substrate for chitin synthesis, is present in zoospores in sufficient concentration to inhibit the first hexosamine pathway-specific enzyme activity. Net chitin accumulates in register with dry weight during exponential growth, but does not accumulate appreciably during the succeeding sporulation phase. Predicted relationships among net rates of chitin synthesis, UDPGlcNAc concentrations, and UDP plus UTP concentrations throughout the life cycle are explored, as are the assumptions upon which the predictions were based. We find that the sensitivity of the first hexosamine pathway-specific enzyme to endproduct inhibition is not constant throughout the life cycle; sensitivity is very high in the zoospore phase, decreases dramatically during germination, remains very low through the growth phase, and increases gradually to the zoospore level during sporulation. The organism appears to have evolved endproduct regulation in this case as an adaptation to “hard-times” phases of the life cycle—i.e., as a safeguard against overproduction of end product (UDPGlcNAc) when its utilization in cell wall (specifically chitin) synthesis is curtailed. Conversely, the organism effectively discards this mode of regulation during “good times,” when the demands for end product are evidently greater than endproduct inhibition would otherwise permit.
Keywords: enzyme regulation, feedback inhibition, metabolic regulation
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Camargo E. P., Dietrich C. P., Sonneborn D., Strominger J. L. Biosynthesis of chitin in spores and growing cells of Blastocladiella emersonii. J Biol Chem. 1967 Jul 10;242(13):3121–3128. [PubMed] [Google Scholar]
- Endo A., Kakiki K., Misato T. Feedback inhibition of L-glutamine D-fructose 6-phosphate amidotransferase by uridine diphosphate N-acetylglucosamine in Neurospora crassa. J Bacteriol. 1970 Sep;103(3):588–594. doi: 10.1128/jb.103.3.588-594.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farkas V. Biosynthesis of cell walls of fungi. Microbiol Rev. 1979 Jun;43(2):117–144. doi: 10.1128/mr.43.2.117-144.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornfeld R. Studies on L-glutamine D-fructose 6-phosphate amidotransferase. I. Feedback inhibition by uridine diphosphate-N-acetylglucosamine. J Biol Chem. 1967 Jul 10;242(13):3135–3141. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lodi W. R., Sonneborn D. R. Protein degradation and protease activity during the late cycle of Blastocladiella emersonii. J Bacteriol. 1974 Mar;117(3):1035–1042. doi: 10.1128/jb.117.3.1035-1042.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovett J. S. Growth and differentiation of the water mold Blastocladiella emersonii: cytodifferentiation and the role of ribonucleic acid and protein synthesis. Bacteriol Rev. 1975 Dec;39(4):345–404. doi: 10.1128/br.39.4.345-404.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyagi T., Tsuiki S. Effect of phosphoglucose isomerase and glucose 6-phosphate on UDP-N-acetylglucosamine inhibition of L-glutamine-D-fructose 6-phosphate aminotransferase. Biochim Biophys Acta. 1971 Oct;250(1):51–62. doi: 10.1016/0005-2744(71)90119-7. [DOI] [PubMed] [Google Scholar]
- Myers R. B., Cantino E. C. The gamma particle. A study of cell-organelle interactions in the development of the water mold Blastocladiella emersonii. Monogr Dev Biol. 1974;8:1–117. [PubMed] [Google Scholar]
- REISSIG J. L., STORMINGER J. L., LELOIR L. F. A modified colorimetric method for the estimation of N-acetylamino sugars. J Biol Chem. 1955 Dec;217(2):959–966. [PubMed] [Google Scholar]
- Ruiz-Herrera J., Bartnicki-Garcia S. Proteolytic activation and inactivation of chitin synthetase from Mucor rouxii. J Gen Microbiol. 1976 Dec;97(2):241–249. doi: 10.1099/00221287-97-2-241. [DOI] [PubMed] [Google Scholar]
- Selitrennikoff C. P., Allin D., Sonneborn D. R. Chitin biosynthesis during Blastocladiella zoospore germination: evidence that the hexosamine biosynthetic pathway is post-translationally activated during cell differentiation. Proc Natl Acad Sci U S A. 1976 Feb;73(2):534–538. doi: 10.1073/pnas.73.2.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selitrennikoff C. P., Sonneborn D. R. Alkaline phosphatase of Blastocladiella emersonii: partial purification and characterization. J Bacteriol. 1977 Apr;130(1):249–256. doi: 10.1128/jb.130.1.249-256.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selitrennikoff C. P., Sonneborn D. R. Post-translational control of de novo cell wall formation during Blastocladiella emersonii zoospore germination: feedback regulation of hexosamine biosynthesis. Dev Biol. 1976 Nov;54(1):37–51. doi: 10.1016/0012-1606(76)90284-0. [DOI] [PubMed] [Google Scholar]
- Van Brunt J., Harold F. M. Ionic control of germination of Blastocladiella emersonii zoospores. J Bacteriol. 1980 Feb;141(2):735–744. doi: 10.1128/jb.141.2.735-744.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vessal M., Hassid W. Z. Partial Purification and Properties of l-Glutamine d-Fructose 6-Phosphate Amidotransferase from Phaseolus aureus. Plant Physiol. 1972 Jun;49(6):977–981. doi: 10.1104/pp.49.6.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterburn P. J., Phelps C. F. Binding of substrates and modifiers to glucosamine synthetase. Biochem J. 1971 Feb;121(4):721–730. doi: 10.1042/bj1210721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterburn P. J., Phelps C. F. Purification and some kinetic properties of rat liver glucosamine synthetase. Biochem J. 1971 Feb;121(4):701–709. doi: 10.1042/bj1210701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterburn P. J., Phelps C. F. Studies on the control of hexosamine biosynthesis by glucosamine synthetase. Biochem J. 1971 Feb;121(4):711–720. doi: 10.1042/bj1210711. [DOI] [PMC free article] [PubMed] [Google Scholar]



