Abstract
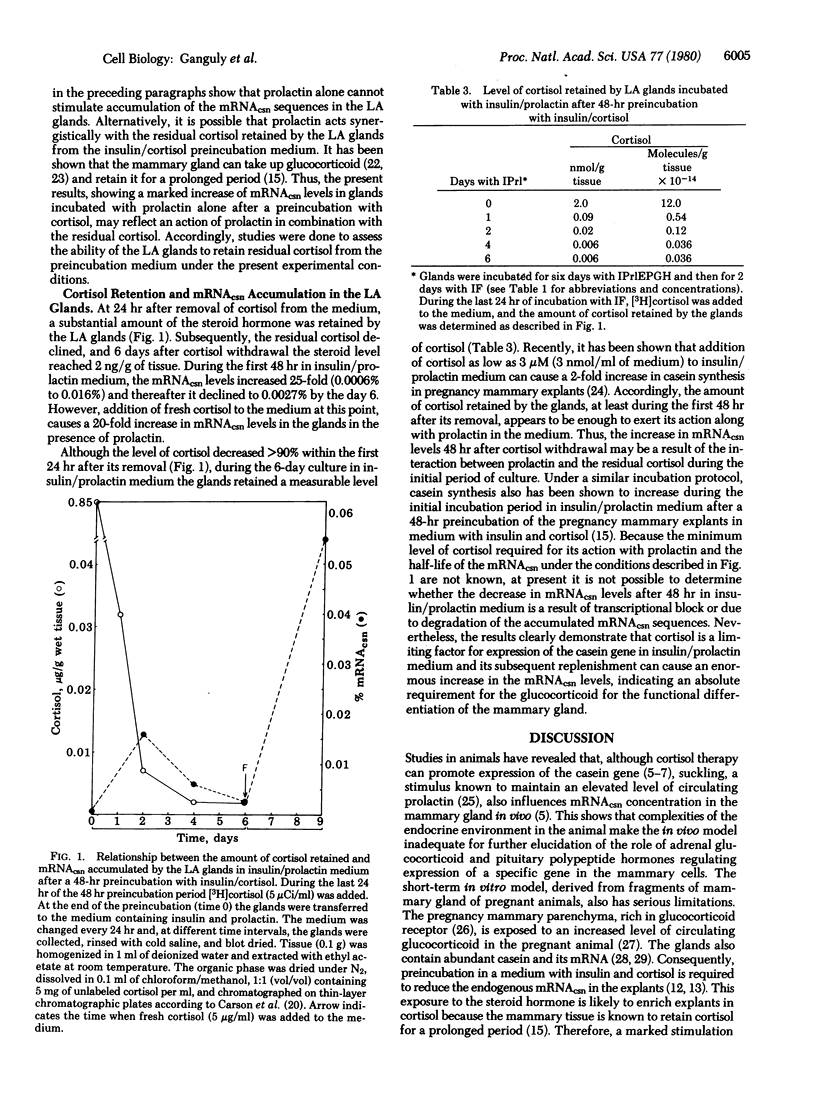
Second thoracic mammary glands of immature BALB/c female mice were stimulated to pregnancy-like lobuloalveolar (LA) development aftr 6 days of incubation in a corticosteroid-free step I culture medium containing insulin, prolactin, estradiol, progesterone, and growth hormone. A low basal level (0.0009%) of casein mRNA (mRNAcsn) sequences was detectable in the LA glands by a specific cDNA probe. Subsequent incubation of the LA glands for 3 days in medium containing insulin and prolactin or insulin and cortisol failed to elicit mRNAcsn above the basal level, indicating that neither prolactin nor cortisol alone can support casein gene expression. However, an increase in mRNAcsn levels was observed when the 3-day incubation with insulin and cortisol or insulin and prolactin was followed by 3 days of culture in presence of insulin, prolactin, and cortisol. When a 3-day incubation with insulin and prolactin was followed by 3 days in insulin and cortisol medium, mRNAcsn levels in the gland remained similar to the basal level. However, a 20-fold increase in the mRNAcsn levels ensued when the LA glands were sequentially incubated for 3 days in insulin and cortisol and then for another 3 days in insulin and prolactin medium. After a preincubation in insulin and cortisol medium, the LA glands retained residual cortisol during subsequent incubation in insulin and prolactin medium, and the mRNAcsn levels in these glands were related to the level of residual cortisol present. When mRNAcsn and the residual cortisol level reached a minimum, addition of fresh cortisol to the medium caused a 20-fold increase in the mRNAcsn levels. This indicates that cortisol is a limiting factor in insulin and prolactin medium and its presence is absolutely required for casein gene expression.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banerjee M. R., Terry P. M., Sakai S., Lin F. K., Ganguly R. Hormonal regulation of casein messenger RNA (mRNA). In Vitro. 1978 Jan;14(1):128–139. doi: 10.1007/BF02618179. [DOI] [PubMed] [Google Scholar]
- Bolander F. F., Jr, Nicholas K. R., Topper Y. J. Retention of glucocorticoid by isolated mammary tissue may complicate interpretation of results from in vitro experiments. Biochem Biophys Res Commun. 1979 Nov 14;91(1):247–252. doi: 10.1016/0006-291x(79)90610-7. [DOI] [PubMed] [Google Scholar]
- Carson G. D., Bolla J. D., Challis J. R. The availability of cortisol in amniotic fluid to the fetus and chorionic and amniotic membranes. Endocrinology. 1979 Apr;104(4):1053–1058. doi: 10.1210/endo-104-4-1053. [DOI] [PubMed] [Google Scholar]
- Gala R. R., Westphal U. Corticosteroid-binding activity in serum of mouse, rabbit and guinea pig during pregnancy and lactation: possible involvement in the initiation of lactation. Acta Endocrinol (Copenh) 1967 May;55(1):47–61. doi: 10.1530/acta.0.0550047. [DOI] [PubMed] [Google Scholar]
- Ganguly R., Mehta N. M., Ganguly N., Banerjee M. R. Glucocorticoid modulation of casein gene transcription in mouse mammary gland. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6466–6470. doi: 10.1073/pnas.76.12.6466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyette W. A., Matusik R. J., Rosen J. M. Prolactin-mediated transcriptional and post-transcriptional control of casein gene expression. Cell. 1979 Aug;17(4):1013–1023. doi: 10.1016/0092-8674(79)90340-4. [DOI] [PubMed] [Google Scholar]
- Ichinose R. R., Nandi S. Influence of hormones on lobulo-alveolar differentiation of mouse mammary glands in vitro. J Endocrinol. 1966 Aug;35(4):331–340. doi: 10.1677/joe.0.0350331. [DOI] [PubMed] [Google Scholar]
- Juergens W. G., Stockdale F. E., Topper Y. J., Elias J. J. Hormone-dependent differentiation of mammary gland in vitro. Proc Natl Acad Sci U S A. 1965 Aug;54(2):629–634. doi: 10.1073/pnas.54.2.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LYONS W. R., LI C. H., JOHNSON R. E. The hormonal control of mammary growth and lactation. Recent Prog Horm Res. 1958;14:219–254. [PubMed] [Google Scholar]
- Matusik R. J., Rosen J. M. Prolactin induction of casein mRNA in organ culture. A model system for studying peptide hormone regulation of gene expression. J Biol Chem. 1978 Apr 10;253(7):2343–2347. [PubMed] [Google Scholar]
- Mehta N. M., Ganguly N., Ganguly R., Banerjee M. R. Hormonal modulation of the casein gene expression in a mammogenesis-lactogenesis culture model of the whole mammary gland of the mouse. J Biol Chem. 1980 May 25;255(10):4430–4434. [PubMed] [Google Scholar]
- Mena F., Enjalbert A., Carbonell L., Priam M., Kordan C. Effect of suckling on plasma prolactin and hypothalamic monoamine levels in the rat. Endocrinology. 1976 Aug;99(2):445–451. doi: 10.1210/endo-99-2-445. [DOI] [PubMed] [Google Scholar]
- Normann T. C. Neurosecretion by exocytosis. Int Rev Cytol. 1976;46:1–77. [PubMed] [Google Scholar]
- Ono M., Oka T. The differential actions of cortisol on the accumulation of alpha-lactalbumin and casein in midpregnant mouse mammary gland in culture. Cell. 1980 Feb;19(2):473–480. doi: 10.1016/0092-8674(80)90522-x. [DOI] [PubMed] [Google Scholar]
- Paterson J. Y., Linzell J. L. The secretion of cortisol and its mammary uptake in the goat. J Endocrinol. 1971 Jul;50(3):493–499. doi: 10.1677/joe.0.0500493. [DOI] [PubMed] [Google Scholar]
- Peters A. R., Mepham T. B. Effects of exogenous cortisol on the uptake of corticosteroid by the isolated perfused guinea-pig mammary gland. J Endocrinol. 1979 Mar;80(3):357–364. doi: 10.1677/joe.0.0800357. [DOI] [PubMed] [Google Scholar]
- RIVERA E. M., BERN H. A. Influence of insulin on maintenance and secretory stimulation of mouse mammary tissues by hormones in organ-culture. Endocrinology. 1961 Aug;69:340–353. doi: 10.1210/endo-69-2-340. [DOI] [PubMed] [Google Scholar]
- Rillema J. A., Anderson L. D. Rapid interaction of prolactin with mouse mammary gland explants. Mol Cell Endocrinol. 1976 Feb;4(3):131–137. doi: 10.1016/0303-7207(76)90032-0. [DOI] [PubMed] [Google Scholar]
- Rosen J. M., O'Neal D. L., McHugh J. E., Comstock J. P. Progesterone-mediated inhibition of casein mRNA and polysomal casein synthesis in the rat mammary gland during pregnancy. Biochemistry. 1978 Jan 24;17(2):290–297. doi: 10.1021/bi00595a016. [DOI] [PubMed] [Google Scholar]
- Strobl J. S., Lippman M. E. Prolonged retention of estradiol by human breast cancer cells in tissue culture. Cancer Res. 1979 Sep;39(9):3319–3327. [PubMed] [Google Scholar]
- Terry P. M., Ball E. M., Ganguly R., Banerjee M. R. An indirect radioimmunoassay for mouse casein using 125I-labeled antigen. J Immunol Methods. 1975 Dec;9(2):123–134. doi: 10.1016/0022-1759(75)90102-7. [DOI] [PubMed] [Google Scholar]
- Terry P. M., Banerjee M. R., Lui R. M. Hormone-inducible casein messenger RNA in a serum-free organ culture of whole mammary gland. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2441–2445. doi: 10.1073/pnas.74.6.2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry P. M., Lin F. K., Banerjee M. R. Responses of mouse mammary gland casein mRNA to corticosteroid action and suckling. Mol Cell Endocrinol. 1977 Dec;9(2):169–182. doi: 10.1016/0303-7207(77)90118-6. [DOI] [PubMed] [Google Scholar]
- Turkington R. W., Majumder G. C., Kadoama N., MacIndoe J. H., Frantz W. L. Hormonal regulation of gene expression in mammary cells. Recent Prog Horm Res. 1973;29:417–455. doi: 10.1016/b978-0-12-571129-6.50015-4. [DOI] [PubMed] [Google Scholar]



