Abstract
Perfluoroalkyl acid carboxylates and sulfonates (PFAAs) have many consumer and industrial applications. Developmental toxicity studies in animals have raised concern about potential reproductive/developmental effects of perfluorooctanoic acid (PFOA) and perfluorooctane sulfonate (PFOS); however, in humans conflicting results have been reported for associations between maternal PFAA levels and these outcomes. Risk assessments and interpretation of available human data during gestation and lactation are hindered due to lack of a framework for understanding and estimating maternal, fetal, and neonatal pharmacokinetics (PK). Physiologically-based pharmacokinetic (PBPK) models were developed for PFOA & PFOS for the gestation and lactation life stages in humans to understand how the physiological changes associated with development affect pharmacokinetics of these compounds in the mother, fetus, and infant. These models were derived from PBPK models for PFOA/PFOS that were previously developed for adult humans and rats during gestation and lactation and from existing human pregnancy and lactation models developed for other chemicals. The models simulated PFOA and PFOS concentrations in fetal, infant, and maternal plasma and milk, were compared to available data in humans, and also used to estimate maternal exposure. The models reported here identified several research needs, which include: 1) the identification of transporters involved in renal resorption to explain the multi-year half-lives of these compounds in humans, 2) factors affecting clearance of PFOA/PFOS during gestation and lactation, and 3) data to estimate clearance of PFOA/PFOS in infants. These models may help address concerns regarding possible adverse health effects due to PFOA/PFOS exposure in the fetus and infant and may be useful in comparing pharmacokinetics across life stages.
Keywords: PFOA, PFOS, perfluoroalkyls, gestation, lactation, physiologically based pharmacokinetic model, PBPK
Introduction
Perfluoroalkyl acids (PFAA) are fully fluorinated man-made carboxylates and sulfonates. The most prevalent PFAA are perfluorooctanoic acid (PFOA) and perfluorooctane sulfonate (PFOS). The unique chemical properties imparted by the C–F bond allows for their use in a variety of both industrial and consumer applications, such as surfactants, paper and packaging treatments for grease and stain resistance, and as processing aids in the manufacture of fluoropolymers (Olsen et al. 2008; Raymer et al. 2012). Compounds in this class have been produced since the 1950s, and the use, distribution, manufacture, and disposal of these and other chemicals that break down to form PFOA and PFOS have resulted in the widespread exposure of both environmental media and humans (Lindstrom et al. 2011). Due to the stability of the C–F bond, these compounds are highly resistant to biodegradation and metabolism and thus are persistent (Lau 2009). The average serum half-life in humans is estimated at 3.8 years for PFOA and 5.4 years for PFOS (Olsen et al. 2007). Biomonitoring studies detected PFOA and PFOS in serum of both occupationally and non-occupationally exposed humans, including pregnant and lactating women, fetal cord blood, and infants (Olsen et al. 2009; 2007). The long half-lives in humans are of particular concern because this indicates that these compounds are bioaccumulative, which may result in higher body burdens, allowing for a greater potential for adverse health effects. Pathways for human exposure to PFAA have not been well-characterized, although it was suggested that dietary exposure is the main contributing pathway in adults (Fromme et al. 2009; Vestergren and Cousins 2009). Other exposure sources include drinking water, dust, and household/consumer products treated with PFOA and PFOS (Trudel et al. 2008; Fromme et al. 2009). Communities with high levels of PFOA in their drinking water have also been found to have serum PFOA levels significantly higher than those reported for the general population (Emmett et al. 2006; Holzer et al. 2008). Exposure to PFOA and PFOS can also occur as the result of the breakdown of other larger fluoropolymers (Vestergren et al. 2008; Paul et al. 2009; Prevedouros et al. 2006).
Reviews on the reproductive and developmental outcomes resulting from exposure to PFOA and PFOS in experimental animals have been published (Lau et al. 2007; 2004). Although the toxicity observed in the animal studies occurred at much higher doses and higher blood levels than the levels observed in human serum from biomonitoring studies, maternal exposure to PFOA and PFOS has resulted in dose-dependent developmental effects in rats, mice, and rabbits. These effects include reduced birth weight, reduced postnatal survival and growth of the offspring, and delayed lung maturation (Case et al. 2001; Grasty et al. 2005; Lau et al. 2003; Luebker et al. 2005a; 2005b; Thibodeaux et al. 2003). Maternal effects include reduced food intake and reduced weight gain (Thibodeaux et al. 2003). Postnatal survival is similar for PFOS exposure in rats and mice; however, there are marked differences in PFOA effects, which may be due to species and gender differences in pharmacokinetics of PFOA. Most notably, the female rat has a short serum half-life for PFOA (approximately 2-4 hours), while the half-lives observed in male rats and other species (e.g., mouse, monkey, and humans) are several days to years (Seacat et al. 2002; Kudo et al. 2002; Olsen et al. 2007; Lou et al. 2009). The variation in half-life is most likely due to differences in expression of organic anion transporters in the proximal tubule (Kudo et al. 2002; Weaver et al. 2009), which are thought to be responsible for renal resorption of PFOA and thus the long half-lives observed across species (Andersen et al. 2006; Loccisano et al. 2011; 2012b). Several renal transporters were found to transport PFOA (Katakura et al. 2007; Kato et al. 2002; Kudo et al. 2002; Nakagawa et al. 2007; Weaver et al. 2009; Yang et al. 2009), and although no study examined PFOS transport explicitly, PFOS is also ionized at physiological pH and is most likely subject to uptake by the same transporters. Elimination kinetics of PFOA and PFOS may also be influenced by branching on the carbon chain (Beesoon et al. 2011; Benskin et al. 2009) or by binding to plasma proteins (PFOA and PFOS are >97% bound to plasma albumin in the rat, monkey, and human (Han et al. 2003; Kerstner-Wood et al. 2003)). Animal studies have shown that these chemicals are well-absorbed orally, poorly eliminated, not metabolized, and that they are distributed mainly to liver, plasma, and kidney (Johnson et al. 1984; Kuslikis et al. 1992; Ophaug and Singer 1980; Vanden Heuvel et al. 1991). The pharmacokinetics of PFOA and PFOS have been reviewed by several authors (Han et al. 2012; Lau et al. 2004; Andersen et al. 2008; Post et al. 2012).
Due to (1) widespread use, (2) bioaccumulative properties of PFAAs, (3) developmental effects observed in animals, and because (4) PFOA and PFOS have been found in pregnant women, cord blood, breast milk, and infants, epidemiologists began to focus on reproductive and developmental outcomes in humans in relation to exposure to PFOA and PFOS. Measurement of PFOA and PFOS in cord blood samples confirmed placental transfer, and measurement in breast milk shows exposure of the infant via this route is also possible. Epidemiology studies reported associations between PFAA exposure and various reproductive and developmental outcomes (Olsen et al. 2009), including time-to-pregnancy and birth weight, though the findings are inconsistent. Many of these outcomes may also be influenced by or correlated with maternal factors including body mass index (BMI), maternal size, nutrition, volume expansion, glomerular filtration rate, preexisting maternal conditions, and other physiological variables.
Pharmacokinetic data for PFOA and PFOS in pregnant and lactating women, the fetus, and infant are limited. However, the use of a physiologically based pharmacokinetic (PBPK) model may aid in understanding the determinants of human pharmacokinetics; the model might also provide an improved understanding of how the physiological changes associated with development affect tissue distributions of these compounds in the mother, fetus, and infant, thus improving the scientific basis of age- and life stage-specific risk assessment for PFAA. In order to estimate maternal exposure from blood or milk concentrations of PFOA and PFOS and help define the relationship between maternal exposure, internal tissue concentrations in the mother, fetus, and infant, PBPK models were developed for PFOA and PFOS for both the gestation and lactation life stages in the human. The gestation and lactation models also allow for examining integrative exposure and internal dose during both the in utero and postnatal periods. Not only can the model be used to predict the internal dose in the fetus or neonate resulting from maternal exposure, but the model can also be used to examine whether the reproductive associations observed in epidemiologic studies might be able to be predicted on the basis of pharmacokinetics. The latter application will be presented in a subsequent manuscript.
Methods
Previously developed adult rat, monkey, and human PBPK models for both PFOA and PFOS have been described (Loccisano et al. 2011; 2012b). In addition, pregnancy and lactation models for PFOA and PFOS were developed and described for the rat (Loccisano et al. 2012a). In this study, the adult human model was extended to the gestation and lactation life stages in order to provide estimates of maternal, fetal, and infant exposure to PFOA and PFOS. These have been documented elsewhere (Loccisano et al. 2011; Loccisano et al. 2012b; Loccisano et al. 2012a); only the development of the human gestation and lactation models is described here.
The pregnancy and lactation models retained all the features used to describe PFOA and PFOS kinetics in the adult human, including a constant free fraction of chemical in plasma and renal resorption in the filtrate compartment. The pregnancy and lactation models also retained all the tissue compartments of the adult human, which included plasma, liver, kidneys, filtrate, fat, skin, and a lumped compartment for the remaining body tissues. The descriptions for pregnancy and lactation in humans were based on previously published pregnancy and lactation models (Gentry et al. 2003; Clewell et al. 1999) and physiological data on the changes that occur during these life stages (Salas et al. 2006; Sims and Krantz 1958; Beall et al. 2007; Dewey et al. 1991; Dunlop 1981; Duvekot et al. 1995; Wosje and Kalkwarf 2004). The models were coded in the acslX program (version 3.0; AEgis Technologies Group, Huntsville, AL). The model code can be found in the Supporting Information.
Model Structures
The human gestation and lactation models simulated environmental exposure to PFAA during pregnancy and lactation for the mother, fetus, and infant. The basic structures (Figures 1 and 2) are based on the rat gestation and lactation models for PFAA. The structural changes made to the adult human model were the addition of mammary tissue, placenta, and fetal compartments, and a milk compartment for lactation. The physiological and PFAA-specific parameters are adapted from the adult human model and modified for the gestation and lactation life stages as outlined below. Only the mother was assumed to have direct exposure to PFAA, and only the free fraction of chemical in plasma is assumed to be available for uptake into tissues. Transport into tissues was flow-limited. Clearance of PFAA in the mother occurs by urinary elimination from the filtrate compartment in both models; clearance in the infant is described with a generic first-order rate constant from the central compartment.
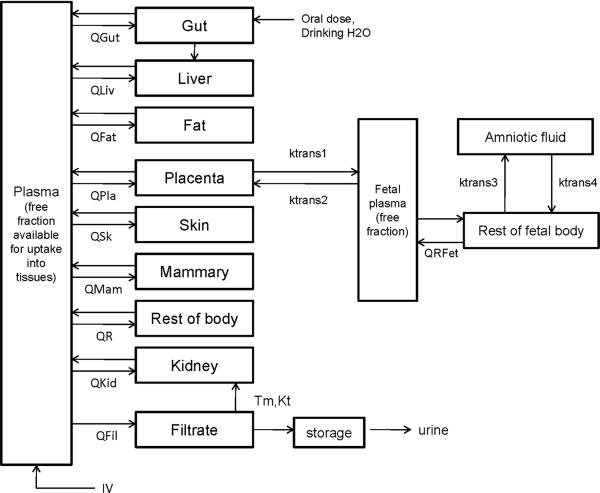
Figure 1.
PBPK model structure for simulating PFOA and PFOS exposure during pregnancy in humans (maternal, left; fetal, right). Fetal plasma circulation is separate from the maternal circulation. Only the mother is exposed directly; chemical is transferred to the fetus through the placenta. Placental transfer is described by a simple diffusion process (ktrans1 and ktrans2). Q's represent blood flows to and from the tissues; only the free fraction of chemical in plasma is available for uptake into tissues. Because contributions to various exposure sources are unknown, the IV route is used as a generic input to plasma; maternal exposure can also be through drinking water.
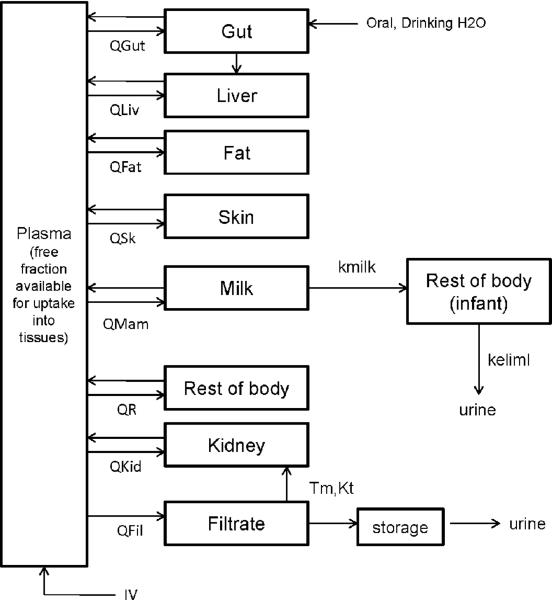
Figure 2.
PBPK model structure for simulating PFOA/PFOS exposure during lactation in humans (maternal, left; infant, right). Maternal exposure is by direct intake; the infant exposure is through milk. No data were available for PFOA or PFOS partitioning into mammary tissue; chemical in plasma was assumed to be in direct contact with the milk compartment. Transfer from the plasma to the milk compartment was assumed to be flow-limited. Limited data was available for concentrations of PFAAs in the infant; thus, the infant was treated as a single compartment. Only the free fraction of chemical in plasma was available for uptake into tissues or milk.
Fetal exposure to PFAA is through placental transfer, which is described as a bidirectional transfer process (from mother to fetus and fetus to mother) between free chemical in the placenta and fetal plasma. Placental transfer was described as a simple diffusion process (ktrans1C and ktrans2C; Table 3). However, just because simple diffusion appears to be sufficient, the possibility that placental transport is also facilitated by an active transport process cannot be ruled out because PFOA and PFOS will be mostly ionized at physiological pH. For example, organic anion transporter 4 (OAT4) is expressed in human kidney proximal tubule cells (apical side) and plays a role in resorption of organic anions (Nakagawa et al. 2007; 2009). This transporter is also expressed in human placenta (Cha et al. 2000) and may also play a role in transport of PFOA and PFOS there. However, the possible roles that these transporters play in placental transport of PFOA and PFOS remains to be elucidated; thus, a description using simple diffusion was used in the current model. Data in rats showed that PFAAs are present in amniotic fluid (Hinderliter et al. 2005); thus, an amniotic fluid compartment was included in the rat models and also in the human models presented here. Transfer rates between the amniotic fluid compartment and the fetal body are described as simple diffusion (ktrans3C and ktrans4C; Table 3). Transport of chemical from fetal plasma into the rest of fetal body compartment was also flow-limited.
Table 3.
Chemical parameters for PBPK models for PFOA and PFOS in pregnant and lactating women, fetus, and infant.
| PFOA | PFOS | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Parameter | mother | fetus | infant | mother | fetus | infant |
| Free fraction of chemical in plasma (Free) | 0.02 | 0.02 | 0.02 | 0.025 | 0.025 | 0.025 |
| Volume of distribution (Vdc; infant only, scaled to BWKid) | - | - | 0.17 | - | 0.22 | |
| Renal resorption Parameters | ||||||
| transporter maximum, Tmc (ug/h/kg0.75) | 10 | - | - | 3.5 | - | - |
| affinity constant, Kt (ug/L) | 0.055 | - | - | 0.023 | - | - |
| Partition Coefficients | ||||||
| Gut:plasma (Pgut) | 0.05 | - | - | 0.57 | - | - |
| Liver:plasma (Pliv) | 2.2 | - | - | 3.72 | - | - |
| Kidney: plasma (Pkid) | 1.05 | - | - | 0.8 | - | - |
| Fat: plasma (Pfat) | 0.04 | - | - | 0.13 | - | - |
| Mammary Tissue: plasma (Pmam; gestation only) | 0.13 | - | - | 0.16 | - | - |
| Rest of body tissues: plasma (Ptis) | 0.12 | 0.12 | - | 0.20 | 0.20 | - |
| Placenta:Plasma (Ppla) | 0.28 | - | - | 0.41 | - | - |
| Skin: plasma (PSk) | 0.1 | - | - | 0.29 | - | - |
| Mammary tissue:plasma (PMam; gestation only) | 0.13 | - | 0.16 | - | - | |
| Milk: plasma (PMilk; lactation only) | 0.038 | - | - | 0.0122 | - | - |
| Uptake/Elimination Rate Constants | ||||||
| urinary elimination rate constant, kurinec (/h/kg-0.25); keliml for infant (/h) | 0.1 | - | 0.005 | 0.005 | - | 0.001 |
| Placental transfer (gestation only) | ||||||
| mother to fetus, ktrans1C (L/h/kg0.75) | 0.46 | - | - | 0.46 | - | - |
| fetus to mother, ktrans2C (L/h/kg0.75) | - | 0.46 | - | - | 1.01 | - |
| Amniotic fluid transfer (gestation only) | ||||||
| fetus to fluid, ktrans3C (L/h/kg0.75) | - | 0.008 | - | - | 0.006 | - |
| fluid to fetus, ktrans4C (L/h/kg0.75) | - | 0.001 | - | - | 0.001 | - |
In the lactation model, no data were available for PFAA partitioning into mammary tissue, so chemical in plasma was placed in direct contact with the milk compartment, and transfer from the plasma to the milk compartment was flow-limited. Only the free fraction of chemical in plasma was available for transfer into milk. The infant was exposed only through milk, and breastfeeding lasted for 6 months postpartum.
Gestation model–physiology
The modifications for pregnancy to the adult human model were based on previously published human pregnancy models for methylmercury (Clewell et al. 1999), isopropanol (Gentry et al. 2002), and various environmental toxicants (Gentry et al. 2003) well as other physiological data for changes that occur during pregnancy (Beall et al. 2007; Dunlop 1981; Duvekot et al. 1995; Salas et al. 2006; Sims and Krantz 1958). Equations were added to the adult human PFAA model to account for changing volumes of fat, mammary tissue, and placenta, expanding plasma volume and the growing fetus. The physiological parameters are given in Table 1; all of the equations used can be found in the model code in the Supporting Information. Initial fractional tissue volumes for fat and mammary tissue were from Gentry et al.(2003). The equations describing the expanding fat and mammary tissue, placental growth, and fetal growth were adapted from Gentry et al. (2003). Plasma volume expansion during pregnancy was based on Salas et al.(2006). Volumes of liver, kidney, filtrate, and gut remained constant fractions of the prepregnancy (non-pregnant) maternal body weight (BWinit); fractional volumes of these tissues for nonpregnant women was taken from Brown et al. (1997). The filtrate compartment, as in the adult human model, was set at 10% of the kidney volume (the model is not sensitive to this value). Increasing maternal body weight (BW) was calculated as the sum of the changing tissue volumes and the prepregnancy (non-pregnant) body weight. The volume of remaining body tissues (VR) was calculated by subtracting the sum of all other tissues from the total maternal BW; this takes into account the increasing BW and other changing tissue volumes.
Table 1.
Physiological parameters for PBPK models for PFOA and PFOS in pregnant woman and fetus.
| Parameter | Mother | Fetus | source |
|---|---|---|---|
| Maternal body weight (BW; kg) and fetal weight (VFet; kg) | 56.9 – 70 (initial); 65.3 – 79.6 (increasing) | 0 – 3.8* | Clewell, et al 1999; Gentry, et al 2003 |
| Tissue volumes (fraction of BW or VFet) | |||
| Gut, VGutC | 0.0171 | - | Brown, et al 1997 |
| Liver, VLivC | 0.026 | - | Brown, et al 1997 |
| Kidney, VKidC | 0.004 | - | Brown, et al 1997 |
| Filtrate, VFilC | 0.0004 | - | 10% of kidney volume |
| Fat, VFatC (initial volume) | 0.214 | - | Brown, et al 1997 |
| Mammary tissue, VMamC (initial volume) | 0.0062 | - | Gentry, et al 2002 |
| Fetal plasma, VPIasFC | - | 0.0428 | Davies & Morris, 1993 |
| Tissue volume (L, age-dependent) | |||
| Fat, VFat | 14.0 – 19.5* | - | Gentry, et al 2002 |
| Mammary tissue, VMam | 0.41 – 0.79* | - | Gentry, et al 2002 |
| Placenta, VPIa | 0 – 0.63* | - | Gentry, et al 2002 |
| Plasma, VPIas | 2.7 – 3.2* | - | Salas, et al 2006 |
| Remaining tissues, VR | 34.5 – 31.9* | difference between BW and sum of other tissues | |
| Amniotic fluid (L, one fetus) | - | 0 – 0.51* | Beall, et al 2007 |
| Blood flows (fraction of QC) | |||
| Cardiac output (QCC, L/h/kg0.75; initial) | 20.0 | - | Clewell, et al 1999 |
| fetal cardiac output (L/h/kg blood) | - | 54.0 | Clewell, et al 1999 |
| Gut, QGutC | 0.181 | - | Brown, et al 1997 |
| Liver, QLivC | 0.25 | - | Brown, et al 1997 |
| Skin, QSkC | 0.058 | - | Brown, et al 1997 |
| hematocrit, Htc | 0.38 – 0.367* | 0.5 | Milman, et al 1999 (maternal); Sisson, et al 1959 (value for newborn) |
| Blood flows (L/h, age-dependent) | |||
| Cardiac output, increasing, QC | 295 – 355* | 0 – 4.8* | Gentry, et al 2003 |
| Fat, QFat | 14.8 – 21.2* | - | Gentry, et al 2002 |
| Mammary tissue, QMam | 7.7 – 15.4* | - | Gentry, et al 2002 |
| Placenta, QPIa | 0 – 23.4* | - | Clewell, et al, 2002 |
| Kidney, QKid ; plasma flow | 24.4 – 40.3* | - | Sims & Krantz, 1958 |
| Filtrate, QFilC—glomular filtration rate (L/h) | 6.0 – 9.1* | - | Sims & Krantz 1958; Dunlop 1981; Duvekot 1995 |
| Remaining tissue, QR | 108 – 115* | 0 – 4.0* | difference between total CO and flows to other tissues |
range of values from beginning to end of gestation
Increasing maternal cardiac output (QC) was calculated by the method of Gentry et al. (2003). The prepregnancy (non-pregnant) cardiac output was taken from Clewell et al. (1999). Because PFOA and PFOS do not partition into red blood cells (Ehresman et al. 2007), the cardiac output was modified for plasma flows by multiplying QC by 1-hematocrit; the maternal hematocrit was 0.41 (Milman et al. 1999). The fraction of cardiac output to the liver, gut, and skin were calculated by multiplying the fractional blood flows to these tissues for nonpregnant women by the prepregnancy (non-pregnant) cardiac output. Fractional blood flows to these tissues were obtained from Brown et al. (1997). Increasing blood flows to the fat, mammary tissue, and placenta were calculated by the method of Gentry et al. (2003). Changing renal plasma flow was adapted from the data of Sims & Krantz (1958). Increasing glomerular filtration rate (QFil) during pregnancy was adapted and combined from several data sets (Dunlop 1981; Duvekot et al. 1995; Sims and Krantz 1958). Plasma flow to the rest of body tissues (QR) was calculated by subtracting the sum of the plasma flows to all other tissues from the total cardiac output.
The fetal part of the model consisted of a fetal plasma compartment, a rest of body compartment, and an amniotic fluid compartment. Fetal growth was described by the method of Gentry et al. (2003). Fetal plasma volume was a constant fraction of fetal body weight and the same as that for the nonpregnant adult human (Davies and Morris 1993). The volume of fetal tissues was calculated as the total fetal body weight minus the weight of the plasma compartment. Changing amniotic fluid volume was taken from the data of Beall et al. (2007), and the data points were interpolated using a TABLE function in acslX. Fetal cardiac output (QFet) was defined as a function of fetal blood volume (Clewell et al. 1999), and, as for the maternal cardiac output, the fetal cardiac output was modified for plasma flows by multiplying QFet by (1-hematocrit) for the fetus (Sisson et al. 1959). The plasma flow to the fetal tissues was equal to the total fetal cardiac output as no fetal tissues were explicitly represented.
Lactation Model–physiology
In order to simulate changing PFAA concentrations in the mother, breast milk, and the nursing infant, the adult human model was modified in a similar manner to the rat model for lactation (Loccisano et al. 2012a). The changes were based on those of Gentry et al.(2003), and other physiological data for changes occurring during lactation in the mother and growing infant. The structure of the lactation model is in Figure 2. Equations were added to describe the changing volume of fat tissue, maternal BW and growing infant, and changing blood flows to tissues. The physiological parameters for the lactation model are given in Table 2, and all of the equations are in the Supporting Information. Decreasing maternal BW for up to 6 months postpartum was obtained from Wosje & Kalkwarf (2004), and data points were interpolated using a TABLE function in acslX. Fractional values for fat as a % maternal BW for lactating women up to 6 months postpartum were also obtained from Wosje & Kalkwarf (2004). Fat volume was then calculated by scaling to decreasing maternal BW. A mammary compartment is not explicitly represented in the lactation model, as no human data were available for PFAA partitioning from mammary tissue into milk. Chemical in plasma was placed in direct contact with the milk compartment as there are human concentration data in plasma and in milk (Fromme et al. 2010; Karrman et al. 2007; Thomsen et al. 2010). Plasma flow to the milk compartment was assumed to be the same as the plasma flow to a mammary tissue compartment, which was adapted from Gentry et al. (2003). Milk:plasma partition coefficients (PMilk) were estimated from three human studies where the concentrations of PFOA and PFOS were measured in both maternal plasma and milk (Karrman et al. 2007; Fromme et al. 2010; Haug 2010). Milk production rates (kmilk) were from Dewey et al (1991) and were assumed to be equal to infant suckling rates. Residual milk volume was obtained from Gentry et al.(2003). Tissue volumes for liver, kidney, filtrate, and gut were constant fractions of pre-pregnancy (non-pregnant) BW. Decreasing plasma volume was adapted from data of Salas et al.(2006). Volume of the remaining maternal tissues was calculated as the total maternal BW minus the remaining tissue volumes.
Table 2.
Physiological parameters for PBPK models for PFOA and PFOS in lactating woman and nursing infant.
| Parameters | Mother | Infant | source |
|---|---|---|---|
| BW (kg) (changing) | 72 – 67.5* | 3.5 – 8* | Wosje, et al, 2004 (maternal); CDC, 2002 (infant) |
| Tissue volume (fraction of BW) | |||
| Gut, VGutC | 0.0171 | - | Brown, et al 1997 |
| Liver, VLivC | 0.026 | Brown, et al 1997 | |
| Fat, VFatC | 0.30 – 0.285* | - | Wosje, et al 2004 |
| Kidney, VKidC | 0.004 | - | Brown, et al 1997 |
| Filtrate, VFilC | 0.0004 | - | 10% of kidney volume |
| Remaining tissues, VR (L) | 31.4 – 30.2* | difference between BW and sum of other tissues | |
| Residual milk volume, Vmilk (L) | 0.25 | - | Gentry, et al 2003 |
| Plasma, VPIas (L) | 3.2 – 2.4* | - | Salas, et al 2006 (mother) |
| Mammary tissue (L) | 0.82 – 1.37* | - | Gentry, et al 2003 |
| Cardiac output | |||
| Maternal (QCC; L/h/kg0.75; nonpregnant) | 20.0 | Gentry, et al 2003 | |
| Maternal (changing; L/h) | 335–330* | ||
| Blood flows (fraction of cardiac output) | |||
| Gut, QGutC | 0.181 | - | Brown, et al 1997 |
| Liver, QLivC | 0.25 | Brown, et al 1997 | |
| Skin, QSkC | 0.058 | - | Brown, et al 1997 |
| Kidney, QKid (L/h) | 38.5 – 30* | - | GFR ~20% of renal plasma flow |
| Filtrate, QFilC (glomerular filtration rate; L/h) | 7.7 – 6.0* | - | Sims & Krantz, 1958 |
| Fat, QFat (L/h) | 21.4 – 20.3* | - | Gentry, et al 2003 |
| Mammary, QMam (L/h) | 13.7 – 21.0* | Gentry, et al 2003 | |
| Remaining tissues, QR (L/h) | 96.7 – 96.6* | - | difference between total CO and flows to other tissues |
| Hematocrit | 0.38 | - | Davies & Morris 1993 |
| Milk production rate, kmilk (L/hr) | 0.0 – 0.037* | milk production rate = suckling rate | Dewey, et al 1983 |
range of values from start of lactation to 6 months postpartum
Changing maternal cardiac output (QC) was calculated by the method of Gentry et al.(2003). As in the gestation model, the cardiac output was modified for plasma flows rather than blood flows by multiplying QC by (1-hematocrit). The fraction of cardiac output to the liver, gut, and skin were calculated by multiplying the fractional blood flows to these tissues for nonpregnant women by the prepregnancy (non-pregnant) cardiac output. Fractional blood flows to these tissues were obtained from Brown et al. (1997). Increasing blood flows to the fat and mammary tissue were calculated by the method of Gentry et al. (2003). Although a separate mammary tissue compartment was not included in the model, a blood flow to the milk compartment was needed. The blood flow to the milk compartment was assumed to be the same as what it would be for a blood flow to the mammary tissue; thus, the blood flow to the mammary tissue (QMam) was still calculated. In order to derive QMam, the volume of mammary tissue had to be calculated, and this was also done according to the methods of Gentry et al.(2003). Because the volume of mammary tissue changes during lactation, these volume changes were accounted for by lumping this change in volume in with the remaining maternal tissue volume. Changing glomerular filtration rate (QFil) after pregnancy was adapted from data from Sims & Krantz (1958) and returned to the pre-pregnancy level at 6 weeks postpartum. Since there were no available data on renal plasma flow changes during lactation, renal plasma flow was assumed to be approximately 5 times that of the GFR (GFR is normally approximately 20% of renal plasma flow). Plasma flow to the rest of body tissues (QR) was calculated by subtracting the sum of the plasma flows to all other tissues from the total cardiac output.
Due to limited data on PFAA in the infant, the infant part of the model was treated as a single compartment with a volume of distribution. The volume of distribution for PFOA and PFOS were the volumes of distribution estimated for compartment models for the monkey (Tan et al. 2008) and then scaled to infant body weight. Infant growth was obtained from the CDC's growth charts (CDC 2002), and data points were interpolated using a TABLE function in acslX.
Gestation and Lactation Models—Chemical Parameters
Chemical-specific parameters used in the human gestation and lactation models for PFOA and PFOS were the same as those used in the adult human model, with the exception of those that were calibrated using available biomonitoring data in pregnant and lactating women, which include the placental transfer rate constants, milk:plasma partition coefficients, and the elimination rate constant for the infant. Further details on chemical-specific parameters and model calibration are outlined below.
Mother
Chemical-specific parameters used in the human gestation and lactation models for PFOA and PFOS are given in Table 3. The chemical-specific parameters for the pregnant and lactating mother are the same as those used in the adult human model (Loccisano et al. 2011). The parameters from the adult model allowed for good agreement with gestation and lactation data when they were used in the pregnancy and lactation models, and as there were no available data on perfluorinated compound (PFC)-specific parameters in pregnant or lactating women, the adult values were retained in the life stage models presented here. Any changes made for the fetus or infant are outlined in the text below. Free fractions of chemical in plasma. Only the free concentration of chemical partitions into tissues. As in the monkey and rat, PFOA and PFOS are highly bound to plasma proteins in the human (>97% bound)(Kerstner-Wood et al. 2003; Han et al. 2003). The species differences in half-lives of these compounds does not appear to be due to differences in protein binding (Han et al. 2003), so the same free fractions estimated for monkeys for PFOA and PFOS were used in the adult human model (Loccisano et al. 2011), and these values were subsequently used in the gestation and lactation models reported here. Although the overall concentration of serum proteins decreases during gestation due to increased plasma volume (Notarianni 1990; Perucca and Crema 1982; Krauer et al. 1980), there are no data available on PFAA binding to plasma proteins during pregnancy nor are there available data to provide evidence that the free fraction of PFAAs change during pregnancy. Thus, explicit binding of PFAAs to plasma proteins is not simulated (as in the monkey and adult human models (Loccisano et al. 2011)), but a constant free fraction of chemical in plasma is accounted for, as the free fraction will determine how much chemical is available for uptake into tissues. Urinary elimination rate constants were scaled as BW−0.25 and estimated by fitting the model to the data of Harada et al. (2005), where serum and urine PFOA and PFOS concentrations were measured.
Renal Resorption
The estimated geometric mean serum elimination half-life in the human is approximately 3.5 years for PFOA (arithmetic mean 3.8 years) and 4.8 years for PFOS (arithmetic mean 5.4 years) based on a study of retired fluorochemical workers (Olsen et al. 2007). A more recent study conducted on a population that had previous exposure to PFOA in drinking water estimated a median plasma half-life for PFOA of 2.3 years (Bartell et al. 2010). It is postulated that renal resorption is responsible for the persistence in humans (Andersen et al. 2006). Renal resorption in the model is described by a transporter maximum (Tm) and an affinity constant (Kt). For a more detailed discussion of how the Tm and Kt values were derived for the adult human model, please see Loccisano et al. (2011). Although renal function is altered during pregnancy (Dunlop 1979; 1981), no data were available on the changes in renal resorption of PFAA during this life stage. Thus, the same values used for Tm and Kt in the adult human model were used for the mother in the gestation and lactation models.
Partition Coefficients (PC)
For PFOA, the PC for liver, kidney, skin, fat, gut, and rest of body tissues were estimated from tissue concentration data reported by Kudo et al.(2007) for a single IV dose of PFOA to male Wistar rats. For PFOS, PC were estimated from tissue concentration data resulting from 5 days of dietary dosing in male C57Bl/6 mice (DePierre 2009). There was no available data on concentrations of PFAA in the human placenta. For PFOA, the PC for the placenta was estimated from a developmental study in rats (Hinderliter et al. 2005). For PFOS, the PC for the placenta was that estimated for rapidly perfused tissues from mouse feeding data (DePierre 2009). A mammary tissue:plasma PC is used in the gestation model (PMam). For PFOA, this was estimated from mouse tissue data from Fenton et al.(2009); for PFOS, this was estimated from concentrations in slowly perfused tissues from mouse feeding data (DePierre 2009) as no concentration data for PFOS in mammary tissue could be found. Fiserova-Bergerova (1975), examined partition coefficients for two compounds (Forane and methylene chloride) in several species (man, monkey, dog, and rat). Tissue:blood partition coefficients were similar for the tissues examined across species; thus, it is reasonable to use the same partition coefficients in the human models. Milk:plasma partition coefficients were calibrated from three human biomonitoring studies where the concentrations of PFOA and PFOS were measured in both maternal plasma and milk (Karrman et al. 2007; Fromme et al. 2010; Haug 2010). These estimates yielded predicted milk or plasma concentrations similar to those observed in epidemiology studies (Liu et al. 2011; Volkel et al. 2008; von Ehrenstein et al. 2009; Thomsen et al. 2010; Kim et al. 2011b; Kim et al. 2011a).
Fetus and infant
Transfer of both PFOA and PFOS across the placenta was described as a simple diffusion process between free chemical in the placenta and free chemical in plasma. Due to lack of data in humans, the rate constants (ktrans1C and ktrans2C) used in the human models were the same as those estimated for the pregnant rat (Loccisano et al. 2012a). In the case of PFOS, the rate constants for the transfer of chemical from mother to fetus and fetus to mother were the same values as those used for the rat (ktrans1C = 0.46 and ktrans2C = 1.01). When compared to available biomonitoring data, these values yield predicted maternal:fetal plasma concentration ratios similar to those observed in epidemiology studies (observed ratios = approximately 2–3; predicted ratios = approximately 2.2). However, for PFOA, symmetric transfer (ktrans1C = ktrans2C = 0.46), rather than the asymmetric transfer that was used for PFOS, predicted maternal:fetal plasma concentration ratios that were more similar to the observed ratios than asymmetric transfer did (observed ratios = approximately 0.6 – 1.5; predicted ratios = approximately 1.0). The placental transfer rate constants were adjusted (calibrated) to yield the maternal:fetal ratios observed in several of the datasets that reported maternal or cord blood PFC levels (Apelberg et al. 2007; Fei et al. 2007; Midasch et al. 2007; Washino et al. 2009). The parameters for placental transfer were scaled to fetal body weight0.75. There is no available human data on concentration of PFAA in amniotic fluid; parameters for transfer to and from the amniotic fluid (ktrans3c and ktrans4c) for both chemicals were the same as those estimated for the rat (Loccisano et al. 2012a), and scaled to fetal BW0.75. Fetal tissue:blood partition coefficients for PFOA and PFOS and the free fraction of chemical in fetal/infant plasma were the same values as used for the mother (the fetal/infant rest of body tissues:blood PC was the same as the maternal rest of body tissues:blood PC). Neither the fetus nor the infant includes a description of renal resorption. The kidneys (GFR, resorption, and secretion) do not function at the level of the adult (Alcorn and McNamara 2003), and there are no available data on resorption or secretion of PFAA for these life stages; thus, resorption was not included for the fetus or infant. The elimination rate constant for the infant was calibrated by fitting the model to the plasma concentration data of Fromme, et al.(2010).
Model simulations
Exposure before pregnancy
Exposure to PFAA started for the mother before becoming pregnant so that steady-state concentrations of PFAA would have been achieved in the tissues. To obtain the steady-state tissue concentrations at the start of pregnancy, the non-pregnant adult human model was run for 30 years in order to reach a steady-state concentration in plasma. The daily intake used in the adult model in order to achieve the steady-state concentrations of PFAA in the blood after 30 years of exposure was that estimated to yield the first concentration reported during pregnancy or lactation (see text below), and the daily intake was scaled to BW. The tissue concentrations at 30 years were then used as the starting tissue concentrations of PFAA for the pregnancy model.
Obviously, pregnancy could occur at an age before or after 30, but for simplicity and the purposes of the simulations reported here, pregnancy was assumed to occur at 30 years old because the plasma PFAA concentrations reached steady state at 30 years. When running the adult (pre-pregnancy) human model with exposures that yield the plasma concentrations of PFOA or PFOS reported for the general population (~1e-04 to 2e-03 ug/kg BW/day), it took approximately 30 years to reach a steady-state plasma concentration. At exposures near those of the general population for women much younger than 30, these women's plasma PFAA concentrations simply would not have reached steady state before they became pregnant.
For lactation, the tissue concentrations at the delivery/birth of the baby (embryonic week 39) were used as the starting tissue concentrations for lactation. The daily dose (ug/kg BW/day) before, during, and after pregnancy stayed the same; however, the daily intake was scaled to maternal BW; thus, as energy requirements change, so does PFAA intake. During lactation, the energy requirements remain increased during breastfeeding; however, maternal BW decreases during this period. To adjust for a higher energy requirement and thus higher daily intake of PFAA, the daily dose was increased by 25% during the first 6 months postpartum and then decreased again at 6 months postpartum to the daily dose used before and during pregnancy (scaled to maternal BW). The energy cost of lactation in the first 6 months after delivery is 500 kcal/day (Otten et al. 2006). The mean energy intake of US women of reproductive age in 1999–2000 was 2028 kcal/day (CDC 2004). Thus, the energy intake was assumed to increase 25% during lactation, and the dose of PFCs was increased proportionally.
Model Evaluation
The human data where more than one concentration during pregnancy or lactation was available was used for model evaluation. For example, if a pregnancy study reported a maternal blood concentration at a time during pregnancy and also at delivery or in cord blood, the concentration during pregnancy was used for exposure estimation and the model was evaluated by assessing the consistency between the maternal concentrations at delivery or cord blood. For the lactation model, if a plasma concentration was reported, the exposure was estimated to yield this concentration and then the resulting predicted milk or infant blood concentration was compared to the observed value to evaluate the model structure and parameters. The goodness of the model fit to the latter data points was considered to reflect the validity of the model structure and parameterization approach. Agreement of model predictions with reported data was examined graphically. Exposure estimation was an iterative process; if the model did not correctly predict the reported concentration at the specified time during pregnancy or lactation, a new exposure was tried, and the whole sequence was repeated (the concentrations in the adult model were allowed to reach steady state, the end concentrations were input to the pregnancy model, and the end pregnancy tissue concentrations were input to the lactation model) until the model predicted the reported mean or median concentration. For exposure estimations, agreement between predicted and observed concentrations was considered satisfactory if the predicted value was within 1% of the observed value. If the difference was more than that, exposure was re-estimated.
Sensitivity Analysis
A normalized sensitivity analysis was performed on the gestation and lactation models for both chemicals in order to examine the influences of each model parameter on the model output. The influence of each model parameter on maternal plasma, fetal plasma, and infant concentrations was examined by calculating the area under the curve (AUC) for maternal, fetal, or infant plasma PFAA concentration that resulted from increasing each parameter by 1%. Each parameter was increased separately. The normalized sensitivity coefficients (SC) were calculated by the following equation:
A is the AUC resulting from the 1% increase in parameter value, B is the AUC resulting from the original parameter value, C is the parameter value increased by 1%, and D is the original parameter value. Thus, an SC with the absolute value of 1 means that there is a 1:1 relationship between the change in the parameter and the dose metric. For parameters that describe the growth or changes (and change during the simulation time), a 1% increase in the parameter was calculated by the following equation:
This equation will allow for the parameter value to increase by 1% of the original value during the entire simulation.
The relative impact of each model parameter was examined for the dose metrics of interest, which were the total plasma AUC in the mother and fetus (at embryonic week 39) for the gestation models or the total maternal plasma AUC and infant body AUC (at 6 months postpartum) for the lactation models. Normalized SC's were calculated for all model parameters, but only those with an SC ≥ 1 or SC ≤ −1 are reported here. For PFOA, sensitivity analysis was performed assuming an estimated maternal exposure of 1.9e-4 ug/kg BW/day for both gestation and lactation; for PFOS, the analysis was performed assuming an estimated maternal exposure of 4.0e-4 ug/kg BW/day for both the gestation and lactation models. These exposures were those estimated for the data of Fromme et al.(2010), which were the only data available for the mother and infant pairs during both pregnancy and lactation.
Model Predictions of Exposure
Exposure estimation and predictions of internal dose for general population
In order to estimate maternal exposure for the various general population studies, maternal PFOA and PFOS exposure was modeled as a generic input to the plasma compartment (designated as “IV” in Figures 1 and 2) as contributions of PFAA exposure from different exposure routes are not well understood (Fromme et al. 2009). When considering IV versus oral exposure, a first-pass effect will not be an issue with these chemicals. When administered orally to animals, PFAA are well-absorbed and are not metabolized appreciably, so most of the compound reaches systemic circulation (Johnson et al. 1984; Kuslikis et al. 1992; Ophaug and Singer 1980; Vanden Heuvel et al. 1991). Several studies were available that reported concentrations of PFAA in maternal plasma, fetal or infant plasma, and/or milk at specified time points during pregnancy or lactation (Apelberg et al. 2007; Fei et al. 2007; Fromme et al. 2010; Hanssen et al. 2010; Inoue et al. 2004; Kim et al. 2011b; Kim et al. 2011a; Midasch et al. 2007; Monroy et al. 2008; Tittlemier et al. 2004; Karrman et al. 2007; Liu et al. 2011; Thomsen et al. 2010; von Ehrenstein et al. 2009; Volkel et al. 2008). For each study, the gestation and lactation models were used to estimate maternal exposure and the resulting maternal, fetal, or infant internal dose. Some reports provided paired maternal and fetal observations at delivery (Fei et al. 2007; Fromme et al. 2010; Hanssen et al. 2010; Kim et al. 2011b; Kim et al. 2011a; Midasch et al. 2007; Monroy et al. 2008; Tittlemier et al. 2004); for these data, the exposure was estimated to yield the mother's plasma concentration at delivery. If the mother's concentration at a time during pregnancy was reported (before delivery) (Fei et al. 2007; Fromme et al. 2010; Monroy et al. 2008), the exposure was estimated to yield this concentration. For the lactation model, maternal exposures were estimated to give the reported mean or median PFAA concentration in milk or maternal plasma at a specific time point after the birth of the baby.
Two estimates of serum half-lives of PFOA are reported in the literature (3.8 years and 2.3 years) (Olsen et al. 2007; Bartell et al. 2010). Based on model predictions with the adult human model, either half-life appeared to be likely (Loccisano et al. 2011). For the PFOA models reported here, when an observed serum concentration was used to estimate maternal exposures, the exposures were based on both half-lives estimated for PFOA. The renal resorption parameters (Tm, Kt) control the half-life of the chemical, i.e., the higher Tm is, the more efficient resorption is and thus, there would be a longer half-life. With a shorter half-life, a higher exposure would be needed to yield the same plasma concentration at a specified time point than with a longer half-life. When exposures were estimated using the pregnancy model, both the 3.8 and 2.3 year half-lives were used in the model (by adjusting Tm), and an exposure was estimated that would yield the reported plasma concentrations at the specified time points for each half-life.
Prediction of internal dose for Little Hocking and Arnsberg
There are human data available for two populations (Little Hocking (LH), Ohio and Arnsberg, Germany) exposed to high levels of PFOA in their drinking water (Emmett et al. 2006; Holzer et al. 2008); however, there are no data available for pregnant or lactating women or for infants from these populations. This group constitutes a potentially sensitive subpopulation, and one use of the model is the prediction of internal dose in the mother, fetus, and infant resulting from drinking the contaminated water. For women exposed to high levels of PFOA through drinking water, simulated exposure was from drinking water only. The reported concentrations for PFOA in drinking water were used (3.55 ppb for Little Hocking)(Emmett et al. 2006) and 0.519 ppb for Arnsberg (Holzer et al. 2008)), and drinking water rates for pregnant and lactating women were adapted from the data of Ershow et al. (1991). For non-pregnant, pregnant, and lactating women, the drinking water rates used were 19.1, 18.3, and 21.4 mL/kg BW/day, respectively. These simulations were performed in the same way as for the general population: exposure was assumed to start before pregnancy, so the adult human model was run for 30 years in order to reach steady state, and the resulting tissue concentrations were used as the starting concentrations for the gestation model. When simulating the women in these communities, the concentration of PFOA in the water was held constant while the drinking water intake changed based on life stage. Drinking water exposure lasted for the entire length of gestation (39 embryonic weeks) and for 6 months after birth. Breast milk was the only exposure assumed for the infant; no further exposure was assumed for the child when breastfeeding stopped for these simulations.
Results
Model Predictions
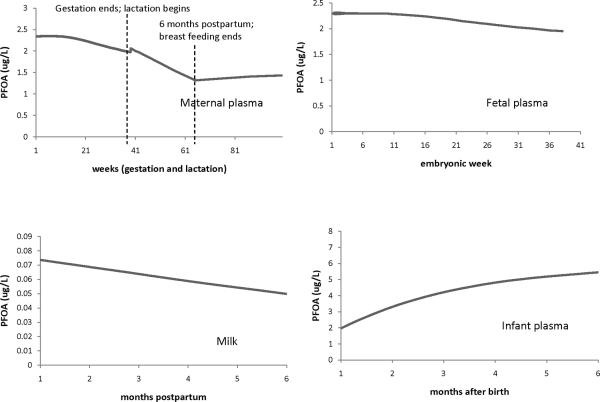
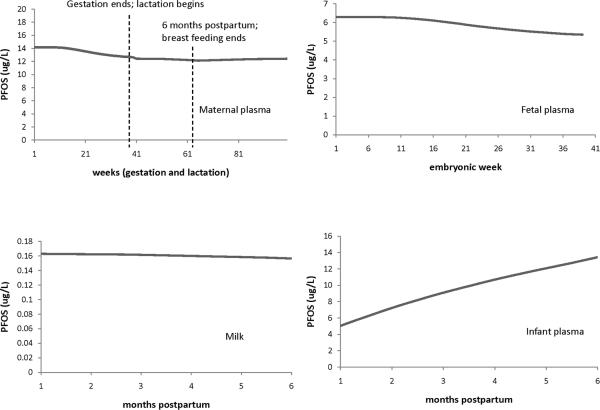
Figures 3 and 4 illustrate the general features and trends of the gestation and lactation model predictions for PFOA (Figure 3) and PFOS (Figure 4). The top left plots in each figure show the PFC concentration in maternal plasma during pregnancy and lactation. As pregnancy progresses, the maternal PFC concentration decreases, and there is an increase right at the time of delivery. For the decrease in PFC concentration during pregnancy, the model is line with existing data (Fei et al. 2007; Fromme et al. 2010; Monroy et al. 2008) where maternal blood concentration has been measured at 2 time points during pregnancy and the concentration was observed to decrease. While there are data on the maternal plasma concentrations of PFCs at delivery and during lactation (see Figures 5 and 6 for these studies), no data on plasma concentration during the first few hours to days after birth could be found (the earliest time point found was 7 days postpartum (Liu et al. 2011)). One use of the model is to be able to use it to predict PFAA concentrations at time points where data is lacking, such as this one. The fetus and milk are routes of excretion for the mother, and the increase in PFC concentration predicted by the model could be due to these extra routes of excretion not being present (the fetus is gone and the mother does not start producing milk until a few days after birth (Neville and Morton 2001), which is also reflected in the current models). The mother's PFC concentration decreases during the first 6 months postpartum when she is breastfeeding, and then increases when breastfeeding stops. Thus, the fetus and milk are equivalent to routes of excretion for the mother. The top right plots in Figures 3 and 4 show the PFC concentration in the fetus during gestation; as for the mother, the fetal concentration decreases as gestation progresses. For PFOA, the levels in maternal plasma are about the same as in the fetus, while for PFOS, maternal blood levels are about twice fetal levels. The bottom left plots in Figure 3 and 4 show the PFC concentrations in milk for up to 6 months of breastfeeding. As lactation progresses, the concentration in milk decreases for both chemicals (0.03 ug/L for PFOA and 0.01 ug/L for PFOS). The plots on the bottom right in Figures 3 and 4 show the PFC concentration in infant plasma for up to 6 months after birth. For simulation purposes, lactation lasted 6 months; the infants' only exposure to PFC was through breast milk. During the first 6 months of life, for infants who are exclusively breastfed, the rise in PFC concentration during this time is most likely due to exposure from breast milk.
Figure 3.
Example of the general features/trends of the model predictions for PFOA during gestation and lactation (maternal exposure was 1.5e-4 ug/kg BW/day). The plots show the model predictions in maternal plasma during pregnancy and lactation, fetal plasma, milk, and infant plasma at up to 6 months after birth. Infant exposure to PFCs was assumed to be only through milk and breastfeeding was assumed to last 6 months postpartum.
Figure 4.
Example of the general features/trends of the model predictions for PFOS during gestation and lactation (maternal dose was 1.35e-3 ug/kg BW/day). The plots show the model predictions in maternal plasma during pregnancy and lactation, fetal plasma, milk, and infant plasma at up to 6 months postpartum. Infant exposure to PFCs was through milk and breastfeeding lasted 6 months postpartum.
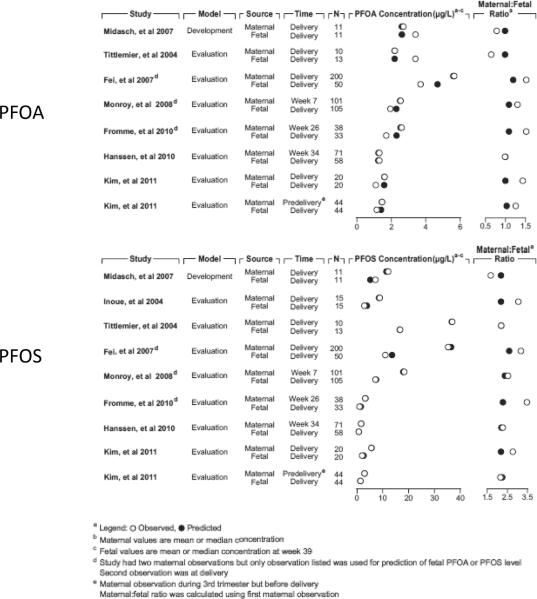
Figure 5.
Comparison of model-predicted and observed values for PFAAs in maternal and fetal plasma during gestation. The figure compares the predicted and observed concentrations and maternal:fetal plasma concentration ratios for PFOA (top) and PFOS (bottom) from several pregnancy studies in which there were paired maternal and fetal observations available.
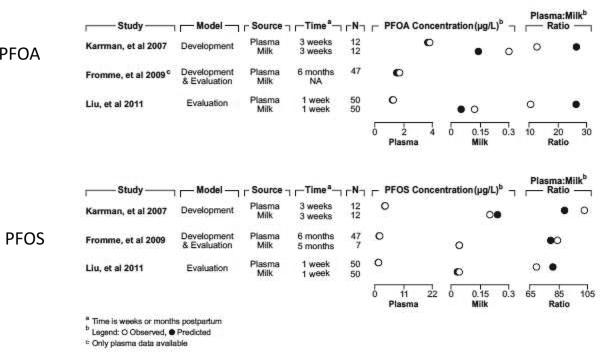
Figure 6.
Comparison of model-predicted and observed values for PFAAs in maternal plasma and milk during lactation. The figure compares the predicted and observed concentrations and the plasma:milk concentration ratios for PFOA (top) and PFOS (bottom) from several lactation studies.
Evaluation by comparing modeled results with available human data
Figure 5 compares the observed and model-predicted maternal and fetal plasma PFC concentrations and maternal:fetal (cord blood) PFC plasma concentration ratios. For PFOA, the observed maternal:fetal ratios range from 0.65 – 1.45. For PFOS, the observed ratios exhibit more variability than for PFOA and range from 1.03 – 3.5. The larger value of the PFOS ratio compared to PFOA indicates that there is probably more efficient placental transfer of PFOA (Olsen et al. 2009). Good agreement between the model-predicted and reported concentrations (Fei et al. 2007; Fromme et al. 2009; Hanssen et al. 2010; Inoue et al. 2004; Midasch et al. 2007; Monroy et al. 2008; Tittlemier et al. 2004; Kim et al. 2011a; Kim et al. 2011b) was observed; the model predicted concentrations and maternal:fetal ratios are all within a factor of 2 of the reported values. Maternal and fetal predictions were compared to the reported standard deviations, interquartile ranges, or concentration ranges if theses data were available; all predicted values were within one standard deviation of the reported mean, were within the interquartile range, or were within the reported concentration ranges of the observed data. Thus, based on the model-estimated maternal exposures of PFOA and PFOS, the model can reasonably predict the internal dose in the fetus.
Figure 6 compares the predicted and reported milk and plasma concentrations (and milk:plasma ratios where available) during lactation. Good agreement is observed between the model and the available human lactation data; the predicted concentrations and ratios for both PFOA and PFOS are all within a factor of 2.3 of the observed concentrations and ratios. Not all studies reported observed concentrations for both milk and plasma during lactation (Thomsen et al. 2010; Volkel et al. 2008; von Ehrenstein et al. 2009; Kim et al. 2011b; Kim et al. 2011a). For some of the studies that examined milk concentration, PFOA was below the limit of detection (LOD) so no comparison could be made between the model predictions and data (Fromme et al. 2010; Volkel et al. 2008). All maternal exposure estimates for the studies listed (Fromme et al. 2010; Karrman et al. 2007; Liu et al. 2011) were made using maternal plasma concentrations either during pregnancy or lactation; the agreement between predicted and observed milk concentrations indicates that the model can reasonably predict the resulting PFAA concentrations in milk. This in turn can provide information on PFAA exposure in nursing infants. For the studies of von Ehrenstein et al. (2009) and Thomsen et al. (2010), no plasma concentrations were measured; thus, maternal exposure estimates had to be made using the observed milk concentrations (first observation). For both PFOA and PFOS, the model predictions for the second observations for both studies were within a factor of 2 of the observed milk concentrations, indicating good agreement with the data (comparison not shown in Figure 6). When predicted plasma and milk concentrations were compared to any available reported standard deviations, interquartile ranges, and concentration ranges, all predicted values were within one standard deviation of the reported mean, were within the interquartile range, or were within the reported concentration ranges of the observed data.
The only study that provided information on infant plasma concentrations was Fromme et al. (2010)(they measured infant plasma concentrations at 6 months postpartum); thus, these were the only data that could be used for comparison of observed and model-predicted data for the PFAA levels in the infant. The model predictions are in good agreement with the reported values (within one standard deviation of the mean), indicating that the model can reasonably predict the internal dose in the infant several months after birth based solely on exposure through breast milk. The observed concentration for PFOS in infants at 6 months of age was 3.3 ug/L, and the predicted value is 3.5 ug/L. For PFOA, the observed concentration is 8.0 ug/L, while the predicted value is 8.2 ug/L.
Sensitivity Analysis
The normalized sensitivity coefficients for both the gestation and lactation models for PFOA and PFOS are shown in Table 4 (gestation, top; lactation, bottom). Several parameters affect the maternal and fetal plasma AUC during gestation. For the mother, these include parameters such as BW, the flow to the filtrate compartment (QFil; analogous to GFR), and the free fraction of chemical in plasma. All of the tissue volumes in the mother are scaled to BW (and maternal BW increases by approximately 25 kg during pregnancy); the flow to the filtrate compartment will affect how quickly the chemical is eliminated; and the free fraction of chemical controls how much chemical will be available for tissue uptake or filtration. Tm and Kt, the renal resorption parameters, determine how much chemical will get taken back into the plasma or eliminated through urinary excretion. Many of the other physiological parameters to which the maternal plasma AUC exhibits sensitivity determine how much chemical is available for uptake into tissues. Liver volume (VLC), fat volume (VFatC), liver:plasma partition coefficient (PL), fat:plasma partition coefficient (PFat), and the rest of tissues:plasma partition coefficient (PR) also control how much chemical gets into liver, fat, and rest of body tissues. Fat volume also constitutes a large % BW in humans (approximately 20% of BW in non-pregnant women), especially in pregnant women (approximately 30% of BW) as this increases as the pregnancy progresses.
Table 4.
Calculated maternal, fetal, and infant sensitivity coefficients for PBPK model parameters for PFOA and PFOS models for human gestation (top) and lactation (bottom) with respect to plasma PFOA or PFOS AUC. For gestation, the AUC was calculated at embryonic week 39; for lactation, the AUC was calculated at 6 months postpartum. Only parameters that have SCs > 0.07 or <−0.07 for the mother, fetus, or infant are shown in the table.
| PFOA gestation | PFOS gestation | ||||
|---|---|---|---|---|---|
|
| |||||
| parameter | maternal SC | fetal SC | parameter | maternal SC | fetal SC |
| initial BW (maternal) | −0.75 | −0.74 | initial BW (maternal) | −0.79 | −0.79 |
| liver volume (fraction of BW) | −0.36 | −0.37 | liver volume (fraction of BW) | −0.34 | −0.33 |
| liver: plasma PC | −0.38 | −0.39 | liver:plasma PC | −0.34 | −0.34 |
| rest of body:plasma PC | −0.39 | −0.39 | fat:plasma PC | −0.12 | −0.13 |
| resorption maximum (Tmc) | 0.35 | −0.35 | rest of body: plasma PC | −0.32 | −0.32 |
| affinity constant (Kt) | −0.35 | −0.35 | resorption maximum (Tmc) | 0.07 | 0.07 |
| free fraction in plasma | −0.11 | 0.88 | affinity constant (Kt) | −0.07 | −0.07 |
| free fraction (fetus) | -- | −0.98 | free fraction in plasma | −0.08 | 0.92 |
| placental transfer rate constant (m to f) | −0.01 | 0.98 | free fraction (fetus) | -- | −0.98 |
| placental transfer rate constant (f to m) | 0.01 | −0.98 | placental transfer rate constant (m to f) | -- | 0.99 |
| GFR | −0.19 | −0.19 | placental transfer rate constant (f to m) | -- | −0.98 |
| fat volume | 0.10 | 0.10 | GFR | −0.15 | −0.15 |
| fat volume (fraction of BW) | 0.11 | 0.10 | fat volume | −0.16 | −0.16 |
| PFOA lactation | PFOS lactation | ||||
|---|---|---|---|---|---|
|
| |||||
| parameter | maternal SC | infant SC | parameter | maternal SC | infant SC |
| liver volume (fraction of BW) | −0.18 | −0.22 | liver volume (as fraction of BW) | −0.23 | −0.28 |
| liver:plasma PC | −0.19 | −0.23 | liver:plasma PC | −0.24 | −0.30 |
| rest of body: plasma PC | −0.21 | −0.22 | fat:plasma PC | −0.03 | −0.12 |
| milk:plasma PC | −0.38 | 0.56 | rest of body: plasma PC | −0.34 | −0.28 |
| Free fraction in plasma | −0.17 | −0.04 | milk:plasma PC | −0.07 | 0.79 |
| resorption maximum (Tmc) | 0.16 | 0.04 | Free fraction in plasma | −0.28 | −0.03 |
| affinity constant (Kt) | −0.16 | −0.04 | Resorption maximum (Tmc) | 0.13 | 0.03 |
| milk production rate | −0.38 | 0.56 | Affinity constant (Kt) | −0.13 | −0.03 |
| maternal BW (decreasing) | −0.20 | −0.40 | milk production rate | −0.07 | 0.77 |
| BW of infant | -- | −0.98 | maternal BW (decreasing) | −0.61 | −0.70 |
| Infant elimination rate constant (keliml) | -- | −0.59 | BW of infant | -- | −0.98 |
| Volume of distribution in infant (Vdc) | -- | −0.99 | Volume of distribution in infant (Vdc) | -- | −0.99 |
| free fraction in infant compartment | -- | −0.72 | GFR | −0.25 | −0.06 |
| GFR | −0.33 | −0.08 | |||
In the fetus, maternal body weight and flow to the filtrate compartment affect the fetal plasma AUC in addition to the free fraction of chemical in maternal and fetal plasma (Free and FreeF) and the bidirectional placental transfer rate constants (ktrans1c and ktrans2c). The free fraction of chemical in maternal and fetal plasma and the placental transfer rates determine how much chemical is available for tissue uptake in both mother and fetus and how quickly chemical is transferred from the mother to the fetus (and from the fetus back to the mother). Maternal parameters such as liver volume, fat volume, liver:plasma PC, fat:plasma PC, and rest of body:plasma PC affect how much chemical can get into the maternal liver and fat versus being transferred to the fetus. The fetal plasma AUC is also affected by the maternal renal resorption parameters (Tm and Kt) as these will determine how much chemical is eliminated from the maternal system rather than being available for transfer to the fetus.
In the lactation model, the maternal plasma AUC at 6 months postpartum is affected by many of the same maternal parameters as during gestation. BW is still a sensitive parameter as all of the tissue volumes and cardiac output are scaled to this parameter, and there is a decrease in BW during the lactation period. QFil (GFR) controls how quickly the chemical is filtered and is thus eliminated versus staying in the blood and being transferred to breast milk. Parameters such as liver volume, liver:plasma PC, fat:plasma PC, and rest of body:plasma PC all determine how much chemical is available for uptake into these tissues (liver, fat, and rest of body). Two parameters specific to the lactation model that affect the maternal plasma AUC are the milk:plasma partition coefficient (PmilkP) and the milk production/suckling rate (kmilkC). Because the model is constructed so that milk is in direct contact with plasma, and PmilkP will determine how much chemical is available for uptake into breast milk from maternal plasma. KmilkC will determine how quickly the chemical is then transferred from breast milk to the nursing infant, and the infant AUC is more sensitive to this parameter than the mother's plasma AUC. For the infant, some of the parameters that affect the fetal plasma AUC also affect the infant AUC at 6 months postpartum. These include the infant BW and several maternal parameters, such as GFR, liver volume, liver:plasma PC, fat:plasma PC, and rest of body:plasma PC; these maternal factors control how quickly the chemical is eliminated from the mother or partitioned into other tissues rather than partitioning into breast milk and transferring chemical to the infant. Parameters for milk production and partitioning into milk also affect the infant AUC. The infant's only exposure to PFAA is through milk, and kmilkC and PMilkP will control how much chemical reaches the breast milk and how much chemical is then received by the infant. The sensitivity of plasma AUC to PmilkP and kmilkC is in line with observations from epidemiology studies; milk appears to be a significant route of excretion of PFAA for the mother and a significant exposure source for the infant.
Estimated exposures to PFOA and PFOS for the general population
Table 5 lists each pregnancy and lactation study used for maternal exposure estimation. For most gestation studies, exposure was estimated based on the maternal plasma concentration at delivery, but a few were estimated based on concentrations observed earlier in pregnancy. During lactation, estimated exposures were based on maternal plasma concentration at a specified time point during lactation, or if that was not reported, estimated exposures were based on milk PFAA concentrations.
Table 5.
Estimated maternal PFOA and PFOS exposures for pregnant and lactating women. The women in these studies were considered “general population” in that they were not known to be exposed to unusually high concentrations of PFOA or PFOS (i.e., not occupationally exposed or drinking water with high concentrations). Because contributions from various exposure sources are not known, intake for the general population exposure was simulated as a direct input to blood. Exposures of the women were estimated to yield the plasma PFAA concentration at delivery or a point during pregnancy or lactation (noted in table). The adult human model was run for 30 years in order to reach steady state at each estimated exposure, and the tissue concentrations at 30 years were used as starting concentrations in the pregnancy model. The tissue concentrations at the end of pregnancy were then used as starting concentrations for the lactation model. Women were assumed to be exposed for the entire length of pregnancy and lactation. For comparison, the exposures estimated for the general population from our previously developed adult human model are shown at the bottom of the table.
| Studies used for exposure estimation (media; time for which exposure was estimated) | estimated PFOA exposure (ug/kg/day); 3.8 yr half-life | estimated PFOA exposure (ug/kg/day); 2.3 yr half-life | estimated PFOS exposure (ug/kg/day); 5.4 yr half-life |
|---|---|---|---|
| gestation | |||
| Fromme, et al 2010 (plasma; week 34) | 1.9E-04 | 3.0E-04 | 3.8E-04 |
| Apelberg, et al 2007 (plasma; delivery) | 1.2E-04 | 2.0E-04 | 5.8E-04 |
| Inoue, et al 2004 (plasma; delivery) | 1.15E-04 | 1.9E-04 | 1.2E-03 |
| Midasch, et al 2007 (plasma; delivery) | 2.0E-04 | 3.4E-04 | 1.35E-03 |
| Fei, et al 2007 (plasma; week 7) | 3.60E-04 | 6.9E-04 | 3.7E-03 |
| Washino, et al 2009 (plasma; delivery) | 1.25E-04 | 2.2E-04 | 7.4E-04 |
| Monroy, et al 2008 (plasma; week 26) | 1.8E-04 | 3.0E-04 | 2.0E-03 |
| Hanssen, et al 2010 (plasma; delivery) | 1.15E-04 | 1.7E-04 | 1.9E-04 |
| Kim, et al 2011 (plasma; delivery) | 1.3E-04 | 2.3E-04 | 6.9E-04 |
| Kim, et al 2011 (plasma; 3rd trimester) | 1.15e-04 | 2.05E-04 | 3.5e-04 |
| Tittlemier, et al 2004 (plasma; delivery) | 1.6E-04 | 2.8E-04 | 4.4E-03 |
| lactation | |||
| Karrman, et al 2007 (plasma; 3 weeks postpartum) | 2.8E-04 | 2.3E-03 | |
| von Ehrenstein, et al 2009 (plasma; 4 weeks postpartum) | 2.4E-04 | 2.25E-03 | |
| Volkel, et al 2008 (milk; 4 weeks postpartum) | NA (PFOA < LOD in milk) | 3.5E-04 | |
| Fromme, et al 2010 (plasma; 6 months postpartum) | 2.4e-4 (1st 6 months postpartum); PFOA < LOD in milk | 4.75e-4 (1st 6 months); 3.8e-04 (after 6 months) | |
| Kim, et al 2011 (milk; 7 days postpartum) | 1.63e-4 (1st 6 months); 1.3e-4 (after 6 months) | 8.6e-4 (1st 6 months); 6.9e-4 (after 6 months) | |
| Kim, et al 2011 (plasma during pregnancy; milk collected 1 month postpartum) | 1.44e-04 (1st 6 months postpartum); 1.15e-04 (after 6 months) | 4.4e-04 (1st 6 months); 3.5e-4 (after 6 months) | |
| Thomsen, et al 2010 (milk; birth) | 1.7E-04 | 1.4E-03 | |
| Liu, et al 2011 (plasma; 1 week postpartum) | 1.4e-4 (1st 6 months); 1.1e-4 (after 6 months) | 4.6e-4 (1st 6 months); 3.7e-4(after 6 months) | |
| General population (estimated using adult human model) | |||
| Calafat, et al 2007 (NHANES 2003–04) | 2.50E-04 | 4.20E-04 | 1.75E-03 |
| Olsen, et al 2008 (Red Cross blood donors) | 2.2e-04-4.4e-04 | 1.3e-04-2.65e-04 | 8.5e-04-1.8e-03 |
For PFOA exposure during pregnancy, estimated maternal exposures ranged from 1.1e−04 to 6.9e−04 ug/kg BW/day, and for PFOS, estimated maternal exposures ranged from 1.9e−04 to 4.4e−03 ug/kg BW/day. For PFOA during lactation, estimated exposures ranged from 1.1e−04 to 2.8e−04 ug/kg BW/day. However, PFOA concentrations in milk were < LOD in some studies (Fromme et al. 2010; Volkel et al. 2008), so no PFOA exposure could be estimated for the women in these studies based on milk concentrations. PFOS was detected in all studies in plasma or milk, and estimated maternal PFOS exposures during lactation ranged from 3.5e−04 to 2.3e−03 ug/kg BW/day. These estimates were obtained by modeling exposure as a generic input to the plasma compartment (see Methods) and assuming constant exposure over a 24 hour time period. Although exposure routes are not well understood, the women most likely have exposure to PFAAs throughout the day through food, dust, and in some cases, drinking water, and while exposure could be turned off while they are sleeping, etc., these compounds have such a long half-life that turning off exposure for a few hours probably will not make much difference in their blood levels.
Predicted PFOA concentrations in populations exposed via contaminated drinking water
One use of the PBPK models reported here was to predict the maternal, fetal, and infant PFOA concentrations resulting from high maternal exposure to PFOA in the drinking water. For the Little Hocking population, it is unknown for how long the residents were exposed to the contaminated water, but it is thought that some residents may have been exposed for long periods of time (Emmett et al. 2006). For the Arnsberg population, the exposure duration is also unknown, but unlike LH, it is suspected that the exposure was for only on the order of a few years (Holzer et al. 2008). The concentration of PFOA in the drinking water was approximately 7 times lower than that measured in Little Hocking (0.519 ppb vs. 3.55 ppb). The shorter exposure duration and lower PFOA water concentration is not sufficient for the plasma PFOA concentration to reach steady state, so when the gestation model was started, the women from LH had reached steady state but the women from Arnsberg had not.
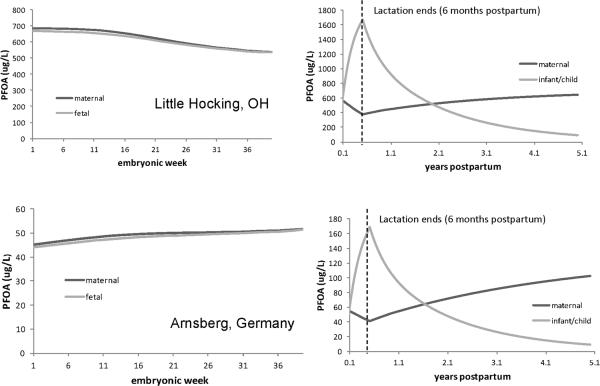
Figure 7 (left panel) shows the model predicted PFOA concentrations in maternal and fetal plasma during gestation for LH (top) and Arnsberg (bottom). For LH, the PFOA plasma concentrations resulting from drinking water exposure resemble the model predicted concentrations for the general population (see Figure 3); both the maternal and fetal concentrations decrease as gestation progresses, and the concentrations are similar in magnitude in the mother and fetus. In the Arnsberg population, like LH, maternal and fetal concentrations are similar in magnitude. However, unlike for LH, where the maternal and fetal concentration decreases as gestation progresses, the concentrations in both mother and fetus in Arnsberg continue to increase over the course of gestation and then level off around 50 ppb towards the end of pregnancy. This is most likely because the mother's plasma concentration did not reach steady state before pregnancy, and since she is still exposed during pregnancy, her plasma concentration is still rising to achieve steady state.
Figure 7.
Predicted PFOA concentrations in maternal, fetal, and infant plasma in Little Hocking (top) and Arnsberg, Germany (bottom) through pregnancy (left panel), lactation, and up to 5 years after birth (right panel).
The predicted maternal plasma concentrations for both communities was higher than the concentrations reported by Emmett et al. (2006) and Holzer et al. (2008) for men and non-pregnant women (448 ppb reported by Emmett vs. a median of 618 ppb predicted by the model for LH during the whole length of gestation and 26.7 ± 13.8 ppb reported by Holzer vs. a median concentration of 48 ppb predicted by the model for Arnsberg during gestation). This could be due to differences in tap water intake; intake rates reported for pregnant women by Ershow et al. (1991) may be higher than those for the LH or Arnsberg populations, resulting in higher model predicted maternal PFOA concentrations. The reported range for the LH water supply is 1.5 – 7.2 ppb, with a median concentration of 3.55 ppb. If a PFOA water concentration in the range of 2 –3 ppb is used in the pregnancy model in combination with the mean tap water intake rate reported by Ershow et al. (1991), the maternal PFOA concentration over the length of pregnancy is within the range of blood PFOA concentrations reported by Emmett et al. (2006) for the LH cohort (interquartile range was 221–576 ug/L). In addition to variability in tap water intake rates and PFOA water concentration, it is likely that there is high variability the serum half-life of PFOA, and in how much exposure comes from drinking water vs. other sources (Loccisano et al. 2011).
For lactation in both communities, the tissue concentrations at the end of embryonic week 39 (9 months) were used as starting tissue concentrations for the lactation model. The reported water concentrations in both communities were used, and the reported tap water intake rate for lactating women reported by Ershow et al. (1991) was used (21.4 mL/kg BW/day). The women breast fed the infants for 6 months postpartum and breast milk was the only exposure source of PFOA for infants. The model-predicted maternal and infant plasma PFOA concentrations are shown in Figure 7 (right panel). The trends observed in the concentrations are similar to what is observed in the data of Fromme et al.(2010): the infant concentration is higher than that of the mother, but then starts to decrease at 6 months postpartum when breastfeeding stops. Fromme et al.(2010) reported low concentrations of PFOA in breast milk relative to plasma concentration, but that the intake of breast milk led to a higher body burden of PFOA in infants than in the mother (Fromme et al. 2010). This phenomenon is also observed in the LH and Arnsberg mothers and infants. During the first 6 months of life, breast milk intake by infants ranges from approximately 400 – 1200 g/day (Dewey et al. 1991), so the rise in PFOA concentration during this time is probably due to exposure from breast milk. Thomsen et al.(2010) showed that even though the concentrations PFCs in breast milk are low compared to the levels in plasma, the infant intake through breast milk is comparable to the dietary intake for Norwegian adults (Thomsen et al. 2010). After 6 months, the intake of breast milk decreases, and thus the infants' plasma concentration decreases, although they most likely receive PFOA from other sources as they begin to eat other foods (Fromme et al. 2010; Llorca et al. 2010). At the same time that the infants' plasma concentrations are rising, the mothers' plasma concentration decreases, indicating that milk is a source of excretion of PFOA. Once breastfeeding stops, the mother's concentration starts to rise again because she is no longer excreting PFOA in milk.
Although the gestation and lactation models appear to yield reasonable predictions of plasma PFOA concentration in pregnant and lactating women, the fetus, and infants in LH and Arnsberg, variability exists in several areas, including drinking water intake rates, variability in other exposure sources, and serum half-lives of PFOA, which all must be considered when using the model to make predictions. When simulating these populations, drinking water was assumed to be the only source of exposure, when in reality, the women and infants are probably receiving additional exposure from food consumed and dust in their environment. However, because these populations had very high levels of PFOA in their water, the contribution from food or dust would be very small compared to what they are exposed to from water and probably would not contribute greatly to their overall blood PFOA concentration in these cases.
Filtration systems have since been installed in both the LH and Arnsberg communities in order to reduce PFOA exposure (and subsequently serum PFOA concentrations are not as high as they once were). However, this exercise demonstrates the predictive ability of the model and where it can be used where data on potentially susceptible individuals is lacking.
Discussion
Gestation and lactation models enable understanding of how physiologic changes during development may affect disposition of chemicals in the mother, fetus, and infant. The models presented here provide a framework for understanding the relationship between maternal exposure and target tissue dose in the fetus or infant. These human PBPK models were used to estimate maternal exposure to PFAA for women in the general population, and based on the estimated exposure the models are also able to simulate PFOA and PFOS concentrations in the fetus, milk, and infant. Limited data are available on the internal dose in the fetus and infant and the models provide indirect estimates in these sensitive populations where risk is a concern.
Gestation and Lactation Models
The agreement between the predicted and observed concentrations of PFOA and PFOS in maternal plasma or milk, or in infant or cord plasma was good, with the ratios of predicted to observed concentrations all within a factor of 2. While this agreement was reassuring, the predicted values were often based on observed values that were temporally close, thus the prediction task was only modestly challenging. The observed concentrations of PFCs in maternal plasma, cord plasma, or milk were reported at time points that were only several days to weeks apart. Because these chemicals have such long half-lives and exposures probably will not change appreciably over time periods of this length, the magnitude of the concentrations in plasma or milk probably will not change greatly; however, if observations had been reported over several years, exposures and thus plasma levels may change, and it may be more difficult to obtain model predictions that are in close agreement with the reported data. Validation data based on paired samples with greater temporal separation or with greater expected variation, such as in women who did or did not breast feed, would support a more informative validation.
Another use of the models is the comparison of internal dose in the pregnant mother and fetus and lactating mother and nursing infant. During gestation, maternal and fetal levels of PFOA are similar and both decrease as gestation progresses. For PFOS, the same trend of decreasing concentration is observed; however, the maternal levels of PFOS are about twice that of what is observed in the fetus (see Figures 5 and 6). During lactation, however, the concentration of PFAA in the infant is equal to or higher than the mother's concentration (which decreases during lactation), indicating that milk is a route of excretion for the mother and also that intake in breast milk is a substantial source of exposure for the infant. This conclusion is in line with the findings of Haug et al. and Thomsen et al.; they found that although the concentrations of PFAA in milk are low relative to blood, the intake from milk by the infant is comparable to dietary intake by adults (Haug et al. 2011; Thomsen et al. 2010).
One application of our human models presented here was to estimate maternal exposure to PFOA and PFOS using observed maternal blood PFAA concentrations from biomonitoring studies in pregnant or lactating women. The women in these studies were considered “general population” in that they were not known to be exposed to unusually high concentrations of PFOA or PFOS (for example, not occupationally exposed or drinking water with higher than average concentrations). Because contributions from various exposure sources were not known, intake for the general population exposure was simulated as a direct input to blood (effectively an IV dose). For PFOA during pregnancy and lactation, estimated maternal exposures ranged from 1.1e−04 to 6.9e−04 ug/kg BW/day; for PFOS, estimated maternal exposures ranged from 1.9e−04 to 4.4e−03 ug/kg BW/day. These estimates are similar to those estimated for the US general population using the adult human model (1.3e−04 – 4.4e−04 ug/kg BW/day for PFOA and 8.5e−04 – 1.8e−03 ug/kg BW/day for PFOS)(Loccisano et al. 2011), and depend on the region in which the women lived or in which years the blood was collected. These estimates were based on PFAA blood levels measured in participants in the NHANES 2003–2004 study and in US Red Cross blood donors (Calafat et al. 2007; Olsen et al. 2008). In 2002, 3M completely ceased production of PFOS-producing chemicals, but in some of the cohorts, the women's blood was sampled over a period of several years, including the years before the phase-out. The overall means or medians reported in these studies may reflect higher past exposure for some women. From the exposures estimated from the models, it appears that pregnant and lactating women do not have appreciably higher exposures to PFOA or PFOS than the general population, even though the dose was scaled to increasing maternal BW. Increased food intake due to increased caloric needs in pregnant and lactating women was accounted for by scaling the daily dose to the increasing maternal BW and then increasing the dose by 25% during the first 6 months after delivery. For studies that had points during pregnancy and lactation, the exposure was estimated for pregnancy, increased by 25% during for the first 6 months postpartum, and decreased back to the pre-pregnancy estimate after 6 months.
For studies with only a plasma or milk observation during lactation, exposure was estimated to yield this observed concentration. Thus, the estimated intake may not reflect recent increases that occur with the onset of lactation. These compounds have a long half-life; it will take some time to reach a steady state plasma concentration. With a higher intake during this short period, the blood concentration probably has not reached steady state. However, this is the best exposure estimate that can be obtained without knowing more specific information about their intake. Because food intake has been suggested to be the main exposure route to PFAA in adult general populations (Fromme et al. 2009; Trudel et al. 2008; Vestergren and Cousins 2009; Tittlemier et al. 2007), food intake by women in these life stages is probably the most important exposure route to consider when estimating exposure.
In LH and Arnsberg the gestation and lactation models yield reasonable predictions of plasma PFOA concentration in pregnant and lactating women, the fetus, and infants, although variability exists in several areas, including drinking water intake rates, other exposure sources, and serum half-lives of PFOA that should be considered when using the model to make predictions. Also, when simulating these populations, drinking water was the only source of exposure. Women and infants may receive additional exposure from food and from dust in their environment; however, because the drinking water levels of PFOA were so high for these populations, the contribution from food would be very small compared to what they are exposed to from water and probably would not contribute much to their overall blood PFOA concentration in these cases. Filtration systems have since been installed in both the LH and Arnsberg communities to reduce PFOA exposure (and subsequently serum PFOA concentrations are not as high as they once were). However, this exercise with only drinking water as an exposure source demonstrates the predictive capacity of the model and situations where it can be used even though data are lacking on potentially susceptible individuals.
A recent study on PFAA intake in the Netherlands, based on analysis of PFAA concentrations in food, estimated the median intake to be 0.2 ng/kg BW/day for PFOA and 0.3 ng/kg BW/day for PFOS (Noorlander et al. 2011). The model-predicted intake of PFOA is in excellent agreement with their estimate; for a 3.8-yr half-life, our median estimated intake was 0.15 ng/kg BW/day. For PFOS, the model-estimated intake is high; our median estimated intake was 0.98 ng/kg BW/day. This discrepancy could arise for several reasons: the intake was estimated for studies where the samples were taken from a range of years, some starting in 1994 (before the phase-out of PFOS/PFOS products). The Noorlander et al. study analyzed food samples from 2009, so it does not reflect the decreasing amount of PFOS exposure in foods over time. The studies used in the current work were conducted in several geographic areas (Noorlander was Netherlands only), and this geographic variation might contribute to the difference. PFOA and PFOS can be created by the breakdown of fluoropolymers, to which people are exposed. Here only direct exposure to PFOA and PFOS was considered; a more complete estimate of exposure would include the contribution of breakdown products in estimates of internal exposure.
The same PFAA-specific parameters that were used in the adult human model were used when extending the adult model to pregnancy and lactation. Good agreement was observed between the model predictions and the observed values for the gestation and lactation models using the same chemical-specific parameters that worked in the adult model. The same PFAA-specific parameters that were used in the adult human model could be used in the pregnancy/lactation models, indicating that tissue partitioning, protein binding, an renal resorption of these chemicals do not change significantly during pregnancy. Modification of the physiology to account for the life stage differences appears to be sufficient to describe the pharmacokinetics of PFAA in pregnant/lactating women, the fetus, and the infant. Possible physiology changes that may alter the PK of PFAAs during these life stages include the fat volume increase and growth of the placenta and fetus during pregnancy and the addition of the milk compartment during lactation. Although PFAAs do not partition appreciably into fat, this compartment serves as an exclusionary volume that may allow for higher concentrations or greater distribution in the plasma or other maternal tissues. The placenta and fetus contain lower concentrations of PFAAs relative to maternal tissues, but they are additional compartments where PFAAs are found, thus lowering concentrations in the mother. In addition, the higher GFR during pregnancy may allow for faster excretion of PFAAs. During lactation, milk serves as a route of excretion for the mother, thus changing the normal clearance kinetics of PFAAs. Maternal exposures estimated during pregnancy allow for good model predictions of cord blood concentrations at delivery and maternal plasma and milk concentrations during lactation. Although some additional physiological variables are altered during pregnancy such as levels of serum PFAA-binding proteins (Perucca and Crema 1982), there are no available data on how this modification may affect PFAA binding during pregnancy, so the non-pregnant values were used.
As in the rat models, placental transfer of chemical was described as a simple diffusion process, and still provided good agreement between predicted cord blood concentrations and available data. There is evidence from in vitro data that human organic anion transporter 4 (OAT4) in the kidney can mediate saturable cellular uptake of PFAA (Yang et al. 2010); there is also evidence that these same transporters are present in the human placenta (Nakagawa et al. 2009). OAT4 is expressed in the fetal-facing membrane and thought to be responsible for the elimination and detoxification of harmful anionic substances from the fetus (Nakagawa et al. 2009; Cha et al. 2000). No data on a Km for hOAT4 in the placenta were found. Based on the fact that OAT4 can transport PFAA and that OAT4 is found in the placenta, this may be a possible transport mechanism for removal of PFAA from the fetus in addition to simple diffusion across the placenta. If this is a detoxification mechanism, saturation of this transporter may mean that PFAA could accumulate in the fetus and the fetus would thus experience increased PFAA exposure.
Unlike the rat lactation models, which had simple diffusion for transport of PFAA from mammary tissue into milk, the human models had flow-limited transport of PFAA into milk. No human data were found for PFAA concentrations in mammary tissue, and the flow-limited description appears to be sufficient. However, limited data were available for estimating the milk:plasma partition coefficient, especially for PFOA. In several of the available studies that measured the concentration of PFAA in milk, the concentration of PFOA was below the detection limit; thus, only a limited number of measurements were available with which to estimate the PFOA milk:plasma partition coefficient. Additional biomonitoring data on matched serum and milk concentrations may improve the lactation model predictions.
For renal resorption, the same parameters used in the adult human model were used for the pregnant and lactating woman. There were no available data on how resorption of PFAA may be altered during pregnancy; however, use of the non-pregnant adult values gave model predictions in good agreement with observed data. Similarly, for the fetus or newborn, there were little available data for PFAA-specific kinetics, such as plasma protein binding or renal reabsorption. Additional data on renal resorption of PFAA in the mother, fetus, and infant (and thus how long the chemicals will be retained in the body) should improve the ability of the models to recapitulate human studies.
Another factor to be considered in risk assessments for PFAA is the difference in renal reuptake and half-lives across species. Renal reabsorption is thought to be responsible for the long half-lives observed in humans and other species (Andersen et al. 2006; Han et al. 2012; Loccisano et al. 2011). The female rat does not express high levels of the renal transporter (Oatp1a1) thought to be responsible for resorption and has a very short half-life. In developmental toxicity studies in rats with PFAAs (PFOA especially), no remarkable findings are observed in the mother or fetuses even at very high maternal doses (a NOAEL of 150 mg/kg/day PFOA was reported for developmental toxicity by Gortner et al. (1981), and maternal toxicity at this level was indicated by a decrease in body weight). This species difference is attributed to the fact that the chemical is eliminated quickly by the female rat and thus adverse effects are not observed. In mice, the half-life of PFAA is longer (12–20 days (Rodriguez et al. 2009)), a pronounced sex difference in clearance is not observed (Lou et al. 2009), and developmental effects (increased neonatal mortality, eye opening delays, and alterations in mammary gland development) have been observed at lower doses than in rats, indicating that mice may be a more sensitive species than rats (Abbott et al. 2007; Lau et al. 2006; White et al. 2007). Currently, it is not clear if the diminished sex difference and slower clearance of PFAA mice (and thus greater sensitivity to PFAA exposure) is related to renal transporters such as Oatp1a1 for PFAA (Tatum-Gibbs et al. 2011). Although the mouse half-life is longer than the female rat half-life and mice appear to be a more sensitive species based on administered dose, both half-lives are much shorter than the multi-year half-life estimated for humans. The chemical is most likely resorbed much more efficiently in humans than in the female rat or in mice, and this difference in renal reuptake and extended half-lives should be considered in risk assessments of PFAAs. Because the PBPK model described here implements renal resorption and can thus account for the differential half-lives across species, use of the model can improve the scientific basis of risk assessment for PFAAs by reducing some of the uncertainty associated with extrapolating rat or mouse data to humans.
Further efforts to refine the model will require more detailed kinetic studies in pregnant and lactating women and infants. The sensitivity analysis identified the model parameters for which additional data would be most helpful. Data on plasma protein binding of PFAA and how that is affected by pregnancy, placental transfer kinetics, and renal resorption of PFAA in pregnant and lactating women, the fetus, and infant would improve model predictions. Also, more data on PFAA concentrations in the fetus and infant would be helpful. The gestation model currently includes a compartment for amniotic fluid, which was included in the rat gestation model because concentration data were available for the rat. Amniotic fluid is a matrix that can be monitored during pregnancy; however, as yet there are no data on PFAA levels in human amniotic fluid. Concentrations of PFAA in the infant after cessation of breastfeeding and intake of solid food begins would be helpful in determining how much exposure infants receive from breast milk vs. other foods. As the child grows and begins to eat other foods, exposure to PFAA will most likely come from additional sources.
These models may help to compare doses and observed effects in animals to the human setting and support risk assessments. By incorporating known developmental physiology changes and chemical transport processes that are capable of describing the kinetics of PFAA, it is possible to estimate the levels of PFAA in susceptible populations where these data are limited or not easily measured, such as the fetus and infant. Given that these populations are exposed to PFAA through food intake, drinking water, etc., an approach to determine their internal dose and how that relates to the observed effects in animals is needed. In this work, a set of models of describing PFAA kinetics that incorporate the necessary physiology and processes to address a variety of species and life stages has been developed.
Supplementary Material
Acknowledgements
The authors would like to thank Dr. John Butenhoff at 3M Company for helpful discussions, comments, and revisions. We would also like to thank Dr. Sofia Salas at Diego Portales University in Chile for generously sharing her data on maternal plasma volume expansion.
Funding Financial support was provided by the 3M and DuPont Companies. This research was also supported in part by the Intramural Research Program of the National Institutes of Health (NIH), National Institute of Environmental Health Sciences (NIEHS).
Abbreviations used
- PFAAs
collectively refers to perfluoroalkyl acid carboxylates and sulfonates
- PFCs
perfluorinated compounds
- PBPK model
physiologically based pharmacokinetic model
- PFOA
perfluorooctanoate
- PFOS
perfluorooctane sulfonate
Footnotes
Conflict of interest The authors declare that there are no conflicts of interest.
References
- Abbott BD, Wolf CJ, Schmid JE, Das KP, Zehr RD, Helfant L, Nakayama S, Lindstrom AB, Strynar MJ, Lau C. Perfluorooctanoic acid induced developmental toxicity in the mouse is dependent on expression of peroxisome proliferator activated receptor-alpha. Toxicol. Sci. 2007;98:571–581. doi: 10.1093/toxsci/kfm110. [DOI] [PubMed] [Google Scholar]
- Alcorn J, McNamara PJ. Pharmacokinetics in the newborn. Adv. Drug Deliv. Rev. 2003;55:667–686. doi: 10.1016/s0169-409x(03)00030-9. [DOI] [PubMed] [Google Scholar]
- Andersen ME, Butenhoff JL, Chang SC, Farrar DG, Kennedy GL, Lau C, Olsen GW, Seed J, Wallace KB. Perfluoroalkyl acids and related chemistries--toxicokinetics and modesl of action. Toxicol. Sci. 2008;102:3–14. doi: 10.1093/toxsci/kfm270. [DOI] [PubMed] [Google Scholar]
- Andersen ME, Clewell HJ, Tan Y-M, Butenhoff JL, Olsen G. Pharmacokinetic modeling of saturable, renal absorption of perfluoroalkylacids in monkeys--probing the determinants of long plasma half-lives. Toxicology. 2006;227:156–164. doi: 10.1016/j.tox.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Apelberg BJ, Witter FR, Herbstman JB, Calafat AM, Halden RU, Needham LL, Goldman LR. Cord serum concentrations of perfluorooctane sulfonate (pfos) and perfluooctanoate (pfoa) in relation to weight and size at birth. Environ. Health. Persp. 2007;115:1670. doi: 10.1289/ehp.10334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartell SM, Calafat AM, Lyu C, Kato K, Ryan PB, Steenland K. Rate of decline in serum pfoa concentrations after granular activated carbon filtration at two public water systems in ohio and west virginia. Environ. Health Persp. 2010;118:222–228. doi: 10.1289/ehp.0901252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beall MH, van den Wijngaard JPHM, van Gemert MJC, Ross MG. Regulation of amniotic fluid volume. Placenta. 2007;28:824–832. doi: 10.1016/j.placenta.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Beesoon S, Webster GM, Shoeib M, Harner T, Benskin JP, Martin JW. Isomer profiles of perfluorochemicals in matched maternal, cord, and house dust samples: Manufacturing sources and transplacental transfer. Environ. Health Persp. 2011;119:1659–1664. doi: 10.1289/ehp.1003265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benskin JP, De Silva AO, Martin LJ, Arsenault G, McCrindle R, Riddell N, Mabury SA, Martin JW. Disposition of perfluorinated acid isomers in sprague-dawley rats; part 1: Single dose. Environ. Toxicol. Chem. 2009;28:542–554. doi: 10.1897/08-239.1. [DOI] [PubMed] [Google Scholar]
- Brown RP, Delp MD, Lindstedt SL, Rhomberg LR, Beliles RP. Physiological parameter values for physiologically based pharmacokinetic models. Toxicol. Ind. Health. 1997;13:407–484. doi: 10.1177/074823379701300401. [DOI] [PubMed] [Google Scholar]
- Calafat AM, Wong L-Y, Kuklenyik Z, Reidy JA, Needham LL. Polyfluoroalkyl chemicals in the u.S. Population: Data from the national health and nutrition examination survey (nhanes) 2003–2004 and comparisons with nhanes 1999–2000. Environ. Health. Persp. 2007;115:1596–1602. doi: 10.1289/ehp.10598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case MT, York RG, Christian MS. Rat and rabbit oral developmental toxicology studies with two perfluorinated compounds. Int. J. Toxicol. 2001;20:101–109. doi: 10.1080/10915810151115236. [DOI] [PubMed] [Google Scholar]
- CDC . Vital and Health Statistics, Series 11, Number 246. DEPARTMENT OF HEALTH AND HUMAN SERVICES, Centers for Disease Control and Prevention, National Center for Health Statistics; 2002. 2000 cdc growth charts for the united states: Methods and development. [PubMed] [Google Scholar]
- CDC . MMWR Morb Mortal Wkly Rep. DEPARTMENT OF HEALTH AND HUMAN SERVICES, Centers for Disease Control and Prevention, National Center for Health Statistics; 2004. Trends in intake of energy and macronutrients--united states, 1971–2000. [Google Scholar]
- Cha SH, Sekine T, Kusuhara H, Yu E, Kim JY, Kim DK, Sugiyama Y, Kanai Y, Endou H. Molecular cloning and characterization of multispecific organic anion transporter 4 expressed in the placenta. J. Biol. Chem. 2000;275:4507–4512. doi: 10.1074/jbc.275.6.4507. [DOI] [PubMed] [Google Scholar]
- Clewell HJ, Gearhart JM, Gentry PR, Covington T, VanLandingham CB, Crump KS, Shipp A. Evaluation of the uncertainty in an oral reference dose for methylmercury due to interindividual variability in pharmacokinetics. Risk Ana. 1999;19:547–558. doi: 10.1023/a:1007017116171. [DOI] [PubMed] [Google Scholar]
- Davies B, Morris T. Physiological parameters in laboratory animals and humans. Pharm. Re. 1993;10:1093–1095. doi: 10.1023/a:1018943613122. [DOI] [PubMed] [Google Scholar]
- DePierre JW. Personal communication. 2009.
- Dewey KG, Heinig MJ, Nommsen LA, Lonnerdal B. Adequacy of energy intake among breast-fed infants in the darling study: Relationships to growth velocity, morbidity, and activity levels. J. Pedia. 1991;119:538–547. doi: 10.1016/s0022-3476(05)82401-1. [DOI] [PubMed] [Google Scholar]
- Dunlop W. Renal physiology in pregnancy. Postgrad. Me. 1979;55:329–332. doi: 10.1136/pgmj.55.643.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlop W. Serial changes in renal hemodynamics during normal human pregnancy. Br. J. Obstet. Gyneco. 1981;88:1–9. doi: 10.1111/j.1471-0528.1981.tb00929.x. [DOI] [PubMed] [Google Scholar]
- Duvekot JJ, Cheriex EC, Pieters FAA, Menheere PPCA, Schouten HJA, Peeters LLH. Maternal volume homeostasis in early pregnancy in relation to fetal growth restriction. Obstet. Gynecol. 1995;85:361–367. doi: 10.1016/0029-7844(94)00417-C. [DOI] [PubMed] [Google Scholar]
- Ehresman DJ, Froehlich JW, Olsen GW, Chang S-C, Butenhoff JL. Comparison of human whole blood, plasma, and serum matrices for the determination of perfluorooctanesulfonate (pfos), perfluorooctanoate (pfoa), and other fluorochemicals. Environ. Res. 2007;103:176–184. doi: 10.1016/j.envres.2006.06.008. [DOI] [PubMed] [Google Scholar]
- Emmett EA, Shofer FS, Zhang H, Freeman D, Desai C, Shaw LM. Community exposure to perfluorooctanoate: Relationships between serum concentrations and exposure sources. J. Occup. Environ. Med. 2006;48:759–770. doi: 10.1097/01.jom.0000232486.07658.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ershow AG, Brown LM, Cantor KP. Intake of tapwater and total water by pregnant and lactating women. Am. J. Public Healt. 1991;81:328–334. doi: 10.2105/ajph.81.3.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fei C, McLaughlin JK, Tarone RE, Olsen J. Perfluorinated chemicals and fetal growth: A study within the danish national birth cohort. Environ. Health. Persp. 2007;115:1677. doi: 10.1289/ehp.10506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenton SE, Reiner JL, Nakayama SF, Delinsky AD, Stanko JP, Hines EP, White SS, Lindstrom AB, Strynar MJ, Petropoulou S-SE. Analysis of pfoa in dosed cd-1 mice. Part 2: Disposition of pfoa in tissues and fluids from pregnant and lactating mice and their pups. Reprod. Toxicol. 2009;27:365–372. doi: 10.1016/j.reprotox.2009.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiserova-Bergerova V. Biological-mathematical modeling of chronic toxicity: Wright-Patterson Air Force Base. Aerospace Medical Research Laboratory; OH: 1975. [Google Scholar]
- Fromme H, Mosch C, Morovitz M, Alba-Alejandre I, Boehmer S, Kiranoglu M, Faber F, Hannibal I, Genzel-Boroviczeny O, Koletzko B, Volkel W. Pre- and postnatal exposure to perfluorinated compounds (pfcs) Environ. Sci. Technol. 2010;44:7123–7129. doi: 10.1021/es101184f. [DOI] [PubMed] [Google Scholar]
- Fromme H, Tittlemier SA, Volkel W, Wilhelm M, Twardella D. Perfluorinated compounds--exposure assessment for the general population in western countries. Int. J. Hyg. Environ. Healt. 2009;212:239–270. doi: 10.1016/j.ijheh.2008.04.007. [DOI] [PubMed] [Google Scholar]
- Gentry PR, Covington T, Andersen ME, Clewell HJ. Application of a physiologically based pharmacokinetic model for isopropanol in the derivation of a reference dose and reference concentration. Reg. Toxicol. Pharmacol. 2002;36:51–68. doi: 10.1006/rtph.2002.1540. [DOI] [PubMed] [Google Scholar]
- Gentry PR, Covington T, Clewell HJ. Evaluation of the potential impact of pharmacokinetic differences on tissue dosimetry in offspring during pregnancy and lactation. Reg. Toxicol. Pharmacol. 2003;38:1–16. doi: 10.1016/s0273-2300(03)00047-3. [DOI] [PubMed] [Google Scholar]
- Gortner EG. Oral teratology study of t-2998coc in rats. Riker Laboratories, Inc.; St. Paul, Mn: 1981. Subsidiary of 3M. [Google Scholar]
- Grasty RC, Bjork JA, Wallace KB, Lau C, Rogers JM. Effects of prenatal perfluorooctane sulfonate (pfos) exposure on lung maturation in the perinatal rat. Birth Defects Res. Part B. 2005;74:405–416. doi: 10.1002/bdrb.20059. [DOI] [PubMed] [Google Scholar]
- Han X, Nabb DL, Russell MH, Kennedy GL, Rickard RW. Renal elimination of perfluorocarboxylates (pfcas) Chem. Res. Toxicol. 2012;25:35–46. doi: 10.1021/tx200363w. [DOI] [PubMed] [Google Scholar]
- Han X, Snow TA, Kemper RA, Jepson GW. Binding of perfluorooctanoic acid to rat and human plasma proteins. Chem. Res. Toxicol. 2003;16:775–781. doi: 10.1021/tx034005w. [DOI] [PubMed] [Google Scholar]
- Hanssen L, Rollin H, Odland JO, Moe MK, Sandanger TM. Perfluorinated compounds in maternal serum and cord blood from selected areas of south africa: Results of a pilot study. J. Environ. Monitor. 2010;12:1355–1361. doi: 10.1039/b924420d. [DOI] [PubMed] [Google Scholar]
- Harada K, Inoue K, Morikawa A, Yoshinaga T, Saito N, Koizumi A. Renal clearance of perfluorooctane sulfonate and perfluorooctanoate in humans and their species-specific excretion. Environ. Res. 2005;99:253–261. doi: 10.1016/j.envres.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Haug L. Personal communication. 2010.
- Haug L, Huber S, Becher G, Thomsen C. Characterization of human exposure pathways to perfluorinated compounds--comparing exposure estimates with biomarkers of exposure. Environ. Int. 2011;37:687–693. doi: 10.1016/j.envint.2011.01.011. [DOI] [PubMed] [Google Scholar]
- Hinderliter PM, Mylchreest E, Gannon SA, Butenhoff JL, Kennedy GL., Jr. Perfluorooctanoate: Placental and lactational transport pharmacokinetics in rats. Toxicolog. 2005;211:139–148. doi: 10.1016/j.tox.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Holzer J, Midasch O, Rauchfuss K, Kraft M, Reupert R, Angerer J, Kleeschulte P, Marschall N, Wilhelm M. Biomonitoring of perfluorinated compounds in children and adults exposed to perfluorooctanoate-contaminated drinking water. Environ. Health Persp. 2008;116:651–657. doi: 10.1289/ehp.11064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue K, Okada F, Ito R, Kato S, Sasaki S, Nakajima S, Uno A, Saijo Y, Sata F, Yoshimura Y, Kishi R, Nakazawa H. Perfluorooctane sulfonate (pfos) and related perfluorinated compounds in human maternal and cord blood samples: Assessment of pfos exposure in a susceptible population during pregnancy. Environ. Health. Persp. 2004;112:1204–1207. doi: 10.1289/ehp.6864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JD, Gibson SJ, Ober RE. Cholestyramine-enhanced fecal elimination of carbon-14 in rats after administration of ammonium [14c]perfluorooctanoate or potassium [14c]perfluorooctanesulfonate. Fund. Appl. Toxicol. 1984;4:972–976. doi: 10.1016/0272-0590(84)90235-5. [DOI] [PubMed] [Google Scholar]
- Karrman A, Ericson I, van Bavel B, Darnerud PO, Aune M, Glynn A, Lignell S, Lindstrom G. Exposure of perfluorinated chemicals through lactation: Levels of matched human milk and serum and a temporal trend, 1996-2004, in sweden. Environ. Health. Persp. 2007;115:226–230. doi: 10.1289/ehp.9491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katakura M, Kudo N, Tsuda T, Hibino Y, Mitsumoto A, Kawashima Y. Rat organic anion transporter 3 and organic anion transporting polypeptide 1 mediate perfluorooctanoic acid transport. J. Health Sci. 2007;53:77–83. [Google Scholar]
- Kato Y, Kuge K, Kusuhara H, Meier PJ, Sugiyama Y. Gender differences in the urinary excretion of organic anions in rats. J. Pharmacol. Exp. Ther. 2002;302:483–489. doi: 10.1124/jpet.102.033878. [DOI] [PubMed] [Google Scholar]
- Kerstner-Wood C, Coward L, Gorman G. Protein binding of perfluorobutane sulfonate, perfluorohexane sulfonate, perfluorooctane sulfonate, and perfluorooctanoate to plasma (human, rat, and monkey) and various human-derived plasma protein fractions. Southern Research Corporation; 2003. [Google Scholar]
- Kim S-K, Lee KT, Kang CS, Tao L, Kannan K, Kim K-R, Kim C-K, Lee JS, Park PS, Yoo YW, Ha JY, Shin Y-S, Lee J-H. Distribution of perfluorochemicals between sera and milk from the same mothers and implications for prenatal and postnatal exposures. Environ. Pollut. 2011a;159:169–174. doi: 10.1016/j.envpol.2010.09.008. [DOI] [PubMed] [Google Scholar]
- Kim S, Choi K, Ji K, Seo J, Kho Y, Park J, Kim S, Park S, Hwang I, Jeon J, Yang H, Giesy JP. Trans-placental transfer of thirteen perfluorinated compounds and relations with fetal thyroid hormones. Environ. Sci. Technol. 2011b;45:7465–7472. doi: 10.1021/es202408a. [DOI] [PubMed] [Google Scholar]
- Krauer B, Krauer F, Hytten FE. Drug disposition and pharmacokinetics in the maternal-placental-fetal unit. Pharmacol. Ther. 1980;10:301–328. doi: 10.1016/0163-7258(80)90085-6. [DOI] [PubMed] [Google Scholar]
- Kudo N, Katakura M, Sato Y, Kawashima Y. Sex hormone-regulated renal transport of perfluorooctanoic acid. Chem.-Biol. Interact. 2002;139:310–316. doi: 10.1016/s0009-2797(02)00006-6. [DOI] [PubMed] [Google Scholar]
- Kudo N, Sakai A, Mitsumoto A, Hibino Y, Tsuda T, Kawashima Y. Tissue distribution and hepatic subcellular distribution of perfluorooctanoic acid at low doses are different from those at high doses. Biol. Pharm. Bull. 2007;30:1535–1540. doi: 10.1248/bpb.30.1535. [DOI] [PubMed] [Google Scholar]
- Kuslikis BI, Vanden Heuvel JP, Peterson RE. Lack of evidence for perfluorodecanoyl- or perfluorooctanoyl-coenzyme a formation in male and female rats. J. Biochem. Toxicol. 1992;7:25–29. doi: 10.1002/jbt.2570070106. [DOI] [PubMed] [Google Scholar]
- Lau C. Perfluoroalkyl acids: Recent activities and research progress. Reprod. Toxicol. 2009;27:209–211. doi: 10.1016/j.reprotox.2009.02.011. [DOI] [PubMed] [Google Scholar]
- Lau C, Anitole K, Hodes C, Lai D, Pfahles-Hutchens A, Seed J. Perfluoroalkyl acids: A review of monitoring and toxicological findings. Toxicol. Sci. 2007;99:366–394. doi: 10.1093/toxsci/kfm128. [DOI] [PubMed] [Google Scholar]
- Lau C, Butenhoff JL, Rogers JM. The developmental toxicity of perfluoroalkyl acids and their derivatives. Toxicol. Appl. Pharmacol. 2004;198:231–241. doi: 10.1016/j.taap.2003.11.031. [DOI] [PubMed] [Google Scholar]
- Lau C, Thibodeaux JR, Hanson RG, Narotsky MG, Rogers JM, Lindstrom AB, Strynar MJ. Effects of perfluorooctanoic acid exposure during pregnancy in the mouse. Toxicol. Sc. 2006;90:510–518. doi: 10.1093/toxsci/kfj105. [DOI] [PubMed] [Google Scholar]
- Lau C, Thibodeaux JR, Hanson RG, Rogers JM, Grey BE, Stanton ME, Butenhoff JL, Stevenson LA. Exposure to perfluorooctane sulfonate during pregnancy in rat and mouse. 2.: Postnatal evaluation. Toxicol. Sci. 2003;74:382–392. doi: 10.1093/toxsci/kfg122. [DOI] [PubMed] [Google Scholar]
- Lindstrom AB, Strynar MJ, Libelo EL. Polyfluorinated compounds: Past, present, and future. Environ. Sci. Technol. 2011;45:7954–7961. doi: 10.1021/es2011622. [DOI] [PubMed] [Google Scholar]
- Liu J, Li J, Liu Y, Chan HM, Zhao Y, Cai Z, Wu Y. Comparison on gestation and lactation exposure of perfluorinated compounds for newborns. Environ. Int. 2011;37:1206–1212. doi: 10.1016/j.envint.2011.05.001. [DOI] [PubMed] [Google Scholar]
- Llorca M, Farre M, Pico Y, Teijon ML, Alvarez JG, Barcelo D. Infant exposure to perfluorinated compounds: Levels in breast milk and commercial baby food. Environ. Int. 2010;36:584–592. doi: 10.1016/j.envint.2010.04.016. [DOI] [PubMed] [Google Scholar]
- Loccisano AE, Campbell JL, Andersen ME, Clewell HJ. Evaluation and prediction of pharmacokinetics in the monkey and human using a pbpk model. Reg. Toxicol. Pharmacol. 2011;59:157–175. doi: 10.1016/j.yrtph.2010.12.004. [DOI] [PubMed] [Google Scholar]
- Loccisano AE, Campbell JL, Butenhoff JL, Andersen ME, Clewell HJ. Evaluation of placental and lactational pharmacokinetics of pfoa and pfos in the pregnant, lactating, fetal, and neontal rat using a physiologically based pharmacokinetic model. Reprod. Toxicol. 2012a;33:468–490. doi: 10.1016/j.reprotox.2011.07.003. [DOI] [PubMed] [Google Scholar]
- Loccisano AE, Campbell JL, Butenhoff JL, Andersen ME, Clewell HJ. Comparison and evaluation of pharmacokinetics of pfoa and pfos in the adult rat using a physiologically based pharmacokinetic model. Reprod. Toxicol. 2012b;33:452–467. doi: 10.1016/j.reprotox.2011.04.006. [DOI] [PubMed] [Google Scholar]
- Lou I, Wambaugh JF, Lau C, Hanson RG, Lindstrom AB, Strynar MJ, Zehr RD, Setzer RW, Barton HA. Modeling single and repeated dose pharmacokinetics of pfoa in mice. Toxicol. Sci. 2009;107:331–341. doi: 10.1093/toxsci/kfn234. [DOI] [PubMed] [Google Scholar]
- Luebker DJ, Case MT, York RG, Moore JA, Hansen KJ, Butenhoff JL. Two-generation reproduction and cross-foster studies of perfluorooctanesulfonate (pfos) in rats. Toxicology. 2005a;215:126–148. doi: 10.1016/j.tox.2005.07.018. [DOI] [PubMed] [Google Scholar]
- Luebker DJ, York RG, Hansen KJ, Moore JA, Butenhoff JL. Neonatal mortality from in utero exposure to perfluorooctanesulfonate (pfos) in sprague-dawley rats: Dose-response, and biochemical and pharmacokinetic parameters. Toxicology. 2005b;215:149–169. doi: 10.1016/j.tox.2005.07.019. [DOI] [PubMed] [Google Scholar]
- Midasch O, Drexler H, Hart N, Beckmann MW, Angerer J. Transplacental exposure of neonates to perfluorooctanesulfonate and perfluorooctanoate: A pilot study. Int. Arch. Occup. Environ. Health. 2007;80:643–648. doi: 10.1007/s00420-006-0165-9. [DOI] [PubMed] [Google Scholar]
- Milman N, Bergholt T, Byg K-E, Eriksen L, Graudal N. Iron status and iron balance during pregnancy. A critical reappraisal of iron supplements. Acta Obstet. Gynecol. Scand. 1999;78:749–757. [PubMed] [Google Scholar]
- Monroy R, Morrison K, Teo K, Atkinson S, Kubwabo C, Stewart B, Foster WG. Serum levels of perfluoroalkyl compounds in human maternal and umbilical cord blood samples. Environ. Res. 2008;108:56–62. doi: 10.1016/j.envres.2008.06.001. [DOI] [PubMed] [Google Scholar]
- Nakagawa H, Hirata T, Terada T, Jutabha P, Miura D, Harada K, Inoue K, Anzai N, Endou H, Inui K, Kanai Y, Koizumi A. Roles of organic anion transporters in the renal excretion of perfluorooctanoic acid. Basic Clin. Pharmacol. Toxicol. 2007;103:1–8. doi: 10.1111/j.1742-7843.2007.00155.x. [DOI] [PubMed] [Google Scholar]
- Nakagawa H, Terada T, Harada KH, Hitomi T, Inoue K, Inui K, Koizumi A. Human organic anion transporter hoat4 is a transporter of perfluorooctanoic acid. Basic Clin. Pharmacol. Toxicol. 2009;105:136–138. doi: 10.1111/j.1742-7843.2009.00409.x. [DOI] [PubMed] [Google Scholar]
- Neville MC, Morton J. Physiology and endocrine changes underlying human lactogenesis ii. J Nutr. 2001;131:3005S–3008S. doi: 10.1093/jn/131.11.3005S. [DOI] [PubMed] [Google Scholar]
- Noorlander CW, van Leeuwen SPJ, t Biesebeek JD, Mengelers MJB, Zeilmaker MJ. Levels of perfluorinate compounds in food and dietary intake of pfos and pfoa in the netherlands. J. Agric. Food Chem. 2011;59:7496–7505. doi: 10.1021/jf104943p. [DOI] [PubMed] [Google Scholar]
- Notarianni LJ. Plasma protein binding of drugs in pregnancy and neonates. Clin. Pharmacokinet. 1990;18:20–36. doi: 10.2165/00003088-199018010-00002. [DOI] [PubMed] [Google Scholar]
- Olsen GW, Burris JM, Ehresman DJ, Froehlich JW, Seacat AM, Butenhoff JL, Zobel LR. Half-life of serum elimination of perfluorooctanesulfonate, perfluorohexanesulfonate, and perfluorooctanoate in retired fluorochemical production workers. Environ. Health Persp. 2007;115:1298–1305. doi: 10.1289/ehp.10009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen GW, Butenhoff JL, Zobel LR. Perfluoroalkyl chemicals and human fetal development: An epidemiologic review with clinical and toxicological perspectives. Reprod. Toxicol. 2009;27:212–230. doi: 10.1016/j.reprotox.2009.02.001. [DOI] [PubMed] [Google Scholar]
- Olsen GW, Mair DC, Church TR, Ellefson ME, Reagen WK, Boyd TM, Herron RM, Medhdizadehkashi Z, Nobiletti JB, Rios JA, Butenhoff JL, Zobel LR. Decline in perfluorooctane sulfonate and polyfluoroalkyl chemicals in american red cross adult blood donors, 2000–2006. Environ. Sci. Technol. 2008;42:4989–4995. doi: 10.1021/es800071x. [DOI] [PubMed] [Google Scholar]
- Ophaug RH, Singer L. Metabolic handling of perfluorooctanoic acid in rats. Proc. Soc. Exp. Biol. Med. 1980;163:19–23. doi: 10.3181/00379727-163-40715. [DOI] [PubMed] [Google Scholar]
- Otten JJ, Hellwig JP, Myers LD, editors. Dietary reference intakes: The essential guide to nutrient requirements. The National Academies Press; Washington, DC: 2006. [Google Scholar]
- Paul AG, Jones KC, Sweetman AJ. A first global production, emission, and environmental inventory for perfluorooctane sulfonate. Environ. Sci. Technol. 2009;15:386–392. doi: 10.1021/es802216n. [DOI] [PubMed] [Google Scholar]
- Perucca E, Crema A. Plasma protein binding of drugs in pregnancy. Clin. Pharmacokinet. 1982;7:336–352. doi: 10.2165/00003088-198207040-00004. [DOI] [PubMed] [Google Scholar]
- Post GB, Cohn PD, Cooper KR. Perfluorooctanoic acid (pfoa), an emerging drinking water contaminant: A critical review of recent literature. Environ. Res. 2012;116:93–117. doi: 10.1016/j.envres.2012.03.007. [DOI] [PubMed] [Google Scholar]
- Prevedouros K, Cousins IT, Buck RC, Korzeniowski SH. Sources, fate, and transport of perfluorocarboxylates. Environ. Sci. Technol. 2006;40:32–43. doi: 10.1021/es0512475. [DOI] [PubMed] [Google Scholar]
- Raymer JH, Michael LC, Studabaker WB, Olsen GW, Sloan CS, Wilcosky T, Walmer DK. Concentrations of perfluorooctane sulfonate (pfos) and perfluorooctanoate (pfoa) and their associations with human semen quality measurements. Reprod. Toxicol. 2012;33:419–427. doi: 10.1016/j.reprotox.2011.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez CE, Setzer RW, Barton HA. Pharmacokinetic modeling of perfluorooctanoic acid during gestaiton and lactation in the mouse. Reprod. Toxicol. 2009;27:373–386. doi: 10.1016/j.reprotox.2009.02.009. [DOI] [PubMed] [Google Scholar]
- Salas SP, Marshall G, Gutierrez BL, Rosso P. Time course of maternal plasma volume and hormonal changes in women with preeclampsia or fetal growth restriction. Hypertension. 2006;47:203–208. doi: 10.1161/01.HYP.0000200042.64517.19. [DOI] [PubMed] [Google Scholar]
- Seacat AM, Thomford PJ, Hansen KJ, Olsen GW, Case MT, Butenhoff JL. Subchronic toxicity studies on perfluorooctanesulfonate potassium salt in cynomolgus monkeys. Toxicol. Sci. 2002;68:249–264. doi: 10.1093/toxsci/68.1.249. [DOI] [PubMed] [Google Scholar]
- Sims EAH, Krantz KE. Serial studies of renal function during pregnancy and the puerperium in normal women. J. Clin. Invest. 1958;37:1764–1774. doi: 10.1172/JCI103769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisson TRC, Lund CJ, Whalen LE, Telek A. The blood volume of infants, 1. The full-term infant in the first year of life. J. Pediat. 1959:163–179. doi: 10.1016/s0022-3476(59)80084-6. [DOI] [PubMed] [Google Scholar]
- Tan Y-M, Clewell HJ, Andersen ME. Time dependencies in perfluorooctylacids disposition in rat and monkeys: A kinetic analysis. Toxicol. Lett. 2008;177:38–47. doi: 10.1016/j.toxlet.2007.12.007. [DOI] [PubMed] [Google Scholar]
- Tatum-Gibbs K, Wambaugh JF, Das KP, Zehr RD, Strynar MJ. Comparative pharmacokinetics of perfluorononanoic acid in rat and mouse. Toxicology. 2011;281:48–55. doi: 10.1016/j.tox.2011.01.003. [DOI] [PubMed] [Google Scholar]
- Thibodeaux JR, Hanson RG, Rogers JM, Grey BE, Barbee BD, Richards JH, Butenhoff JL, Stevenson LA, Lau C. Exposure to perfluorooctane sulfonate during pregnancy in rat and mouse. 1.: Maternal and prenatal evaluations. Toxicol. Sci. 2003;74:369–381. doi: 10.1093/toxsci/kfg121. [DOI] [PubMed] [Google Scholar]
- Thomsen C, Haug LS, Stigum H, Froshaug M, Broadwell SL, Becher G. Changes in concentrations of perfluorinated compounds, polybrominated diphenyl ethers, and polychlorinated biphenyls in norwegian breast-milk during twelve months of lactation. Environ. Sci. Technol. 2010;15:9550–9560. doi: 10.1021/es1021922. [DOI] [PubMed] [Google Scholar]
- Tittlemier SA, Pepper K, Seymore C, Moisey J, Bronson R, Cao XL, Dabeka RW. Dietary exposure of canadians to perfluorinated carboxylates and perfluorooctane sulfonate via consumption of meat, fish, fast foods, and food items prepared in their packaging. J. Agric. Food Chem. 2007;55:3203–3210. doi: 10.1021/jf0634045. [DOI] [PubMed] [Google Scholar]
- Tittlemier SA, Ryan JJ, Van Oostdam J. Presence of anionic perfluorinated organic compounds in serum collected from northern canadian populations. Organohalogen Compounds. 2004;66:3959–3964. [Google Scholar]
- Trudel D, Horowitz L, Wormuth M, Scheringer M, Cousins IT, Hungerbuhler K. Estimating consumer exposure to pfos and pfoa. Risk Anal. 2008;28:251–269. doi: 10.1111/j.1539-6924.2008.01017.x. [DOI] [PubMed] [Google Scholar]
- Vanden Heuvel JP, Kuslikis BI, Rafelghem MJ, Peterson RE. Tissue distribution, metabolism, and elimination of perfluorooctanoic acid in male and female rats. J. Biochem. Toxicol. 1991;6:83–92. doi: 10.1002/jbt.2570060202. [DOI] [PubMed] [Google Scholar]
- Vestergren R, Cousins IT. Tracking the pathways of human exposure to perfluorocarboxylates. Environ. Sci. Technol. 2009;43:5565–5575. doi: 10.1021/es900228k. [DOI] [PubMed] [Google Scholar]
- Vestergren R, Cousins IT, Trudel D, Wormuth M, Scheringer M. Estimating the contribution of precursor compounds in consumer exposure to pfos and pfoa. Chemosphere. 2008;73:1617–1624. doi: 10.1016/j.chemosphere.2008.08.011. [DOI] [PubMed] [Google Scholar]
- Volkel W, Genzel-Boroviczeny O, Demmelmair H, Gebauer C, Koletzko B, Twardella D, Raab U, Fromme H. Perfluorooctane sulphonate (pfos) and perfluorooctanoic acid (pfoa) in human breast milk: Results of a pilot study. Int. J. Hyg. Environ. Health. 2008;211:440–446. doi: 10.1016/j.ijheh.2007.07.024. [DOI] [PubMed] [Google Scholar]
- von Ehrenstein OS, Fenton SE, Kato K, Kuklenyik Z, Calafat AM, Hines EP. Polyfluoroalkyl chemicals in the serum and milk of breastfeeding women. Reprod. Toxicol. 2009;27:239–245. doi: 10.1016/j.reprotox.2009.03.001. [DOI] [PubMed] [Google Scholar]
- Washino N, Saijo Y, Sasaki S, Kato S, Ban S, Konishi K, Ito R, Nakata A, Iwasaki Y, Saito K, Nakazawa H, Kishi R. Correlations between prenatal exposure to perfluorinated chemicals and reduced fetal growth. Environ. Health. Persp. 2009;117:660–667. doi: 10.1289/ehp.11681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver YM, Ehresman DJ, Butenhoff JL, Hagenbuch B. Roles of rat renal organic anion transporters in transporting perfluorinated carboxylates with different chain lengths. Toxicol. Sci. 2009;113:305–314. doi: 10.1093/toxsci/kfp275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White SS, Calafat AM, Kuklenyik Z, Villanueva L, Zehr RD, Helfant L, Strynar MJ, Lindstrom AB, Thibodeaux JR, Wood C, Fenton SE. Gestational pfoa exposure of mice is associated with altered mammary gland development in dams and female offspring. Toxicol. Sci. 2007;96:133–144. doi: 10.1093/toxsci/kfl177. [DOI] [PubMed] [Google Scholar]
- Wosje KS, Kalkwarf HJ. Lactation, weaning, and calcium supplementation: Effects on body composition in postpartum women. Am. J. Clin. Nutr. 2004;80:423–429. doi: 10.1093/ajcn/80.2.423. [DOI] [PubMed] [Google Scholar]
- Yang C-H, Glover KP, Han X. Characterization of cellular uptake of perfluoroctanoate via organic anion-transporting polypeptide 1a2, organic anion transporter 4, and urate transporter 1 for their potential roles in mediating human renl reabsorption of perfluorocarboxylates. Toxicol. Sci. 2010;117:294–302. doi: 10.1093/toxsci/kfq219. [DOI] [PubMed] [Google Scholar]
- Yang C, Glover KP, Han X. Organic anion transporting polypeptide (oatp) 1a1-mediated perfluorooctanoate transport and evidence for a renal reabsorption mechanism of oatp1a1 in renal elimination of perfluorocarboxylates in rats. Toxicol. Lett. 2009;190:163–171. doi: 10.1016/j.toxlet.2009.07.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.