Abstract
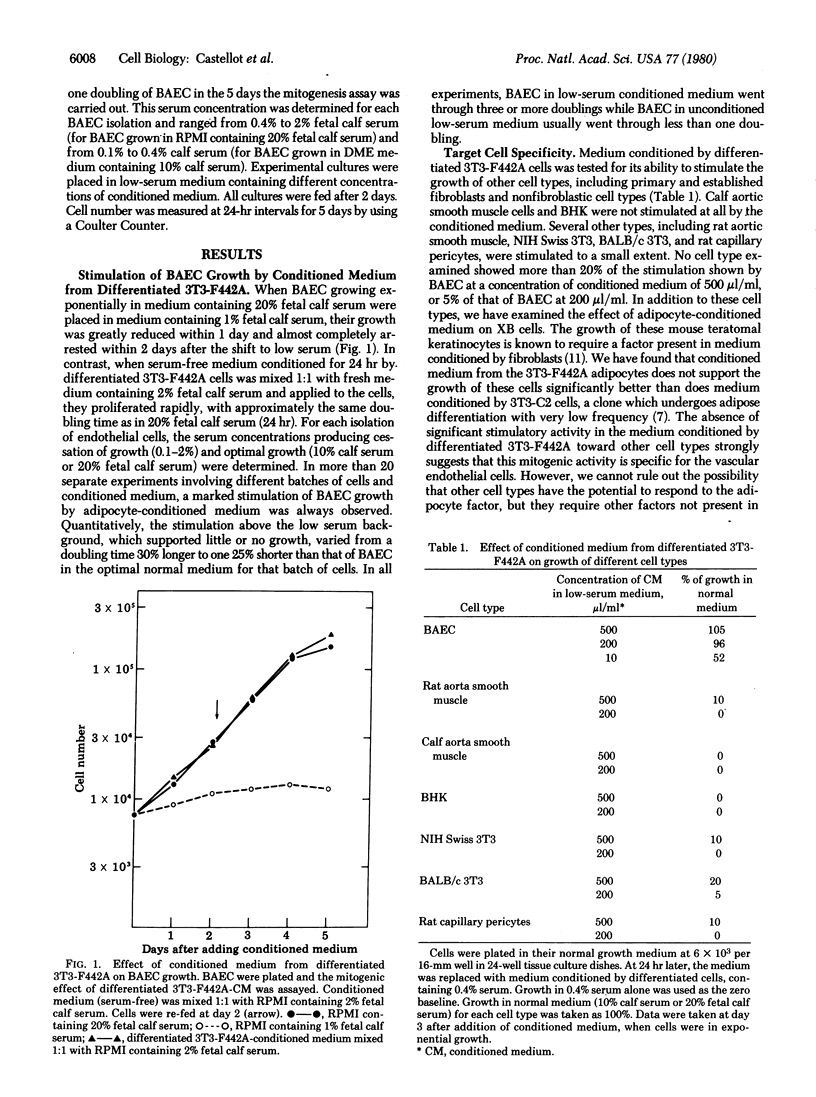
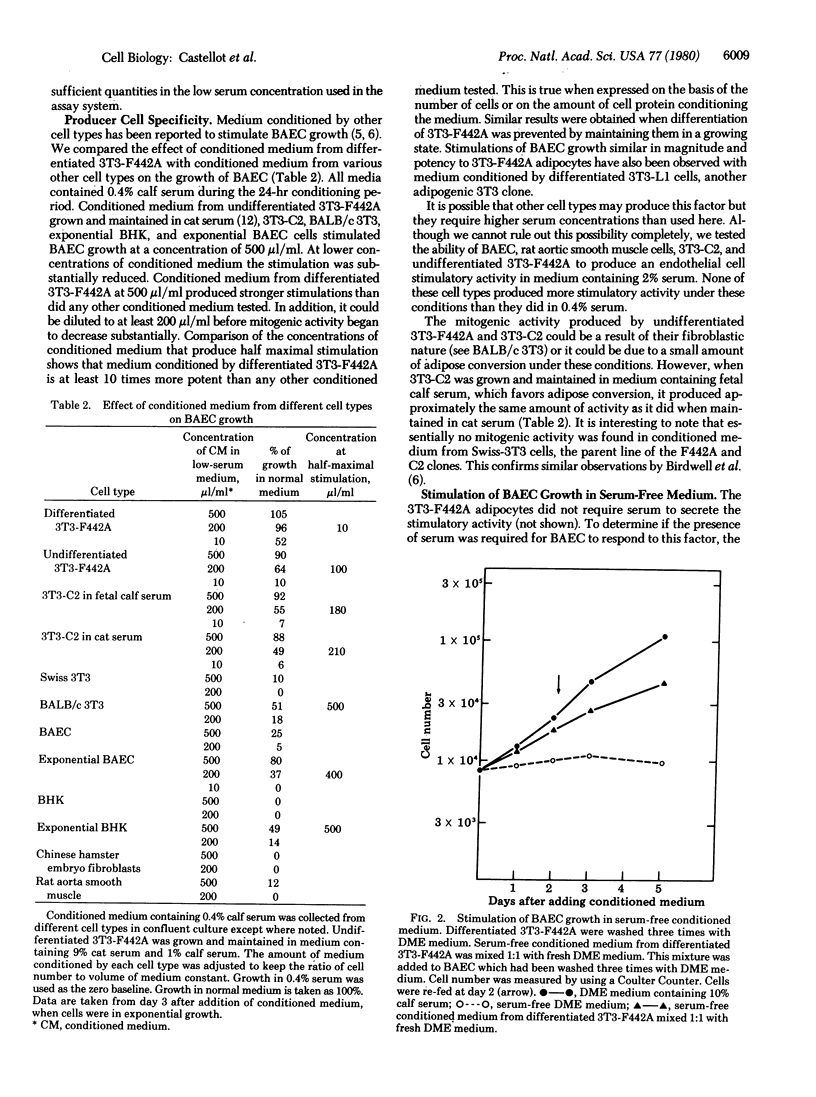
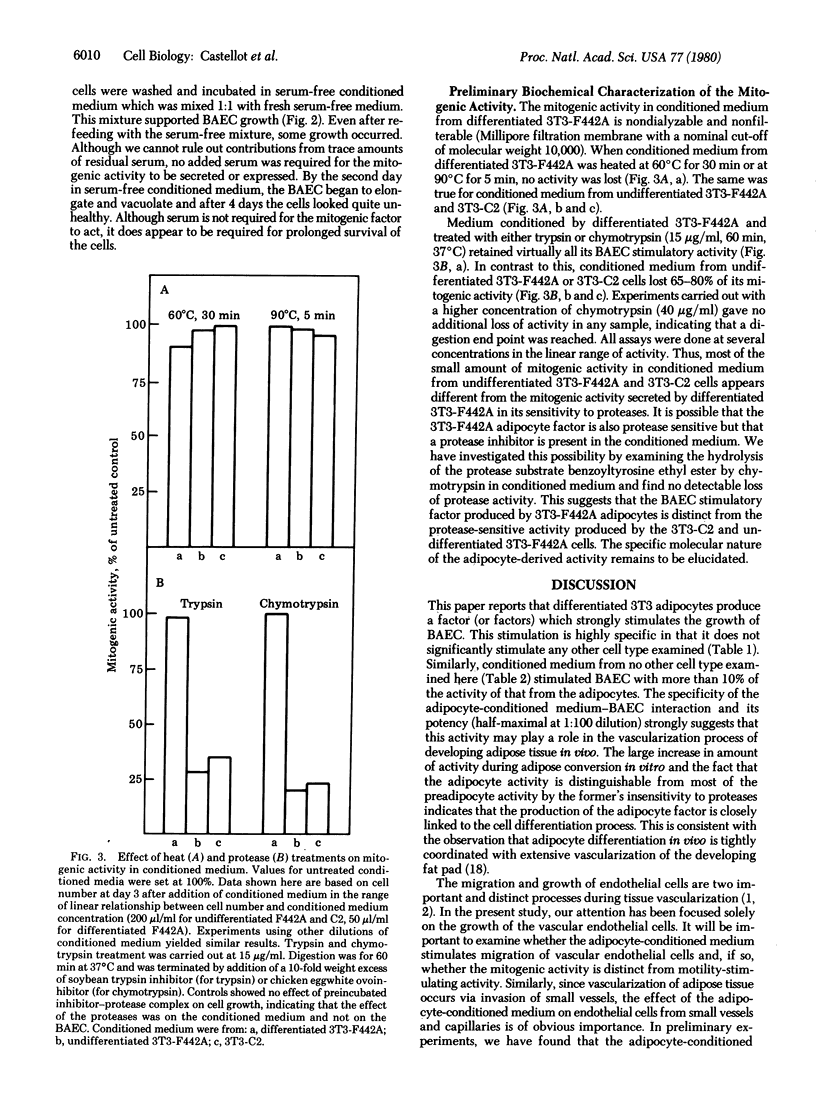
3T3 cells that have undergone adipose differentiation in vitro secrete into the culture medium a potent growth stimulatory activity for bovine aortic endothelial cells. When medium containing 2% fetal calf serum, which does not support significant endothelial cell growth, is conditioned by 3T3-F442A adipocytes, the endothelial cells grow rapidly (doubling time, 24 hr) at a rate equal to the growth rate in 20% fetal calf serum. The potency of the conditioned medium is further shown by the fact that it can be diluted 1:5 with little apparent loss of activity and shows a half-maximal stimulation at 10 microliter/ml. Serum is not required for either the secretion of this mitogen by the adipocytes or its action on the endothelial cells, as shown by the fact that the latter are stimulated to divide in serum-free medium conditioned by the adipocytes. The growth stimulatory activity appears to be specific for vascular endothelial cells in that no other cell type examined, including vascular smooth muscle cells and pericytes, are significantly stimulated by medium conditioned by 3T3-F442A cells. Similarly, medium conditioned by no other cell type examined has more than 10% of the activity of medium conditioned by the adipocytes. The specificity and potency of the adipocyte-derived factor suggest that it may play a role in the vascularization of this tissue during development. Preliminary biochemical analysis indicates that the adipocyte factor is nondialyzable and is not inactivated by heat or proteases. The protease insensitivity distinguishes the adipocyte growth stimulatory activity from the low levels of activity secreted by fibroblasts and preadipocytes, suggesting that the adipocyte mitogen is a product specifically related to the differentiation process.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ausprunk D. H., Folkman J. Migration and proliferation of endothelial cells in preformed and newly formed blood vessels during tumor angiogenesis. Microvasc Res. 1977 Jul;14(1):53–65. doi: 10.1016/0026-2862(77)90141-8. [DOI] [PubMed] [Google Scholar]
- Birdwell C. R., Gospodarowicz D., Nicholson G. L. Factors from 3T3 cells stimulate proliferation of cultured vascular endothelial cells. Nature. 1977 Aug 11;268(5620):528–531. doi: 10.1038/268528a0. [DOI] [PubMed] [Google Scholar]
- Booyse F. M., Sedlak B. J., Rafelson M. E., Jr Culture of arterial endothelial cells: characterization and growth of bovine aortic cells. Thromb Diath Haemorrh. 1975 Dec 15;34(3):825–839. [PubMed] [Google Scholar]
- Brown R. A., Weiss J. B., Tomlinson I. W., Phillips P., Kumar S. Angiogenic factor from synovial fluid resembling that from tumours. Lancet. 1980 Mar 29;1(8170):682–685. [PubMed] [Google Scholar]
- Fenselau A., Mello R. J. Growth stimulation of cultured endothelial cells by tumor cell homogenates. Cancer Res. 1976 Sep;36(9 PT1):3269–3273. [PubMed] [Google Scholar]
- Folkman J., Haudenschild C. C., Zetter B. R. Long-term culture of capillary endothelial cells. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5217–5221. doi: 10.1073/pnas.76.10.5217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkman J., Merler E., Abernathy C., Williams G. Isolation of a tumor factor responsible for angiogenesis. J Exp Med. 1971 Feb 1;133(2):275–288. doi: 10.1084/jem.133.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajdusek C., DiCorleto P., Ross R., Schwartz S. M. An endothelial cell-derived growth factor. J Cell Biol. 1980 May;85(2):467–472. doi: 10.1083/jcb.85.2.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser B. M., D'Amore P. A., Michels R. G., Patz A., Fenselau A. Demonstration of vasoproliferative activity from mammalian retina. J Cell Biol. 1980 Feb;84(2):298–304. doi: 10.1083/jcb.84.2.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gospodarowicz D., Thakral K. K. Production a corpus luteum angiogenic factor responsible for proliferation of capillaries and neovascularization of the corpus luteum. Proc Natl Acad Sci U S A. 1978 Feb;75(2):847–851. doi: 10.1073/pnas.75.2.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green H., Kehinde O. Formation of normally differentiated subcutaneous fat pads by an established preadipose cell line. J Cell Physiol. 1979 Oct;101(1):169–171. doi: 10.1002/jcp.1041010119. [DOI] [PubMed] [Google Scholar]
- Green H., Kehinde O. Spontaneous heritable changes leading to increased adipose conversion in 3T3 cells. Cell. 1976 Jan;7(1):105–113. doi: 10.1016/0092-8674(76)90260-9. [DOI] [PubMed] [Google Scholar]
- HAUSBERGER F. X., WIDELITZ M. M. Distribution of labeled erythrocytes in adipose tissue and muscle in the rat. Am J Physiol. 1963 Apr;204:649–652. doi: 10.1152/ajplegacy.1963.204.4.649. [DOI] [PubMed] [Google Scholar]
- Jaffe E. A., Nachman R. L., Becker C. G., Minick C. R. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest. 1973 Nov;52(11):2745–2756. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuri-Harcuch W., Green H. Adipose conversion of 3T3 cells depends on a serum factor. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6107–6109. doi: 10.1073/pnas.75.12.6107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAuslan B. R., Hoffman H. Endothelium stimulating factor from Walker carcinoma cells. Relation to tumor angiogenic factor. Exp Cell Res. 1979 Mar 1;119(1):181–190. doi: 10.1016/0014-4827(79)90347-1. [DOI] [PubMed] [Google Scholar]
- Polverini P. J., Cotran P. S., Gimbrone M. A., Jr, Unanue E. R. Activated macrophages induce vascular proliferation. Nature. 1977 Oct 27;269(5631):804–806. doi: 10.1038/269804a0. [DOI] [PubMed] [Google Scholar]
- Rheinwald J. G., Green H. Formation of a keratinizing epithelium in culture by a cloned cell line derived from a teratoma. Cell. 1975 Nov;6(3):317–330. doi: 10.1016/0092-8674(75)90183-x. [DOI] [PubMed] [Google Scholar]
- Ross R. The smooth muscle cell. II. Growth of smooth muscle in culture and formation of elastic fibers. J Cell Biol. 1971 Jul;50(1):172–186. doi: 10.1083/jcb.50.1.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zetter B. R. Migration of capillary endothelial cells is stimulated by tumour-derived factors. Nature. 1980 May 1;285(5759):41–43. doi: 10.1038/285041a0. [DOI] [PubMed] [Google Scholar]



