Abstract
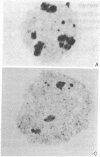
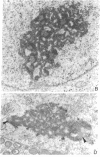
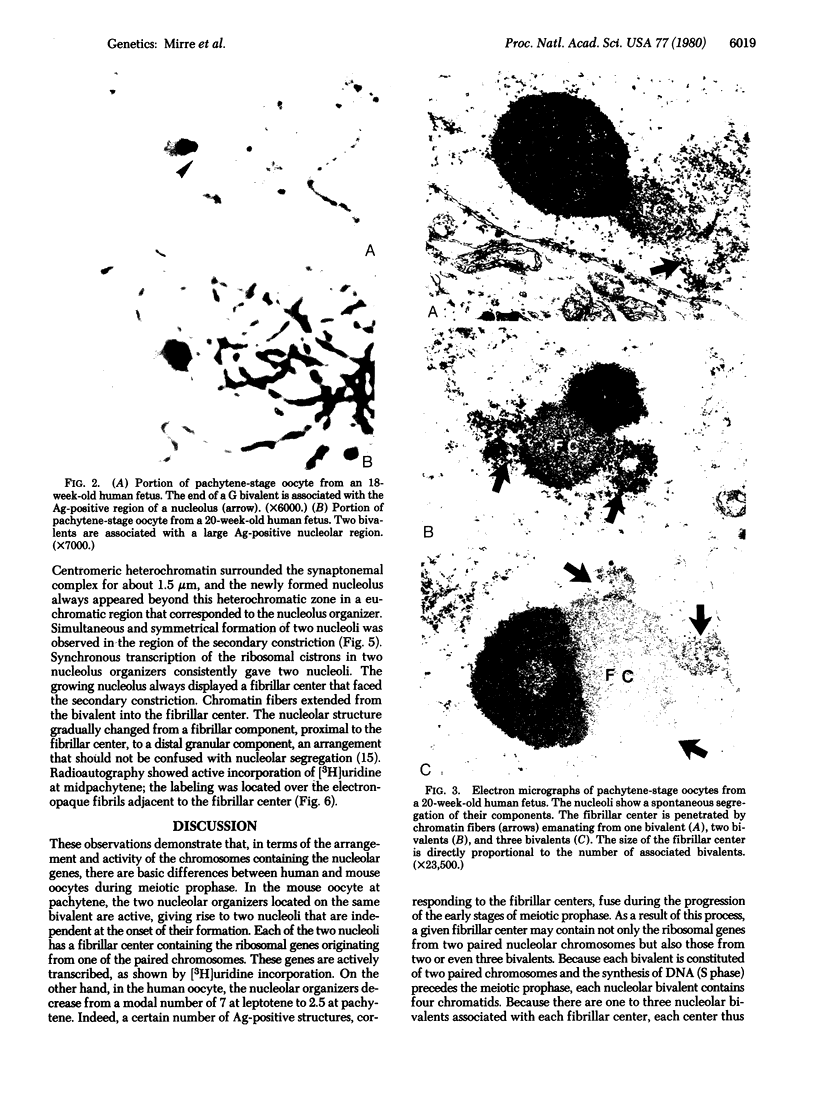
Prophase I meiosis was studied in the human oocyte obtained from 16- to 24-week-old fetuses. Electron microscopy and silver stainihg showed that, at pachytene, the ribosomal genes belonging to several chromosomes are gathered in the same nucleolar fibrillar center, where they are embedded in an argyrophilic protein. The nucleolus showed spontaneous segregation of its components due to temporary inactivation of the ribosomal genes. The fibrillar center, separated from the other nucleolar components, was penetrated as midpachytene by chromatin fibers containing rDNA emanating from one to three nucleolar bivalents. Thus, the ribosomal genes from 4-12 chromatids are temporarily juxtaposed inside the same structure. Such a structural arrangement is completely different from that observed in the pachytene-stage mouse oocyte, where two independent and active nucleoli, each displaying its own fibrillar center, were formed on the bivalents containing paired ribosomal genes. These different structural patterns are correlated with the high frequency of nondisjunction in the human oocyte and the relative infrequency of such in the mouse oocyte. The pattern observed in the human oocyte may be a cause of translocations.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bourgeois C. A., Hernandez-Verdun D., Hubert J., Bouteille M. Silver staining of NORs in electron microscopy. Exp Cell Res. 1979 Oct 15;123(2):449–452. doi: 10.1016/0014-4827(79)90498-1. [DOI] [PubMed] [Google Scholar]
- Boué A., Boué J. Le rôle des anomalies chromosomiques dans les échecs de la reproduction. J Gynecol Obstet Biol Reprod (Paris) 1977 Jan;6(1):5–21. [PubMed] [Google Scholar]
- Boué J. G., Boué A., Lazar P. Les aberrations chromosomiques dans les avortements. Ann Genet. 1967 Dec;10(4):179–187. [PubMed] [Google Scholar]
- Carr D. H. Chromosome anomalies as a cause of spontaneous abortion. Am J Obstet Gynecol. 1967 Feb 1;97(3):283–293. doi: 10.1016/0002-9378(67)90488-7. [DOI] [PubMed] [Google Scholar]
- Evans H. J. The nucleolus, virus infection, and trisomy in man. Nature. 1967 Apr 22;214(5086):361–363. doi: 10.1038/214361a0. [DOI] [PubMed] [Google Scholar]
- FERGUSON-SMITH M. A., HANDMAKER S. D. Observations on the satellited human chromosomes. Lancet. 1961 Mar 25;1(7178):638–640. doi: 10.1016/s0140-6736(61)91655-5. [DOI] [PubMed] [Google Scholar]
- FERGUSON-SMITH M. A., HANDMAKER S. D. THE ASSOCIATION OF SATELLITED CHROMOSOMES WITH SPECIFIC CHROMOSOMAL REGIONS IN CULTURED HUMAN SOMATIC CELLS. Ann Hum Genet. 1963 Nov;27:143–156. doi: 10.1111/j.1469-1809.1963.tb00207.x. [DOI] [PubMed] [Google Scholar]
- FERGUSON-SMITH M. A. THE SITES OF NUCLEOLUS FORMATION IN HUMAN PACHYTENE CHROMOSOMES. Cytogenetics. 1964;3:124–134. doi: 10.1159/000129803. [DOI] [PubMed] [Google Scholar]
- Goessens G. Etude autoradiographique au microscope électronique des nucléoles de cellules tumorales d'Ehrlich. Mise en évidence d'ADN au niveau des centres fibrillaires. C R Acad Sci Hebd Seances Acad Sci D. 1974 Sep 16;279(12):991–993. [PubMed] [Google Scholar]
- Goodpasture C., Bloom S. E. Visualization of nucleolar organizer regions im mammalian chromosomes using silver staining. Chromosoma. 1975 Nov 20;53(1):37–50. doi: 10.1007/BF00329389. [DOI] [PubMed] [Google Scholar]
- Hansmann I., El-Nahass E. Incidence of nondisjunction in mouse oocytes. Cytogenet Cell Genet. 1979;24(2):115–121. doi: 10.1159/000131364. [DOI] [PubMed] [Google Scholar]
- Hartung M., Mirre C., Stahl A. Nucleolar organizers in human oocytes at meiotic prophase I, studied by the silver-NOR method and electron microscopy. Hum Genet. 1979;52(3):295–308. doi: 10.1007/BF00278679. [DOI] [PubMed] [Google Scholar]
- Hartung M., Stahl A. Autoradiographic study of RNA synthesis during meiotic prophase in the human oocyte. Cytogenet Cell Genet. 1978;20(1-6):51–58. doi: 10.1159/000130839. [DOI] [PubMed] [Google Scholar]
- Hofgärtner F. J., Schmid M., Krone W., Zenzes M. T., Engel W. Pattern of activity of nucleolus organizers during spermatogenesis in mammals as analyzed by silver-staining. Chromosoma. 1979 Feb 21;71(2):197–216. doi: 10.1007/BF00292823. [DOI] [PubMed] [Google Scholar]
- Hubbell H. R., Rothblum L. I., Hsu T. C. Identification of a silver binding protein associated with the cytological silver staining of actively transcribing nucleolar regions. Cell Biol Int Rep. 1979 Oct;3(7):615–622. doi: 10.1016/0309-1651(79)90060-2. [DOI] [PubMed] [Google Scholar]
- Jacobs P. A., Morton N. E. Origin of human trisomics and polyploids. Hum Hered. 1977;27(1):59–72. doi: 10.1159/000152852. [DOI] [PubMed] [Google Scholar]
- Jordan E. G., Luck B. T. The nucleolus organizer and the synaptonemal complex in Endymion non-scriptus (L.). J Cell Sci. 1976 Oct;22(1):75–86. doi: 10.1242/jcs.22.1.75. [DOI] [PubMed] [Google Scholar]
- Kajii T., Oama K., Niikawa N., Ferrier A., Avirachan S. Banding analysis of abnormal karyotypes in spontaneous abortion. Am J Hum Genet. 1973 Sep;25(5):539–547. [PMC free article] [PubMed] [Google Scholar]
- Luciani J. M., Devictor-Vuillet M., Gagné R., Stahl A. An air-drying method for first meiotic prophase preparations from mammalian ovaries. J Reprod Fertil. 1974 Feb;36(2):409–411. doi: 10.1530/jrf.0.0360409. [DOI] [PubMed] [Google Scholar]
- Mattei J. F., Mattei M. G., Ayme S., Giraud F. Origin of the extra chromosome in trisomy 21. Hum Genet. 1979 Jan 19;46(1):107–110. doi: 10.1007/BF00278908. [DOI] [PubMed] [Google Scholar]
- Mattei M. G., Mattei J. F., Ayme S., Giraud F. Dicentric Robertsonian translocation in man. 17 cases studied by R,C, and N banding. Hum Genet. 1979;50(1):33–38. doi: 10.1007/BF00295586. [DOI] [PubMed] [Google Scholar]
- Mikkelsen M., Hallberg A., Poulsen H. Maternal and paternal origin of extra chromosome in trisomy 21. Hum Genet. 1976 Apr 15;32(1):17–21. doi: 10.1007/BF00569972. [DOI] [PubMed] [Google Scholar]
- Miller D. A., Tantravahi R., Dev V. G., Miller O. J. Frequency of satellite association of human chromosomes is correlated with amount of Ag-staining of the nucleolus organizer region. Am J Hum Genet. 1977 Sep;29(5):490–502. [PMC free article] [PubMed] [Google Scholar]
- Mirre C., Stahl A. Peripheral RNA synthesis of fibrillar center in nucleoli of Japanese quail oocytes and somatic cells. J Ultrastruct Res. 1978 Sep;64(3):377–387. doi: 10.1016/s0022-5320(78)90045-x. [DOI] [PubMed] [Google Scholar]
- Mirre C., Stahl A. Ultrastructure and activity of the nucleolar organizer in the mouse oocyte during meiotic prophase. J Cell Sci. 1978 Jun;31:79–100. doi: 10.1242/jcs.31.1.79. [DOI] [PubMed] [Google Scholar]
- OHNO S., TRUJILLO J. M., KAPLAN W. D., KINOSITA R. Nucleolus-organisers in the causation of chromosomal anomalies in man. Lancet. 1961 Jul 15;2(7194):123–126. doi: 10.1016/s0140-6736(61)92647-2. [DOI] [PubMed] [Google Scholar]
- Recher L., Whitescarver J., Briggs L. The fine structure of a nucleolar constituent. J Ultrastruct Res. 1969 Oct;29(1):1–14. doi: 10.1016/s0022-5320(69)80052-3. [DOI] [PubMed] [Google Scholar]
- Schwarzacher H. G., Mikelsaar A. V., Schnedl W. The nature of the Ag-staining of nucleolus organizer regions. Electron- and light-microscopic studies on human cells in interphase, mitosis, and meiosis. Cytogenet Cell Genet. 1978;20(1-6):24–39. doi: 10.1159/000130837. [DOI] [PubMed] [Google Scholar]
- Stahl A., Luciani J. M. Nucleoli and chromosomes: their relationships during the meiotic prophase of the human fetal oocyte. Humangenetik. 1972;14(4):269–284. doi: 10.1007/BF00290169. [DOI] [PubMed] [Google Scholar]
- Warburton D., Henderson A. S. Sequential silver staining and hybridization in situ on nucleolus organizing regions in human cells. Cytogenet Cell Genet. 1979;24(3):168–175. doi: 10.1159/000131373. [DOI] [PubMed] [Google Scholar]