Abstract
BACKGROUND
Fibrodysplasia ossificans progressiva is a rare and disabling genetic condition characterized by congenital malformation of the great toes and by progressive heterotopic ossification in specific anatomic patterns. Most patients with fibrodys-plasia ossificans progressiva are misdiagnosed early in life before the appearance of heterotopic ossification and undergo diagnostic procedures that can cause lifelong disability. Recently, the genetic cause of fibrodysplasia ossificans progressiva was identified, and definitive genetic testing for fibrodysplasia ossificans progressiva is now available before the appearance of heterotopic ossification.
METHODS
We recently evaluated 7 children for diagnosis of fibrodysplasia ossificans progressiva before the onset of heterotopic ossification. A medical history, physical examination, and skeletal survey were obtained on all of the patients, as well as clinical genetic testing for the canonical fibrodysplasia ossificans progressiva mutation.
RESULTS
All 7 of the children (4 girls and 3 boys; ages 3 months to 6 years) had congenital malformations of the great toes, but none had radiographic evidence of heterotopic ossification at the time of evaluation. Five of the 7 children had soft tissue lesions of the neck and back, suggestive of early fibrodysplasia ossificans progressiva flare-ups, 3 of whom had undergone invasive diagnostic procedures that exacerbated their condition. Two children had no history or signs of soft tissue swelling or flare-ups. DNA sequence analysis found that all 7 of the children had the recurrent fibrodysplasia ossificans progressiva missense mutation, a single nucleotide substitution (c.617G>A) at codon 206 in the glycine-serine activation domain of activin receptor IA, a bone morphogenetic protein type 1 receptor.
CONCLUSION
Clinical suspicion of fibrodysplasia ossificans progressiva early in life on the basis of malformed great toes can lead to early clinical diagnosis, confirmatory diagnostic genetic testing, and the avoidance of additional harmful diagnostic and treatment procedures. This is the first report of genetic confirmation of fibrodysplasia ossificans progressiva before the appearance of heterotopic ossification. Pediatricians should be aware of the early diagnostic features of fibrodysplasia ossificans progressiva, even before the appearance of heterotopic ossification. This awareness should prompt early genetic consultation and testing and the institution of assiduous precautions to prevent iatrogenic harm.
Keywords: fibrodysplasia ossificans progressiva, heterotopic ossification, malformed great toes, bone morphogenetic protein, ACVR1
Fibrodysplasia ossificans progressiva (FOP) is a rare and disabling genetic disorder that is the most catastrophic disorder of heterotopic ossification in humans. 1–6 Classic FOP is characterized by congenital malformations of the great toes and by progressive heterotopic ossification in specific anatomic patterns, usually beginning in the first decade of life (Fig 1).5 Despite the distinct constellation of defining clinical features, FOP is poorly recognized by most clinicians. Most patients with FOP are misdiagnosed during childhood as having cancer or aggressive fibromatosis after presentation with vascular fibroproliferative soft tissue lesions that appear in the muscles, tendons, and aponeuroses before the definitive appearance of heterotopic bone.7,8 Nearly 90% of FOP patients worldwide are misdiagnosed, and 67% undergo dangerous and unnecessary diagnostic procedures that lead to permanent harm and lifelong disability in >50% of all affected individuals.8
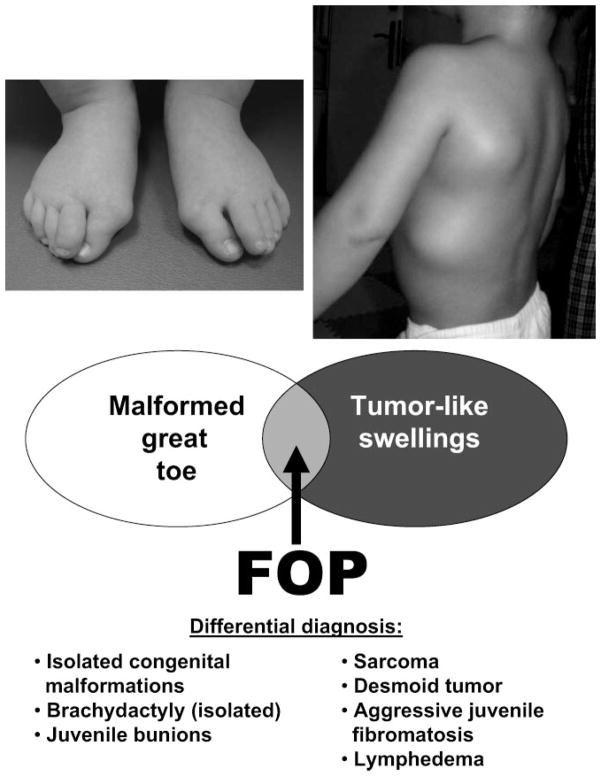
FIGURE 1.
Critical early diagnostic features of FOP. When malformations of great toes (note the hallux valgus deformity) and preosseous tumor-like swellings are present, the diagnosis is FOP. Cartoon illustration of the differential diagnosis. (Adapted with permission from Kitterman JA, Kantanie S, Rocke DM, Kaplan FS. Iatrogenic harm caused by diagnostic errors in fibrodysplasia ossificans progressiva. Pediatrics. 2005;116:).•••
Few clinicians are aware of the classic features of FOP or of the clinical significance of the congenital malformations of the great toes. Even fewer are aware of the specific association of preosseous soft tissue lesions with FOP that are commonly mistaken for sarcomas or aggressive fibromatosis.8 These rapidly forming preosseous soft tissue lesions are often confounding to physicians who are perplexed by their extraordinarily rapid appearance and growth, often over hours.5,7 Most patients who have FOP undergo dangerous, unnecessary, and harmful diagnostic procedures, such as incisional and excisional biopsies and amputations that inevitably exacerbate progression of the disease and lead to permanent lifelong disability.8
The prevalence of FOP is ~1 affected individual in 2 million worldwide, without any racial, ethnic, gender, or geographic predilection.9 Although FOP can be inherited in an autosomal dominant manner with complete penetrance, reproductive fitness is low, and only a few multigenerational families have been identified worldwide.9,10 Most patients develop FOP as a result of a spontaneous new mutation. The genetic mutation causing FOP was recently found to be a recurrent single nucleotide substitution causing a missense mutation in codon 206 (c.617G>A; R206H) in the glycine-serine activation domain of the gene encoding activin receptor IA (ACVR1), a bone morphogenetic protein type 1 receptor.11 This mutation has been found in all of the familial and sporadically affected individuals with classic FOP and is, therefore, one of the most specific disease-causing mutations in the human genome.11
The identification of a single recurrent mutation in patients with classic FOP makes it particularly amenable to clinical genetic testing. The unique and readily detectable congenital toe malformation in FOP before the formation of heterotopic bone provides an opportunity for early diagnosis of this condition. Here we report the first genetic confirmation of FOP before the appearance of heterotopic ossification in 7 children. This finding has important implications for the diagnosis and long-term management of patients with FOP.
METHODS
Patients
Patients were not recruited for this study but were accrued based on physician referral and family inquiries. Patients were evaluated for a possible diagnosis of FOP based on the presence of congenital malformations of the great toes with or without soft tissue swelling but without any evidence of heterotopic ossification. Six patients were referred by their primary physician or consultants; one was brought to see us after her parents read a newspaper article and saw a television documentary on the discovery of the FOP gene. A routine FOP questionnaire was completed by the parents of each child. A routine physical examination was performed, and a radiographic skeletal survey was obtained on each child. Studies were approved by the investigational review board of the University of Pennsylvania.
ACVR1 Mutational Analysis
Polymerase chain reaction (PCR) evaluation and DNA sequence analysis were conducted according to standard techniques from genomic DNA obtained from routine venipuncture. Genomic DNA was screened for mutations in ACVR1 by PCR amplification using primers that flank the recurrent c.617G>A mutation identified in patients with classic FOP, as described previously.11
PCR amplification was performed using Amplitaq Gold enzyme on PE2700 automated thermocyclers (Per-kin Elmer, ABI, Waltham, MA). The PCR volume was 25 μL, with 60 ng of genomic DNA, 10 pmol each of forward and reverse primer, 200 μM of deoxyribonucleotide triphosphate, 1.5 mM of MgCl2, and 10× PCR buffer. The cycling conditions were 94°C for 5 minutes, followed by 30 cycles of 94°C for 30 seconds, 60°C for 30 seconds, and 72°C for 60 seconds, then 1 cycle of 72°C for 5 minutes. The PCR product amplification was verified by agarose gel electrophoresis. The amplicons were subsequently treated with Shrimp Alkaline Phosphatase and Exonuclease (USB, Cleveland, OH) to eliminate unincorporated nucleotides and primers before DNA sequence analysis. The products were purified on a 96-well purification plate (Edge Biosystems, Gaithersburg, MD), dissolved in water, and analyzed by dye terminator cycle sequencing with an ABI 3100 sequencer (ABI) using either the reverse or forward PCR primer.
RESULTS
During the 6-month period (May 2006 to October 2006) after the publication of the discovery of the FOP gene, we clinically evaluated 7 children (4 girls and 3 boys; ages 3 months to 6 years) with congenital malformations of the great toes for the possible diagnosis of FOP. All of the patients were evaluated clinically, with detailed medical histories obtained from observant parents, followed by physical examinations and skeletal surveys (Table 1).
TABLE 1.
Early Diagnosis of FOP
| No. | Age, y | Gender | Congenital Malformation of Great Toes | Soft Tissue Lesions in Characteristic Anatomic Patterns | Additional Radiographic Abnormalitiesa | Radiographic Evidence of Heterotopic Ossification | Invasive Diagnostic Procedure (s) | ACVR1 Mutation (c.617G>A;R206H) |
|---|---|---|---|---|---|---|---|---|
| 1 | 6.00 | M | + | − | + | − | − | + |
| 2 | 3.00 | F | + | + | + | − | + | + |
| 3 | 1.00 | F | + | + | + | − | + | + |
| 4 | 0.25 | M | + | + | + | − | + | + |
| 5 | 2.00 | F | + | + | + | − | − | + |
| 6 | 2.00 | M | + | − | + | − | − | + |
| 7 | 3.00 | F | + | + | + | − | − | + |
M indicates male; F, female.
Data may include any or all of the following radiographic findings, commonly observed in individuals who have FOP: orthotopic fusions of facet joints of the cervical spine; osteochondromas of the proximal medial tibias; short, broad, femoral necks; and/or malformations of the thumbs.
All 7 of the children had the congenital malformations of the great toes that are characteristic of classic FOP, but none had heterotopic ossification at the time of evaluation. Five of the 7 children had soft tissue lesions of the neck and back. The lesions occurred rapidly and spontaneously in 4 children and were associated with a recent viral illness in 1 child. Three of the 5 children with soft tissue lesions had undergone an invasive diagnostic procedure before their referral to us and were diagnosed with benign nodular fascitis, aggressive juvenile fibromatosis, or soft tissue sarcoma on the basis of the histopathologic evaluation of the biopsy specimen. Subsequent to their biopsies, all 3 of the children experienced a rapid postoperative increase in soft tissue swelling at the biopsy site. Two of the 7 children had no signs of soft tissue swellings at the time of evaluation by us. One of those children was referred to us by his parents after they had read a newspaper article and saw a television documentary about FOP. The other child was brought to our attention by an astute geneticist who noted the malformed great toes.
All 7 of the children had been evaluated by orthopedic surgeons for their malformed great toes, but none of the orthopaedic surgeons were aware of the possible association of the toe malformation or soft tissue lesions (when present) with FOP. There was no family history of malformed toes or heterotopic ossification.
Radiographic skeletal surveys were obtained on all 7 of the children and were specifically examined for the presence of malformed thumbs, congenital anomalies of the cervical vertebra, short broad femoral necks, and proximal medial tibial osteochondromas, associated but nonspecific radiographic features commonly seen in patients who have FOP (Fig 2). All 7 of the children had ≥2 associated radiographic features of FOP.
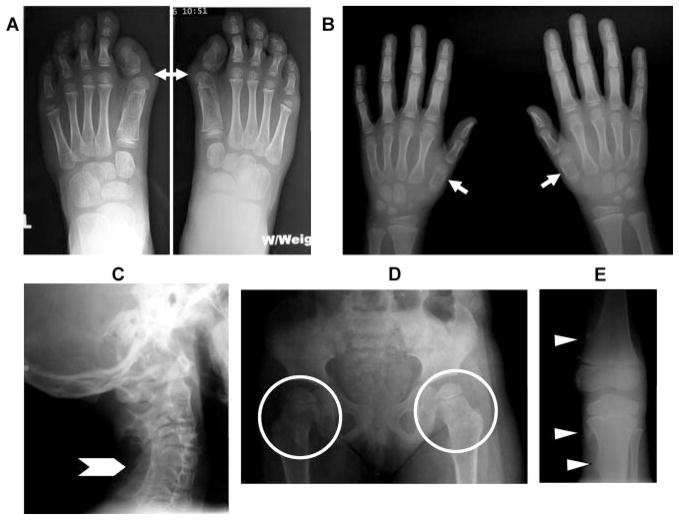
FIGURE 2.
Common radiographic features of FOP. Composite antero-posterior radiographs of the feet (A), hands (B), pelvis (D), knee (E), and lateral radiograph of the cervical spine (C) from a child with FOP. Note the short great toes with malformed first metatarsals, monophalangism, and hallux valgus deformity (A, arrows), short first metatarsals (B, arrows), orthotopic fusion of posterior elements of multiple subaxial vertebrae (C, arrowhead), short, broad femoral necks (D, circled areas), and small sessile distal femoral and proximal medial tibial osteochondromas (E, arrowheads). All are common radiographic features in patients with FOP.
Clinical genetic testing for the canonical FOP mutation was performed on DNA isolated from peripheral blood lymphocytes after venipuncture. All 7 of the children had the classic FOP heterozygous missense mutation (c.617G>A; R206H), a single nucleotide substitution at codon 206 in the glycine-serine activation domain of ACVR1. Representative DNA sequencing chromatograms are shown in Fig 3. No false-negatives were detected; the recurrent c.617G>A mutation was identified in 100% (75 of 75) of patients with classic FOP. No false-positives have been detected by screening of >150 unaffected control subjects.
FIGURE 3.
DNA diagnostic testing of genomic DNA for mutations in the ACVR1 gene. DNA sequence analysis of the ACVR1 gene identified the identical heterozygous mutation (G →A) at cDNA nucleotide position c.617 in all 7 FOP patients. Representative DNA sequencing chromatograms for control and FOP patient genomic DNAs are shown. The upper two panels show DNA sequencing results from complementary DNA sequence strands (forward and reverse sequences) from control DNA (c.617G). The lower two panels show forward and reverse sequence results from patient DNA (c617G →A mutation). In the nucleotide sequence, R =A and G (heterozygous) at position c.617 in the forward direction and Y =C and T at c.617 (complementary base pairing) in the reverse direction.
DISCUSSION
We show here that reliable molecular diagnosis of FOP is possible before the appearance of heterotopic ossification. Pediatricians and pediatric orthopedic surgeons are often among the first physicians to see a child who has congenital malformation of the great toes, a classic, characteristic feature of FOP.5,11 All 7 of the children evaluated in this study had been seen by a pediatric orthopedic surgeon for evaluation of malformed great toes, but none of the orthopedic surgeons recognized the association of malformed great toes with a possible diagnosis of FOP.
Toe malformations may arise from a variety of causes, including isolated congenital malformations, brachydactyly, synostosis and symphalangism syndromes, and juvenile bunions, as well as FOP.5 However, in addition to malformations of the great toes, children with FOP often have other less penetrant skeletal malformations, including malformations of the thumbs (~50% of patients), orthotopic fusions of the posterior elements of cervical spine (~80% of patients), short broad femoral necks (~50% of patients), and proximal medial tibial osteochondromas (~90% of patients).5,12 Although the absence of these skeletal anomalies in a patient with malformed great toes does not exclude the diagnosis of FOP, their presence individually or in combination further strengthens a clinical diagnosis of FOP.
In addition to the congenital toe malformations and other skeletal abnormalities, soft tissue swellings characteristically precede heterotopic ossification.5,13 However, the clinical presentation of rapidly appearing soft-tissue lesions in FOP has led many physicians to consider neoplasm as the underlying diagnosis. Cancer and other tumors were the most common initial diagnosis in a recent study of FOP patients worldwide who had undergone biopsy of a soft tissue lesion.8 All 3 of our patients who underwent excisional biopsies before seeing one of us were diagnosed with sarcoma, aggressive fibromatosis, or nodular fascitis. However, most neoplasms tend to grow in a slowly progressive manner. By contrast, FOP lesions appear suddenly and then change size and shape rapidly, often in a matter of hours, far greater than the rate of change seen in most tumors.7,13
Although some children with FOP develop soft tissue lesions in the first year of life, most children begin to develop these rapidly growing connective tissue lesions (or flare-ups) between 2 and 5 years of age.5,7,13 It is understandable that these flare-ups cause great alarm, first among parents, then among unsuspecting primary practitioners and consultants who are usually unfamiliar with the natural history of classic FOP or with the connection of axial soft tissue swellings and malformations of the great toes. In the 13% of patients worldwide where the correct diagnosis of FOP was made promptly, a parent, primary practitioner, or consultant recognized the diagnostic relationship between the rapidly appearing soft tissue lesions in the neck and back and the presence of malformed great toes.8 In fact, the parents of 1 of our patients had read a newspaper article and saw a television documentary about FOP prompting them to seek direct consultation with one of us after using the Google search engine with the entries “malformed toes and soft tissue swellings” and found the Web site of the International FOP Association (www.ifopa.org).
Although the diagnosis of FOP can be made on the basis of the 2 classic clinical features (malformation of the great toes and progressive heterotopic ossification), identification of the recurrent single nucleotide mutation that causes FOP now provides a level of molecular certainty to the diagnosis at an early stage in the disease process. Late diagnosis or misdiagnosis can lead to dangerous and unnecessary procedures and to catastrophic lifelong disability.8
Definitive molecular diagnosis is now possible for FOP before the appearance of heterotopic ossification and even before the appearance of soft tissue lesions at early stages of disease development when misdiagnosis is most likely to occur. However, the correct diagnosis of FOP cannot be made if it is not first suspected. Clinical awareness of the early preosseous features of FOP will ensure its proper and timely diagnosis. Once suspected, on the basis of congenital malformation of the toes and associated radiographic findings, molecular genetic studies can be performed rapidly to confirm or exclude the diagnosis of classic FOP. Such early diagnostic certainty at the molecular level will allow clinicians and parents together to approach the preventative aspects of FOP in a timely and rational manner.14 Although presymptomatic genetic testing of children has generated much ethical debate,15 there is broad consensus that such testing is justified when it can benefit the child through either medical or preventative measures,16 as is clearly the case in FOP. Once a definitive diagnosis of FOP is made, unambiguous avoidance of deep soft issue trauma, intramuscular injections, preschool intramuscular immunizations, injections into the jaw for dental work, and dangerous and unnecessary lesional biopsies can be instituted.17–20
Given the rare occurrence of FOP in the general population, prenatal testing would not be appropriate for general mutation screening; however, it could be considered within a family in which a child with FOP had been diagnosed previously. Because the great majority of FOP cases are because of de novo spontaneous mutation, and because the c.617G>A ACVR1 mutation appears to be fully penetrant, families with a child who has been diagnosed with FOP and who has 2 unaffected parents are highly unlikely to have a second child with FOP. However, rare cases of gonadal mosaicism for FOP may occur,9,11,21 and prenatal genetic testing could be considered to offer assurance and preparation.
Among all physicians, pediatricians and pediatric orthopaedic surgeons are likely to be consulted to evaluate a child with malformed toes, often soon after birth and usually a year or more before soft tissue swellings are likely to appear. Although all children with malformed great toes will not have FOP, the consideration of FOP must be part of the differential diagnosis. Once soft tissue swelling occurs, the clinical diagnosis is certain.5
Attention to easily identifiable signs and symptoms of FOP early in life, before the appearance of disabling heterotopic ossification, can limit the disability and lifelong harm resulting from diagnostic errors and inappropriate invasive procedures. Although definitive therapies for FOP are not yet available, early clinical diagnosis and genetic testing can provide a high degree of diagnostic certainty that can be used to prevent iatrogenic harm.
What’s Known on This Subject
FOP is the most catastrophic disorder of heterotopic ossification known to humankind and leads to the metamorphosis of soft connective tissue into a second skeleton of heterotopic bone. A recently discovered mutation in a morphogen receptor is responsible for FOP.
What This Study Adds
We report genetic confirmation of FOP before the appearance of heterotopic ossification. Early clinical suspicion of FOP on the basis of malformed great toes can lead to early clinical diagnosis, confirmatory genetic testing, and avoidance of iatrogenic harm.
Acknowledgments
This work was supported in part by the International FOP Association, the Ian Cali FOP Endowment, the Wel-don Family FOP Endowment, the Isaac and Rose Nassau Professorship of Orthopaedic Molecular Medicine, and the National Institutes of Health (R01-AR41916).
We thank Jennifer Richards, Lori Swanson, and Lynn Godmilow for their help in developing the DNA diagnostic test.
Abbreviations
- FOP
fibrodysplasia ossificans progressiva
- ACVR1
activin receptor IA
- PCR
polymerase chain reaction
Footnotes
The authors have indicated they have no financial relationships relevant to this article to disclose.
References
- 1.Cohen RB, Hahn GV, Tabas J, et al. The natural history of heterotopic ossification in patients who have fibrodysplasia ossificans progressiva. J Bone Joint Surg. 1993;75(2):215– 219. doi: 10.2106/00004623-199302000-00008. [DOI] [PubMed] [Google Scholar]
- 2.Rocke DM, Zasloff M, Peeper J, Cohen RB, Kaplan FS. Age and joint-specific risk of initial heterotopic ossification in patients who have fibrodysplasia ossificans progressiva. Clin Orthop Rel Res. 1994;301:243–248. [PubMed] [Google Scholar]
- 3.Kaplan FS, Strear CM, Zasloff MA. Radiographic and scinti-graphic features of modeling and remodeling in the heterotopic skeleton of patients who have fibrodysplasia ossificans progressiva. Clin Orthop Rel Res. 1994;304:238–247. [PubMed] [Google Scholar]
- 4.Mahboubi S, Glaser DL, Shore EM, Kaplan FS. Fibrodysplasia ossificans progressiva. Pediatr Radiol. 2001;31(5):307–314. doi: 10.1007/s002470100447. [DOI] [PubMed] [Google Scholar]
- 5.Kaplan FS, Glaser DL, Shore EM, et al. The phenotype of fibrodysplasia ossificans progressiva. Clin Rev Bone Miner Metab. 2005;3(3– 4):183–188. [Google Scholar]
- 6.Kaplan FS, Glaser DL. Thoracic insufficiency syndrome in patients with fibrodysplasia ossificans progressiva. Clin Rev Bone Miner Metab. 2005;3(3– 4):213–216. [Google Scholar]
- 7.Kaplan FS, Tabas J, Gannon FH, Finkel G, Hahn GV, Zasloff MA. The histopathology of fibrodysplasia ossificans progressiva: an endochondral process. J Bone Joint Surg. 1993;75(2):220–230. doi: 10.2106/00004623-199302000-00009. [DOI] [PubMed] [Google Scholar]
- 8.Kitterman JA, Kantanie S, Rocke DM, Kaplan FS. Iatrogenic harm caused by diagnostic errors in fibrodysplasia ossificans progressiva. Pediatrics. 2005:116. doi: 10.1542/peds.2005-0469. Available at: www.pediatrics.org/cgi/content/full/116/5/e654. [DOI] [PubMed]
- 9.Shore EM, Feldman GJ, Xu M, Kaplan FS. The genetics of fibrodysplasia ossificans progressiva. Clin Rev Bone Miner Metab. 2005;3(3– 4):201–204. [Google Scholar]
- 10.Kaplan FS, McCluskey W, Hahn G, Tabas J, Muenke M, Zasloff MA. Genetic transmission of fibrodysplasia ossificans progressiva. J Bone Joint Surg. 1993;75(8):1214–1220. doi: 10.2106/00004623-199308000-00011. [DOI] [PubMed] [Google Scholar]
- 11.Shore EM, Xu M, Feldman GJ, et al. A recurrent mutation in the BMP type I receptor ACVR1 causes inherited and sporadic fibrodysplasia ossificans progressiva. Nat Genet. 2006;38(5):525–527. doi: 10.1038/ng1783. [DOI] [PubMed] [Google Scholar]
- 12.Schaffer AA, Kaplan FS, Tracy MR, et al. Developmental anomalies of the cervical spine in patients with fibrodysplasia ossificans progressiva are distinctly different from those in patients with Klippel-Feil syndrome. Spine. 2005;30(12):1379–1385. doi: 10.1097/01.brs.0000166619.22832.2c. [DOI] [PubMed] [Google Scholar]
- 13.Pignolo RJ, Suda RK, Kaplan FS. The fibrodysplasia ossificans progressiva lesion. Clin Rev Bone Miner Metab. 2005;3(3– 4):195–200. [Google Scholar]
- 14.Ravitsky V. Disclosing individual genetic results to research participants. Am J Bioethics. 2006;6(6):8–17. doi: 10.1080/15265160600934772. [DOI] [PubMed] [Google Scholar]
- 15.Pelias MK. Genetic testing of children for adult-onset diseases: is testing in the child’s best interest? Mt Sinai J Med. 2006;73(3):605– 608. [PubMed] [Google Scholar]
- 16.Borry P, Stultiens L, Nys H, Cassiman JJ, Dierickx K. Presymptomatic and predictive genetic testing in minors: a systematic review of guidelines and position papers. Clin Genet. 2006;70(5):374–381. doi: 10.1111/j.1399-0004.2006.00692.x. [DOI] [PubMed] [Google Scholar]
- 17.Lanchoney TF, Cohen RB, Rocke DM, Zasloff MA, Kaplan FS. Permanent heterotopic ossification at the injection site after diphtheria-tetanus-pertussis immunizations in children who have fibrodysplasia ossificans progressiva. J Pediatrics. 1995;126(5 pt 1):762–764. doi: 10.1016/s0022-3476(95)70408-6. [DOI] [PubMed] [Google Scholar]
- 18.Luchetti W, Cohen RB, Hahn GV, et al. Severe restriction in jaw movement after routine injection of local anesthetic in patients who have fibrodysplasia ossificans progressiva. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996;81(1):21–25. doi: 10.1016/s1079-2104(96)80141-7. [DOI] [PubMed] [Google Scholar]
- 19.Glaser DL, Rocke DM, Kaplan FS. Catastrophic falls in patients who have fibrodysplasia ossificans progressiva. Clin Orthop Rel Res. 1998;346:110–116. [PubMed] [Google Scholar]
- 20.Glaser DL, Kaplan FS. Treatment considerations for the management of fibrodysplasia ossificans progressiva. Clin Rev Bone Miner Metab. 2005;3(3– 4):243–250. [Google Scholar]
- 21.Janoff HB, Muenke M, Johnson LO, et al. Fibrodysplasia ossificans progressiva in two half sisters: evidence for maternal mosaicism. Am J Med Genet. 1996;61(4):320–324. doi: 10.1002/(SICI)1096-8628(19960202)61:4<320::AID-AJMG4>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]