Abstract
To observe the inhibitory effects of an attenuated S. typhimurium strain carrying IL-2 gene (TPI) on hepatoma cell line (HepG2) and transplanted tumors in mice. TPI, TPG (an attenuated S. typhimurium strain carrying green fluorescent protein gene), and TP (an attenuated S. typhimurium strain) strains were transfected into HepG2 cells. At 48h after transfecting, the transfection rate was 82.58 ± 1.74%. The expression level of IL-2 was (99.5 ± 12.2) ng/1 × 106 cells. Compared with TPG, TP, and normal mouse groups, the proportion of CD4+ T and CD8+ T cells in the blood from the TPI group was higher, the levels of IgM and IgG1 were significantly increased, and the proliferation activity of splenic lymphocyte was significantly stronger. The transplanted tumor weight in the TPI group was significantly smaller than that in the other two groups. The infiltration of lymphocytes increased in the tumor from TPI group mice. TPI was effectively transfected into cancer cells, which expressed the protein of interest. Oral administration of TPI prolonged survival of mice transplanted with hepatoma cell tumours.
1. Introduction
Carcinoma is one of the most deadly diseases of the 21st century. Traditional cancer therapies, such as surgery, radiotherapy, and chemotherapy, are limited in terms of their effectiveness. Many of these therapies have serious side effects, including damage to normal cells (especially hematopoietic and immune cells), anemia, bleeding, infection, gastrointestinal disorders, and alopecia. Furthermore, these therapies do not ensure complete remission and are less likely to be effective against metastatic cancers [1].
Cells stimulated by mitogens produce interleukin-2 (IL-2), which influences the immune system, impedes the viral load, and has an effect on cancer cells [2]. Currently, IL-2 has few side effects and may be used locally to treat various cancers, including gastric cancer [3], renal cancer [4], lymphoma [5], ovarian cancer [6], pancreatic cancer [7], lung cancer [8], melanoma [9], and bladder cancer [10]. However, it is difficult to make the orally administered IL-2 because of its short half-life and expensive cost of production, which is directly related to the complex separation and purification processes. As a result, some researchers have started to focus on IL-2-based gene therapy.
The attenuated S. typhimurium vector has many advantages in gene therapy. It can be used to transfer exogenous genes into cells due to its high invasiveness and low pathogenicity [11]. Additionally, it has the ability to selectively congregate around the tumor tissue [12]. The attenuated S. typhimurium strain Ty21a, which was used as a vector in this study, has been extensively studied with regard to its safety and immunogenicity in human vaccines for many years [13]. The main purpose of our study is to prepare the stable attenuated S. typhimurium strain expressing the IL-2 gene and to observe the effects of this strain on reducing HepG2 burden resulting in prolonged survival in mice. The results suggest that oral administration of attenuated S. typhimurium containing the IL-2 gene has the potential to inhibit hepatic cellular tumors.
2. Material and Methods
2.1. Reagents, Cells, and Animals
The plasmid pcDNA4-green fluorescent protein (pcDNA4-GFP) and PBV220-IL-2 were maintained in our laboratory. The attenuated S. typhimurium Ty21a (strain no. 50218) was purchased from the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China). The human liver carcinoma cell line HepG2 was from ATCC (American Type Culture Collection). RPMI1640 and FBS were purchased from Gibco (Gibco, Grand Island, N.Y.). FITC labeled rat anti-mouse IgM, IgG1, and IgA (IgM-FITC, IgG1-FITC, IgA-FITC) antibodies, and FITC and PE double-labeled rat anti-mouse CD4 and CD8 (CD4-FITC/CD8-PE) antibodies were purchased from BD (BD Biosciences Pharmingen, San Diego, CA, USA). BALB/c mice were purchased from the Animal Centre of Gansu Chinese Medical College and raised under pathogen-free conditions before use. The Gansu Chinese Medical College Animal Studies Committee approved all experimental procedures.
3. Methods
3.1. Construction of Eukaryotic Expression Vector Carrying the IL-2 Gene
The following primer pair was designed according to the human IL-2 gene sequence: the forward primer was 5′-GAATTCCAATGTACAGGATGCACCTCC-3′, and the reverse primer was 5′-CTCGAGAGTTAGTGTTGAGATGATGCT-3′. The PBV220-IL-2 plasmid was used as a template for amplification of IL-2 cDNA by PCR, and the sequencing and accrediting were performed after ligating the PCR product with the T vector (Promega Corporation, 2800 Woods Hollow Road. Madison, WI 5371–5399, USA), which a convenient vector for the cloning of PCR products. The constructed T-IL-2 plasmid and eukaryotic expression vector pcDNA4 were double digested with the enzymes EcoRI and XhoI, ligated and transformed. The positive clone was identified by enzyme digestion and named pcDNA4-IL-2.
3.2. Preparation of Attenuated S. typhimurium Strain TPI (Containing Plasmid Expressing the IL-2 Gene), TPG (Containing Plasmid Expressing the GFP Gene), and TP (Containing Plasmid Vector)
The attenuated S. typhimurium Ty21a strain was inoculated in 50 mL LB medium and cultured for about 6 h (A525 nm was 0.6). It was then collected by centrifugation at 4000 r/min at 4°C. It was washed twice with precooled sterile deionized water, and then suspended in 1 mL precooled sterile deionized water. 0.2 μg pcDNA4-IL-2 plasmid, pcDNA4-GFP, and pcDNA4 plasmid were added into 200 μL of the above-mentioned strain and then transformed by electroporation (Multiporator electroporation system, Eppendoff, Germany). 1 mL SOC culture was added and mixed at 37°C for 45 min. The mixture was spread onto a plate containing ampicillin.
Five colonies were picked from each culture plate and identified. The positive strain containing pcDNA4-IL-2 plasmid (named as TPI) was assessed by PCR for amplification of CMV and IL-2 genes, and EcoRI/XhoI double enzyme digestion after extracting plasmid. PCR for amplification of the CMV gene and fluorescence microscope observation were performed in screening of the positive strain containing pcDNA4-GFP plasmid (named as TPG). PCR for amplification of the CMV gene was performed in screening of the positive strain containing pcDNA4 plasmid (named as TP). The forward primer of cytomegalovirus (CMV) was 5′-CCCAGTACATGACCTTATGGG-3′ and the reverse primer of CMV was 5′-GGAGACTTGGAAATCCCCGT-3′.
3.3. Expression of Target Protein after HepG2 Cell Transfection with TPG and TPI
The HepG2 cells were cultured in 6-well plates at 1 × 106 cells/well and washed twice with RPMI1640 (no antibiotics) the next day. 1 × 108 cfu of TPG and TPI were added into every well and incubated at 37°C for 30 min. After washing twice with serum-free RPMI1640 containing gentamicin (50 mg/L), the HepG2 cells were incubated with RPMI1640 containing 10% FBS at 37°C for 4 h, then administered tetracycline (the final concentration was 10 mg/L) for incubation for an additional 48 h. The expression of green fluorescence protein was observed by fluorescence microscopy, the transfection rate analyzed by flow cytometry, and ELISA was used to detect the expression level of IL-2 protein.
3.4. Repressive Effect of TPI on Transplanted Tumors in Mice
3.4.1. Oral Administration and HepG2 Cell Inoculation
Forty healthy BALB/c mice were randomly divided into four groups (10 mice in each group). TPI group treated with 0.1 mL 1 × 109 cfu/mL TPI; TPG group treated with 0.1 mL 1 × 109 cfu/mL TPG; TP group treated with 0.1 mL 1 × 109 cfu/mL TP; Normal group treated with 0.1 mL 10% NaHCO3. The solvent used was 10% NaHCO3. All mice were fed via a gastric tube once a week for a total of 3 times. Next, 5 × 105 HepG2 cells were suspended in PBS and injected subcutaneously into the left back of mice.
3.5. Detection of Lymphocyte Subsets and Immunoglobulin by Flow Cytometry
One week after finishing oral administration, whole-blood samples were taken from the eyes of the mice in each group. T-cell subsets and immunoglobulin were measured by flow cytometry. In brief, 10 μL CD4-FITC/CD8-PE monoclonal antibodies (BD Biosciences Pharmingen, San Diego, CA, USA), 5 μL IgG1-FITC monoclonal antibody (BD Biosciences Pharmingen, San Diego, CA, USA), 5 μL IgM-FITC monoclonal antibody (BD Biosciences Pharmingen, San Diego, CA, USA), or 2 μL IgA-FITC monoclonal antibody (BD Biosciences Pharmingen, San Diego, CA, USA) were thoroughly mixed with 100 μL whole blood, respectively, and then placed the tubes in the dark for 15 min. Afterwards, 450 μL of erythrocyte lysing solution (BD, Franklin Lakes, NJ, USA) was added and mixed thoroughly followed by another 15 min incubation under the same conditions. At least 1 × 104 cells were counted and analyzed by flow cytometry (CellQuest software) for the percentage of cells that were positive for CD4+ T, CD8+ T, IgM, IgG1, or IgA.
3.6. Detection of Splenic Lymphocyte Proliferation by MTT
One week after finishing oral administration, the spleens of three immunized mice from each group were harvested under aseptic conditions. Spleen single-cell suspensions were prepared and adjusted to 1 × 106 cell/mL using RPMI1640. Then the cells were cultured in 96-well plates (100 μL/well) for detecting proliferation of the splenic lymphocytes by MTT. Different stimulators were added as follows: 5 × 105 cfu/mL TP, 5 × 105 cfu/mL TPI, or 5 × 105 cfu/mL TPG. PHA and LPS served as positive controls. Solvent PBS served as negative control. The OD was detected at 570 nm and the stimulation index (SI, SI = the average OD570 of stimulated group/the average OD570 of the negative control group) was calculated. This was used to scale the lymphocyte proliferation.
3.7. Expression of Target Protein after Oral Administration with TPG and TPI
The second week after HepG2 cell inoculation, three mice in each group were sacrificed for detection of target protein expression. Serum, liver, spleen, kidney, lung, intestine, and cancer tissues of these mice from the TPI and TP groups were collected for detection of IL-2 protein expression by ELISA. The liver, spleen, kidney, lung, intestine, and cancer tissues of mice from the TPG group were prepared for observation of GFP expression by viewing frozen sections under the fluorescence microscope. At the same time, eukaryotic expression vector promoter CMV was detected from the liver, spleen, kidney, lung, intestine, and cancer tissues of mice from three groups by PCR. Thirty cycles of amplification were carried out at 94°C for 30 s, 55°C for 30 s, and 72°C for 60 s, and then extended at 72°C for 7 min.
3.8. Observation, Inoculation, and Tumor Detection of Mice after Oral Administration with TPI
After inoculating HepG2 cells, the general condition of the mice was observed and mouse weight was measured on alternate days. Two weeks after tumor challenge, the tumors were taken from the mice and weighed on an analytical balance and the infiltration of lymphocytes was observed in the tumor. The infiltration of lymphocytes was observed in the tumor section from mice treated with recombinant attenuated S. typhimurium strains by microscopical examination of sections from representative areas that had the biggest number of lymphocytes. Coded specimens were evaluated quantitatively by two investigators unaware of the code. The medium number of lymphocyte was calculated by counting on five microscopic fields with an objective of ×200 (IX 71, Olympus).
3.9. Statistical Analysis
All data are expressed as the mean ± standard deviation (SD) unless otherwise stated. The repeated and dual factorial ANOVA was used to analyze the data, which was obtained after 3 treatments and 6 treatments, from each group. A P < 0.05 was considered significant. SPSS, version 11.5, was used for the statistical analysis.
4. Results
4.1. Preparation of Recombinant Attenuated S. typhimurium Strain TPI, TPG, and TP
The clones of the TPI strain were identified by amplifying the CMV gene and IL-2 gene by PCR. The positive clone showed that both 140 and 490 bp DNA fragments were observed in agarose gel electrophoresis. The clones of TPG strain showed smooth round green translucent S type colonies on the LB plate containing ampicillin. The CMV fragment was amplified from a single colony by PCR. The GFP expression was observed under a fluorescence microscope after smearing a single colony (Figure 1). TP contained the plasmid pcDNA4 and was successfully transformed into Ty21a bacterium.
Figure 1.
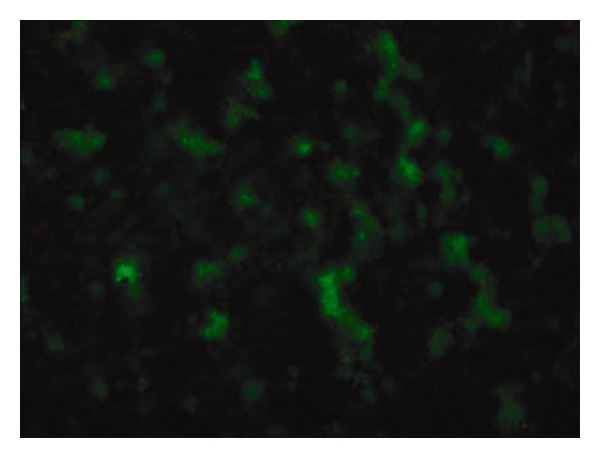
Screening of positive clone of TPG strain (attenuated S. typhimurium containing pcDNA4-GFP plasmid). The green fluorescence of the positive clone was observed under a fluorescence microscope (×1000).
4.2. Expression of the Target Gene in Tumor Cells Mediated by Recombinant Attenuated S. typhimurium TPG and TPI
1 × 108 cfu recombinant attenuated S. typhimurium TPG were transfected into HepG2 cells (1 × 106 cells). The strong green fluorescence of the cells was observed under a fluorescence microscope at both 24 h and 48 h after transfection, and the transfection rate was 82.6 ± 1.7% (Figure 2). 1 × 108 cfu TPI were transfected into HepG2 cells (1 × 106 cells). The expression of IL-2 protein in the supernatant was detected by ELISA at 48 h after transfection. The results showed that 1 × 106 HepG2 cells could express 99.5 ± 12.2 ng IL-2 protein.
Figure 2.
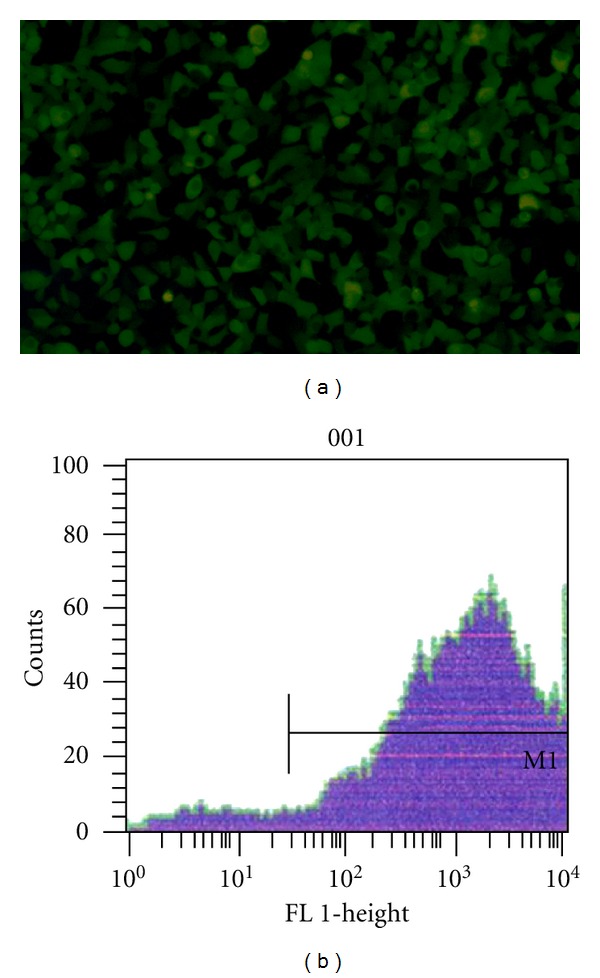
HepG2 cell transfection with TPG for 48 h. (a) The GFP expression was observed under a fluorescence microscope (×100); (b) The transfection rate was detected by flow cytometry.
4.3. Analysis of T Lymphocyte Subsets and Immunoglobulin
To further elucidate the immune effects of the TPI administration, T-cell subsets and immunoglobulin levels were detected in blood samples from each mouse group by flow cytometry one week after finishing administration of the recombinant attenuated S. typhimurium strains. Compared with the TPG, TP, and normal groups, the proportion of CD4+ and CD8+ T cells in the blood of the mice in the TPI group was higher (P < 0.05). In comparison with the normal group, the proportion of CD4+, CD8+ in the blood of the mice in the TP and TPG groups was also markedly increased (P < 0.05). No significant difference was observed in the CD4+/CD8+ ratio from blood samples among the four groups (Table 1). Compared with the normal group, the levels of IgM and IgG1 of the TPI, TP, TPG groups were significantly higher (P < 0.01). Compared with the TP and TPG groups, the levels of IgM and IgG1 of the TPI group were significantly higher (P < 0.01). Although the levels of IgA increased in the TPI group, it was not statistically significant (P > 0.05) (Table 2).
Table 1.
Subset of T lymphocytes in the peripheral blood (mean ± SD, %).
| Groups | CD4+ | CD8+ | CD4+/CD8+ |
|---|---|---|---|
| Normal | 30.90 ± 3.76 | 20.82 ± 5.50 | 1.58 ± 0.51 |
| TP | 35.08 ± 3.02** | 23.67 ± 3.83** | 1.48 ± 0.51 |
| TPG | 33.90 ± 2.22** | 23.42 ± 2.64** | 1.45 ± 0.27 |
| TPI | 46.04 ± 3.52* | 30.30 ± 3.52* | 1.52 ± 0.42 |
*P < 0.05, TPI versus TP, TPG, Normal groups; **P < 0.05, TP versus Normal group, TPG versus Normal group.
TP: attenuated S. typhimurium with the eukaryotic expression vector; TPI: attenuated S. typhimurium with the eukaryotic expression plasmid carrying IL-2 gene; TPG: attenuated S. typhimurium with the eukaryotic expression plasmid carrying GFP gene.
Table 2.
Blood levels of IgM, IgG1 ,and IgA (mean ± SD) (mg/dL).
| Groups | IgM | IgG1 | IgA |
|---|---|---|---|
| Normal | 3.07 ± 0.47 | 0.56 ± 0.15 | 1.28 ± 0.44 |
| TP | 10.52 ± 0.76* | 2.58 ± 0.43* | 2.01 ± 0.73 |
| TPG | 8.56 ± 0.76* | 2.37 ± 0.61* | 1.86 ± 0.12 |
| TPI | 20.37 ± 1.11** | 4.47 ± 0.51** | 2.55 ± 0.34 |
*P < 0.01, TP, TPG versus Normal group; **P < 0.01, TPI versus TP, TPG and Normal group.
TP: attenuated S. typhimurium with the eukaryotic expression vector; TPI: attenuated S. typhimurium with the eukaryotic expression plasmid carrying IL-2 gene; TPG: attenuated S. typhimurium with the eukaryotic expression plasmid carrying GFP gene.
4.4. The Proliferation Effect of Splenic Lymphocyte
The stimulation index (SI) of splenic lymphocytes from each group changed when the splenic lymphocytes were stimulated by LPS, PHA, and other immunogens. Compared with the normal group and TP group, the proliferation activity of splenic lymphocytes was significantly higher in the TPI group (P < 0.05) when these cells were stimulated by TP, TPI, LPS, and PHA. Meanwhile, compared with the normal group, the proliferation activity of splenic lymphocytes significantly rose in the TP group (P < 0.05) when these cells were stimulated by TP, TPI, LPS, and PHA (Table 3).
Table 3.
Proliferation effect of splenic lymphocyte (mean ± SD).
| Groups | TP | TPI | LPS | PHA |
|---|---|---|---|---|
| Normal | 1.141 ± 0.021 | 1.170 ± 0.025 | 1.100 ± 0.063 | 1.074 ± 0.036 |
| TP | 1.266 ± 0.039* | 1.254 ± 0.095* | 1.273 ± 0.046* | 1.282 ± 0.051* |
| TPI | 1.374 ± 0.035∗# | 1.402 ± 0.033∗# | 1.353 ± 0.053∗# | 1.383 ± 0.025∗# |
*P < 0.05, TPI versus Normal group, TP versus Normal group; #P < 0.05, TPI versus TP group.
TP: attenuated S. typhimurium with the eukaryotic expression vector; TPI: attenuated S. typhimurium with the eukaryotic expression plasmid carrying IL-2 gene.
4.5. Expression of Target Protein after Oral Administration with TPG, TP, and TPI
At the second week after HepG2 cell inoculation, the liver, spleen, kidney, lung, intestine, and tumor tissues of mice from the normal group and the TPG group were taken to prepare for observation of green fluorescent protein (GFP) expression by viewing frozen sections under the fluorescence microscope. Although the green fluorescence was observed in liver, kidney, lung, spleen, intestine, and tumor tissues of mice in the TPG group, it was strongest in the spleen and tumor (Figure 3). No GFP was expressed in the normal group tissues.
Figure 3.
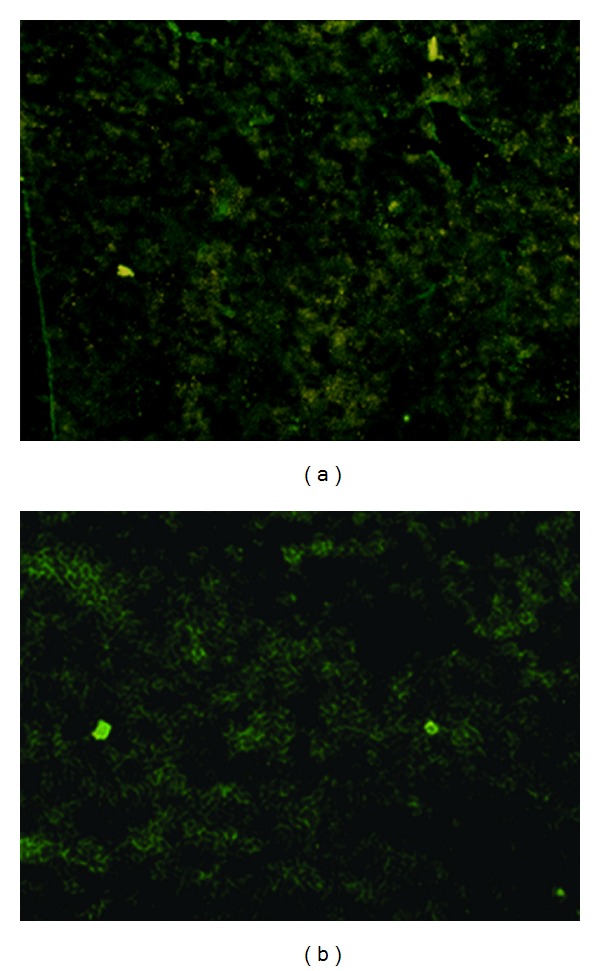
The expression of green fluorescence protein in the tissues of mice after TPG administration (×100). (a) spleen tissue; (b) tumor tissue.
At the same time, three mice in the TPI, TP, and Normal groups were sacrificed for detecting target protein expression. Serum, liver, spleen, kidney, lung, intestine, and tumor tissues of these mice from the TPI and TP groups were collected for detecting IL-2 protein expression by ELISA. Compared with the TP group and normal group, the expression levels of IL-2 protein were significantly higher in the TPI group (P < 0.01). Meanwhile, compared with the normal group, the IL-2 expression level was significantly higher in the TP group (P < 0.01) (Table 4).
Table 4.
Expression level of IL-2 protein in tissues of mice (mean ± SD).
| Groups | Serum (ng/mL) | Liver (ng/g) | Spleen (ng/g) | Kidney (ng/g) | Lung (ng/g) | Intestine (ng/g) | Tumor (ng/g) |
|---|---|---|---|---|---|---|---|
| TPI | 19.17 ± 10.6* | 17.89 ± 1.0* | 57.22 ± 6.19* | 15.17 ± 1.41* | 16.31 ± 1.46* | 15.32 ± 1.56* | 36.72 ± 7.55* |
| TP | 8.48 ± 0.34* | 8.78 ± 0.36** | 10.34 ± 0.64* | 7.89 ± 0.45** | 7.50 ± 0.44** | 7.38 ± 0.43** | 9.30 ± 0.51** |
| Normal | 2.06 ± 0.10 | 2.56 ± 0.12 | 3.12 ± 0.21 | 1.97 ± 0.10 | 1.69 ± 0.10 | 2.08 ± 0.11 | 3.37 ± 0.13 |
*P < 0.01, TPI versus TP and Normal group; **P < 0.01, TP versus Normal group.
TP: attenuated S. typhimurium with the eukaryotic expression vector; TPI: attenuated S. typhimurium with the eukaryotic expression plasmid carrying IL-2 gene.
4.6. Distribution of the Eukaryotic Expression Vector Promoter CMV
At the second week after HepG2 cell inoculation, the eukaryotic expression vector promoter CMV was detected from the liver, spleen, kidney, lung, intestine, and tumor tissues of mice from three groups by PCR. CMV was regarded as the promoter of the eukaryotic expression vector pcDNA4. The result demonstrated that a CMV fragment with the length of 140 bp was amplified from the liver, spleen, kidney, lung, intestine, and tumor tissues of mice from each group. The figure showed the result of TPI group (Figure 4).
Figure 4.
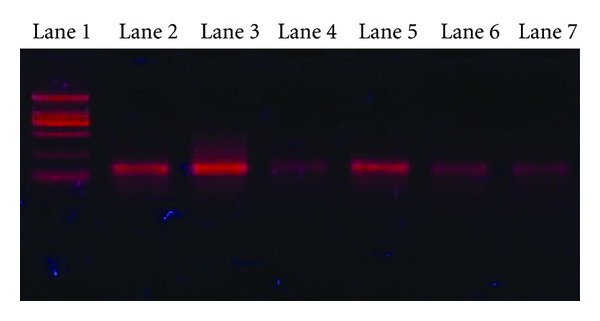
Distribution of the eukaryotic expression vector promoter CMV in tissues of TPI group mice treated with recombinant attenuated S. typhimurium strains. Lane 1: DNA marker DL2000; lane 2: liver tissue as template; lane 3: spleen tissue as template; lane 4: kidney tissue as template; lane 5: tumor tissue as template; lane 6: lung tissue as template; lane 7: intestine tissue as template.
4.7. Inhibitory Effect of TPI on Transplanted Tumors in Mice
After inoculating HepG2 cells for one week, the mice in the TPG and TP groups became thin and appeared to have diarrhea. In the TPI group, the symptoms of the mice were not as severe. Compared with mice in the normal group, the body weight of the mice in the TPI, TP, and TPG groups was slightly decreased, but no significant difference or other obvious toxic effects were observed in mice from each group. Two weeks after tumor challenge, the transplanted tumor weight in the TPI group (0.54 ± 0.12 g) was significantly smaller than that in the other two groups (TP group: (1.27 ± 0.09)g; TPG group (1.23 ± 0.13)g, P < 0.01). Compared with the TP and TPG groups, the infiltration of lymphocytes increased in the tumor from the TPI group mice (Figure 5).
Figure 5.
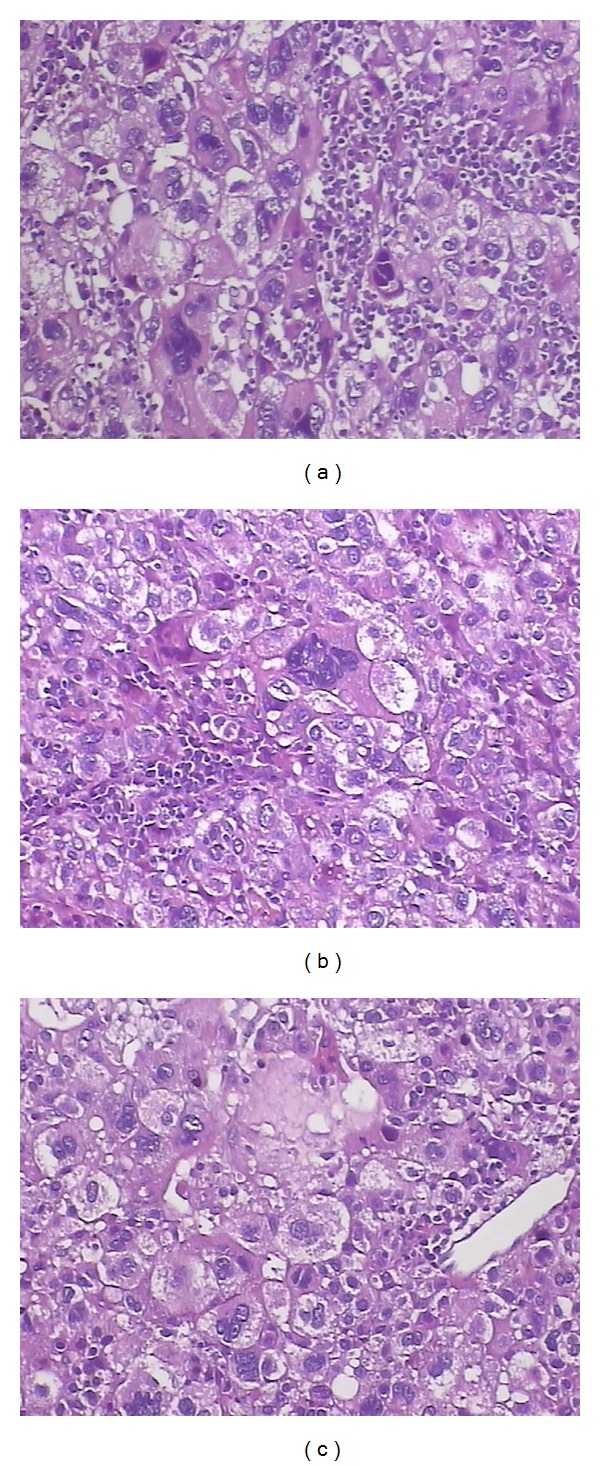
The infiltration of lymphocytes in the tumor from the mice (×100, H.E staining). The infiltration of lymphocytes was observed in the tumor section from mice treated with recombinant attenuated S. typhimurium strains. It showed that the number of lymphocytes was obviously more in the tumor from TPI group (a) than in the tumor from TP (b) and TPG group (c).
5. Discussion
Cytokine gene therapy of cancer is one of the most promising biological therapies for cancer therapeutics. IL-2 cytokine can be used alone or in combination with other cytokines and is currently being adopted in the current research of gene therapy for cancer therapeutics. IL-2 cytokine, produced by T cells, is one of the most important lymphokines that may adjust the immune system and inhibit cancer and infection. Its ability to induce various immunocytes to proliferate and help them differentiate into T lymphocytes, B lymphocytes, natural killer cells (NK), and macrophages could have multiple uses. IL-2 could increase the IL-2R expression and the secretion function of macrophages. And enhance the killing effect of macrophages on pathogenic microorganisms. It also has the ability to induce the cells to secrete other cytokines, such as IFN-γ, TNF, and IL-4, and subsequently promote cell locomotion and reinforce cell-cell contact [13, 14]. But Recent evidence reported that an IL-2 monoclonal antibody administration could inhibited metastasis of murine osteosarcoma [15].
The attenuated S. typhimurium is a Gram-negative intracellular facultative anaerobe transmitted via the fecal-oral route. Upon oral administration, S. typhimurium migrate to the gastrointestinal tract and colonize Peyer's patches of the small intestine, and enters the lymphatics and bloodstream by infecting inactivated macrophage and dendritic cells. The bacteria then escapes to systemic tissues including the liver, spleen, and lungs. The attenuated S. typhimurium is very compatible to serve as the vector for oral gene medicines because it may selectively congregate around the cancer tissue. Under the hypoxic environment, intermediate metabolites that are needed by the bacterium are present around the cancer tissue [16]. Furthermore, the cancer tissue produces immunosuppression factors, which inhibit the clearance of the bacterium by the immune system [17]. The kinetics of colonization of various tissues, examined in both mice and monkeys, provide some insight into to the mechanism of preferential accumulation of bacteria in tumors [18]. The mechanisms responsible for the initial infection of tumor, followed by preferential accumulation of bacteria to high levels, compared with normal tissues, are not completely understood [19]. Differences between tumor and normal vasculature and blood flow patterns could be involved in favoring entry into and entrapment of bacteria within tumors. Similarly, bacteria may be delivered to tumor by macrophages or monocytes, or perhaps other cells invaded by bacteria.
In this study, we found that the target protein could be strongly expressed in the spleen and the transplanted tumor after administrating the attenuated S. typhimurium carrying the target gene. It demonstrated that the recombinant attenuated S. typhimurium containing a eukaryotic expression plasmid could transform the exogenous gene into the tissues of mice and be expressed in these tissues. Attenuated S. typhimurium is one of the prevalent bacterial vectors in gene therapy and oral DNA vaccines, which could transfer multiple exogenous gene into host cells effectively [20, 21]. In vivo, live attenuated S. typhimurium strains penetrate into the intestinal epithelial barrier via M cells and macrophages, and reach the Peyer's patches, gain access to the gut-associated lymphoid tissue, migrate to the mesenteric lymph nodes (MLNs) and disseminate to the liver and spleen [22].
The malignant tumor produces many immunosuppression factors which are able to suppress the cellular immunity of the patient. Cellular immunity is one of the most important elements in antineoplastic processes, and the T lymphocytes play an important role in this process. The CD4+ cell is one of the main response cells of immune response which may produce lymphokines and differentiate into response-assisted cells (TH), killer T cells, or B cells. It may also promote humoral immune function to inhibit tumorigenesis. We found that the proportion of CD4+ and CD8+T cells in the blood of the mice in the TPI group was higher than that in the TPG, TP, and normal groups. The levels of IgM and IgG1 of the TPI group was significantly higher than that in the TPG, TP, and normal groups. The splenic lymphocyte proliferation activity of the TPI group increased under the condition of IL-2, LPS and PHA, compared with the TP group. In other words, the oral administration of the IL-2 gene mediated by attenuated S. typhimurium could really improve the level of cellular immunity and humoral immunity, thereby inhibiting the proliferation and pervasion of cancer cells.
When the cell supernatant containing IL-2 protein was directly administered to HepG2 cells, the effect on proliferation and migration was minimal. IL-2 protein mainly produced a marked effect on cancer in vivo by the various immune pathways. We found in the mice that were orally immunized with the attenuated S. typhimurium containing IL-2 gene after cancer inoculation, the transplanted tumor weight was much smaller compared to the TP and TPG group. And the infiltration of lymphocytes increased in the tumor from the TPI group mice. It suggests that the immune effect of TPI may be stronger than that of TP. The oral attenuated S. typhimurium recombined IL-2 (TPI) gene could really prevent the proliferation of cancer. And recent evidence suggested that oral administration of an attenuated S. typhimurium to express a truncated human IL-2 resulted in a maximal increase of splenic NK cell populations and decreased metastatic hepatic tumor burden. Its safety and the immunogenicity are investigated [23]. The oral gene therapy mediated by attenuated S. typhimurium could develop into a novel anticancer method, and it may encourage the research of other cancer vaccines.
6. Conclusion
The main purpose of our study is to prepare the stable attenuated S. typhimurium strain expressing the IL-2 gene (TPI) and to observe the effects of this strain on reducing HepG2 burden resulting in prolonged survival in mice. The results showed that oral administration of TPI has the potential to inhibit hepatic cellular tumors in mice. TPI was targetly transfected into cancer cells, which expressed the protein of interest, and resulted in hepatic cellular tumor regression. It implies that TPI may be a potential gene therapy regent to inhibit hepatic cellular tumors.
Conflict of Interests
The authors declare that they have no conflict of interests.
Acknowledgments
The authors thank the Animal Center in Gansu College of Traditional Chinese Medicine for mice feeder. They thank the support of the grant from the Postdoctoral Fund (no. 200603901920).
References
- 1.Yennurajalingam S, Dev R, Walker PW, Reddy SK, Bruera E. Challenges associated with spinal opioid therapy for pain in patients with advanced cancer: a report of three cases. Journal of Pain and Symptom Management. 2010;39(5):930–935. doi: 10.1016/j.jpainsymman.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 2.Teng MWL, Von Scheidt B, Duret H, Towne JE, Smyth MJ. Anti-IL-23 monoclonal antibody synergizes in combination with targeted therapies or IL-2 to suppress tumor growth and metastases. Cancer Research. 2011;71(6):2077–2086. doi: 10.1158/0008-5472.CAN-10-3994. [DOI] [PubMed] [Google Scholar]
- 3.Hara M, Nakanishi H, Tsujimura K, et al. Interleukin-2 potentiation of cetuximab antitumor activity for epidermal growth factor receptor-overexpressing gastric cancer xenografts through antibody-dependent cellular cytotoxicity. Cancer Science. 2008;99(7):1471–1478. doi: 10.1111/j.1349-7006.2008.00821.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dudek AZ, Yee RT, Manivel JC, Isaksson R, Yee HO. Carbonic anhydrase IX expression is associated with improved outcome of high-dose interleukin-2 therapy for metastatic renal cell carcinoma. Anticancer Research. 2010;30(3):987–992. [PubMed] [Google Scholar]
- 5.Kasprzak A, Spachacz R, Wachowiak J, Stefańska K, Kaczmarek E, Zabel M. Tissue expression of interleukin 2 (IL-2) and IL-2 receptor (IL-2Rα/CD25) in non-Hodgkin B-cell lymphomas in children correlations with clinical data. Journal of Pediatric Hematology/Oncology. 2010;32(6):462–471. doi: 10.1097/MPH.0b013e3181e33f9c. [DOI] [PubMed] [Google Scholar]
- 6.Zhang X, Feng J, Ye X, Yao Y, Zhou P, Chen X. Development of an immunocytokine, IL-2-183B2scFv, for targeted immunotherapy of ovarian cancer. Gynecologic Oncology. 2006;103(3):848–852. doi: 10.1016/j.ygyno.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 7.Uggeri F, Caprotti R, De Grate L, et al. Short-term preoperative IL-2 immunotherapy in operable pancreatic cancer: a randomized study. Hepato-Gastroenterology. 2009;56(91-92):861–865. [PubMed] [Google Scholar]
- 8.Ridolfi L, Bertetto O, Santo A, et al. Chemotherapy with or without low-dose interleukin-2 in advanced non-small cell lung cancer: results from a phase III randomized multicentric trial. International Journal of Oncology. 2011;39(4):1011–1017. doi: 10.3892/ijo.2011.1099. [DOI] [PubMed] [Google Scholar]
- 9.Ridolfi L, Fiorentini G, Guida M, et al. Multicentre, open, noncomparative Phase II trial to evaluate the efficacy and tolerability of fotemustine, cisplatin, alpha-interferon and interleukin-2 in advanced melanoma patients. Melanoma Research. 2009;19(2):100–105. doi: 10.1097/CMR.0b013e328328f7ec. [DOI] [PubMed] [Google Scholar]
- 10.Li YG, Wang ZP, Tian JQ, et al. Dendritic cell transfected with secondary lymphoid-tissue chemokine and/or interleukin-2 gene-enhanced cytotoxicity of t-lymphocyte in human bladder tumor cell s in vitro. Cancer Investigation. 2009;27(9):909–917. doi: 10.3109/07357900802375746. [DOI] [PubMed] [Google Scholar]
- 11.Antony GK, Dudek AZ. Interleukin 2 in cancer therapy. Current Medicinal Chemistry. 2010;17(29):3297–3302. doi: 10.2174/092986710793176410. [DOI] [PubMed] [Google Scholar]
- 12.Den Otter W, Jacobs JJL, Battermann JJ, et al. Local therapy of cancer with free IL-2. Cancer Immunology, Immunotherapy. 2008;57(7):931–950. doi: 10.1007/s00262-008-0455-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kilhamn J, Lundin SB, Brevinge H, Svennerholm AM, Jertborn M. T- and B-cell immune responses of patients who had undergone colectomies to oral administration of Salmonella enterica serovar Typhi Ty21a vaccine. Clinical and Diagnostic Laboratory Immunology. 2003;10(3):426–430. doi: 10.1128/CDLI.10.3.426-430.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang Z, Dauer DJ, Ha GK, Lewis MH, Petitto JM. Interleukin-2 deficiency-induced T cell autoimmunity in the mouse brain. Neuroscience Letters. 2009;463(1):44–48. doi: 10.1016/j.neulet.2009.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kohyama K, Sugiura H, Kozawa E, et al. Antitumor activity of an interleukin-2 monoclonal antibody in a murine osteosarcoma transplantation model2012. Anticancer Research. 2012;32(3):779–782. [PubMed] [Google Scholar]
- 16.Wilson M, Davies NP, Brundler MA, McConville C, Grundy RG, Peet AC. High resolution magic angle spinning 1H NMR of childhood brain and nervous system tumours. Molecular Cancer. 2009;8, article 6 doi: 10.1186/1476-4598-8-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fox AC, Robertson CM, Belt B, et al. Cancer causes increased mortality and is associated with altered apoptosis in murine sepsis. Critical Care Medicine. 2010;38(3):886–893. doi: 10.1097/CCM.0b013e3181c8fdb1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sznol M, Lin SL, Bermudes D, Zheng LM, King I. Use of preferentially replicating bacteria for the treatment of cancer. Journal of Clinical Investigation. 2000;105(8):1027–1030. doi: 10.1172/JCI9818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clairmont C, Bermudes D, Low KB, et al. VNP20009, a genetically modified Salmonella typhimuriumml: anti-tumor efficacy, toxicology, and biodistribution in preclinical models. Clinical Cancer Research. 1999;5, supplement:p. 102. [Google Scholar]
- 20.Fu W, Lan H, Li S, et al. Synergistic antitumor efficacy of suicide/ePNP gene and 6-methylpurine 2′-deoxyriboside via Salmonella against murine tumors. Cancer Gene Therapy. 2008;15(7):474–484. doi: 10.1038/cgt.2008.19. [DOI] [PubMed] [Google Scholar]
- 21.Qi H, Li YH, Zheng SB. Oral gene therapy via live attenuated Salmonella leads to tumor regression and survival prolongation in mice. Journal of Southern Medical University. 2006;26(12):1738–1741. [PubMed] [Google Scholar]
- 22.Jones BD, Falkow S. Salmonellosis: host immune responses and bacterial virulence determinants. Annual Review of Immunology. 1996;14:533–561. doi: 10.1146/annurev.immunol.14.1.533. [DOI] [PubMed] [Google Scholar]
- 23.Sorenson B, Banton K, Augustin L, et al. Safety and immunogenicity of Salmonella typhimurium expressing C-terminal truncated human IL-2 in a murine model. Biologics. 2009;4:61–73. doi: 10.2147/btt.s9121. [DOI] [PMC free article] [PubMed] [Google Scholar]


